Abstract
Membrane proteins are very important in controlling bioenergetics, functional activity, and initializing signal pathways in a wide variety of complicated biological systems. They also represent approximately 50% of the potential drug targets. EPR spectroscopy is a very popular and powerful biophysical tool that is used to study the structural and dynamic properties of membrane proteins. In this article, a basic overview of the most commonly used EPR techniques and examples of recent applications to answer pertinent structural and dynamic related questions on membrane protein systems will be presented.
Keywords: Membrane proteins, Electron paramagnetic resonance spectroscopy, Site-directed spin labeling, DEER, Structural topology and dynamics
Introduction
Membrane proteins
Membrane proteins are intermediates to the cells and play an essential role in controlling the cell function, ion movement across a cell, and signal transduction within cell membranes. Genes encoding membrane proteins consists of ~30 % of human and E. coli genomes [1–3]. Mutations in genes and misfolding of membrane proteins are linked to several human dysfunctions, disorders and diseases, e. g., rhodopsin misfolding causes retinitis pigmentosa, and mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) can cause a potentially fatal disease in children [4,5]. More than 50% of membrane proteins are potential drug targets [6,7]. Detailed structural and dynamic information is very important to understand the proper functions and regulations of membrane proteins [8–10]. However structure and dynamic information on membrane proteins is still lagging behind those of soluble proteins. Challenges in studying membrane proteins arise due to the hydrophobic nature of membrane proteins making overexpression, purification, and crystallization more difficult and lacking of suitable solubilizing membrane mimetics [11]. Membrane proteins are incorporated into a lipid bilayer in several different fashions or orientations. The membrane bound helices may be short, long, kinked, or interrupted in the middle of the lipid bilayer. They may cross the membrane at different angles, lie flat on membrane surface or form re-entrant loops. Figure 1 shows an illustration of a membrane peptide (acetylcholine receptor (AchR) M2δ, PDB entry 1EQ8) incorporated into lipid bilayers (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)) [12]. Figure 1 was prepared using visual molecular dynamics (VMD) and molecular modeling was performed using CHARMM-GUI (http://www.charmm-gui.org) [13,14].
Figure 1.
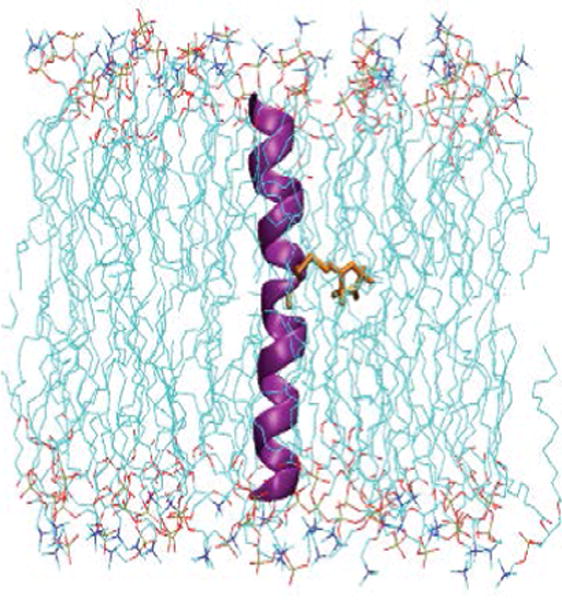
Cartoon representation of a membrane peptide (acetylcholine receptor (AchR) M2δ, PDB entry 1EQ8) incorporated into lipid bilayers (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)). Methanethiosulfonate spin label (MTSL) (orange color) has been attached at 11th position. Figure was prepared using visual molecular dynamics (VMD) and molecular modeling was performed using CHARMM-GUI (http://www.charmm-gui.org).
X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy are the two most widely used biophysical techniques for obtaining detailed structural information on biological systems. NMR spectroscopy can also provide dynamic information for a variety of biological systems. These methods have their own advantages and limitations. Solution NMR can provide structural information in a physiologically relevant environment, however, it is limited due to size restrictions (≤ ~50 kD) [15–18]. NMR structural studies on membrane proteins are also challenging due to the size of the micelle complex and increased spectral linewidth [19]. X-ray crystallography provides highly resolved structural information, but cannot provide detailed dynamic information [20]. Additionally, the hydrophobic surfaces of membrane protein often complicate the crystallization process, limiting the use of X-ray crystallographic techniques for many membrane protein systems [16]. Electron paramagnetic resonance (EPR) spectroscopy has been developed as a powerful biophysical technique to resolve these limitations and provide prominent solutions to obtain structural and dynamic information on peptides, proteins, macromolecules, and nucleic acids [9,10,21–27]. EPR spectroscopy measures an absorption of microwave radiation corresponding to the energy splitting of an unpaired electron when it is placed in a strong magnetic field. The simplest EPR active system consists of a single unpaired electron spin residing in a molecular orbital. In a typical continuous wave (CW)-EPR experiment, a fixed microwave frequency is applied and the magnetic field (B0) is varied. The EPR transition occurs when the energy separation between the two electron spin states matches the constant microwave energy. This phenomenon is known as resonance [28]. In addition to varying B0, the field is also modulated to improve the signal to noise of the spectra. This gives rise to the derivative lineshape typically observed in most EPR spectra.
