Abstract
BACKGROUND/OBJECTIVES
It has been shown that vitamin A supplementation has different effects on skeletal health and the antioxidant system. Deficiency or excess of this vitamin can lead to health problems. Vitamin A can work as either an antioxidant or prooxidant depending on its concentration. The present study was conducted to investigate the effects of different doses of vitamin A supplementation on the antioxidant system in rats.
MATERIALS/METHODS
Forty Spargue-Dawley male rats were divided into four groups according to the dose of vitamin A received: 0 (A0), 4,000 (A1), 8,000 (A2), and 20,000 (A3) IU retinyl palmitate/kg diet. After a feeding period of 4 wks, lipid peroxide levels, glutathione concentration, antioxidant enzyme activities, and vitamins A and E concentrations were measured. Histopathological changes were observed in rat liver tissue using an optical microscope and transmission electron microscope.
RESULTS
Lipid peroxide levels in plasma were significantly decreased in the A1 and A2 groups compared to the A0 rats. Erythrocyte catalase and hepatic superoxide dismutase activities of the A2 group were significantly higher than those of the A0 group. Hepatic glutathione peroxidase activity was significantly lower in the A3 group compared to the other groups. Total glutathione concentrations were significantly higher in the A1 and A2 groups than in the A0 group. Histological examination of liver tissue showed that excessive supplementation of vitamin A might lead to lipid droplet accumulation and nuclear membrane deformation.
CONCLUSIONS
These results indicate that appropriate supplementation of vitamin A might have a beneficial effect on the antioxidant system in rats.
Keywords: Vitamin A supplementation, vitamin A deficiency, antioxidant, rat
INTRODUCTION
As the dietary pattern of South Koreans changes, excess energy and unbalanced nutrient intake has become a serious problem that can cause chronic diseases including obesity, hypertension, and cardiovascular diseases [1]. According to the results of the 2010 Korea National Health and Nutrition Examination Survey, a high portion of people consumed lower amounts of calcium, iron, potassium, riboflavin, and vitamin A than the recommended levels reported in the Korea Dietary Reference Intakes 2010 [2]. About 38% of South Koreans consume less than the estimated average requirement of vitamin A while about 3.6% of South Koreans consume a higher quantity of this vitamin than the tolerable upper intake level which can induce toxicity in the body [3]. These findings indicate that imbalanced consumption of vitamin A poses a health problem for the South Korean population.
Low intake of vitamin A is still a significant nutritional problem in underdeveloped countries [4]. de Oliveros et al. [5] showed that fatty acid oxidation in the mitochondria can be elevated in rats fed a vitamin A-deficient diet and affect lipid metabolism in heart tissue. Another study indicated that low intake of vitamin A may lead to a reduction of retinoid X receptor (RXR) α, β expression in heart tissue, which can interfere with the regulation of oxidative stress [6]. A recent review study reported that insufficient intake of vitamin A is associated with poor bone health [7]. On the other hand, excessive accumulation of vitamin A in the body can cause an imbalance of reactive nitrogen species and subsequently increase the risk of cardiovascular disease [8]. In infants and children, excessive vitamin A consumption may cause leukemia, necrotizing vasculitis, and melancholia [9,10].
According to a study by Pasquali [11] that focused on excessive intake of vitamin A in rats, high levels of this vitamin increased thiobarbituric acid reactive species (TBARS) levels and promoted the accumulation of lipid peroxides in lung tissue. High levels of vitamin A supplementation can also increase oxidative stress through protein carbonylation, oxidation of protein thiol groups, and lipid peroxidation in a rat model [11]. In another previous investigation, supplementation of vitamin A at clinical levels increased oxidative damage as well as antioxidant enzymes activities in rat liver [12].
Few studies have been performed to clarify the connection between vitamin A intake levels and the antioxidant defense systems. Therefore, more research is needed to determine the appropriate amount of vitamin A that can optimize the antioxidant defense systems. The current study was thus carried out to investigate the effects of various levels of vitamin A supplementation on the antioxidant system in rats.
MATERIALS AND METHODS
Experimental animals and diets
Male Sprague-Dawley rats (70-90 g, n = 40) 4 wk old were purchased from Central Lab. Animal Inc., (Seoul, South Korea). During the first week, the animals were housed individually in stainless steel cages with a 12-h light/dark cycle at 25 ± 3℃ and 40 ± 15% humidity. The rats were given a control pellet diet (Central Lab. Animal Inc.) and water ad libitum during the acclimatization period.
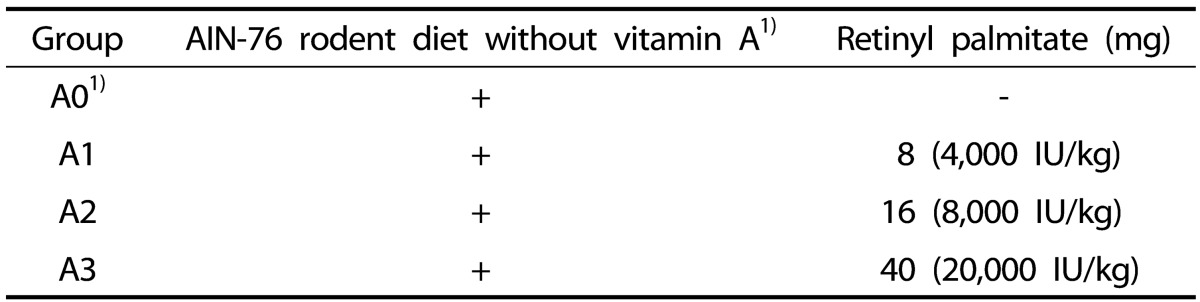
The animals were divided into four groups of 10 rats each after 1 wk of acclimatization. The experimental diet (Research Diets, Inc., New Brunswick, NJ, USA) based on the AIN-76A diet without vitamin A was used as a basal diet for the study. The four groups of rats were fed the A0, A1, A2, and A3 experimental diets mixed with 0 IU/kg, 4,000 IU/kg, 8,000 IU/kg, and 20,000 IU/kg diet of vitamin A, respectively, for 4 wk (Table 1). Recommended vitamin A level in AIN-76A rodent diet is 4,000 IU/kg diet. Based on the level, 8,000 IU/kg (two times) and 20,000 IU/kg (five times) were used to observe whether these amounts of vitamin A have a different effect in rat model, and thus to present the effect of supplementary levels to induce antioxidant system in rats. Vitamin A was supplied as retinyl palmitate (Sigma, St.Louis, MO, USA).
