Abstract
Background
The widespread use of cannabis, the increasing legalization of “medical” cannabis, the increasing potency of cannabis and the growing recreational use of synthetic cannabinoid 1 receptor (CB1R) full agonists underscores the importance of elucidating the effects of cannabinoids on the CB1R system. Exposure to cannabinoids is known to result in CB1R downregulation. However, the precise time course of changes in CB1R availability in cannabis dependent subjects (CDs) following short and intermediate term abstinence has not been determined.
Methods
Using High Resolution Research Tomography (HRRT) and [11C]OMAR, CB1R availability as indexed by the volume of distribution (VT) [11C]OMAR was measured in male CDs (n=11) and matched healthy controls (HCs) (n=19). CDs were scanned at baseline (while they were neither intoxicated nor in withdrawal), and after 2 days and 28 days of monitored abstinence. HCs were scanned at baseline and a subset (n=4) was rescanned 28 days later.
Results
Compared to HCs, [11C]OMAR VT was 15% lower in CDs (effect size Cohen’s d=−1.11) at baseline in almost all brain regions. However, these group differences in CB1R availability were no longer evident after just 2 days of monitored abstinence from cannabis. There was a robust negative correlation between CB1R availability and withdrawal symptoms after 2 days of abstinence. Finally, there were no significant group differences in CB1R availability in CDs after 28 days of abstinence.
Conclusions
Cannabis dependence is associated with CB1R downregulation, which begins to reverse surprisingly rapidly upon termination of cannabis use and may continue to increase over time.
Keywords: Cannabis, cannabinoids, CB1R, tolerance, dependence, withdrawal, downregulation, upregulation, recovery
INTRODUCTION
Cannabis is the most commonly used illicit drug by adults in the U.S. (1). “Medical” marijuana is being legalized increasingly across the US and some states have legalized recreational cannabis use too. The average Δ9-tetrahydrocannabinol (THC) content of cannabis has increased (2), and highly potent, synthetic full cannabinoid 1 receptor (CB1R) agonists (e.g., Spice and K-2) are being used recreationally (3). Collectively, this underscores the importance of elucidating the effects of cannabinoids on the CB1R system.
Cannabinoids (CBs) including both synthetic CB1R agonists and those present in cannabis produce their psychoactive effects via activation of brain CB1Rs (4). Repeated exposure to CBs is associated with the development of tolerance and dependence (5, 6) which likely reflect adaptive changes in the CB1R system. In animals, chronic CB exposure is associated with a reduction in the number and function of CB1Rs (7–11). These changes have a distinct regional and temporal course and are related to the duration and magnitude of exposure; with greater downregulation in cortical versus subcortical regions (12, 13). Discontinuation of chronic, heavy CB exposure and the administration of CB1R antagonists to CB-dependent animals are associated with a withdrawal syndrome (14–16). Finally, prolonged abstinence in CB-dependent animals results in normalization of both the number and function of CB1Rs over 2 weeks, with faster recovery in subcortical versus cortical regions (10).
Hirvonen et al. (2012), using positron emission tomography (PET) imaging and the CB1R agonist ligand [18F]FMPEP-d2, demonstrated that chronic, heavy cannabis users showed 20% lower CB1R availability relative to controls (17). Consistent with animal studies, this reduction occurred in cortical but not in subcortical regions. A subset of cannabis users (n=14) who were scanned again after 13–32 days of monitored inpatient abstinence (17) showed an increase in CB1R availability in the same regions that had shown decreased CB1R availability at baseline. Collectively these findings were interpreted as evidence of CB1R downregulation with cannabis dependence that reversed with abstinence. This important first in vivo study also raises several questions. First, it remains unclear how quickly reversal of downregulation occurs. Second, since the sample consisted of subjects with very heavy cannabis use (10 ± 6 joints/day) for 12 (±7) years, whether modest cannabis use is associated with a similar magnitude of downregulation is not clear. Third, given the known interplay between CB1R and nicotinic receptor systems (18, 19), since the majority (>80%) of subjects were tobacco smokers, the extent to which tobacco use influenced the results could not be conclusively determined. Fourth, since healthy controls were not scanned twice, there was no control for interscan variability. Finally, the variability in the duration between the first and second scans (13–32 days) did not afford precision in characterizing the temporal profile of CB1R upregulation. A second study using [18F]MK-9470, a different PET tracer, found reduced global CB1R receptor availability (by 11.7%) in cannabis dependence (n=10) relative to controls (20). However, the characteristics of [18F]MK-9470, which shows primarily irreversible receptor binding, poses challenges. Furthermore, the validity of the simplified data analysis technique (mSUV) used has been challenged by several groups (21, 22).