Spin Labeling EPR
Earlier EPR studies were restricted to EPR active transition metals and the biological samples containing naturally occurring radicals. Molecular biology techniques have been developed to incorporate stable radicals at specific locations on biological systems. These techniques are known as spin labeling. Spin labeling techniques have extended the application of EPR spectroscopy to nearly any biological system. The site-specific incorporation of unpaired electrons into biomolecules in the form of spin labels is known as site-directed spin labeling (SDSL) [29,30]. In SDSL experiments, all native nondisulfide bonded cysteines are removed by replacing them with another amino acid such as alanine. A unique cysteine residue is then introduced into a recombinant protein using site-directed mutagenesis, and subsequently reacted with a sulfhydryl-specific nitroxide reagent to generate a stable spin label side-chain [26,30,31]. Figure 2 shows the structure of the most commonly used spin label, methanethiosulfonate spin label (MTSL), and the resulting side-chain produced by reaction with the cysteine residue of the protein [10].
Figure 2.
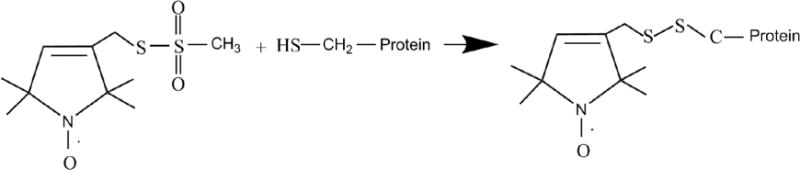
Structure of MTSL (Methanethiosulfonate spin label) and the resulting side-chain produced by reaction with the cysteine residue of the protein.
EPR spectroscopy is a very sensitive technique when compared to NMR spectroscopy. It offers up to three orders of magnitude higher sensitivity than that of NMR and does not rely on expensive isotopic labels [24]. It can be applied to any size protein and is not limited to the optical properties of the sample. EPR experiments can be performed on samples ranging from proteins in solution to highly packed membrane suspensions, tissue samples, ammonium sulfate-precipitated solids, or samples frozen and maintained at cryogenic temperatures [32]. EPR experiments are routinely performed from ~ 70 nanoliter of sample at W-band (94 GHz) to several mL of sample or even small animals at L-band (1–2 GHz) [33,34]. EPR spectroscopy can answer pertinent structural and dynamic questions related to both solution and membrane bound proteins that are difficult to be solved by traditional methods [9,22,27,35]. Continuous wave EPR (CW-EPR) spectroscopy of spin-labeled molecules reveals structural and dynamic information about the motion of the nitroxide side-chain, solvent accessibility, the polarity of its surrounding environment, and intra- or intermolecular distances between two nitroxides or a single nitroxide and another paramagnetic center in the system [26]. The lineshape analysis of the EPR data for a series of spin-labeled protein sequences can probe structural model of the protein with a spatial resolution at the backbone level [36–39]. While EPR has several advantages over the existing biophysical techniques, it relies on unpaired electrons which might not be relevant for all the systems to be studied. Some of the EPR experiments need temperatures as low as 4 K for signal detection to investigate certain biological systems (e.g., metal complexes) and may be an expensive constraint.
EPR spectroscopy applied to membrane proteins
SDSL coupled with EPR spectroscopy has been widely used to study membrane proteins. This is a very broad topic, which will be discussed in an introductory fashion with recent examples in the following sections. For a more in depth background, the following are excellent reviews [9,10,35,40].
Structural topology and dynamics of membrane proteins
The basic information contained within the EPR spectrum is the motion of spin label, which reflects the environment surrounding it. For spin labels that are moving very rapidly in solution, the spectrum reduces to three sharp peaks. Conversely, if the spin label motion is very slow such that it is motionless, the spectrum is in the rigid limit [41]. In a rigid limit spectrum, it is as if the sample has been frozen and the full orientation dependent parameters are observed. For systems in which the spin label movement falls between these two regions, a correlation time (τc) can be determined that indicates the dynamics properties of the spin label located at the specific site [41]. Figure 3 shows a series of EPR spectra simulated corresponding to different rotational correlation times [42]. When the spin label motion is fast (EPR timescale), the EPR spectrum posses three sharp lines of approximately similar height (Figure 3(A)). As the motion slows down, the EPR lines broaden resulting in decrease in their amplitudes (see Figures 3(B) and (C)). For the motionless spin label, a powder pattern lineshape is observed (see Figure 3(D)), also known as rigid limit spectrum. The overall mobility of the nitroxide spin label attached to the protein or peptide is a superposition of the contributions from the motion of the label relative to the peptide backbone, fluctuations of the α-carbon backbone, and the rotational motion of the entire protein or peptide. Under experimental conditions, these motions can be isolated from the EPR spectrum. The inverse linewidth of the central line of the EPR spectrum provides a measure of relative mobility [9,43,44]. The inverse linewidth mobility against the amino acid sequence can produce a periodic data profile, which can be used to predict the local secondary structure of the proteins and peptides [9,10,45]. There are several biologically important membrane proteins (e.g., prokaryotic potassium channel KcsA, KCNE1, lactose permease protein, and KvAP voltage-sensing domain) that have used CW-EPR spectroscopy to probe their secondary structure [43,44,46,47]. This method can also be used to indentify functional domains in high molecular weight proteins, supramolecular complexes and membrane proteins [20].