Table 1. Classification of experimental groups.
1) Research Diets, Inc., New Brunswick, NJ, USA
The amount of feed intake was recorded three times per week and body weight was measured every week; these data were used to calculate the feed efficiency ratio (FER). This animal experiment was performed according to the guidelines provided by the Experimental Animal Care Ethical Committee of Yeungnam University and approved by the committee (2012-001).
Sample preparation
At the end of the 4-wk feeding period, the rats were anesthetized with diethyl ether after an overnight fast, and blood samples were collected from the inferior vena cava using heparin-treated syringes (Jung Rim Medical Co., Seoul, South Korea). Plasma was separated from blood by centrifugation (MF-300; Hanil, Seoul, South Korea) at 1,700 × g for 15 min. Erythrocytes were isolated as previously described by Mccord and Fridovich [13]. Liver tissue was removed, washed rapidly with ice-cold saline, and then frozen in liquid nitrogen. The method by Hogeboom [14] was used to isolate mitochondria, microsomes, and cytosol from the liver tissue. All samples were stored at -70℃ in a deep freezer until use.
Lipid peroxides assay
The concentration of hepatic TBARS, a bio-parameter used to estimate lipid peroxide levels, was determined as previously described by Ohkawa et al. [15]. In brief, 0.1 mL liver tissue homogenate was mixed with 0.2 mL of 8.1% sodium dodecylsulfate (SDS), 1.5 mL of 20% acetic acid buffer (pH 3.5), 1.5 mL of 0.8% thiobarbituric acid (TBA; Sigma, St.Louis, MO, USA), and 0.7 mL distilled water. The solution was mixed well and incubated for 60 min in a water bath at 95℃. After cooling under tap water, 5 mL of an n-butanol and pyridine solution (15:1, v:v) along with 1 mL of distilled water was added and the solution was mixed thoroughly. The mixture was centrifuged at 1700 × g for 10 min. Absorbance of the supernatant was read spectrophotometrically at 532 nm (U-2900; Hitachi, Tokyo, Japan). 1,1,3,3-Tetraethoxy propane (Sigma, St. Louis, MO, USA) was used as a reference to calculate the lipid peroxide levels.
The lipid peroxide concentration in plasma was measured as previously described by Yagi [16]. To extract the lipid peroxide, 0.1 mL plasma was mixed with 4 mL of 1/12 N H2SO4 and centrifuged at 1700 × g for 10 min to pellet the precipitate. Next, 1 mL of TBA and 4 mL of distilled water were added, and the solution was heated at 95℃ for 10 min. After cooling in tap water, 0.5 mL of butanol was added to the mixture and centrifuged as described above. The plasma TBARS concentration was determined fluorometrically using a multilabel plate reader (Victor™ X3; Perkin Elmer, Waltham, MA, USA) at an excitation wavelength of 515 nm and emission wavelength of 553 nm.
Total glutathione and oxidized/reduced (GSH/GSSG) glutathione assay
Glutathione concentration in liver tissue was measured using a glutathione kit (BioVision Inc., Milpitas, CA, USA). Liver tissue (40 mg) was homogenized with glutathione assay buffer (100 µL) using a tissue grinder (Wheaton, Millville, NJ, USA) on ice. Next, 10 µL of a reducing agent mixture was added to the 40 µL of homogenized sample to convert GSSG into GSH. Levels of GSH were analyzed directly in the homogenate sample. Absorbance of GSH and total glutathione was measured with a multilabel plate reader (Victor™ X3) at 515 nm (excitation) and 553 nm (emission). The GSH concentration was calculated using a GSH standard curve. GSSG contents were determined by subtracting the amount of GSH from the level of total glutathione.
Measurement of antioxidant enzyme activities
Catalase activity in liver mitochondria and erythrocytes was assayed by spectrophotometrically analyzing the change of absorbance at 240 nm that can reflect the rate of H2O2 decomposition [17]. Superoxide dismutase (SOD) activity in the cytosolic fraction from liver tissues and erythrocytes was measured as previously described by Marklund and Marklund [18]. Color change of pyrogallol autoxidation was spectrophotometrically monitored at 420 nm for 3 min. The results were expressed as unit/min/mg protein or hemoglobin and one unit was defined as 50% inhibition of pyrogallol autoxidation. Glutathione reductase (GR) activity of the hepatic cytosolic fraction was determined as previously described by Pinto and Bartley [19]. One unit of GR was defined as reduction of 1 µmol oxidized glutathione/min/mg protein. Glutathione peroxidase (GSH-Px) activity in the mitochondrial fraction of liver tissue and erythrocytes was assayed using a modified method described by Paglia and Valentine [20]. The activity is expressed as nmol of reduced NADPH/min/mg protein or hemoglobin. Glutathione-S-transferase (GST) activity of the hepatic cytosolic fraction was measured according to the method of Habig et al. [21].
Measurement of retinol and α-tocopherol in plasma
The levels of retinol and α-tocopherol in plasma were analyzed simultaneously according to the HPLC method by Bieri et al. [22]. For this, 100 µL retinyl acetate (Sigma, St. Louis, MO, USA) and 200 µL tocopherol acetate (Sigma, St. Louis, MO, USA) were added as internal standards to 200 µL plasma sample to calculate the extraction yield. Next, 400 µL of hexane extraction solvent and 100 µL of 0.01% 2, 6-di-tert-butyl-4-methylphenol as an antioxidant were added to the solution, mixed thoroughly, and centrifuged for 5 min at 220 × g. After repeating this procedure three times, the supernatant was concentrated under nitrogen gas and the residue was redissolved in a mixture of diethyl ether : methanol (1:3, v:v). The sample was passed through a 0.45-µm membrane filter (Millipore, Bedford, MA, USA) and 20 µL was injected into an SPD-10Avp HPLC system with a UV detector and column (3.9 × 300 mm, W12461N; Waters, Milford, MA, USA). A solution of methanol : water (95:5, v:v) was used as the mobile phase and absorbance was read at 280 nm. The flow rate was 1.0 mL/min and the run time was 30 min.
