Abstract
Damage to hippocampus can occur through many causes including head trauma, ischemia, stroke, status epilepticus and Alzheimer’s disease. Certain changes such as increased levels of neurogenesis and elevated concentrations of multiple neurotrophic factors that ensue in the acute phase after injury seem beneficial for restraining hippocampal dysfunction. However, many alterations that arise in the intermediate to chronic phase after injury such as abnormal migration of newly born neurons, aberrant synaptic reorganization, progressive loss of inhibitory gamma-amino butyric acid positive interneurons including those expressing reelin, greatly declined neurogenesis and sustained inflammation are detrimental. Consequently, the net effect of post-injury plasticity in the hippocampus remains inadequate for promoting significant functional recovery. Hence, ideal therapeutic interventions ought to be efficient for restraining these detrimental changes in order to block the propensity of most hippocampal injuries to evolve into learning deficits, memory dysfunction, depression, and temporal lobe epilepsy. Neural stem cell (NSC) grafting into the hippocampus early after injury appears alluring from this perspective because several recent studies have demonstrated therapeutic value of this intervention, especially for preventing/easing memory dysfunction, depresion and temporal lobe epilepsy development in the chronic phase after injury. These beneficial effects of NSC grafting appeared to be mediated through considerable modulation of aberrant hippocampal post-injury plasticity with additions of new inhibitory gamma-amino butyric acid positive interneurons, and astrocytes secreting a variety of neurotrophic factors and anticonvulsant proteins. This review confers advancements made in NSC grafting therapy for treating hippocampal injury in animal models of excitotoxic injury, traumatic brain injury, Alzheimer’s disease and status epilepticus, potential mechanisms of functional recovery mediated by NSC grafts placed early after hippocampal injury, and issues that need to be resolved prior to considering clinical application of NSC grafting for hippocampal injury.
INTRODUCTION
Hippocampus is an area of the brain vital for functions such as learning, memory and mood [1,2]. It is also one of the brain regions that reacts to injury or neurodegeneration with robust plasticity [3–7]. Hippocampal injury can manifest from numerous causes, which comprise head trauma, ischemia, hemorrhagic stroke, acute seizures, status epilepticus (SE), encephalitis, brain tumors, drug withdrawal, exposure to chronic unpredictable stress, and Alzheimer’s disease (AD) [8–12]. Typically, the acute phase after hippocampal injury is exemplified by increased neurogenesis from neural stem cells (NSCs) located in the subgranular zone (SGZ) of the dentate gyrus (DG) and enhanced levels of multiple neurotrophic factors [13–16]. Increased neurogenesis is also associated aberrant migration of newly born neurons into the dentate hilus (DH) and the molecular layer, and projection of axons from newly born neurons into the dentate molecular layer, which eventually lead to significant synaptic reorganization in the hippocampus [17–19].
Although the above mentioned post-injury changes in the hippocampus are likely innate compensatory mechanisms to restrain the overall dysfunction, some of which are not considered beneficial for recovery. For instance, alterations in the migration and connectivity of newly born neurons have been shown to contribute considerably towards DG hyperexcitability and development of chronic epilepsy after injury [17–19]. Furthermore, early post-injury compensatory alterations are inadequate for promoting recovery of function as most injuries to the hippocampus have a predilection for evolving into learning deficits, memory and mood dysfunction, and/or chronic temporal lobe epilepsy (TLE) typified by spontaneous recurrent seizures (SRS) [20–22]. These impairments in the chronic phase after injury are typically linked with greatly waned neurogenesis from NSCs [14], reduced neuronal differentiation of newly born cells [23], altered integration of newly born neurons through their abnormal migration and/or occurrence of synaptogenesis on basal dendrites projecting from newly born neurons into the DH [24], and prominently reduced concentration of neurotrophic factors that are important for NSC proliferation and differentiation as well as maintenance of normal cognitive and mood function. These include the brain-derived neurotrophic factor (BDNF), the fibroblast growth factor-2 (FGF-2) and the glial cell-line derived neurotrophic factor (GDNF) [14,15,25]. From this perspective, intervention strategies that are efficient for preventing or restraining the progression of the original precipitating injury into memory and mood dysfunction and chronic epilepsy development have immense value [26]. Ideal therapeutic interventions are those capable of promoting normal levels of neurogenesis with apt incorporation of newly generated neurons into the injured hippocampal circuitry. These requirements are important because the ongoing hippocampal neurogenesis is widely believed to play vital roles in the formation of hippocampal-dependent memories and the maintenance of mood function [1,27,28], and the likelihood that abnormal neurogenesis that ensues after injury contributes to an aberrant synaptic reorganization in the hippocampus, memory and mood dysfunction, and chronic TLE development [29,30].
NSCs are well suited for treating Hippocampal Injury
NSCs are self-renewing, multipotent cells capable of generating all three central nervous system phenotypes (neurons, astrocytes and oligodendrocytes). Cell therapy using NSCs as donor cells has received great interest as one of the promising therapeutic interventions for restraining hippocampus injury-induced memory and mood dysfunction and chronic TLE development [8,26,31]. This concept is buoyed by multiple characteristics of these cells. First of all, multipotent NSCs can be harvested/generated easily from multiple sources such as fetal, postnatal and adult brain tissues, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs) [32–35]. Second, NSCs and their progeny can survive well in hypoxic conditions prevailing in the injured brain regions [36], migrate and integrate into regions of the brain displaying neuron loss and inflammatory reaction in the form of hypertrophy of astrocytes, activation of microglia and increased concentration of pro-inflammatory cytokines [37,38]. Third, NSCs have the potential to replace significant numbers of lost interneurons that secrete the inhibitory neurotransmitter gamma-amino butyric acid (GABA; Fig. 1) to regulate the activity of excitatory neurons and to maintain normal network function in the hippocampus [25,39,40]. Fourth, considerable fractions of the progeny of NSCs readily differentiate into astrocytes capable of secreting beneficial neurotrophic factors that promote neuroprotection, ease seizures [25,39,40] and enhance neurogenesis via stimulation of the proliferation of endogenous NSCs in the hippocampus [41,42]. Fifth, NSCs give rise to oligodendrocytes after grafting, which can repair myelin sheaths of axons in demyelinated regions of the brain [43]. Sixth, NSCs exhibit considerable anti-inflammatory properties. For example, a single intravenous injection of NSCs can considerably suppress neuroinflammation following experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis [44] and NSC transplantation in an animal model of AD can attenuate inflammatory activity [37]. Additionally, NSCs can be genetically engineered prior to grafting to deliver neuroprotective proteins to the injured brain [45]. These characteristics make NSCs the most versatile type of donor cells for treating brain injury or neurodegenerative diseases.