This study aimed to first compare CB1R availability between cannabis dependent subjects (CDs) and healthy controls (HCs) and then to characterize the temporal course of changes in CB1R availability in CDs following short-(2 days) and intermediate-(28 days) term abstinence. To increase generalizability and also to address gaps in the literature, another aim was to measure CB1R availability in subjects with modest cannabis exposure. In order to address the potential confound in previous studies, this study aimed to measure CB1R availability in subjects who did not use tobacco. Finally, to rule out interscan variability as an explanation for any changes in CB1R availability in CDs, a small subset of HCs were scanned 4 weeks apart. CB1R availability was measured using the reversible ligand [11C]OMAR (23) based on absolute quantification using arterial sampling with metabolite analysis and tracer kinetic modeling (22), a method that does not have the limitations associated with the mSUV outcome measure. CB1R availability was hypothesized to 1) be lower in CDs at baseline, 2) increase after 2 days of abstinence due to compensatory upregulation, and 3) recover to normal levels after 28 days of abstinence.
METHODS
Subjects
Cannabis dependent males aged 18–35 years were studied. Cannabis dependence was operationalized as: 1) use of ≥ 30 joints or equivalents in the past 30 days, ≥ 21 days of cannabis use in the past 30 days, and ≥ 120 days of cannabis use in the past 6 months as estimated by a Timeline Follow Back (TLFB) approach, 2) ≥ 2 year history of regular cannabis use, 3) positive urine screen for cannabinoids but not any other drugs on at least 2 separate screening visits, 4) DSM-IV cannabis dependence, and 5) no self-reported problematic illicit substance use during the past three months as assessed by six-month TLFB. Age-matched (± 3 years) HCs without any DSM-IV Axis I or II disorders served as the comparison group. Common exclusion criteria to both groups were: 1) major DSM-IV diagnosis of Axis I disorder, 2) nicotine dependent tobacco users, 3) weekly alcohol consumption exceeding National Institute on Alcohol Abuse and Alcoholism (NIAAA) guidelines (4 or more drinks on any single day and 14 or more drinks per week), and 4) significant medical or neurological disease. Regulatory approvals, screening process, contingency management and study procedures are detailed in the supplementary materials. Furthermore, subjects were screened for both current and lifetime substance abuse and psychiatric problems using the Structured Clinical Interview for DSM-IV conducted by a research assistant and psychiatric evaluations by psychiatrists.
Magnetic resonance imaging (MRI) scans (3T) were collected during screening. Eligible CDs were instructed not to use cannabis after 6pm on Day-1 and then admitted to a closed inpatient research unit to ensure abstinence from cannabis. Both CDs and HCs underwent a PET scan on Day 0, the first full day of abstinence. CDs remained hospitalized and were scanned again after 2 days of abstinence. They were then discharged and required to attend the outpatient research clinic twice per week for 1) motivational enhancement, 2) escalating contingency management (Text S3), 3) to assess cannabis withdrawal and 4) confirmation of abstinence. The latter was confirmed at every visit by immediate spot urine drug testing and later offsite quantification of THC-COOH by GCMS (threshold of detection 5 ng/mL) (24, 25). To account for deliberate dilution of urine, urine collection was observed on a random basis and urinary THC-COOH:creatinine (T:Cr) ratio was calculated. Subjects were determined to be abstinent if their urinary T:Cr ratio decreased over time and did not increase by more than 50% relative to the prior test (26–28). CDs who demonstrated maintained abstinence (based on progressive decrease in urinary T:Cr ratio and self-reported abstinence) throughout the outpatient phase underwent a third PET scan on Day #28 of abstinence.
Imaging
CDs were scanned with PET and [11C]OMAR 1) no less than 8 hours but no more than 24 hours following last use i.e., while still dependent but neither intoxicated nor in withdrawal, 2) after 2 days of confirmed acute inpatient abstinence, to coincide with the time when cannabis withdrawal was expected to peak and 3) after 28 days of confirmed outpatient abstinence when the CB1R system is expected to recover completely (Table S2 and Text S4). HCs were scanned once and a subsample (n=4) was scanned again within 28 days to confirm long-term test-retest reliability.
PET scans were acquired as subjects rested in the HRRT scanner (207 slices, resolution better than 3 mm FWHM in 3D acquisition mode) (Siemens Medical Systems, Knoxville, TN). A transmission scan was obtained before the emission scan. Motion correction was performed dynamically with measurements from the Vicra (NDI Systems, Waterloo, Ontario) used by a dedicated list-mode reconstruction algorithm (29). A dynamic PET scan of up to 120 min duration was acquired after intravenous (IV) administration of up to 20 mCi of high-specific activity [11C]OMAR. In the first 7 min of the study, the arterial input functions were measured continuously (PBS-101, Veenstra Instruments, Joure, The Netherlands) using a continuous withdrawal system where the radioactivity in whole blood was measured with a calibrated radioactivity monitor. Subsequently, individual blood samples were taken at various time points and counted in a gamma counter (Wizard 1480; Perkin-Elmer, Waltham, MA), and selected samples were assayed for the presence of the parent radiotracer compound that had not been metabolized. These measurements were performed by high pressure liquid chromatography. In addition, the fraction of plasma radioactivity unbound to protein (fp) was determined by ultrafiltration (22).