Measurement of retinol, retinyl palmitate, and α-tocopherol in liver tissue
The levels of retinol, retinyl palmitate, and α-tocopherol in liver tissue were measured using the HPLC method by Furr et al. [23]. Briefly, 0.5 mg liver tissue was ground (Samhwa, Seoul, South Korea) with 1 g of anhydrous sodium sulfate. The sample was combined with 8 mL of dichloromethane, 100 µL of retinyl acetate (Sigma), and 100 µL of tocopherol acetate (Sigma); and the mixture was centrifuged at 690 × g for 10 min. After the extraction procedure was repeated three times, the supernatant was collected and concentrated under nitrogen gas. The residue was redissolved in 200 µL of an ethyl ether : methanol (1:3, v:v) solution and passed through a 0.45-µm membrane filter (Millipore) for HPLC analysis. The analytical method was the same as that used for plasma but the mobile phase was a mixture of methanol : tetrahydrofuran : water (85:10:5, v:v:v).
Histopathological examination
Liver tissue was stored in a 10% formalin solution for 24 h immediately after dissection and then embedded in paraffin. Sections were cut and stained with hematoxylin and eosin [24]. The sections were examined with an optical microscope (Axiostar Plus; Carl Zeiss, Göttingen, Germany).
The liver tissues were fixed in 0.1 M phosphate buffer (pH 7.2) with 2.5% glutaraldehyde at 4℃ for 3 h, and then post-fixed in 0.1 M phosphate buffer (pH 7.2) with 1% osmium tetroxide for 1.5 h. The tissues were dehydrated in a mixture of ethanol and water, embedded in epoxy resin, and incubated at 60℃ for 25 h for polymerization. The blocks were cut into sections 80-nm thick with an ultramicrotome (MT-X; RMC, Tucson, AZ, USA) and stained with uranyl acetate and lead nitrate [25]. The grids were examined with a transmission electron microscope (H-7600; Hitachi, Tokyo, Japan) at a high voltage of 75 kV.
Measurement of protein and hemoglobin
Protein contents of the analytical samples were measured as previously described by Lowry [26]. Hemoglobin levels in each sample were assayed using a commercial hemoglobin analytical kit (Asan Pharm. Co., Ltd., Seoul, South Korea).
Statistical analysis
All results were analyzed with a one-way analysis of variance (ANOVA) followed by Duncan's multiple-range test using the SPSS software package (SPSS ver. 18.0; Chicago, IL, USA). P-values less than 0.05 were considered significant.
RESULTS
Growth performance of the experimental animals
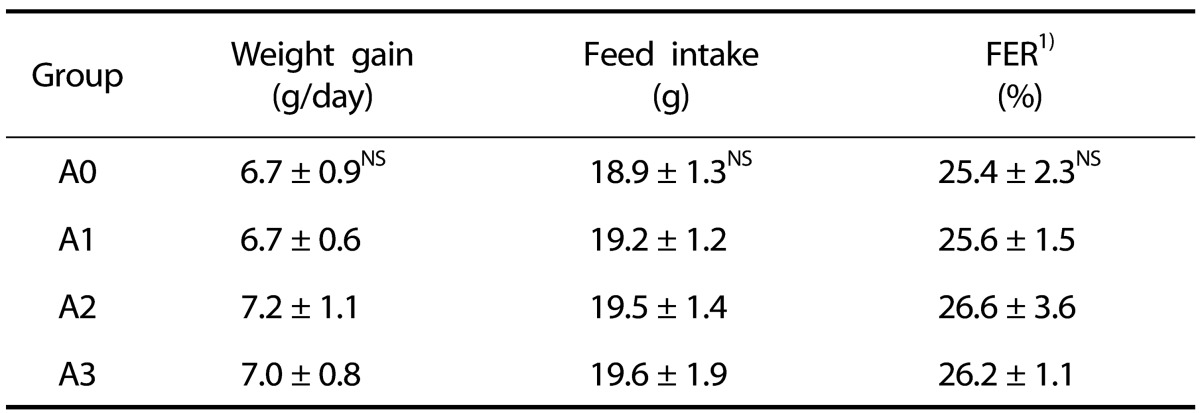
Weight gain, feed intake, and FER of the rats during the feeding period did not significantly vary among all groups (Table 2).
Table 2. Effects of vitamin A supplementation on weight gain, feed intake, and FER in rats fed the experimental diets.
The experimental groups are described in Table 1.
1) Feed efficiency ratio
Values represent the mean ± SD.
NS: Not significant
Effect of vitamin A supplementation on lipid peroxide levels in plasma and liver
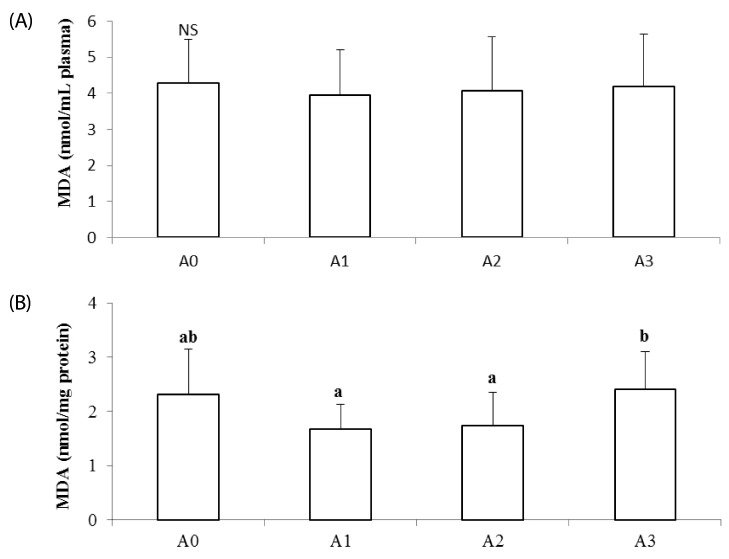
TBARS levels in plasma were not significantly different among the experimental groups (Fig. 1A). In liver homogenates, TBARS levels of the A3 group were significantly higher than those of the A1 and A2 groups (P < 0.05; Fig. 1B), but not significantly different from those of the A0 group.