Figure 3.
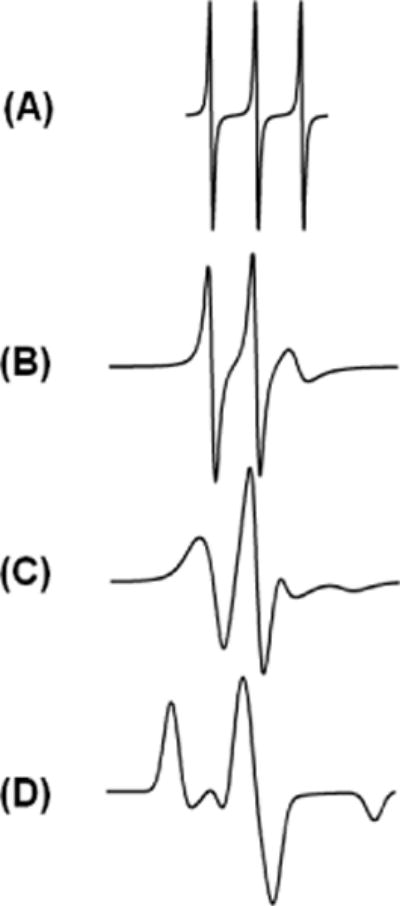
CW-EPR spectra of different spin label side chain motions. Spectra were simulated using EasySpin simulation software [41].
The changes in the spin-label mobility can be used to investigate the peptide-membrane binding activities [40]. In the aqueous phase, a spin-labeled peptide or small protein rapidly tumbling leads to an isotropic spectrum with a rotational correlation time of less than nanosecond. However in a membrane, spin labeled peptides experience restricted mobility, resulting in a broader EPR spectrum with two motional components resulted from the superposition of the signals arising from a free and bound peptide [40,48].
Membrane protein topology can be probed with respect to the membrane using nitroxide based SDSL EPR [10,49,50]. Power saturation EPR experiments can be used to determine a residue’s accessibility to different paramagnetic reagents [50].
A recent example of using nitroxide based site-directed spin labeling EPR is a study of the integrin β1a [49]. Integrins are heterodimeric membrane proteins that regulate essential process related to cell-cell and cell-matrix interactions, i.e., cell migration, cell growth, extracellular matrix assembly and tumor metastasis. Yu et al. used CW-EPR lineshape analysis of 26 consecutive single spin-labeled mutants on the transmembrane (TM) domain of integrin β1a to determine the side-chain mobility and solvent accessibility in detergent micelles and liposomes [49]. The mobility data identified two integrin β1a-TM regions with different motional properties in micelles and a highly immobile non-continuous integrin β1a-TM helix in liposomes. Additionally, the comparison of mobility and accessibility data of transmembrane and cytoplamic domain of integrin β1a identified a monomeric state in detergent micelles and leucine-zipper-like oligomeric clusters in liposomes.
Nitroxide based CW-EPR spectroscopy at X-band can also be used to study membrane topology of membrane proteins/peptides bound to aligned phospholipid bilayers [51,52]. Recently, the Lorigan lab used the membrane alignment technique coupled with dipolar broadening CW-EPR to determine the distance and relative orientation of two nitroxide spin labels on the integral membrane peptide (M2δ segment of the acetylcholine receptor (AchR)) and peripheral membrane peptide (antimicrobial peptide magainin-2) in 1,2-dimyristoyl-sn-glycerol-3-phosphocholine (DMPC) vesicles [52,53].
Distance Measurement of Membrane Proteins
Distance measurement between two spin labels is one of the most commonly and rapidly developing structure biology techniques of EPR spectroscopy. Distances can be measured in the terms of either intramolecular distances on the same protein or intermolecular distances between sites on different proteins [32]. This is a powerful approach for studying protein-protein interactions. Using double labeling site-directed spin labeling EPR techniques, distance measurement can be used to probe secondary, tertiary and quaternary structures [32]. CW dipolar broadening EPR technique can provide pertinent structural and functional dynamic information over an intermediate distance range of 8–30Å [54,55].