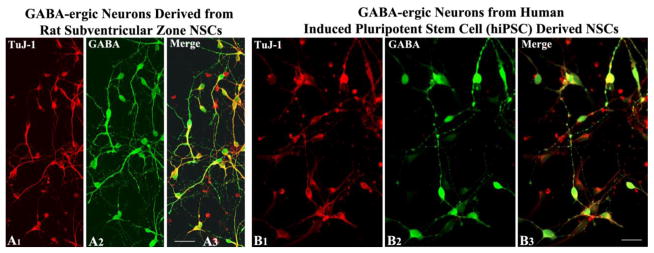
Figure 1.
Gamma-amino butyric acid (GABA) positive neurons derived from postnatal rat subventricular zone neural stem cells (A1–A3) and neural stem cells generated from human induced pluripotent stem cells (B1–B3). A1 and B1, TuJ-1. A2 and B2, GABA. A3 and B3, merged photographs showing both TuJ-1 and GABA expression. Scale bar, A1–A3 = 50 μm. B1–B3, = 25 μm.
NSC grafting early after hippocampal injury can prevent impairments in memory, mood and neurogenesis
Several studies using animal models have shown the promise of NSC grafting intervention for easing hippocampal dysfunction after injury (Table 1). A recent study revealed that grafting of NSCs expanded from the anterior subventricular zone (SVZ) of the postnatal brain into the hippocampus of young adult rats early after injury is an efficacious approach for thwarting memory impairments and depression typically found in the chronic phase after injury [39]. Specifically, NSC grafting preserved ability for making spatial and recognition memories and prevented increased depressive-like behavior. Maintenance of normal memory and mood function in animals receiving SVZ-NSC grafts after hippocampal injury was linked with excellent survival and widespread migration of graft-derived cells, differentiation of significant percentages of NSC graft-derived cells into different subtypes of GABA-ergic interneurons secreting calcium binding proteins calbindin and parvalbumin (PV), and glial cells such as astrocytes, oligodendrocytes and oligodendrocyte progenitors [39; Figure 2].
Table 1.
Outcome of NSC Grafting in Hippocampal Injury/Neurodegeneration Prototypes on Memory and Mood Function
| Type of Injury | Type of NSCs and Species | Region and Timing of grafting | Effect on Memory Function | Effect on Mood Function |
|---|---|---|---|---|
| KA-induced unilateral hippocampal injury in rat [39] | NSCs from the postnatal SVZ | Injured CA3 Region at 5 days after injury | Prevented spatial and recognition memory deficits | Prevented depressive-like behavior |
| KA-induced bilateral hippocampal injury in mouse [60] | NSCs from the postnatal SVZ transduced with IGF-1 | Injured hippocampus at 4 days after injury | Reduced Memory Deficits | Not Examined |
| KA-induced unilateral hippocampal injury in rat [64] | Human NSCs transduced with ChAT | Injured hippocampus at 4 weeks after injury | Improved memory function | Not examined |
| Fluid Percussion prototype of TBI in rat [61] | Human fetal NSCs | Injured hippocampus one-day after TBI | Improved Memory Function | Not examined |
| Triple transgenic AD mouse (3xTg-AD) [38] | NSCs from the entire postnatal day 1 brain | hippocampus of 18-month old mice exhibiting Aβ plaques and NFTs | Improved Memory Function | Not examined |
| Status epilepticus (SE) induced hippocampal injury in rat [40] | NSCs from the postnatal SVZ | Injured hippocampi (bilateral) 7-days after SE | Prevented recognition memory dysfunction | Prevented depressive-like behavior |
Aβ, amyloid beta; AD, Alzheimer’s disease; BDNF, brain derived neurotrophic factor; ChAT, choline acetyltransferase; GD, gestation day; IGF-1, insulin-like growth factor-1; KA, kainic acid; NFTs, neurofibrillary tangles; NSCs, neural stem cells; SE, status epilepticus; SVZ, subventricular zone; TBI, traumatic brain injury.
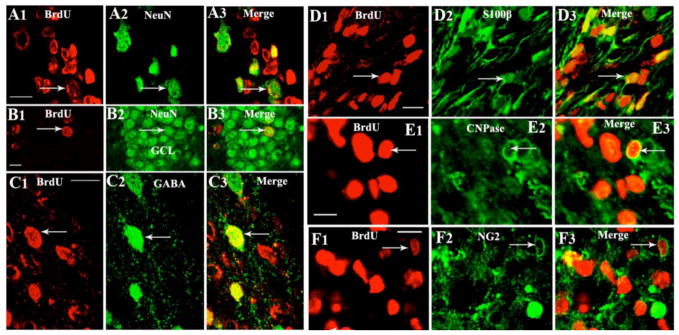
Figure 2.
Differentiation of 5′-bromodeoxyuridine positive (red) cells derived from a neural stem cell graft into: (i) neuron-specific nuclear antigen (NeuN) positive neurons near the graft core (A1–A3) and the granule cell layer (GCL; B1–B3); (ii) gamma-amino butyric acid (GABA) positive neurons (C1–C3), S100β+ astrocytes (D1–D3), 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase (CNPase) positive oligodendrocytes (E1–E3) and NG2+ oligodendrocyte progenitors (F1–F3). Scale bar, A1–A3: 200 μm; B1–B3, C1–C3, D1–D3, E1–E3: 10 μm; F1–F3=20 μm. Figure reproduced from Hattiangady and Shetty, Stem Cells Transl Med, 1:696–708, 2012.