Fig. 1. Effects of vitamin A supplementation on plasma (A) and hepatic (B) lipid peroxides in rats.
The experimental groups are described in Table 1. Values are presented as the mean ± SD. Values with different superscript letter are significantly different (P < 0.05). MDA; malondialdehyde. NS: Not significant.
Effect of vitamin A supplementation on total glutathione levels and the GSSG/GSH ratio in liver
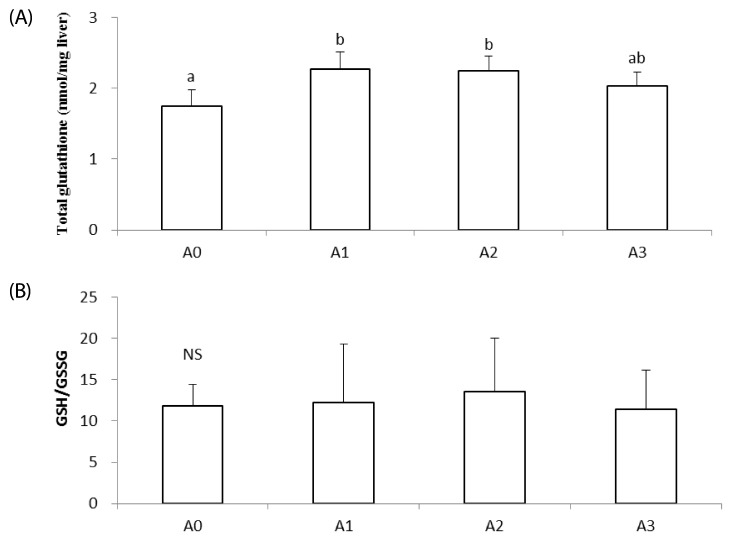
Total glutathione concentration of the A0 group was the lowest among all experimental groups, and this level was significantly increased in the A1 and A2 groups compared to the A0 group (P < 0.05; Fig. 2A). The concentration of total glutathione for the A3 group was not significantly different from that of the other groups. There was no significant difference in GSSG/GSH ratios among all groups (Fig. 2B).
Fig. 2. Effects of vitamin A supplementation on hepatic total glutathione (A) and the GSH/GSSG (B) ratio in rats fed the experimental diets.
The experimental groups are described in Table 1. Values with different superscript letter are significantly different (P < 0.05). NS: Not significant.
Effect of vitamin A supplementation on antioxidant enzymes activities
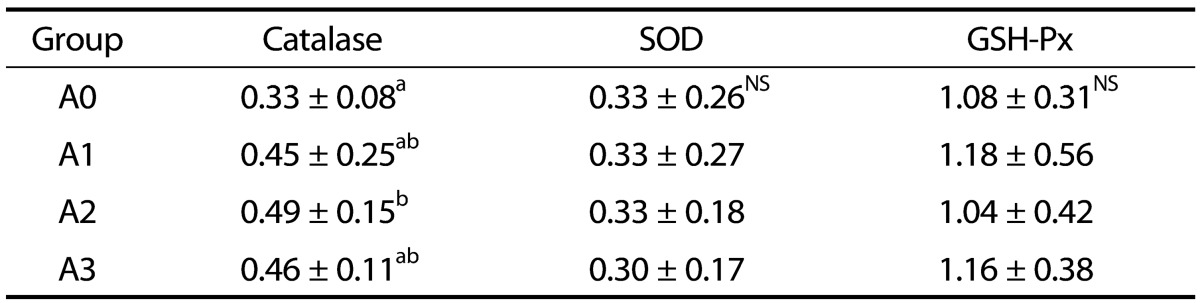
Catalase, SOD, and GSH-Px activities of the rat erythrocytes are presented in Table 3. Catalase activity for the A2 group was significantly higher than that for the A0 group (P < 0.05). Erythrocyte SOD and GSH-Px activities were not significantly different among all groups.
Table 3. Effects of vitamin A supplementation on erythrocyte catalase, SOD, and GSH-Px activities in rats fed the experimental diets (unit/min/mg Hb).
The experimental groups are described in Table 1.
Values represent the mean ± SD.
Values with different superscript letters within a column are significantly different (P < 0.05).
NS: Not significant; SOD: Superoxide dismutase; GSH-Px: Glutathione peroxidase
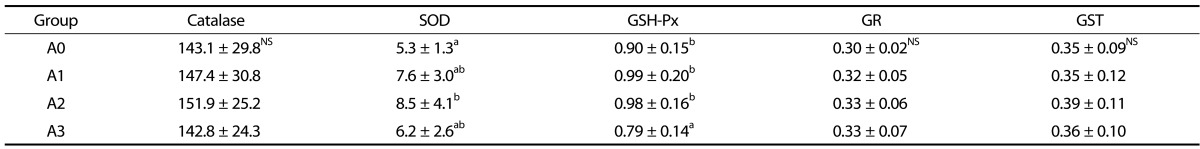
Antioxidant enzyme activities in the liver are presented in Table 4. Hepatic catalase activity was not significantly different among all experimental groups. SOD activity was increased in the vitamin A-supplemented groups compared to the A0 group, but only the level found in the A2 group was significantly different (P < 0.05). The GSH-Px activity of the A3 group was reduced compared to that of the other groups. GR and GST activities in the liver were not significantly different among all experimental groups.