Image Analysis
Summed PET images were registered to the subject’s T1-weighted MR images, which, in turn, were registered to an MR template. Gray matter regions of interest were predefined on a template (Anatomical Automatic Labeling (AAL) for SPM2). This process permitted direct, automatic determination of volume of distribution (VT) values using the multilinear analysis-1 (MA1) method with t*= 30 min (30). Predetermined regions-of-interest (ROIs) included the amygdala, caudate, cerebellum, anterior and posterior cingulate cortex, frontal cortex, hippocampus, hypothalamus, insula, occipital cortex, pallidum, putamen, temporal cortex, and thalamus.
Other Assessments
The Cannabis Withdrawal Assessment Scale (CWAS) (31), weight and vital signs were recorded in CDS periodically.
Statistical analyses
Since [11C]OMAR VT values, heretofore referred to as VT, across brain regions were highly correlated in CDs (r=0.80 to 0.98) and HCs (r=0.81 to 0.98), mean composite VT values were computed by averaging VT values across all brain regions for each individual. Mean composite VT levels at 1) baseline (no more than 24 hours from last exposure to cannabis), 2) early abstinence (2 days from last exposure to cannabis), and 3) prolonged abstinence scans (28 days from last exposure to cannabis) in CDs were compared to the baseline scan in HCs, using separate analysis of variance (ANOVA) models with group (CDs or HCs) as a between-subjects factor. Changes in composite VT levels from baseline to 28-day scan between CDs and HCs were compared using linear mixed models with group as a between-subjects factor and time as a within-subjects factor. Separate linear mixed models were fitted within group to assess changes in VT across the three scans among CDs and the two scans among HCs. Exploratory analyses were conducted for regional VT values by including region as an additional within-subjects factor in the models specified above. For each model described, all main and interaction effects were modeled and the best-fitting variance-covariance structure (e.g., unstructured, compound symmetry (CS), heterogeneous CS, first-order autoregressive (AR[1]) or heterogeneous AR[1]) was selected based on Bayesian Information Criteria (BIC). Age and BMI were in tested as covariates in all models but were not significant, did not change parameter estimates, and thus were dropped for parsimony. Given the exploratory nature of the regional analysis, results were not corrected for multiple comparisons. All comparisons were tested at an alpha=0.05 threshold. Creatinine-corrected urinary THC-COOH levels were analyzed in CDs with time as a within subject factor. Similarly, weight, vital signs and cannabis withdrawal symptoms were analyzed in CDs with time as a within subject factor. For weight and vital signs post hoc comparisons were conducted between the epochs before and after the initiation of abstinence. Spearman correlations were performed between baseline VT and both measures of cannabis exposure in CDs. Similarly, Spearman correlations were performed between VT and cannabis withdrawal both collected after 48 hours of abstinence.
RESULTS
Eleven, 10 and 8 CDs completed the first, second and third scans, respectively (Table 1). Twenty one matched HCs were scanned once a subset (n=4) of whom were rescanned 28 days after their first scan.
Table 1.
Demographic, Clinical And Radiochemical Information Of Subjects
| Cannabis Dependent Subjects (n=11) Means (±S.D) |
Healthy Controls (n=21) Means (±S.D) |
t-value | df | Pr > |t| | |
|---|---|---|---|---|---|
| AGE (YEARS) | 26.2 (5.7) | 29.6 (8.3) | −1.21 | 30 | 0.237 |
| BODY MASS INDEX | 26.8 (5.4) | 27.1 (5) | −0.17 | 30 | 0.866 |
| EDUCATION (YEARS) | 13.4 (1.9) | 14.3 (1.1) | −1.33 | 28 | 0.196 |
| DSM-IV NICOTINE DEPENDENCE | 0 | 0 | |||
| DSM-IV CANNABIS DEPENDENCE | 11 | 0 | |||
| AGE OF FIRST EXPOSURE TO CANNABIS | 16 (1.8) | 17.6 (3.1) | −1.39 | 18 | 0.18 |
| LAST MONTH CANNABIS USE (DAYS) | 24.4 (3.6) | 0.1 (.2) | 31.45 | 30 | <0.0001 |
| LAST MONTH CANNABIS USE (# JOINT EQUIVALENTS) | 59 (60.1) | 0.1 (0.2) | 4.68 | 26 | <0.0001 |
| 6 MONTHS CANNABIS USE (# DAYS) | 148.7 (25) | 0.1 (0.2) | 28.39 | 26 | <0.0001 |
| ALCOHOL USE (# DRINKS PER WEEK) | 5.22 (3.76) | 4.89 (3.03) | 0.2694 | 30 | 0.7895 |
| INJECTED ACTIVITY OF [11C]OMAR (MBq) | 569 (164) | 578(110) | −0.181 | 30 | 0.858 |
| INJECTED MASS OF [11 C]OMAR (μg) | 3.42 (2.80) | 3.17 (3.04) | 0.214 | 30 | 0.832 |
| FRACTION OF FREE [11C]OMAR IN PLASMA | 0.0012 (0.0005) | 0.0010 (0.0004) | 0.871 | 30 | 0.391 |
Abstinence from cannabis
Urinary creatinine-adjusted THC-COOH levels decreased significantly (F16,99=1.92, p<0.027) over time to undetectable levels on day 28 (Figure S1).