Grafting of NSCs also restrained several pathological features that are believed to have adverse effects on memory and mood function in the chronic phase after injury. It appeared that NSC grafting preserved mood and memory function after hippocampal injury via several mechanisms. First, the acute phase after injury typically enhances hippocampal neurogenesis as well as promotes abnormal neurogenesis typified by aberrant migration of newly born neurons into the DH and the molecular layer and projection of basal dendrites from newly born neurons projecting into the DH. Whereas, the chronic phase after injury is characterized by greatly declined production of newly born neurons and persistence of abnormal pattern of neurogenesis, which closely parallel cognitive and mood dysfunction seen after hippocampal injury [14,22,39]. Interestingly, NSC grafting normalized the extent and pattern of neurogenesis in the injured hippocampus (Fig. 3) with proliferation of NSCs in the neurogenic SGZ maintained at par with levels seen in the age-matched intact hippocampus and to greater levels seen in the injured hippocampus receiving no grafts [39]. Moreover, animals receiving NSC grafts to the injured hippocampus also displayed increased neurogenesis in the hippocampus contralateral to injury. Furthermore, early NSC grafting after injury considerably restrained abnormal migration of newly born neurons into the DH and occurrence of basal dendrites from newly born neurons (Fig. 3). This change appeared to be mediated through protection of a subclass of GABA-ergic interneurons (i.e. neurons expressing an extracellular glycoprotein reelin) in the DG that are important for guiding newly born neurons generated from NSCs into the granule cell layer [39,46]. From the perspective of close association between hippocampal neurogenesis and cognitive and mood function [1,27,28,47,48], it is likely that NSC grafting mediated normal extent and pattern neurogenesis in the injured hippocampus and enhanced neurogenesis in the contralateral hippocampus contributed to maintenance of normal memory and mood function in grafted animals.
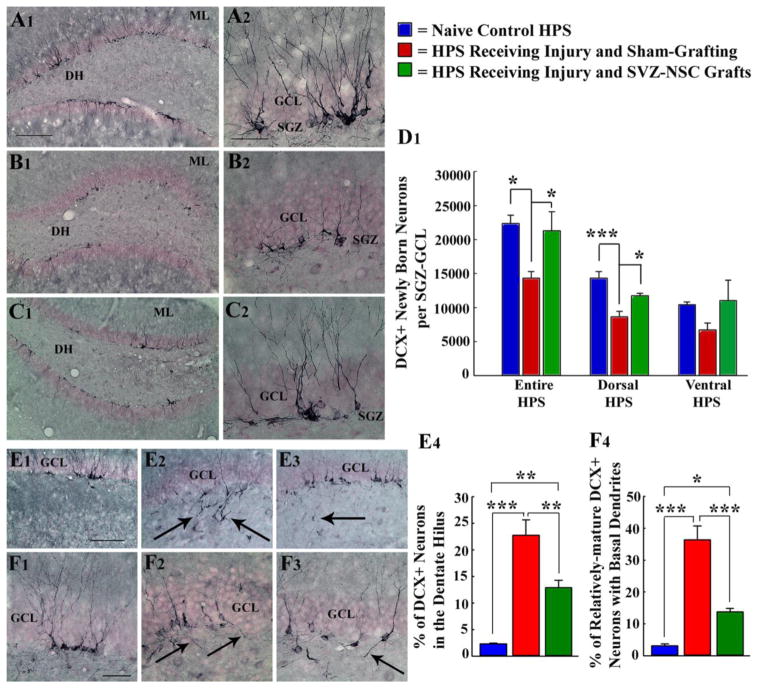
Figure 3.
Grafting of subventricular zone-neural stem cells (SVZ-NSCs) into the hippocampus (HPS) maintains neurogenesis and neural stem cell (NSC) activity at levels comparable to the intact control HPS and preserves reelin+ interneurons in the dentate gyrus. * = p<0.05; ** = p<0.01; ***= p<0.001. A1–D1: Extent of neurogenesis measured through doublecortin (DCX) immunostaining. E1–F4: Pattern of neurogenesis. Note that, in comparison to naïve control rats (A1–A2 & D1; E1 & E4; F1 & F4), rats receiving sham-grafting surgery after hippocampal injury exhibit: (i) decreased neurogenesis (B1, B2, D1); (ii) greater fractions of newly born neurons migrating abnormally into the dentate hilus (E2, E4); (iii) increased occurrences of aberrant basal dendrites from newly born neurons (F2, F4). In contrast, rats receiving SVZ-NSC grafts after hippocampal injury display neurogenesis (C1, C2, D1) to levels observed in naïve control rats. Additionally, both abnormal hilar migration of newly born neurons (E3, E4) and occurrences of aberrant basal dendrites are greatly reduced in these rats (F3, F4). DH, dentate hilus; GCL, granule cell layer; ML, molecular layer. Scale bar, A1, B1, C1: 200 μm; A2, B2, C2, F1–F3: 50μm; E1–E3=100 μm. Figure reproduced from Hattiangady and Shetty, Stem Cells Transl Med, 1:696–708, 2012.
There are other factors, which may have also contributed to conservation of memory and mood function after NSC grafting in this study. First, substantial portions of NSC graft-derived cells expressed a number of valuable neurotrophic factors including the GDNF, BDNF, FGF-2, and vascular endothelial growth factor (VEGF). Apart from having neuroprotective function and the ability for enhancing NSC proliferation and neurogenesis, these factors can also improve cognitive and mood function [49–53]. Additionally, GDNF can suppress seizures [54], and BDNF and FGF-2 can reduce epileptogenesis and hyperexcitability that ensue after hippocampal injury [55]. Second, NSC grafting added significant numbers of GABA-ergic neurons into the injured hippocampus. This may have increased the concentration of GABA and contributed to improved memory and mood function. This is because, hippocampal injury causes considerable loss of GABA-ergic interneurons in the chronic phase [56], major depressive disorders are linked with reduced levels of GABA in brain regions (57), GABAA receptor activation reverses memory deficits in an animal model of schizophrenia [58] and administration of GABA enhancing drugs reverses memory deficits in aged rats [59]. Overall, this study demonstrated that early NSC grafting intervention after hippocampal injury is efficacious for thwarting mood and memory dysfunction as well as reduced and abnormal neurogenesis. Figure 4 illustrates how NSC grafting intervention into the hippocampus in the early phase after injury modulates an abnormal hippocampal post-injury plasticity to promote the maintenance of normal memory and mood function and to restrain the development of chronic epilepsy.