Table 4. Effects of vitamin A supplementation on liver catalase, SOD, GSH-Px, GR, and GST activities in rats fed the experimental diets (unit/min/mg protein).
The experimental groups are described in Table 1.
Values represent the mean ± SD.
Values with different superscript letters within a column are significantly different (P < 0.05).
NS: Not significant; SOD: Superoxide dismutase; GSH-Px: Glutathione peroxidase; GR: Glutathione reductase; GST: Glutathione-S-transferase
Effects of vitamin A supplementation on vitamin A and E concentrations in plasma and liver
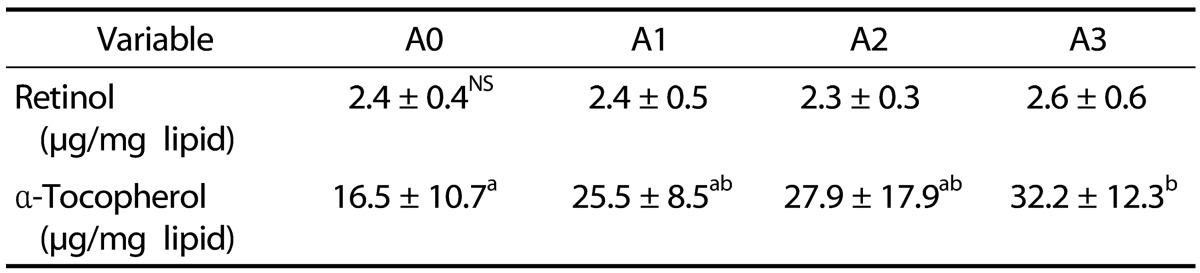
The retinol and α-tocopherol concentrations in plasma are shown in Table 5. Plasma retinol levels did not significantly differ among all groups. The α-tocopherol level of the A3 group was significantly higher than that of the A0 group (P < 0.05).
Table 5. Effects of vitamin A supplementation on plasma concentrations of retinol and α-tocopherol in rats fed the experimental diets.
The experimental groups are described in Table 1.
Values represent the mean ± SD.
Values with different superscript letters within a column are significantly different (P < 0.05).
NS: Not significant
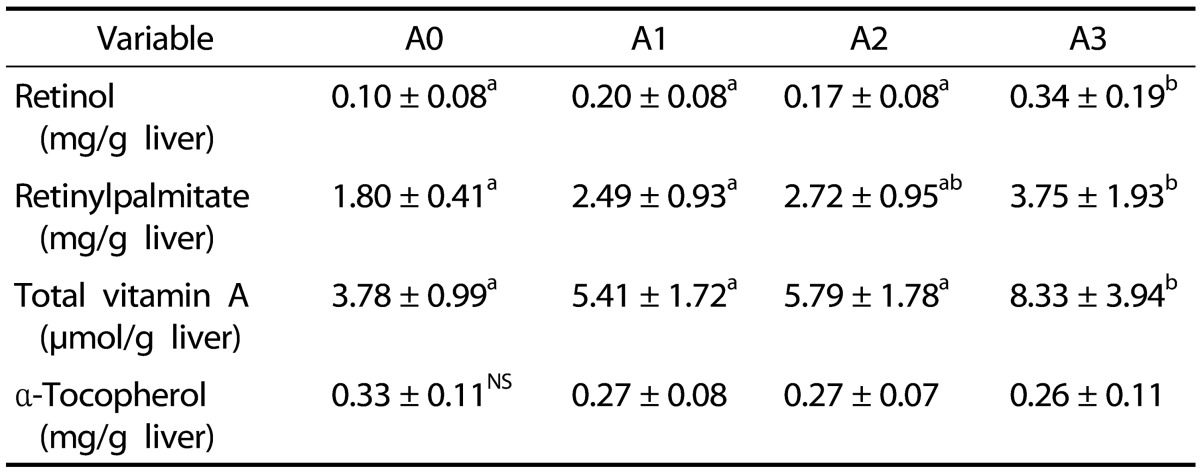
The hepatic retinol concentration for the A3 group was significantly increased compared to that of the other groups (P < 0.05; Table 6). Retinyl palmitate levels in the liver were lowest in the A0 group. This was increased along with elevated concentrations of vitamin A supplementation. The retinyl palmitate level of the A3 group was significantly higher than that of the A1 and A2 groups (P < 0.05). Total vitamin A contents of the A3 group were significantly increased compared to the other groups (P < 0.05). α-Tocopherol concentrations in the liver did not significantly differ among all groups.
Table 6. Effects of vitamin A supplementation on hepatic concentrations of retinol, retinyl palmitate, total vitamin A, and α-tocopherol in rats fed the experimental diets.
The experimental groups are described in Table 1.
Values are presented as the mean ± SD.
Values with different superscript letters within a column are significantly different (P < 0.05).
NS: Not significant
Effect of vitamin A supplementation on histological changes of rat hepatocytes
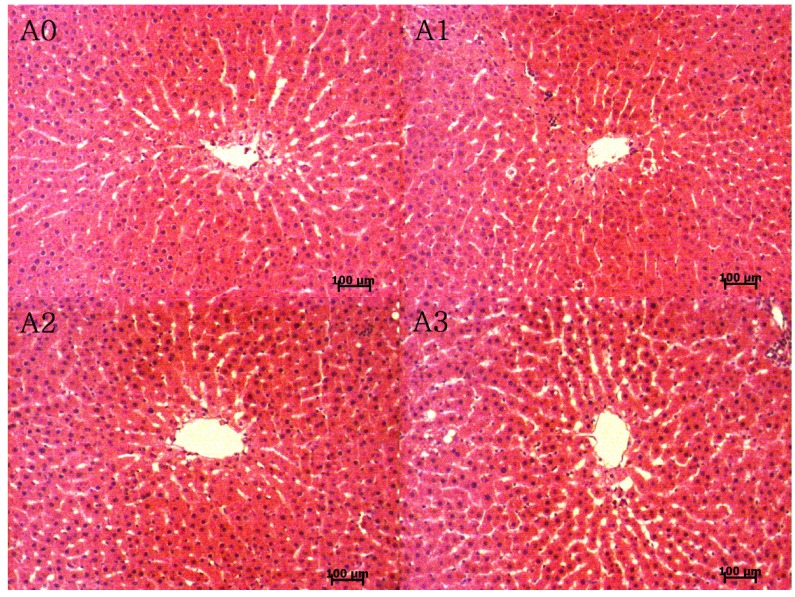
Micrographs of the liver tissues are shown in Fig. 3. The hepatocytes were better proportioned around the central vein in the A1 and A2 groups compared to the A0 and A3 groups. However, the liver sinusoid space was increased in the A2 and A3 groups compared to the A0 and A1 groups.