Double electron-electron resonance (DEER) is also known as pulse electron double resonance (PELDOR). In DEER, the spin echo decay of one set of spin labels is modulated by an intramolecular dipolar interaction with another set of spin labels on the same protein molecule and/or by an intermolecular dipolar interaction with the same set of spins or another set of spins on a separate molecule. The periodicity of the oscillating echo produced by the former process directly reflects the average distance. The latter process is an exponential decay which dampens the oscillation, and is referred to as the background. The background contribution is removed from the echo decay during the data analysis, yielding a distance distribution characterized by the weighted average distance and a standard deviation. DEER spectroscopy is a very powerful and popular structural biology technique for measuring longer range distances (20–80 Å) for probing the structure of wide variety of biological systems [56–64]. Additionally, DEER at high field can be used to measure the relative orientation of the spins [65]. However, the application of DEER spectroscopy to study membrane proteins is very challenging due to much shorter transverse relaxation/phase memory times and poor DEER modulation in more biologically relevant liposomes as compared to water soluble proteins or membrane proteins in detergent micelles. The short phase memory times are due to uneven distribution of the spin labeled proteins within the membrane, which creates local inhomogeneous pockets of high spin concentration [61]. The high effective protein concentration in the proteoliposomes introduces a strong background contribution with severe limits on sensitivity, distance range, and experimental throughput [66]. Great efforts have been made to minimize the above limitations using a low protein/lipid molar ratio, reconstitutions in the presence of unlabeled proteins, bicelles, nanodics, lipodisq nanoparticles, restricted spin label probes and Q-band pulse EPR measurements [63,64,67–73]. The protein backbone dynamics and spin label rotameric motions have a significant contribution to the DEER distribution width. With the development of molecular dynamics simulations, several labs have recently used DEER distance restraints to refine the secondary structure of membrane bound proteins [44,64,74]. These methodological developments have made DEER a powerful spectroscopic tool to study complicated biological systems such as membrane proteins.
A recent example of using DEER spectroscopy is the study of N-terminal microdomain of human dihydroorotate dehydrogenase enzyme (HsDHODH) [75]. The HsDHODH enzyme is an attractive drug target for the potential treatment of several proliferative diseases such as cancer and rheumatoid arthritis. Eduardo et al. used DEER distance data in combination with CD spectroscopy to determine the different conformational states of HsDHODH peptide in micelles and liposomes [75]. The results revealed that the peptide undergoes different conformational states in micelles and liposomes suggesting this mircodomain acts in specific regions or areas of the mitochondria, which can be related with the control of the quinone access to the HsDHODH active site. Figure 4 shows DEER distance data obtained on the N-terminal microdomain of HsDHODH peptide and predicted conformational states in micelles and liposomes.
Figure 4.
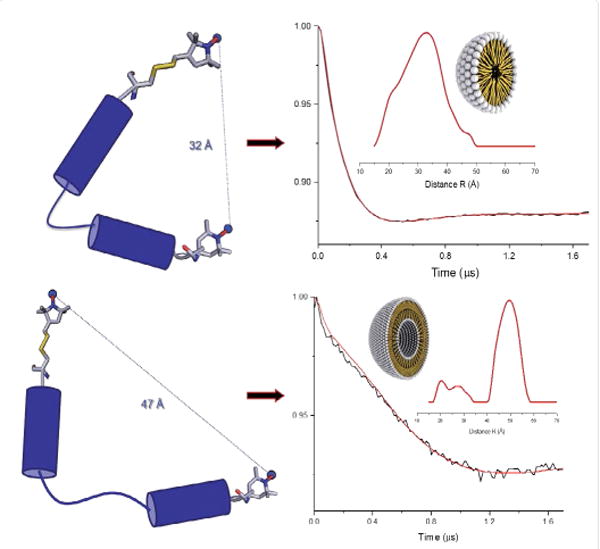
Four pulse Q-band DEER (double electron-electron resonance) data for peptide analogue N-terminal microdomain of HsDHODH peptide and predicted conformational states in micelles (top panel) and POPC liposomes (bottom panel) (Adapted from ref. [75] with permission).
Another excellent recent example of using DEER distance data is a study on the KvAP voltage-sensing domain [44]. Voltage-gated ion channels have a critical role in defining a wide variety of signaling process controlling basic cellular functions as electrical excitability, hormone secretion and osmotic balance. Li et al. used SDSL coupled with EPR spectroscopy to measure inter-helical distances and solvent accessibility. The results suggested that KvAP gates through S4 movements involving an ~ 3 Å upward tilt and simultaneous ~ 2 Å axial shift [44]. This motion supported a new gating model that combines structural rearrangements and electric-field remodeling.
DEER spectroscopy was recently used to investigate the structure of the transmembrane domain of KCNE1 in proteoliposomes [64]. KCNE1 is a transmembrane protein consisting of 129 amino acids that modulates the function of a voltage gated potassium ion channel (Kv). Hereditary mutations in the genes encoding either protein can result in diseases such as congenital deafness, long QT syndrome, ventricular tachyarrhythmia, syncope, and sudden cardiac death. Nine DEER distances were measured along the transmembrane domain (TMD) of KCNE1. The distances were compared in three different membrane environments (micelles, POPC/POPG vesicles and POPC/POPG lipodisq nanoparticles). The nine DEER distance restraints based on MTSL spin labeling sites were used in simulated annealing molecular dynamics simulation using Xplor-NIH and determined the structure of the transmembrane domain of KCNE1. This new structure was validated using experimentally derived DEER distances obtained using more restricted bifunctional spin labels (BSL). The results indicated that the transmembrane domain of KCNE1 is α-helical with a slightly curved conformation consistent with the previously determined solution NMR micelle structure [76].