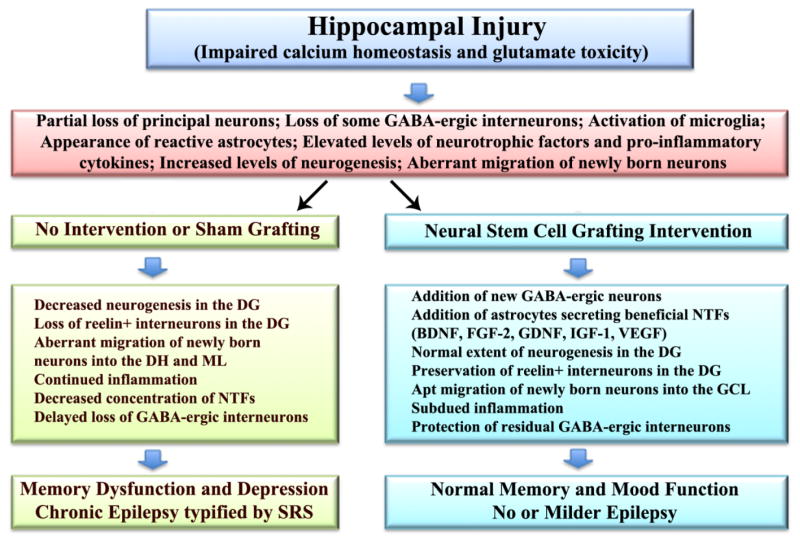
Figure 4.
Potential mechanisms by which neural stem cell grafting intervention into the hippocampus in the early phase after injury modulates hippocampal post-injury plasticity in the intermediate and chronic phases after injury, promotes the maintenance of normal memory and mood function, and restrains the development of chronic epilepsy. An initial injury to hippocampus following head trauma, ischemia, stroke or status epilepticus likely involves impaired neuronal calcium homeostasis and glutamate toxicity. In the acute phase after injury, this leads to partial loss of principal neurons and gamma-amino butyric acid (GABA) positive interneurons, activation of microglia, appearance of reactive astrocytes, increased concentrations of neurotrophic factors and pro-inflammatory cytokines, and enhanced but abnormal neurogenesis typified by aberrant migration of newly born neurons. Absence of therapeutic intervention in the acute phase (left half of the figure) leads to multiple adverse changes in the intermediate and chronic phases of injury, which comprise decreased neurogenesis, loss of reelin+ interneurons in the dentate gyrus (DG), abnormal migration of newly born neurons into the dentate hilus (DH) and the molecular layer (ML), sustained inflammation, decreased concentration of multiple neurotrophic factors (NTFs) and further loss of GABA positive interneurons. All of these changes are known to contribute towards cognitive and mood dysfunction and chronic epilepsy development seen in the chronic phase after injury. However, neural stem cell grafting intervention in the acute phase after injury (right half of figure) considerably modulates these adverse effects with the addition of new GABA-ergic neurons and astrocytes secreting a variety of neurotrophic factors, which in turn contribute to the maintenance of normal memory and mood function and no or milder epilepsy typified by diminished frequency and intensity of spontaneous recurrent seizures (SRS). BDNF, brain derived neurotrophic factor; FGF-2, fibroblast growth factor-2; IGF-1, insulin-like growth factor-1; VEGF, vascular endothelial growth factor.
Another recent study in a mouse model of bilateral hippocampal injury also reported similar findings [60]. Specifically, grafting of NSCs overexpressing the neurotrophic factor insulin-like growth factor-1 (IGF-1) into the hippocampus early after injury eased injury-induced cognitive impairment [60]. Histological characterization showed that early NSC grafting after injury maintained hippocampal neurogenesis to near normal levels and restrained the appearance of reactive astrocytes. Additional analyses showed that functional recovery after grafting was associated with survival of neurons and glia derived from grafted NSCs.
NSC grafting is efficacious for improving hippocampus function after traumatic brain injury (TBI) or AD
Grafting studies in animal models of TBI, stroke and AD have also demonstrated beneficial effects of NSC grafts for alleviating hippocampal injury/neurodegeneration mediated functional deficits. In a model of TBI, NSC grafting prevented cognitive deficits two weeks after TBI by reducing injury-induced progressive axonal degeneration in the fimbria and other brain regions [61,62]. This appeared to be mediated through release of GDNF by graft-derived cells as well as blocking of abnormal accumulation of amyloid precursor protein (APP) in axons and dendrites of hippocampal neurons. APP is a protein that forms conspicuous aggregates in AD brains and increased expression of APP disturbs axonal transport and causes axonal swellings through abnormal accumulation, which is believed to be one of the causes for the evolution of initial TBI-induced neurodegeneration into cognitive dysfunction [62,63]. Furthermore, NSC grafting counteracted TBI induced aberrant accumulation of alpha-smooth muscle actin in hippocampal neurons. In another study, Park and colleagues [64] investigated the effects of grafting of human NSCs that are genetically modified to secrete choline acetyltransferase (the synthesizing enzyme of acetylcholine) into the hippocampus following kainic acid induced injury in the CA3 region. They showed that NSC grafts eased hippocampal injury induced learning and memory deficits. Additional analyses demonstrated that graft-derived cells targeted the injured CA3 region and differentiated into neurons and astrocytes, and increased the overall concentration of acetylcholine in the cerebrospinal fluid. Thus, combined NSC grafting and gene therapy seemed to have increased efficacy in some injury models. However, issues such as timing of grafting intervention and the most suitable brain region for grafting after TBI need to be determined for further enhancing the efficacy of NSC grafts for functional recovery after TBI [65].
An investigation in an animal model of AD has also shown beneficial effects of NSC grafting into the hippocampus [38]. This study investigated the efficacy of NSC grafts placed into the hippocampus of aged transgenic mouse (3xTg-AD mouse) displaying the characteristics of AD. The results demonstrated that in spite of the prevalent and established pathology typified by APP plaques and tau-protein mediated neurofibrillary tangles, NSC grafting alleviated spatial learning and memory deficits seen in Alzheimer’s mice. Of interest is the finding that grafted NSCs did not alter plaques or tangles that were prevalent in the Alzheimer mouse brain but improved cognition via enhancement of hippocampal synaptic density, which was mediated through secretion of BDNF [38]. This was validated through gain-of-function and loss-of-function studies, which revealed that administration of recombinant BDNF reproduced the positive outcomes of NSC grafting but depletion of NSC-derived BDNF failed to alleviate cognitive dysfunction [38]. Overall, this study revealed that NSC grafting could also be efficient for relieving much complex behavioral deficits linked with AD.