Comparison of baseline [11C]OMAR VT between CDs and HCs
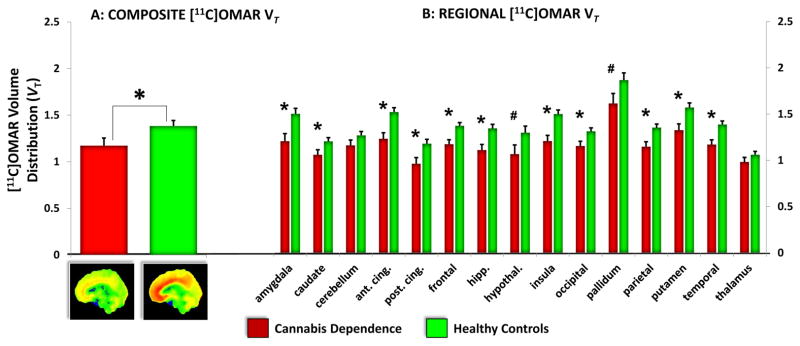
Composite brain VT in CDs who had last been exposed to cannabis no more than 24 hours earlier was significantly lower (group effect: t30=−2.91, p=0.007) compared to HCs (Figure 1). CDs had a mean 15% lower VT compared to HC (effect size Cohen’s d=−1.11) (Figures 1 and S2).
Figure 1. Composite & Regional CB1R Availability in cannabis Dependent subjects compared to Healthy Controls at Baseline.
A: Composite [11C]OMAR VT
The figure on the left shows the mean and standard error bars of composite [11C]OMAR VT in Cannabis Dependent subjects (n=11) (red) compared to Healthy Controls (n=21) (green). Statistically significant group differences are indicated with *. Below the figure on the left are grand averaged Grand Averaged [11C]OMAR VT in CDs (left) vs. HCs (right) at Baseline.
B: Regional [11C]OMAR VT
The figure on the right shows the mean and standard error bars of regional [11C]OMAR VT in Cannabis Dependent subjects (red) compared to Healthy Controls (green) across different brain regions. Statistically significant group differences are indicated with *. Trend level group differences are indicated with #.
The exploratory group by region analysis revealed a significant main effect of group (F1,30=8.07, p=0.008), region (F14,420=65.04, p<0.0001) and group by region interaction (F14,420=3.83, p<0.0001). The effects of group, region and the interaction between group and region survived Bonferroni adjustment for multiple comparisons. Post hoc analyses revealed significant (all p values<0.01) group differences in the amygdala, caudate, anterior and posterior cingulate cortex, frontal cortex, hippocampus, insula, occipital cortex, parietal cortex, putamen, and temporal cortex with trend level differences in the hypothalamus and pallidum (p=0.06). The only regions where there were no significant group differences were the cerebellum and thalamus. Adjusting for multiple comparisons, group differences in the anterior cingulate cortex, frontal cortex, hippocampus, insula, parietal cortexand temporal cortex remained significant with trend level differences in the amygdala and putamen.
Comparison of [11C]OMAR VT between CDs and HCs over time
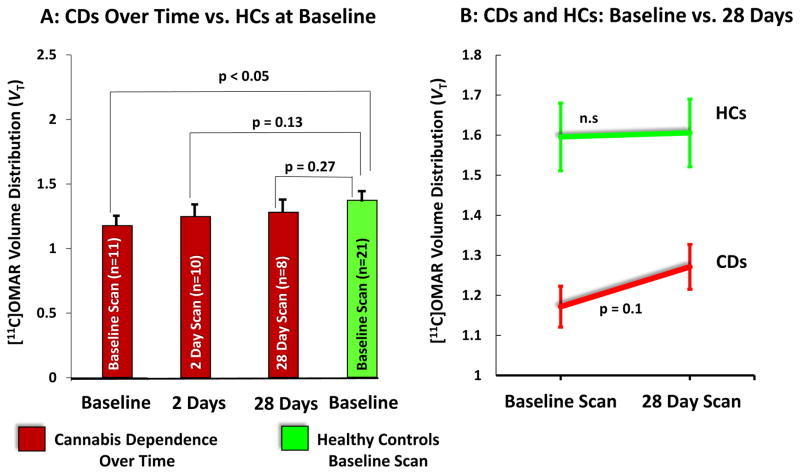
While still lower, there were no statistically significant differences between composite brain or regional VT measured after both 2 days (n=10) and 28 days (n=8) of monitored abstinence from cannabis in CDs compared to the baseline scan in HCs (Figure 2A).