Also, Influenza A M2 protein was recently investigated in liposomes using DEER spectroscopy [77]. Influenza A M2 protein is a 97 amino acid single-pass transmembrane protein, which oligomerizes to form a tetrameric proton-selective channel. This protein plays an essential role in viral adjustment and replication in the host cell and hence it is a target for drug development. Georgieva et al. collected DEER data on the M2 transmembrane protein at different protein to lipid molar ratios to understand the mechanism of transmembrane domain assembly in lipid membranes [77]. The results indicated that the M2 TMD exists as monomers, dimers, and tetramers whose relative populations shift to tetramers with an increase in the protein to lipid molar ratio.
Conclusion
In this brief review, the application of SDSL coupled with EPR spectroscopic methods to study complicated membrane proteins is discussed. Unique structural and dynamic information on membrane proteins can be gleaned using these powerful techniques. Table 1 shows recent examples of some biologically important membrane protein systems studied by using EPR spectroscopy [43,44,49,64,74,75,78,79]. EPR spectroscopy has become complementary to existing biophysical methods to study membrane proteins. Finally, EPR spectroscopy can address important biological questions that other biophysical techniques cannot or are very difficult.
Table 1.
Examples of biologically important membrane protein systems recently studied by using EPR spectroscopy.
| EPR techniques | Recently studied membrane proteins |
|---|---|
| CW-EPR, EPR power saturation, and DEER | KCNE1 |
| CW-EPR, EPR power saturation, and DEER | C99 amyloid precursor protein |
| CW-EPR, EPR power saturation, and DEER | α-Synuclein |
| CW-EPR | Integrin β1a |
| DEER | Human dihydroorotate dehydrogenase enzyme (HsDHODH) |
| CW-EPR, EPR power saturation, and DEER | KvAP voltage-sensing domain |
| CW-EPR | Photosystem II(PSII) |
Acknowledgments
Researchers of this work were generously supported by a National Institute of Health Grant R01GM080542 and a National Science Foundation Award CHE-1011909.
References
- 1.Landreh M, Robinson CV. A new window into the molecular physiology of membrane proteins. J Physiol. 2015;593:355–362. doi: 10.1113/jphysiol.2014.283150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallin E, von Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998;7:1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moraes I, Evans G, Sanchez-Weatherby J, Newstead S, Stewart PD. Membrane protein structure determination - the next generation. Biochim Biophys Acta. 2014;1838:78–87. doi: 10.1016/j.bbamem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung JC, Deber CM. Misfolding of the cystic fibrosis transmembrane conductance regulator and disease. Biochemistry. 2008;47:1465–1473. doi: 10.1021/bi702209s. [DOI] [PubMed] [Google Scholar]
- 5.Conn PM, Ulloa-Aguirre A, Ito J, Janovick JA. G protein-coupled receptor trafficking in health and disease: Lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacological Reviews. 2007;59:225–250. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 6.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 7.von Heijne G. The membrane protein universe: what’s out there and why bother? J Intern Med. 2007;261:543–557. doi: 10.1111/j.1365-2796.2007.01792.x. [DOI] [PubMed] [Google Scholar]
- 8.Engel A, Gaub HE. Structure and mechanics of membrane proteins. Annu Rev Biochem. 2008;77:127–148. doi: 10.1146/annurev.biochem.77.062706.154450. [DOI] [PubMed] [Google Scholar]
- 9.Klug CS, Feix JB. Methods and applications of site-directed spin labeling EPR spectroscopy. Methods Cell Biol. 2008;84:617–658. doi: 10.1016/S0091-679X(07)84020-9. [DOI] [PubMed] [Google Scholar]
- 10.Sahu ID, McCarrick RM, Lorigan GA. Use of electron paramagnetic resonance to solve biochemical problems. Biochemistry. 2013;52:5967–5984. doi: 10.1021/bi400834a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker M. Making membrane proteins for structures: a trillion tiny tweaks. Nat Methods. 2010;7:429–434. doi: 10.1038/nmeth0610-429. [DOI] [PubMed] [Google Scholar]
- 12.Opella SJ, Marassi FM, Gesell JJ, Valente AP, Kim Y, et al. Structures of the M2 channel-lining segments from nicotinic acetylcholine and NMDA receptors by NMR spectroscopy. Nat Struct Biol. 1999;6:374–379. doi: 10.1038/7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jo S, Kim T, Iyer VG, Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 14.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. 27–8. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 15.Wüthrich K. NMR studies of structure and function of biological macromolecules (Nobel Lecture) J Biomol NMR. 2003;27:13–39. doi: 10.1023/a:1024733922459. [DOI] [PubMed] [Google Scholar]