NSC grafting can ease cognitive and mood dysfunction and chronic epilepsy development following SE
SE is a life threatening emergency condition typified by prolonged seizure activity and affects ~150,000 Americans every year with ~20% mortality [66,67]. SE resulting from multiple causes (e.g. head trauma, stroke, tumors, neurodegenerative conditions) is often implicated in initiating epileptogenesis, a progression by which a normal brain tissue is malformed into a tissue that can engender SRS [68]. Hippocampus is highly susceptible to SE and hence hippocampal neurodegeneration is one of the most conspicuous consequences of SE. Although the extent of neurodegeneration varies depending on the duration and intensity of SE, a cascade of morphological and functional changes typically follows this initial precipitating injury (IPI) in the hippocampus over weeks and months, which eventually culminates in the manifestation of TLE typified by SRS, depression, memory dysfunction, and decreased and abnormal hippocampal neurogenesis [14,23,69,70,71]. Most antiepileptic drugs (AEDs) used for controlling seizures after SE are not antiepileptogenic [68], as clinical trials have shown that administration of AEDs such as phenytoin, phenobarbital, carbamazepine or valproate do not prevent epileptogenesis and TLE development after acute brain insults [72–74]. Studies in post-SE animal models of TLE also point out the lack of antiepileptogenic effect of AEDs after SE [68]. Thus, currently available medications simply suppress acute seizures but fail to prevent SE-induced epileptogenesis and development of chronic TLE.
While multiple causes have been proposed for SE-induced SRS, its link to hippocampal alterations such as the loss of different subclasses of GABA-ergic interneurons and failure of inhibition [69], persistent neuroinflammation typified by reactive astrocytes and activated microglia [75], and aberrant neurogenesis in the early phase after SE [19,76,77] have received significant support. On the other hand, SE-induced mood and memory dysfunction are likely linked to progressive loss of hippocampal principal neurons (e.g. hippocampal sclerosis in chronic TLE) and greatly declined DG neurogenesis in the chronic phase after SE [14,23,78,79]. An ideal interventional treatment strategy applied shortly after an episode of SE should therefore be capable of protecting the left over hippocampal principal neurons and subclasses of GABA-ergic interneurons, diminishing inflammation, adding new GABA-ergic interneurons, maintaining the extent and pattern of hippocampal neurogenesis at normal levels. From these viewpoints, NSC grafting appears promising as a treatment for SE because, NSCs have the potential to release a multitude of neuroprotective factors, suppress inflammation, add new GABA-ergic interneurons, and maintain hippocampal neurogenesis at normal levels through addition of fresh NSCs to the neurogenic niche, stimulation of endogenous NSC proliferation and facilitation of increased neuronal differentiation of the progeny of NSCs [26,31,37,44].
Indeed, a recent study suggests that early grafting of NSCs after SE can considerably ease SRS occurring in the chronic phase after SE [40]. SE was induced through graded intraperitoneal injections of kainic acid and bilateral grafting of postnatal SVZ-derived NSCs into the hippocampus was performed seven days after SE. Measurement of behavioral SRS at 3–5 months after SE showed greatly reduced frequency and intensity of SRS in rats receiving NSC grafts after SE, in comparison to rats receiving no grafts or dead NSC grafts [40]. Continuous video-electroencephalographic (video-EEG) recordings also demonstrated reduced frequency and intensity of SRS in rats receiving NSC grafts. It comprised 59% reduction in the frequency of all SRS, 97% reduction in the frequency of stage-V SRS (the most severe form of seizures), 45% reduction in the duration of individual SRS and 72% reduction in the amount of recorded time spent in seizure activity [40]. Analyses of recognition memory function through a novel object recognition test revealed preserved memory function in rats receiving NSC grafts after SE but not in rats that underwent SE alone. Characterization using a forced swim test revealed that depressive-like behavior (another co-morbidity of TLE) was completely abolished in animals receiving NSC grafts after SE, in comparison to animals receiving no grafts after SE. Histological analyses revealed that the yield of graft-derived cells was equivalent to ~89% of injected cells and ~24% of graft-derived cells differentiated into neurons. Significant fractions of grafted cells also migrated into the neurogenic SGZ-granule cell layer of the DG where 75% of them integrated as neurons. Furthermore, graft-derived cells differentiated into GABA-ergic interneurons (~22%) and astrocytes secreting neurotrophic factors (~60%). Additionally, NSC grafting intervention after SE considerably preserved subclasses of host GABA-ergic interneurons synthesizing neuropeptide Y (NPY) and PV in the DG. Protection of these interneurons by NSC grafting has functional relevance. First, NPY can inhibit the activity of excitatory neurons in the DG through hyperpolarization and inhibition of glutamate release (80,81), act as a robust anticonvulsant protein (82), and potent stimulator of NSC proliferation in the hippocampus (83,84). Second, PV (a calcium binding protein expressed conspicuously in fast-spiking GABA-ergic hippocampal interneurons) plays important roles in the synchronization of excitatory neurons during network oscillations and maintenance of cognitive function (85,86). Collectively, the results of this study suggested that grafting of NSCs into the hippocampus early after SE can promote multiple effects that are beneficial not only for restraining the evolution of initial SE-induced injury a state of chronic SRS but also for easing SE-induced cognitive and mood dysfunction.
Issues to resolve prior to considering clinical application of NSC grafting for hippocampal injury
Studies conferred above support that NSC grafting intervention early after unilateral hippocampal injury is efficacious for averting or considerably alleviating impairments in memory and mood function [39]. Studies in SE model suggested that this approach is also beneficial for greatly diminishing the frequency and intensity of SRS that ensue in the chronic phase after an IPI [40]. However, NSC grafting alone did not appear to be adequate for blocking the incidence of chronic TLE after a major IPI causing bilateral hippocampal damage such as after SE [40]. Prevention of chronic TLE development following bilateral hippocampal damage may need approaches that combine NSC grafting with novel antiepileptogenic drug therapy.