Figure 2. Composite CB1R Availability in CDs vs. HCs Over Time.
A: CDs over time vs. HCS at baseline
The figure on the left shows the mean and standard error bars of composite [11C]OMAR VT in Cannabis Dependent subjects (red) at baseline (n=11), and after 2 (n=10) and 28 (n=8) days of abstinence compared to Healthy Controls (n=21) (green).
B: CDs and HCs: baseline vs. 28 Days
The figure on the right shows the differences in composite [11C]OMAR VT between baseline and 28 days in Cannabis Dependent subjects (red) (n=8), and Healthy Controls (n=4) (green).
In the subset of HCs (n=4), there were no significant differences in composite brain or regional VT measured at baseline and 28 days (F1,87=0.01, p=0.91)(Table S3)). In contrast, there was a trend towards an increase in composite brain or regional VT measured at baseline and 28 days in CDs (Figure 2B).
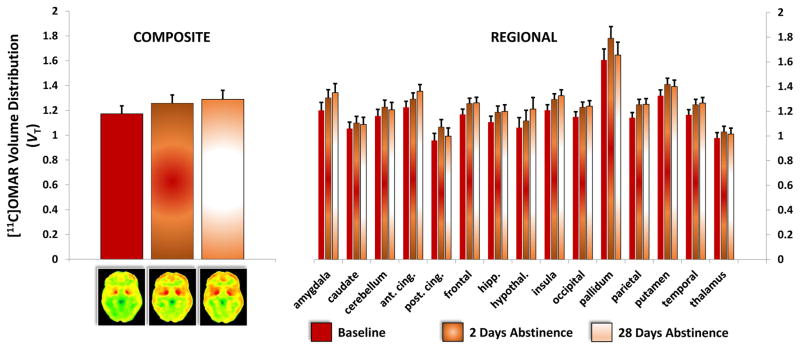
Change in [11C]OMAR VT within CDs over time
While composite VT increased with abstinence (Figure 3), the increase from baseline (1.172 ± 0.137) to after 2 days of abstinence (1.257 ± 0.189) trended to significance (F1,9=3.57, p=0.09). There was an increase in regional VT from baseline to 2 days of abstinence (scan: F1,245=3.64, p=0.056), but no significant scan by region interaction (scan*region: F14,245=0.5, p<0.93).
Figure 3. Composite and Regional CB1R Availability in CDs Over Time.
The figure on the left shows the mean and standard error bars of composite [11C]OMAR VT in Cannabis Dependent subjects at baseline (n=11), after 2 days of abstinence (n=10) and after 28 days of abstinence (n=8). Below the figure on the left are the corresponding grand averaged Grand Averaged [11C]OMAR VT in CDs at baseline (n=11), after 2 days of abstinence (n=10) and after 28 days of abstinence (n=8).
The figure on the left shows the mean and standard error bars of regional [11C]OMAR VT in Cannabis Dependent subjects at baseline (n=11), after 2 days of abstinence (n=10) and after 28 days of abstinence (n=8). See text for statistics.
The increase in composite VT from baseline (1.172 ± 0.137) to after 28 days of monitored inpatient abstinence (1.287 ± 0.148) trended towards significance (F1,9=3.46, p<0.1). There was a trend towards an increase in regional VT from baseline to 28 days of abstinence (scan: F1,245=3.14, p=0.078) with a significant scan by region interaction effects (scan*region: F14,245=6.29, p<0.0001). None of the comparisons across regions survived Bonferroni correction.