- 16.Acharya KR, Lloyd MD. The advantages and limitations of protein crystal structures. Trends Pharmacol Sci. 2005;26:10–14. doi: 10.1016/j.tips.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Klare JP. Site-Directed Spin Labeling and Electron Paramagnetic Resonance (EPR) Spectroscopy: A Versatile Tool to Study Protein-Protein Interaction. In Tech 2012 [Google Scholar]
- 18.Schiemann O, Prisner TF. Long-range distance determinations in biomacromolecules by EPR spectroscopy. Q Rev Biophys. 2007;40:1–53. doi: 10.1017/S003358350700460X. [DOI] [PubMed] [Google Scholar]
- 19.Torres J, Stevens TJ, Samsó M. Membrane proteins: the ‘Wild West’ of structural biology. Trends Biochem Sci. 2003;28:137–144. doi: 10.1016/S0968-0004(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 20.Columbus L, Hubbell WL. A new spin on protein dynamics. Trends Biochem Sci. 2002;27:288–295. doi: 10.1016/s0968-0004(02)02095-9. [DOI] [PubMed] [Google Scholar]
- 21.Fanucci GE, Cafiso DS. Recent advances and applications of site-directed spin labeling. Curr Opin Struct Biol. 2006;16:644–653. doi: 10.1016/j.sbi.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Hubbell WL, Gross A, Langen R, Lietzow MA. Recent advances in site-directed spin labeling of proteins. Curr Opin Struct Biol. 1998;8:649–656. doi: 10.1016/s0959-440x(98)80158-9. [DOI] [PubMed] [Google Scholar]
- 23.Qin PZ, Dieckmann T. Application of NMR and EPR methods to the study of RNA. Curr Opin Struct Biol. 2004;14:350–359. doi: 10.1016/j.sbi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Berliner LJ. From spin-labeled proteins to in vivo EPR applications. Eur Biophys J. 2010;39:579–588. doi: 10.1007/s00249-009-0534-x. [DOI] [PubMed] [Google Scholar]
- 25.Speicher DW. Characterization of protein primary structure. Dev Biol Stand. 1998;96:27–28. [PubMed] [Google Scholar]
- 26.Klare JP, Steinhoff HJ. Spin labeling EPR. Photosynth Res. 2009;102:377–390. doi: 10.1007/s11120-009-9490-7. [DOI] [PubMed] [Google Scholar]
- 27.Hubbell WL, Mchaourab HS, Altenbach C, Lietzow MA. Watching proteins move using site-directed spin labeling. Structure. 1996;4:779–783. doi: 10.1016/s0969-2126(96)00085-8. [DOI] [PubMed] [Google Scholar]
- 28.Weil JA, Bolton JR. Electron paramagnetic resonance: Elementary Theory and Practical Applications. Wiley-Interscience; Hoboken N.J: 2007. [Google Scholar]
- 29.Stone TJ, Buckman T, Nordio PL, McConnell HM. Spin-labeled biomolecules. Proc Natl Acad Sci U S A. 1965;54:1010–1017. doi: 10.1073/pnas.54.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornish VW, Benson DR, Altenbach CA, Hideg K, Hubbell WL, et al. Site-specific incorporation of biophysical probes into proteins. Proc Natl Acad Sci U S A. 1994;91:2910–2914. doi: 10.1073/pnas.91.8.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinhoff HJ. Multi-frequency EPR spectroscopy studies of the structure and conformational changes of site-directed spin labelled membrane proteins. Supramolecular Structure and Function. 2004;8:157–177. [Google Scholar]
- 32.Hustedt EJ, Beth AH. Nitroxide spin-spin interactions: applications to protein structure and dynamics. Annu Rev Biophys Biomol Struct. 1999;28:129–153. doi: 10.1146/annurev.biophys.28.1.129. [DOI] [PubMed] [Google Scholar]
- 33.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, et al. Lithium phthalocyanine - a probe for electron-paramagnetic-resonance oximetry in viable biological-systems. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hustedt EJ, Smirnov AI, Laub CF, Cobb CE, Beth AH. Molecular distances from dipolar coupled spin-labels: The global analysis of multifrequency continuous wave electron paramagnetic resonance data. Biophys J. 1997;72:1861–1877. doi: 10.1016/S0006-3495(97)78832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bordignon E, Steinhoff HJ. Membrane protein structure and dynamics studied by site-directed spin-labeling ESR. ESR Spectroscopy in Membrane Biophysics. 2007;27:129–164. [Google Scholar]
- 36.Jeschke G, Bender A, Schweikardt T, Panek G, Decker H, et al. Localization of the N-terminal domain in light-harvesting chlorophyll a/b protein by EPR measurements. J Biol Chem. 2005;280:18623–18630. doi: 10.1074/jbc.M501171200. [DOI] [PubMed] [Google Scholar]
- 37.Mchaourab HS, Perozo E. Determination of Protein Folds and Conformational Dynamics Using Spin-Labeling EPR Spectroscopy. In: Berliner L, Eaton G, Eaton S, editors. Biological Magnetic Resonance. Springer; US: 2002. pp. 185–247. [Google Scholar]
- 38.Perozo E, Cortes DM, Cuello LG. Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nat Struct Biol. 1998;5:459–469. doi: 10.1038/nsb0698-459. [DOI] [PubMed] [Google Scholar]
- 39.Vásquez V, Sotomayor M, Cortes DM, Roux B, Schulten K, et al. Three-dimensional architecture of membrane-embedded MscS in the closed conformation. J Mol Biol. 2008;378:55–70. doi: 10.1016/j.jmb.2007.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klug CS, Feix JB. SDSL: A survey of biological applications. Biol Magn Reson. 2004;24:269–308. [Google Scholar]