Nonetheless, clinical application of NSC grafting as a prophylaxis against unilateral hippocampal injury mediated memory and mood impairments appears attractive though there are some concerns, which need to be resolved before considering clinical application of NSC grafting. The primary issue is the apt source of adequate numbers of human-derived transplantable NSCs. Although NSCs can be acquired from autopsied tissue samples from NSC enriched regions (e.g. SVZ and hippocampus) of postnatal or adult brains, these sources may not be plentiful for routine NSC grafting therapy for neurological conditions because of snags such as the prerequisite to collect tissues for NSC expansion within specific hours after death, and a possible inconsistency between autopsy samples collected from different age groups and at different time intervals after death. Besides, expansion of human NSCs from autopsied adult brain samples in vitro may not correspond to levels obtained from fresh rodent brains, which may impede the ability to obtain adequate numbers of NSCs for cell therapy. These drawbacks have obligated scientists to consider other sources of NSCs for clinical therapy. From this standpoint, NSCs produced from pluripotent human embryonic stem cells (hESCs) obtained from the blastocyst stage of embryo or human induced pluripotent stem cells (hiPSCs) obtained via reprogramming of somatic cells such as skin fibroblasts appears attractive because both hESCs and hiPSCs can easily generate unrestricted numbers of NSCs for grafting [87–90]. However, there are some major hurdles for the clinical use of NSCs derived from these pluripotent stem cells. First, there is a concern that NSCs expanded from hESCs and hiPSCs may carry genetic and epigenetic abnormalities, which necessitates testing of every batch of NSCs prior to grafting for normal NSC gene signatures [89,90]. Second, it is important that NSCs generated from hESCs and hiPSCs be purified to hundred percent as inclusion of even a single pluripotent stem cell in the NSC suspension prepared for grafting may potentially cause formation of teratoma, a mixed tumor comprising tissue types generated from ectoderm, endoderm and mesoderm lineage cells [90]. Development of innovative techniques that eliminate teratoma formation from cells generated from pluripotent stem cells is needed to avoid tumors from such cells following grafting. Thus, although there are many sources of NSCs that may be used for grafting, because of issues discussed above, advancement towards clinical application of NSC therapy for neurological conditions has been moving slowly but steadily. Even so, there is a lot of enthusiasm and optimism for such therapies to advance into clinic in the coming years because preclinical studies reinforcing the safety and functional competence of NSCs derived from such cells are growing in the literature [89].
Highlights.
Hippocampal injuries typically evolve into cognitive dysfunction, depression, and/or epilepsy.
Neural stem cell grafting early after injury has promise for preventing neurological deficits.
Neural stem cell grafting early after injury modulates aberrant hippocampal post-injury plasticity.
Neural stem cell grafting adds new inhibitory GABA-ergic interneurons into the hippocampus.
Neural stem cell grafting adds new astrocytes secreting a variety of neurotrophic factors.
Issues such as source of neural stem cells need to be resolved prior to clinical application of neural stem cell grafts for hippocampal injury.
Acknowledgments
This work was supported by grants from the Texas A&M Health Science Center (Emerging Technology Funds from the State of Texas to A.K.S.), National Institute of Neurological Disorders and Stroke (R01 NS054780 & R01 NS043507 to A.K.S.), and the Department of Veterans Affairs (VA Merit Review Award to A.K.S.). Author thanks Drs. Bharathi Hattiangady, Ramkumar Kuruba and Bing Shuai for their excellent contributions to SHETTY LAB neural stem cell grafting studies discussed in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- 3.Reeves TM, Steward O. Changes in the firing properties of neurons in the dentate gyrus with denervation and reinnervation: implications for behavioral recovery. Exp Neurol. 1988;102:37–49. doi: 10.1016/0014-4886(88)90076-3. [DOI] [PubMed] [Google Scholar]
- 4.Shetty AK, Turner DA. Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol. 1999;414:238–254. doi: 10.1002/(sici)1096-9861(19991115)414:2<238::aid-cne7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–63. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- 6.Hunt RF, Scheff SW, Smith BN. Synaptic reorganization of inhibitory hilar interneuron circuitry after traumatic brain injury in mice. J Neurosci. 2013;31:6880–90. doi: 10.1523/JNEUROSCI.0032-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perederiy JV, Luikart BW, Washburn EK, Schnell E, Westbrook GL. Neural injury alters proliferation and integration of adult-generated neurons in the dentate gyrus. J Neurosci. 2013;33:4754–4767. doi: 10.1523/JNEUROSCI.4785-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shetty AK, Hattiangady B. Prospects of Stem Cell therapy for Temporal Lobe Epilepsy. Stem Cells. 2007;25:2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa S, Kitao Y, Hori O. Ischemia-induced neuronal cell death and stress response. 2007;9:573–587. doi: 10.1089/ars.2006.1516. [DOI] [PubMed] [Google Scholar]
- 10.Acharya MM, Hattiangady B, Shetty AK. Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol. 2008:363–404. doi: 10.1016/j.pneurobio.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitkänen A, Immonen RJ, Gröhn OH, Kharatishvili I. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia. 2009;50 (Suppl 2):21–9. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 12.Ubhi K, Masliah E. Alzheimer’s disease: recent advances and future perspectives. J Alzheimer’s Dis. 2013;33 (Suppl 1):S185–94. doi: 10.3233/JAD-2012-129028. [DOI] [PubMed] [Google Scholar]