Fig. 3. Micrographs of liver tissue from rats fed the experimental diets (H&E staining, 100 × magnification).
The experimental groups are described in Table 1.
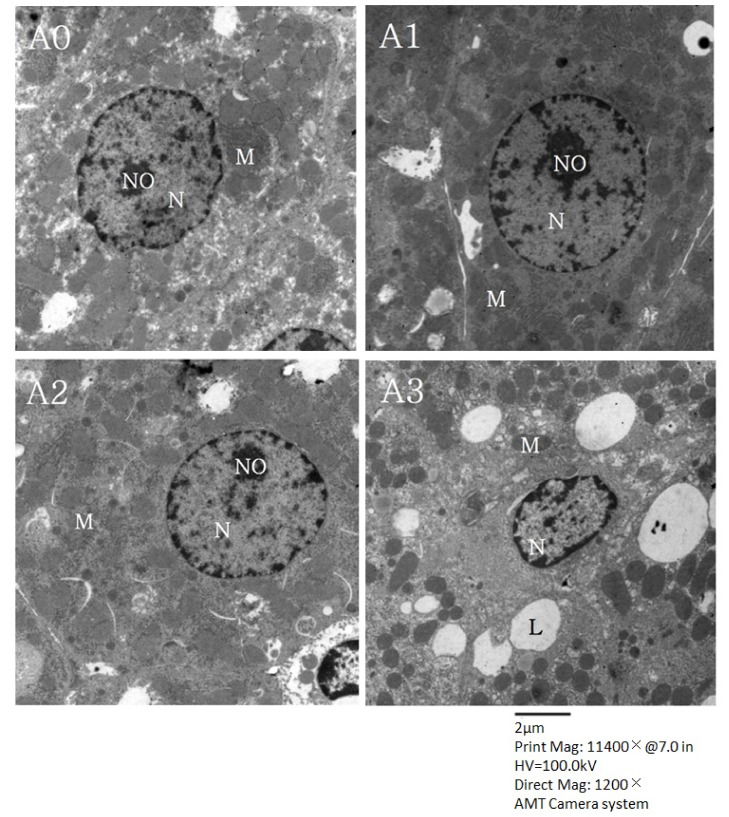
In Fig. 4, micrographs of the hepatocytes observed with transmission electron microscopy are presented. Hepatocytes from the A1 group contained clear nuclei with round nuclear membranes, regular chromatin distribution, and abundant mitochondria. These characteristics were similar to the ones observed for the A2 group. The shape of the nuclear membrane was not perfectly round and a large amount of the rough endoplasmic reticulum was degranulated in hepatocytes from the A0 group. Results for the A3 group were similar to those of the A0 group although some fat droplets were observed in the cytoplasm of a few liver cells from the A3 group.
Fig. 4. Micrographs of hepatocytes from rats fed the experimental diets (transmission electron microscopy, 1200 × magnification).
N, nuclei; NO, nucleoli; M, mitochondria; L, lipid droplets. The experimental groups are described in Table 1.
DISCUSSION
Vitamin A deficiency is one of the most common types of malnutrition in many underdeveloped and developing countries [27]. Vitamin A supplementation is commonly recommended for nutritional purposes in the world [11]. On the other hand, accumulation of vitamin A in the body leads to a high likelihood of vitamin toxicity [8]. In the past few years, this vitamin has been suggested to have an antioxidant effect in tissues such as liver and lungs [28]. However, the effects of vitamin A supplementation differ depending on the dose because this compound can be either act as an antioxidant or prooxidant [11]. The effects of vitamin A supplementation on the antioxidant system thus need to be explored according to concentration.
Supplementation with 1.2 mg retinol/kg (4,000 IU/kg) in mice was not found to have a significant impact compared to a vitamin A-deficient group [29]. Another study reported that vitamin A intake of 4,000 IU/kg significantly increases the body weight of 21-wk-old rats compared to vitamin A-deficient animals during a feeding period of 10 wk [30]. In our study, however, no significant change of body weight was observed, probably due to the short experimental period of 4 wk.
Recent study has shown that appropriate amounts of lipid peroxides help mediate the activation of receptors, nuclear transcription factors, inducers of adaptive responses, and regulators of gene expression [31]. In contrast, excess levels of lipid peroxides can cause various disorders and diseases [31]. When rats were fed diets supplemented with 1,000, 2,500, 4500, and 9,000 IU/kg retinyl palmitate, the level of TBARS significantly increased in rats with 9,000 IU/kg compared to the other groups [32]. These results are similar to our findings from liver tissue of rats with 20,000 IU/kg. These elevated levels of this oxidative marker in the liver tissue are considered as the indicator of hepatocyte damage [33]. A dose of 4,000 IU/kg vitamin A supplementation was found to result in a significantly lower level of lipid peroxides in spleen tissue of rats compared to a vitamin A-deficient group [31].
Indeed there is increasing concern about the excessive use of vitamin A among both children and adults [34]. It is common for infants and children to be provided with a single high-dose vitamin A (300 to 10,000 IU/kg) supplement at regular intervals in many developing countries [9]. Thus it is important to elucidate the effect of vitamin A intake levels on biological system.