- 41.Stoll S, Schweiger A. Easyspin: simulating cw ESR spectra. Biol Magn Reson. 2007;27:299–321. [Google Scholar]
- 42.Stoll S, Schweiger A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J Magn Reson. 2006;178:42–55. doi: 10.1016/j.jmr.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Sahu ID, Craig AF, Dunagan MM, Troxel KR, Zhang R, et al. Probing Structural Dynamics and Topology of the KCNE1 Membrane Protein in Lipid Bilayers via Site-Directed Spin Labeling and Electron Paramagnetic Resonance Spectroscopy. Biochemistry. 2015;54:6402–6412. doi: 10.1021/acs.biochem.5b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Wanderling S, Sompornpisut P, Perozo E. Structural basis of lipid-driven conformational transitions in the KvAP voltage-sensing domain. Nature Structural & Molecular Biology. 2014;21:160–166. doi: 10.1038/nsmb.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuello LG, Cortes DM, Perozo E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science. 2004;306:491–495. doi: 10.1126/science.1101373. [DOI] [PubMed] [Google Scholar]
- 46.Cortes DM, Cuello LG, Perozo E. Molecular architecture of full-length KcsA: role of cytoplasmic domains in ion permeation and activation gating. J Gen Physiol. 2001;117:165–180. doi: 10.1085/jgp.117.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voss J, He MM, Hubbell WL, Kaback HR. Site-directed spin labeling demonstrates that transmembrane domain XII in the lactose permease of Escherichia coli is an alpha-helix. Biochemistry. 1996;35:12915–12918. doi: 10.1021/bi9608774. [DOI] [PubMed] [Google Scholar]
- 48.Victor KG, Cafiso DS. Location and dynamics of basic peptides at the membrane interface: Electron paramagnetic resonance spectroscopy of tetramethyl-piperidine-N-oxyl-4-amino-4-carboxylic acid-labeled peptides. Biophysical Journal. 2001;81:2241–2250. doi: 10.1016/S0006-3495(01)75871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu L, Wang W, Ling S, Liu S, Xiao L, et al. CW-EPR studies revealed different motional properties and oligomeric states of the integrin β1a transmembrane domain in detergent micelles or liposomes. Sci Rep. 2015;5:7848. doi: 10.1038/srep07848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altenbach C, Greenhalgh DA, Khorana HG, Hubbell WL. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1994;91:1667–1671. doi: 10.1073/pnas.91.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inbaraj JJ, Cardon TB, Laryukhin M, Grosser SM, Lorigan GA. Determining the topology of integral membrane peptides using EPR spectroscopy. J Am Chem Soc. 2006;128:9549–9554. doi: 10.1021/ja0622204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahu ID, Hustedt EJ, Ghimire H, Inbaraj JJ, McCarrick RM, et al. CW dipolar broadening EPR spectroscopy and mechanically aligned bilayers used to measure distance and relative orientation between two TOAC spin labels on an antimicrobial peptide. J Magn Reson. 2014;249:72–79. doi: 10.1016/j.jmr.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghimire H, Hustedt EJ, Sahu ID, Inbaraj JJ, McCarrick R, et al. Distance measurements on a dual-labeled TOAC AChR M2δ peptide in mechanically aligned DMPC bilayers via dipolar broadening CW-EPR spectroscopy. J Phys Chem B. 2012;116:3866–3873. doi: 10.1021/jp212272d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiang YW, Zheng TY, Kao CJ, Horng JC. Determination of Interspin Distance Distributions by cw-ESR Is a Single Linear Inverse Problem. Biophys J. 2009;97:930–936. doi: 10.1016/j.bpj.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kittell AW, Hustedt EJ, Hyde JS. Inter-spin distance determination using L-band (1–2 GHz) non-adiabatic rapid sweep electron paramagnetic resonance (NARS EPR) J Magn Reson. 2012;221:51–56. doi: 10.1016/j.jmr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeschke G, Polyhach Y. Distance measurements on spin-labelled biomacromolecules by pulsed electron paramagnetic resonance. Phys Chem Chem Phys. 2007;9:1895–1910. doi: 10.1039/b614920k. [DOI] [PubMed] [Google Scholar]
- 57.Borbat PP, McHaourab HS, Freed JH. Protein structure determination using long-distance constraints from double-quantum coherence ESR: Study of T4 lysozyme. J Am Chem Soc. 2002;124:5304–5314. doi: 10.1021/ja020040y. [DOI] [PubMed] [Google Scholar]
- 58.Schiemann O, Piton N, Mu Y, Stock G, Engels JW, et al. A PELDOR-based nanometer distance ruler for oligonucleotides. J Am Chem Soc. 2004;126:5722–5729. doi: 10.1021/ja0393877. [DOI] [PubMed] [Google Scholar]
- 59.Milov AD, Tsvetkov YD, Formaggio F, Crisma M, Toniolo C, et al. The secondary structure of a membrane-modifying peptide in a supramolecular assembly studied by PELDOR and CW-ESR spectroscopies. J Am Chem Soc. 2001;123:3784–3789. doi: 10.1021/ja0033990. [DOI] [PubMed] [Google Scholar]