Cannabis Withdrawal and Relationship to [11C]OMAR VT
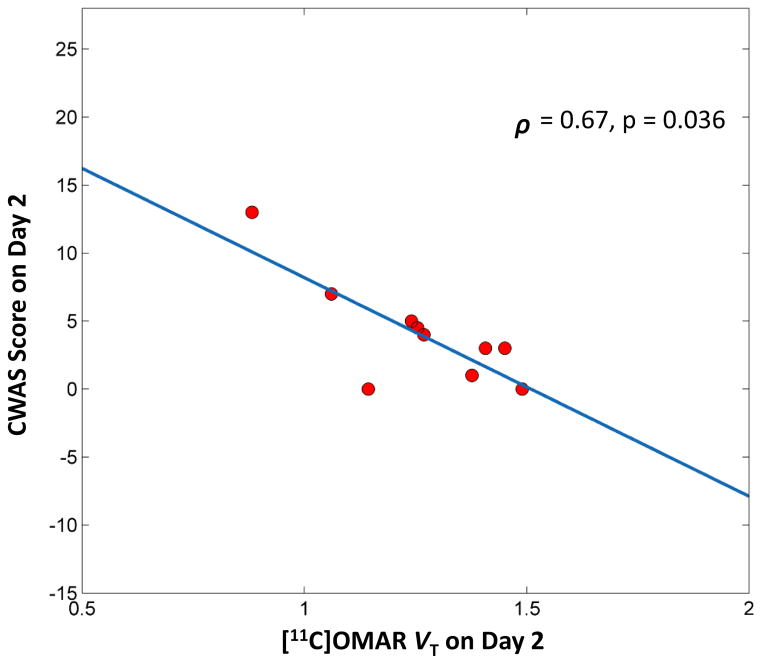
There were significant changes in withdrawal symptoms over time (time: F14,113=1.86, p=0.037) (Figure S3). Interestingly, cannabis withdrawal and composite VT were highly correlated on day 2, when withdrawal symptoms are predicted to peak (ρ =0.665, p=0.036) (Figure 4).
Figure 4. The relationship between Cannabis Withdrawal and Composite CB1R Availability in CDs.
The figure shows the relationship between cannabis withdrawal symptoms measured by the Cannabis Withdrawal Assessment Scale and composite [11C]OMAR VT in Cannabis Dependent subjects after 2 days of abstinence when withdrawal is expected to peak.
Relationship between [11C]OMAR VT and patterns of cannabis use
There were no significant correlations between baseline composite VT and either 1) cannabis use in the past 30 days, or 2) baseline THC/Creatinine ratio.
Weight in CDs over time
There was a significant reduction in weight over time (time: F16,121=1.88, p=0.029) (Figure S4). Post hoc contrasts revealed a trend towards weight loss during the post abstinence period (Pre- vs. Post abstinence: F1,121=3.48, p=0.065).
DISCUSSION
This is the first study to our knowledge examining the temporal course of changes in CB1R availability in cannabis dependence following both short and intermediate-term abstinence from cannabis. CB1R availability as indexed by [11C]OMAR VT was 14.85% lower in CDs (effect size Cohen’s d=−1.11) at baseline, (while they were neither intoxicated nor in withdrawal) compared to HCs. These differences were significant in all brain regions except the thalamus and cerebellum. These findings cannot be attributed to group differences in peripheral distribution and metabolism of [11C]OMAR. The significant group differences in CB1R availability at baseline were no longer statistically significant after just 2 days of monitored abstinence from cannabis. Similarly, relative to HCs scanned at baseline there were no significant differences in CB1R availability in CDs after 28 days of abstinence. Also, while CB1R availability within CDs increased over time with increasing duration of abstinence, these changes were only trend-level changes. Finally, there was a robust negative correlation between CB1R availability and withdrawal symptoms. Taken together, these data suggest that cannabis dependence and withdrawal are associated with CB1R downregulation, which begins to reverse surprisingly rapidly upon termination of cannabis use and may continue to increase over time.
These findings are consistent with and extend those of Hirvonen et al. (32) and Ceccarini et al., (20). However, while we observed reductions in the same regions of a similar magnitude as Hirvonen et al., we also observed decreases in subcortical areas (Table S4) suggesting more widespread decreases in brain CB1R availability.
Residual Cannabinoids
Cannabis users are known to have persistently detectable peripheral levels of cannabinoids (THC and THC-COOH) despite confirmed abstinence (33, 34), and human postmortem studies have detected cannabinoids in brain even though they were undetectable in blood (35). This raises the possibility that lower CB1R availability in CDs may be due to residual cannabinoids that interfere with the binding of CB1R PET tracers.
However, in mice chronically treated with THC, the affinity (Kd) of the CB1R antagonist [3H]SR141716 did not differ between THC- and vehicle-treated mice at any time point (including 1 day) after discontinuation of THC administration, suggesting that there was no residual THC in the brain at any time point after cessation of THC administration (10). Finally, in non-human primates we found that intravenous THC administration at a recreationally relevant dose did not block [11C]OMAR binding (unpublished data); furthermore, the administration of both direct and indirect CB1R agonists to rats did not displace [11C]MePPEP binding (36). Collectively, these findings suggest that decreased CB1R availability in CDs cannot be attributed to the presence of residual cannabinoids in the brain.
Tobacco Use
In contrast to both Hirvonen et al. (80% smokers) and Ceccarini et al., (60% smokers), the current study excluded tobacco smokers demonstrating that decreased CB1R availability in CDs cannot be attributed to tobacco exposure.
Temporal Course of Changes
Group differences in CB1R availability that were present at baseline were no longer evident after just 2 days of abstinence, suggesting that CB1R upregulation begins quickly after abstinence. Similar to these findings Ceccarini et al., studied CDs after ~5 days of abstinence and observed no statistically significant differences in CB1R availability in most areas except for four (Table S5).