Glutathione is an antioxidant that can play an important role in the mammalian antioxidant defense system. This compound mainly exists in the reduced thiol state (GSH) that can interact with many antioxidant enzymes to remove peroxides from biological systems [35]. Vitamin A deficiency decreased GSH concentrations and the GSH/GSSG ratio in rat liver compared to the control group; these effects were reversed after restoring vitamin A administration [36]. These findings from vitamin A deficiency are similar with results of total glutathione from the present study, suggesting that vitamin A deficiency increased the level of lipid peroxides that can disrupt the antioxidant system in rats.
In a study by de Oliveira et al. [32], catalase activity was unchanged when comparing vitamin A supplementary groups (1,000, 2,500, 4,500, and 9,000 IU/kg) and vitamin A-deficient group. SOD activity of the 4,500 and 9,000 IU/kg groups significantly increased compared to the vitamin A-deficient group. These findings coincided with our results, showing that 8,000 IU/kg vitamin A intake can increase liver SOD activity. The relation of some anti-oxidant enzymes between erythrocyte and liver in experimental groups turned out to be a different tendency. Although the SOD and GSH-Px activities in the erythrocyte were not different among experimental groups, those in liver tended to increase in the A2 group and to decrease in the A3 group. It is associated that the plasma lipid peroxide was not different among experimental groups, but liver lipid peroxide was the highest in the A3 group. It may be due to deplete the hepatic SOD and GSH-Px to counter oxidative stress increased by supplementation of excessive vitamin A. Further studies, however, are needed to elucidate the different tendency of anti-oxidant enzymes according to the tissues.
Vitamin A deficiency was shown to significantly reduce vitamin A levels in plasma compared to a 4,000 IU/kg supplementation group [37]. When a vitamin A-deficient diet was fed to 60-d-old rats for 21 d, retinal and retinyl palmitate concentrations were decreased over 95% compared to the vitamin A intake group. Even after 10 d of retinoic acid recovery, the levels of these two compounds did not increase [38]. Vitamin A supplementation did not change the plasma level, but significantly increased hepatic concentration of vitamin A in the present study.
In another previous study, vitamin A treatment significantly alleviated liver injury caused by ischemia/reperfusion compared to a control group [39]. Additionally, Murakami et al. [40] reported that in rats with hepatic fibrosis induced by tetrachloride, histological changes associated with hepatic fibrosis were ameliorated with vitamin A treatment compared to the control group. Similar results were presented in a previous study in which vitamin A deficiency induced hepatic damage that was ameliorated in control rats fed an adequate level of vitamin A [41].
It has been reported that oxidative stress may contribute to the pathogenesis of diffuse lung disease [42]. Rat liver mitochondria are vulnerable to vitamin A, which induces permeability transition from rat liver mitochondria [43]. Overall, vitamin A deficiency can cause liver damage while a high dose of vitamin A can also lead to liver injury. According to the present study, high doses of vitamin A might exert a prooxidant effect on liver hepatocytes. Increased oxidative damage to hepatocytes may culminate in pathological effects [44]. Prooxidant effects observed with vitamin A supplementation at a high dose may be involved in the development of liver diseases associated with redox dysfunction and free radical-induced damage to biomolecules [45]. Considering our findings, 4,000 IU/kg based on vitamin A level of AIN-76 diet and 8,000 IU/kg supplementation might be considered as adequate level to induce the antioxidant system in rats.
In conclusion, appropriate supplementation of vitamin A can affect the antioxidant system of rats. This might be associated with regulation of lipid peroxide production and some antioxidant enzyme activities, and amelioration of hepatic histological changes. Additional studies are needed to further investigate the long-term, dose-dependent effects of vitamin A supplementation in biological systems.
Footnotes
This work was supported by a 2012 Yeungnam University Research Grant.
References
- 1.Lee MJ, Kim JH. The changes of dietary reference intakes for Koreans and its application to the new text book. J Korean Home Econ Educ Assoc. 2008;20:75–94. [Google Scholar]
- 2.The Korean Nutrition Society. Dietary Reference Intakes for Koreans. 1st rev. ed. Seoul: The Korean Nutrition Society; 2010. [Google Scholar]
- 3.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey (KNHANES V-1) Cheongwon: Korea Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 4.Demissie T, Haider J, Tibeb HN, Haile B. Impact evaluation of EPI-PLUS and WIBS approaches in controlling vitamin A deficiency in Tigray and Harari regions, Ethiopia. Ethiop J Health Dev. 2000;14:303–310. [Google Scholar]
- 5.Oliveros L, Vega V, Anzulovich AC, Ramirez D, Giménez MS. Vitamin A deficiency modifies antioxidant defenses and essential element contents in rat heart. Nutr Res. 2000;20:1139–1150. [Google Scholar]
- 6.Vega VA, Anzulovich AC, Varas SM, Bonomi MR, Giménez MS, Oliveros LB. Effect of nutritional vitamin A deficiency on lipid metabolism in the rat heart: Its relation to PPAR gene expression. Nutrition. 2009;25:828–838. doi: 10.1016/j.nut.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Tanumihardjo SA. Vitamin A and bone health: the balancing act. J Clin Densitom. 2013;16:414–419. doi: 10.1016/j.jocd.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Allen LH, Haskell M. Estimating the potential for vitamin A toxicity in women and young children. J Nutr. 2002;132:2907S–2919S. doi: 10.1093/jn/132.9.2907S. [DOI] [PubMed] [Google Scholar]
- 10.Binkley N, Krueger D. Hypervitaminosis A and bone. Nutr Rev. 2000;58:138–144. doi: 10.1111/j.1753-4887.2000.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 11.Pasquali MA, Gelain DP, Oliveira MR, Behr GA, Motta LL, Rocha RF, Klamt F, Moreira JC. Vitamin A supplementation induces oxidative stress and decreases the immunocontent of catalase and superoxide dismutase in rat lungs. Exp Lung Res. 2009;35:427–438. doi: 10.1080/01902140902747436. [DOI] [PubMed] [Google Scholar]