- 13.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem. 2003;87:147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- 16.Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Scharfman HE, McCloskey DP. Postnatal neurogenesis as a therapeutic target in temporal lobe epilepsy. Epilepsy Res. 2009;85:150–61. doi: 10.1016/j.eplepsyres.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokaia M. Seizure-induced neurogenesis in the adult brain. Eur J Neurosci. 2011;33:1133–8. doi: 10.1111/j.1460-9568.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- 19.Hester MS, Danzer SC. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci. 2013;33:8926–36. doi: 10.1523/JNEUROSCI.5161-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorge RE, Acion L, Starkstein SE, Magnotta V. Hippocampal volume and mood disorders after traumatic brain injury. Biol Psychiatry. 2007;62:332–338. doi: 10.1016/j.biopsych.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Potvin O, Allen K, Thibaudeau G, Doré FY, Goulet S. Performance on spatial working memory tasks after dorsal or ventral hippocampal lesions and adjacent damage to the subiculum. Behav Neurosci. 2006;120:413–422. doi: 10.1037/0735-7044.120.2.413. [DOI] [PubMed] [Google Scholar]
- 22.Hattiangady B, Kuruba R, Shetty AK. Acute seizures in old age leads to a greater loss of CA1 pyramidal neurons, an increased propensity for developing chronic TLE and a severe cognitive dysfunction. Aging Disease. 2011;2:1–17. [PMC free article] [PubMed] [Google Scholar]
- 23.Hattiangady B, Shetty AK. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus. 2010;20:97–112. doi: 10.1002/hipo.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez RM, Ribak CE, Shapiro LA. Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy. Epilepsia. 2012;53 (Suppl 1):98–108. doi: 10.1111/j.1528-1167.2012.03481.x. [DOI] [PubMed] [Google Scholar]
- 25.Waldau B, Hattiangady B, Kuruba R, et al. Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells. 2010;28:1153–1164. doi: 10.1002/stem.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shetty AK. Progress in cell grafting therapy for temporal lobe epilepsy. Neurotherapeutics. 2011;8:721–735. doi: 10.1007/s13311-011-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehl M, Abrous DN. A new chapter in the field of memory: adult hippocampal neurogenesis. Eur J Neurosci. 2011;33:1101–1114. doi: 10.1111/j.1460-9568.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- 28.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharfman HE, Gray WP. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia. 2007;48 (Suppl 2):33–41. doi: 10.1111/j.1528-1167.2007.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jessberger S, Nakashima K, Clemenson GD, Mejia E, Mathews E, Ure K, et al. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci. 2007;27:5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shetty AK. Neural Stem Cell Therapy for Temporal Lobe Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 32.Kozhich OA, Hamilton RS, Mallon BS. Standardized Generation and Differentiation of Neural Precursor Cells from Human Pluripotent Stem Cells. Stem Cell Rev. 2012 doi: 10.1007/s12015-012-9357-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu T, Zhang M, Laurent T, Xie M, Ding S. Concise review: chemical approaches for modulating lineage-specific stem cells and progenitors. Stem Cells Transl Med. 2013;2:355–361. doi: 10.5966/sctm.2012-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hattiangady B, Shetty AK. Neural stem cell grafting in an animal model of chronic temporal lobe epilepsy. Curr Protoc Stem Cell Biol. 2011;Chapter 2(Unit2D):7. doi: 10.1002/9780470151808.sc02d07s18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shetty AK, Hattiangady B. Postnatal age governs the extent of differentiation of hippocampal CA1 and CA3 subfield neural stem/progenitor cells into neurons and oligodendrocytes. Int J Dev Neurosci. 2013 Jun 3; doi: 10.1016/j.ijdevneu.2013.05.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santilli G, Lamorte G, Carlessi L, Ferrari D, Rota Nodari L, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PLoS One. 2010;5:e8575. doi: 10.1371/journal.pone.0008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryu JK, Cho T, Wang YT, McLarnon JG. Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed AD brain. J Neuroinflammation. 2009;6:39. doi: 10.1186/1742-2094-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hattiangady B, Shetty AK. Neural stem cell grafting counteracts hippocampal injury-induced impairments in mood, memory and neurogenesis. Stem Cell Transl Med. 2012;1:696–708. doi: 10.5966/sctm.2012-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hattiangady B, Kuruba R, Shuai B, Waldau B, Shetty AK. Neural stem cell grafting after status epilepticus restrains spontaneous recurrent seizures and preserves cognitive and mood function. Society for Neuroscience Abstracts. 2012;35.07 [Google Scholar]
- 41.Hattiangady B, Shuai B, Cai J Coksaygan T, Rao MS, Shetty AK. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- 42.Teng YD, Benn SC, Kalkanis SN, Shefner JM, Onario RC, Cheng B, et al. Multimodal actions of neural stem cells in a mouse model of ALS: a meta-analysis. Sci Transl Med. 2012;4:165ra164. doi: 10.1126/scitranslmed.3004579. [DOI] [PubMed] [Google Scholar]
- 43.Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, et al. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med. 2012;4:155ra136. doi: 10.1126/scitranslmed.3004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao W, Yang Y, Wang Z, Liu A, Fang L, Wu F. Leukemia inhibitory factor inhibits T helper 17 cell differentiation and confers treatment effects of neural progenitor cell therapy in autoimmune disease. Immunity. 2011;35:273–284. doi: 10.1016/j.immuni.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Lee HJ, Lim IJ, Park SW, Kim YB, Ko Y, Kim SU. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012;21:2487–2496. doi: 10.3727/096368912X638964. [DOI] [PubMed] [Google Scholar]
- 46.Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pertusa M, Garcia-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29:1366–1379. doi: 10.1016/j.neurobiolaging.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 52.Graham BM, Richardson R. Acute systemic fibroblast growth factor-2 enhances long-term memory in developing rats. Neurobiol Learn Mem. 2009;91:424–430. doi: 10.1016/j.nlm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Kiyota T, Ingraham KL, Jacobsen MT, Xiong H, Ikezu T. FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer’s disease and has therapeutic implications for neurocognitive disorders. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E1339–1348. doi: 10.1073/pnas.1102349108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanter-Schlifke I, Georgievska B, Kirik D, Kokaia M. Seizure suppression by GDNF gene therapy in animal models of epilepsy. Mol Ther. 2007;15:1106–1113. doi: 10.1038/sj.mt.6300148. [DOI] [PubMed] [Google Scholar]