There were no group differences between CB1R availability measured after 28 days of abstinence. These findings are similar to those of Hirvonen et al., who reported that after 26±5 days of monitored abstinence, CB1R upregulated specifically in those brain regions that had shown decreases at baseline except for the hippocampus. The latter is likely related to the heavier cannabis exposure in the sample of Hirvonen et al., compared to ours (1–2 versus 10±6 joints/day).
Relationship to cannabis exposure
Despite heavier cannabis exposure in the Hirvonen et al., study relative to ours, the magnitude of reductions in CB1R availability were comparable ~15% versus ~20% across studies. We failed to find any relationship between CB1R availability and cannabis use in the last 30 days. Similarly, both Hirvonen et al. and Ceccarini et al., did not find a correlation between CB1R availability and current cannabis use. Collectively, these findings suggest that recent cannabis consumption may not impact the degree of CB1R downregulation.
Relationship to cannabis withdrawal
There was a robust negative correlation between CB1R availability and cannabis withdrawal symptoms, both measured 2 days after abstinence from cannabis. Interestingly, unlike our study, Hirvonen et al. did not measure CB1R availability at a time coincident with the estimated time of peak withdrawal and did not find any correlation between CB1R availability and measures of withdrawal or craving.
Clinical Implications
The animal literature (8–11, 37), the findings of Hirvonen et al., Ceccarini et al., and the current study, provide strong evidence of CB1R downregulation in cannabis dependence, with even moderate daily cannabis use. Furthermore, we and others have shown that minimal cannabis use is associated with a blunted response to an array of CB effects (38–41). Thus, cannabis exposure, tolerance and CB1R downregulation are linked. In the context of “medical” marijuana, the dose of cannabinoid-based medications would need to be adjusted (increased) accordingly.
Furthermore, acute withdrawal may result from dysregulation of the eCB system (i.e., the simultaneous clearance of THC while CB1Rs are upregulating). Given the high negative correlation between CB1R availability and withdrawal symptoms, strategies that activate CB1Rs by increasing levels of anandamide and 2-arachidonoylglycerol through inhibition of their degradation enzymes fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL), respectively, should be tested to treat withdrawal symptoms and dependence. Finally, when individuals who were receiving cannabinoid-based medications stop taking them for more than a few weeks, the dose may need to be adjusted to account for CB1R upregulation during the pause.
Conclusions
CB1R availability is decreased in most brain regions in chronic moderate daily cannabis smokers. Significant CB1R upregulation occurs begins within merely 2 days of abstinence, and continues over 4 weeks. Despite 4 weeks of abstinence, CB1R availability in CDs did not reach healthy control levels. Whether, CB1R upregulation continues beyond 4 weeks is not known as is whether decreased CB1R availability in CDs reflects a state rather than a trait feature. Alternatively the lack of recovery of CB1R availability to healthy control levels following 4 weeks of abstinence may be due to inadequate sample size or that there are reductions in CB1R deficits that are present in CDs that preexist their use of cannabis. Future studies should determine whether sporadic cannabis smokers or CD females show similar downregulation, and characterize the complete temporal profile of CB1R upregulation. Longer follow-up will be necessary to determine to what extent the observed reductions in CB1R availability reflect trait vs. state features. Finally, it will be important to establish the relationship between changes in receptor levels with changes in indices of neural function e.g., cognition.
Supplementary Material
Acknowledgments
The authors wish to acknowledge support from the National Institute of Drug Abuse (21DA030702-01A1 to DCD), National Institute of Mental Health (R21MH094961 to DCD, R01MH096876 to AN, and R25MH071584 supporting RR), the Department of Veterans Affairs, the Yale Center for Clinical Investigation (YCCI) for their contributions to the success of this project. The authors wish to acknowledge the contributions of the Clinical Neuroscience Research Unit (CNRU) staff of the Connecticut Mental Health Center (CMHC) in caring for these subjects during the inpatient phase of the study.