- 12.de Oliveira MR, Moreira JC. Acute and chronic vitamin A supplementation at therapeutic doses induces oxidative stress in submitochondrial particles isolated from cerebral cortex and cerebellum of adult rats. Toxicol Lett. 2007;173:145–150. doi: 10.1016/j.toxlet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 14.Hogeboom GH. Fractionation of cell components of animal tissues. Methods Enzymol. 1955;1:16–19. [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 17.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York (NY): Academic Press; 1974. pp. 673–683. [Google Scholar]
- 18.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 19.Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969;112:109–115. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 21.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 22.Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- 23.Furr HC, Amédée-Manesme O, Olson JA. Gradient reversed-phase high-performance liquid chromatographic separation of naturally occurring retinoids. J Chromatogr. 1984;309:299–307. doi: 10.1016/0378-4347(84)80037-7. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.Mori M. Electron microscopic and new microscopic studies of hepatocyte cytoskeleton: physiological and pathological relevance. J Electron Microsc (Tokyo) 1994;43:347–355. [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Wedner SH, Ross DA. Vitamin A deficiency and its prevention. In: Heggenhougen K, Quah SR, editors. International Encyclopedia of Public Health. Amsterdam: Elsevier; 2008. pp. 526–532. [Google Scholar]
- 28.Palace VP, Khaper N, Qin Q, Singal PK. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic Biol Med. 1999;26:746–761. doi: 10.1016/s0891-5849(98)00266-4. [DOI] [PubMed] [Google Scholar]
- 29.Kim SC, Lee HJ, Joo JH, Yoon JH, Choi JY. Vitamin A deficiency induces fluid hyposecretion from the airway submucosal glands of mice. J Nutr. 2012;142:739–743. doi: 10.3945/jn.111.154047. [DOI] [PubMed] [Google Scholar]
- 30.Arruda SF, Siqueira EM, de Valência FF. Vitamin A deficiency increases hepcidin expression and oxidative stress in rat. Nutrition. 2009;25:472–478. doi: 10.1016/j.nut.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Niki E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012;586:3767–3770. doi: 10.1016/j.febslet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 32.de Oliveira MR, Soares Oliveira MW, Müller Hoff ML, Behr GA, da Rocha RF, Fonseca Moreira JC. Evaluation of redox and bioenergetics states in the liver of vitamin A-treated rats. Eur J Pharmacol. 2009;610:99–105. doi: 10.1016/j.ejphar.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 33.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008;29:9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Lam HS, Chow CM, Poon WT, Lai CK, Chan KC, Yeung WL, Hui J, Chan AY, Ng PC. Risk of vitamin A toxicity from candy-like chewable vitamin supplements for children. Pediatrics. 2006;118:820–824. doi: 10.1542/peds.2006-0167. [DOI] [PubMed] [Google Scholar]
- 35.Vávrová A, Popelová O, Stěrba M, Jirkovský E, Hašková P, Mertlíková-Kaiserová H, Geršl V, Simůnek T. In vivo and in vitro assessment of the role of glutathione antioxidant system in anthracycline-induced cardiotoxicity. Arch Toxicol. 2011;85:525–535. doi: 10.1007/s00204-010-0615-8. [DOI] [PubMed] [Google Scholar]
- 36.Barber T, Borrás E, Torres L, García C, Cabezuelo F, Lloret A, Pallardó FV, Viña JR. Vitamin A deficiency causes oxidative damage to liver mitochondria in rats. Free Radic Biol Med. 2000;29:1–7. doi: 10.1016/s0891-5849(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 37.Gatica L, Alvarez S, Gomez N, Zago MP, Oteiza P, Oliveros L, Gimenez MS. Vitamin A deficiency induces prooxidant environment and inflammation in rat aorta. Free Radic Res. 2005;39:621–628. doi: 10.1080/10715760500072214. [DOI] [PubMed] [Google Scholar]
- 38.Esteban-Pretel G, Marín MP, Renau-Piqueras J, Barber T, Timoneda J. Vitamin A deficiency alters rat lung alveolar basement membrane: reversibility by retinoic acid. J Nutr Biochem. 2010;21:227–236. doi: 10.1016/j.jnutbio.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Elias-Miró M, Massip-Salcedo M, Raila J, Schweigert F, Mendes-Braz M, Ramalho F, Jiménez-Castro MB, Casillas-Ramírez A, Bermudo R, Rimola A, Rodes J, Peralta C. Retinol binding protein 4 and retinol in steatotic and nonsteatotic rat livers in the setting of partial hepatectomy under ischemia/reperfusion. Liver Transpl. 2012;18:1198–1208. doi: 10.1002/lt.23489. [DOI] [PubMed] [Google Scholar]
- 40.Murakami K, Kaji T, Shimono R, Hayashida Y, Matsufuji H, Tsuyama S, Maezono R, Kosai K, Takamatsu H. Therapeutic effects of vitamin A on experimental cholestatic rats with hepatic fibrosis. Pediatr Surg Int. 2011;27:863–870. doi: 10.1007/s00383-011-2853-0. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar RP, Genta S, Oliveros L, Anzulovich A, Giménez MS, Sánchez SS. Vitamin A deficiency injures liver parenchyma and alters the expression of hepatic extracellular matrix. J Appl Toxicol. 2009;29:214–222. doi: 10.1002/jat.1399. [DOI] [PubMed] [Google Scholar]
- 42.Lenz AG, Costabel U, Maier KL. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur Respir J. 1996;9:307–312. doi: 10.1183/09031936.96.09020307. [DOI] [PubMed] [Google Scholar]
- 43.Klamt F, Roberto de Oliveira M, Moreira JC. Retinol induces permeability transition and cytochrome c release from rat liver mitochondria. Biochim Biophys Acta. 2005;1726:14–20. doi: 10.1016/j.bbagen.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, Budillon G, Cimino L, Di Carlo A, Di Marino MP, Morisco F, Picciotto F, Terracciano L, Vecchione R, Verde V, Del Vecchio Blanco C. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J Hepatol. 2001;35:568–574. doi: 10.1016/s0168-8278(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 45.Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011;31:1432–1448. doi: 10.1111/j.1478-3231.2011.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]