- 60.Hilger D, Jung H, Padan E, Wegener C, Vogel KP, et al. Assessing oligomerization of membrane proteins by four-pulse DEER: pH-dependent dimerization of NhaA Na+/H+ antiporter of E. coli. Biophys J. 2005;89:1328–1338. doi: 10.1529/biophysj.105.062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeschke G. DEER distance measurements on proteins. Annu Rev Phys Chem. 2012;63:419–446. doi: 10.1146/annurev-physchem-032511-143716. [DOI] [PubMed] [Google Scholar]
- 62.Banham JE, Timmel CR, Abbott RJ, Lea SM, Jeschke G. The characterization of weak protein-protein interactions: evidence from DEER for the trimerization of a von Willebrand Factor A domain in solution. Angew Chem Int Ed Engl. 2006;45:1058–1061. doi: 10.1002/anie.200503720. [DOI] [PubMed] [Google Scholar]
- 63.Sahu ID, McCarrick RM, Troxel KR, Zhang R, Smith HJ, et al. DEER EPR measurement for Membrane Protein Structures via Bifunctional Spin Labels and Lipodisq Nanoparticles. Biochemistry. 2013;52:6627–6632. doi: 10.1021/bi4009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sahu ID, Kroncke BM, Zhang R, Dunagan MM, Smith HJ, et al. Structural investigation of the transmembrane domain of KCNE1 in proteoliposomes. Biochemistry. 2014;53:6392–6401. doi: 10.1021/bi500943p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reginsson GW, Hunter RI, Cruickshank PA, Bolton DR, Sigurdsson ST, et al. W-band PELDOR with 1 kW microwave power: molecular geometry, flexibility and exchange coupling. J Magn Reson. 2012;216:175–182. doi: 10.1016/j.jmr.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 66.McHaourab HS, Steed PR, Kazmier K. Toward the fourth dimension of membrane protein structure: insight into dynamics from spin-labeling EPR spectroscopy. Structure. 2011;19:1549–1561. doi: 10.1016/j.str.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou P, Bortolus M, McHaourab HS. Conformational Cycle of the ABC Transporter MsbA in Liposomes: Detailed Analysis Using Double Electron-Electron Resonance Spectroscopy. J Mol Biol. 2009;393:586–597. doi: 10.1016/j.jmb.2009.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Endeward B, Butterwick JA, MacKinnon R, Prisner TF. Pulsed Electron-Electron Double-Resonance Determination of Spin-Label Distances and Orientations on the Tetrameric Potassium Ion Channel KcsA. J Am Chem Soc. 2009;131:15246–15250. doi: 10.1021/ja904808n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. Membrane-bound alpha-synuclein forms an extended helix: Long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou P, McHaourab HS. Increased Sensitivity and Extended Range of Distance Measurements in Spin-Labeled Membrane Proteins: Q-Band Double Electron-Electron Resonance and Nanoscale Bilayers. Biophys J. 2010;98:L18–L20. doi: 10.1016/j.bpj.2009.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Q, Ellena JF, Kim M, Cafiso DS. Substrate-dependent unfolding of the energy coupling motif of a membrane transport protein determined by double electron-electron resonance. Biochemistry. 2006;45:10847–10854. doi: 10.1021/bi061051x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polyhach Y, Bordignon E, Tschaggelar R, Gandra S, Godt A, et al. High sensitivity and versatility of the DEER experiment on nitroxide radical pairs at Q-band frequencies. Phys Chem Chem Phys. 2012;14:10762–10773. doi: 10.1039/c2cp41520h. [DOI] [PubMed] [Google Scholar]
- 73.Cunningham TF, Putterman MR, Desai A, Horne WS, Saxena S. The Double-Histidine Cu2+-Binding Motif: A Highly Rigid, Site-Specific Spin Probe for Electron Spin Resonance Distance Measurements. Angew Chem Int Ed Engl. 2015;54:6330–6334. doi: 10.1002/anie.201501968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci U S A. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vicente EF, Sahu ID, Costa-Filho AJ, Cilli EM, Lorigan GA. Conformational changes of the HsDHODH N-terminal Microdomain via DEER Spectroscopy. J Phys Chem B. 2015;119:8693–8697. doi: 10.1021/acs.jpcb.5b01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang C, Tian C, Sönnichsen FD, Smith JA, Meiler J, et al. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Georgieva ER, Borbat PP, Norman HD, Freed JH. Mechanism of influenza A M2 transmembrane domain assembly in lipid membranes. Sci Rep. 2015;5:11757. doi: 10.1038/srep11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barrett PJ, Song Y, Van Horn WD, Hustedt EJ, Schafer JM, et al. The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science. 2012;336:1168–1171. doi: 10.1126/science.1219988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen G, Allahverdiyeva Y, Aro EM, Styring S, Mamedov F. Electron paramagnetic resonance study of the electron transfer reactions in photosystem II membrane preparations from Arabidopsis thaliana. Biochim Biophys Acta. 2011;1807:205–215. doi: 10.1016/j.bbabio.2010.10.010. [DOI] [PubMed] [Google Scholar]