Footnotes
Funding and Disclosure
Jose Cortes, Gina Creatura, Alexander Neumeister, Brian Pittman, Beata Planeta, Patrick D. Skosnik, Marc Normandin, Toral Surti, Drs. Surti, Michael Kapinos, Jim Ropchan and Halle Thurnauer, report no biomedical financial interests or potential conflicts of interest. Mohini Ranganathan has in the past three years or currently receives research grant support administered through Yale University School of Medicine from Eli Lilly Inc. Deepak Cyril D’Souza has in the past three years or currently receives research grant support administered through Yale University School of Medicine from Astra Zeneca, Abbott Laboratories, Eli Lilly Inc., Forest Laboratories, Organon, Pfizer Inc., and Sanofi; he is a consultant for Bristol Meyers Squibb and Johnson and Johnson. Yiyun (Henry) Huang has in the past three years or currently receives research support administered through Yale University from Eli Lilly Inc., Pfizer Inc., and UCB Biopharma SPRL. Richard E. Carson has in the past three years or currently receives research support administered through Yale University from Abbvie, Aptuit, Astellas Pharma, Bristol-Myers Squibb, Eli Lilly Inc., Pfizer Inc., Taisho Pharmaceuticals, and UCB Pharma SA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SAMHSA; Aministration SAaMHS, editor. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 2.Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al. Potency trends of Delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 3.Spaderna M, Addy PH, D’Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl) 2013;228:525–540. doi: 10.1007/s00213-013-3188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mechoulam R, Hanus LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nature reviews Neuroscience. 2014;15:757–764. doi: 10.1038/nrn3811. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. Journal of Clinical Pharmacology. 1981;21:143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 7.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 8.Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- 9.Oviedo A, Glowa J, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- 10.Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70:986–996. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- 11.McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, et al. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero J, Garcia-Palomero E, Castro JG, Garcia-Gil L, Ramos JA, Fernandez-Ruiz JJ. Effects of chronic exposure to delta9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions. Brain Res Mol Brain Res. 1997;46:100–108. doi: 10.1016/s0169-328x(96)00277-x. [DOI] [PubMed] [Google Scholar]
- 13.Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 15.Lichtman AH, Martin BR. Marijuana withdrawal syndrome in the animal model. J Clin Pharmacol. 2002;42:20S–27S. doi: 10.1002/j.1552-4604.2002.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 16.Aceto MD, Scates SM, Lowe JA, Martin BR. Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. Eur J Pharmacol. 1995;282:R1–2. doi: 10.1016/0014-2999(95)00447-s. [DOI] [PubMed] [Google Scholar]
- 17.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 20.Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K. [F]MK-9470 PET measurement of cannabinoid CB receptor availability in chronic cannabis users. Addict Biol. 2013 doi: 10.1111/adb.12116. [DOI] [PubMed] [Google Scholar]
- 21.Hirvonen J, Terry GE, Halldin C, Pike VW, Innis RB. Approaches to quantify radioligands that wash out slowly from target organs. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37:917–919. doi: 10.1007/s00259-010-1426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Normandin MD, Zheng MQ, Lin KS, Mason NS, Narendran R, Lin SF, et al. Imaging the cannabinoid CB1 receptor in human with [11C]OMAR: Assessment of kinetic analysis methods, test-retest reproducibility, and gender differences. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1038/jcbfm.2015.46. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horti AG, Fan H, Kuwabara H, Hilton J, Ravert HT, Holt DP, et al. 11C-JHU75528: a radiotracer for PET imaging of CB1 cannabinoid receptors. J Nucl Med. 2006;47:1689–1696. [PubMed] [Google Scholar]
- 24.D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57:594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 25.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 26.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. Journal of Abnormal Psychology. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 27.Kouri EM, Pope HG., Jr Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8:483–492. doi: 10.1037//1064-1297.8.4.483. [DOI] [PubMed] [Google Scholar]
- 28.Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- 29.Carson RE, Barker WC, Liow J-S, Adler S, Johnson CA. Design of a motion-compensation OSEM List-mode Algorithm for Resolution-Recovery Reconstruction of the HRRT. Conf Record IEEE Nuclear Science Symposium and Medical Imaging; Portland, OR. 2003. [Google Scholar]
- 30.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22:1271–1281. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 31.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, et al. Reduced cannabinoid CB1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psychiatry. 2013;18:916–921. doi: 10.1038/mp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Herning RI, Cadet JL, et al. Implications of plasma Delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol. 2009;33:469–477. doi: 10.1093/jat/33.8.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Pope HG, Herning R, et al. Do Delta9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–2048. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mura P, Kintz P, Dumestre V, Raul S, Hauet T. THC can be detected in brain while absent in blood. J Anal Toxicol. 2005;29:842–843. doi: 10.1093/jat/29.8.842. [DOI] [PubMed] [Google Scholar]
- 36.Terry G, Liow JS, Chernet E, Zoghbi SS, Phebus L, Felder CC, et al. Positron emission tomography imaging using an inverse agonist radioligand to assess cannabinoid CB1 receptors in rodents. NeuroImage. 2008;41:690–698. doi: 10.1016/j.neuroimage.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breivogel CS, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR. The effects of delta9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. European Journal of Pharmacology. 2003;459:139–150. doi: 10.1016/s0014-2999(02)02854-6. [DOI] [PubMed] [Google Scholar]
- 38.D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranganathan M, Braley G, Pittman B, Cooper T, Perry E, Krystal J, et al. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl) 2009;203:737–744. doi: 10.1007/s00213-008-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, et al. Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Delta(9)-THC in humans. Neuropsychopharmacology. 2012;37:1632–1646. doi: 10.1038/npp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.