- 55.Paradiso B, Zucchini S, Su T, Bovolenta R, Berto E, Marconi P, et al. Localized overexpression of FGF-2 and BDNF in hippocampus reduces mossy fiber sprouting and spontaneous seizures up to 4 weeks after pilocarpine-induced status epilepticus. Epilepsia. 2011;52:572–578. doi: 10.1111/j.1528-1167.2010.02930.x. [DOI] [PubMed] [Google Scholar]
- 56.Shetty AK, Hattiangady B, Rao MS. Vulnerability of hippocampal GABA-ergic interneurons to kainate-induced excitotoxic injury during old age. J Cell Mol Med. 2009;13:2408–2423. doi: 10.1111/j.1582-4934.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Damgaard T, Plath N, Neill JC, Hansen SL. Extrasynaptic GABAA receptor activation reverses recognition memory deficits in an animal model of schizophrenia. Psychopharmacology (Berl) 2011;214:403–413. doi: 10.1007/s00213-010-2039-9. [DOI] [PubMed] [Google Scholar]
- 59.Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miltiadous P, Kouroupi G, Stamatakis A, Koutsoudaki PN, Matsas R, Stylianopoulou F. Subventricular zone-derived neural stem cell grafts protect against hippocampal degeneration and restore cognitive function in a the mouse following Intrahippocampal kainic acid administration. Stem Cells Transl Med. 2013:185–198. doi: 10.5966/sctm.2012-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, et al. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol. 2006;201:281–292. doi: 10.1016/j.expneurol.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 62.Wang E, Gao J, Yang Q, Parsley MO, Dunn TJ, Zhang L, et al. Molecular mechanisms underlying effects of neural stem cells against traumatic axonal injury. J Neurotruama. 2012;29:295–312. doi: 10.1089/neu.2011.2043. [DOI] [PubMed] [Google Scholar]
- 63.Bramlett HM, Kraydieh S, Green EJ, Dietrich WD. Temporal and regional patterns of axonal damage following traumatic brain injury: a beta-amyloid precursor protein immunocytochemical study in rats. J Neuropathol Exp Neurol. 1997;56:1132–1141. doi: 10.1097/00005072-199710000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Park D, Joo SS, Kim TK, Lee SH, Kang H, Lee HJ, et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012;21:365–371. doi: 10.3727/096368911X586765. [DOI] [PubMed] [Google Scholar]
- 65.Shear DA, Tate CC, Tate MC, Archer DR, LaPlaca MC, Stein DG, et al. Stem cell survival and functional outcome after traumatic brain injury is dependent on transplant timing and location. Restor Neurol Neurosci. 2011;29:215–225. doi: 10.3233/RNN-2011-0593. [DOI] [PubMed] [Google Scholar]
- 66.Sirven JI, Waterhouse E. Management of status epilepticus. Am Fam Physician. 2003;68:469–76. [PubMed] [Google Scholar]
- 67.Boggs JG. Mortality associated with status epilepticus. Epilepsy Currents. 2004;4:25–27. doi: 10.1111/j.1535-7597.2004.04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Löscher W. Strategies for antiepileptogenesis: Antiepileptic drugs versus novel approaches evaluated in post-status epilepticus models of temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 69.Ben-Ari Y. Kainate and Temporal Lobe Epilepsies: 3 decades of progress. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 70.Kleen JK, Scott RC, Lenck-Santini PP, Holmes GL. Cognitive and Behavioral Co-Morbidities of Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 71.Sankar R, Mazarati A. Neurobiology of Depression as a Comorbidity of Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 72.Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–24. doi: 10.1046/j.1528-1157.2001.28900.x. [DOI] [PubMed] [Google Scholar]
- 73.Temkin NR. Causes and prevention of symptomatic epilepsy. a clinical survey. New Horizons in the development of antiepileptic drugs II: The search for new targets. In: Löscher W, Schmidt D, editors. Epilepsy Res. Vol. 60. 2004. pp. 80–83. [DOI] [PubMed] [Google Scholar]
- 74.Dichter MA. Posttraumatic epilepsy: the challenge of translating discoveries in the laboratory to pathways to a cure. Epilepsia. 2009;50 (Suppl 2):41–45. doi: 10.1111/j.1528-1167.2008.02009.x. [DOI] [PubMed] [Google Scholar]
- 75.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parent JM, Kron MM. Neurogenesis and epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- 77.Scharfman HE, Pierce JP. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia. 2012;53 (Suppl 1):109–15. doi: 10.1111/j.1528-1167.2012.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hattiangady B, Shetty AK. Implications of decreased hippocampal neurogenesis in chronic temporal lobe epilepsy. Epilepsia. 2008;49 (Suppl 5):26–41. doi: 10.1111/j.1528-1167.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, et al. Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain. 2010;133:3359–3372. doi: 10.1093/brain/awq215. [DOI] [PubMed] [Google Scholar]
- 80.Fu LY, van den Pol AN. GABA excitation in mouse hilar neuropeptide Y neurons. J Physiol. 2007;579:445–464. doi: 10.1113/jphysiol.2002.019356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Redrobe JP, Dumont Y, St-Pierre JA, Quirion R. Multiple receptors for neuropeptide Y in the hippocampus: putative roles in seizures and cognition. Brain Res. 1999;848:153–166. doi: 10.1016/s0006-8993(99)02119-8. [DOI] [PubMed] [Google Scholar]
- 82.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 83.Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, et al. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–570. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- 84.Rodrigo C, Zaben M, Lawrence T, Laskowski A, Howell OW, Gray WP. NPY augments the proliferative effect of FGF2 and increases the expression of FGFR1 on nestin positive postnatal hippocampal precursor cells, via the Y1 receptor. J Neurochem. 2010;113:615–627. doi: 10.1111/j.1471-4159.2010.06633.x. [DOI] [PubMed] [Google Scholar]
- 85.Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 87.De Filippis L, Binda E. Self-renewal in the central nervous system: neural stem cells from embryo to adult. Stem Cells Transl Med. 2012;1:298–308. doi: 10.5966/sctm.2011-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ito D, Okano H, Suzuki N. Accelerating progress in induced pluripotent stem cell research for neurological diseases. Ann Neurol. 2012;72:167–174. doi: 10.1002/ana.23596. [DOI] [PubMed] [Google Scholar]
- 89.Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res. 2013;112:523–533. doi: 10.1161/CIRCRESAHA.111.256149. [DOI] [PubMed] [Google Scholar]
- 90.Marr RA, Thomas RM, Peterson DA. Insights into neurogenesis and aging: potential therapy for degenerative disease? Future Neurol. 2010;5:527–541. doi: 10.2217/FNL.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]