Abstract
Most primate species produce offspring that are altricial and highly dependent upon caregivers. As a consequence, a host of developmental trajectories can be dramatically altered by variation in early experiences. We review the impact of early social experiences (in both experimental models and natural contexts) on developmental profiles in three species of nonhuman primates: marmosets, squirrel monkeys, and macaques. Graded exposure to early-life social adversity (ELSA) produces short- to long-term effects on multiple developmental outcomes, including affect, social behavior, cognitive and attentional processes, and in the neural substrates that underlie these sociobehavioral traits.
Introduction
It is scientific and biomedical dogma that exposure to adverse early environments leads to a host of altered biological and behavioral processes, many of which can place organisms at risk for lifetime dysfunction and disease. Given the delayed neurological development and the extensive reliance on caregivers early in life, primates represent a taxon in which the absence of, unpredictability in, and even subtle variation, in early caregiving would be expected to leave neural and behavioral fingerprints on a host of developmental outcomes. We will compare the outcomes of experimental models of early-life social adversity (ELSA) vs. natural variation in ELSA to understand the relative utility of such models in psychiatric and evolutionary research. In this review, we focus on recent findings from nonhuman primates that characterize the ways in which ELSA profoundly shapes subsequent social behavior, emotional processing, and cognition, and alters the neural substrates for the canalization of these traits.
Models of early life social adversity in nonhuman primates
Three approaches are utilized to evaluate the impact of ELSA on developmental outcomes in nonhuman primates. The first involves chronic rearing of offspring in suboptimal rearing environments (SOR) that depart from species-typical norms (e.g., rearing in a nursery vs. rearing with the mother; variable foraging demand on mothers). The second approach exposes young primates to unpredictable short-term separations (STS) from caregivers. The third approach takes advantage of normative variation (NV) in early interactions with caregivers. These strategies are characterized in Table 1. Sufficient data for review are available on three taxa of primates – macaques (Macaca spp.), squirrel monkeys (Saimiri sciureus), and marmosets (Callithrix spp.), and important features of social structure and early offspring care in these species are highlighted in Box 1. Below we review the impact of ELSA on six major developmental outcomes.
Table 1.
Methodological approaches for Early-life Social Adversity (ELSA) in nonhuman primates.
| Methodology | Approach1 | Description | Classification of Cited Studies |
|---|---|---|---|
| Suboptimal rearing (SOR) | Exp | Departure from species-typical rearing pattern throughout early development (e.g., social partners present during rearing; inducing changes in maternal care with enhanced foraging demands) | 1, 4, 6, 10, 19, 20, 26, 28, 29, 31, 32, 33, 41, 42, 43, 44, 45 |
| Short-term separation from caregivers (STS) | Exp | Intermittent and variable length of separation from caregivers during early development | 2, 3, 5, 7, 8, 12, 15, 16, 21, 22, 23, 24, 38 |
| Normative variation in early caregiving (NV) | Corr | Natural variation in early caregiving; ranges from extreme variation (e.g., infant abuse) to subtle variation (e.g., rates of infant rejection) | 9, 11, 13, 14, 17, 18, 27, 34, 35, 36, 39,40 |
Exp = Experimental; Corr = Correlational
Box 1. Species-specific social structure and offspring rearing styles in macaques, squirrel monkeys, and marmosets.
The three most common species for evaluating ELSA in nonhuman primates differ substantially in the nature of their social structure and, as a consequence, the social context in which offspring rearing occurs. Macaques reside in large multimale/multifemale groups, and female social relationships are organized along matrilines. Offspring thus are exposed to mothers, close female kin, but do interact infrequently with adult males. While squirrel monkeys also live in large mixed-sex groups, these groups are sexually-segregated during the birth season and early life of infants; thus, infants interact primarily with mothers and other females. Marmoset monkeys live in extended family groups with minimal contact with non-kin individuals. Mothers, fathers, and older siblings all play a role in caregiving for offspring, and after the first two weeks of life, the majority of caregiving other than nursing is provided by fathers and siblings rather than mothers. primates.
Developmental Outcomes
Affective Behavior
Affective behavior encompasses emotional responses, including distress, fear, and anxiety. Though affective behavior also encompasses positive emotional states, the extant literature focuses primarily on the effects of ELSA on negative affect. Defining and assessing affective behavior in primates is complex and must take into account species-typical behavior. For instance, anxiety behavior is characterized by yawning and scratching in rhesus [1], dorsal contact levels and food consumption in squirrel monkeys [2], and heightened locomotion and contact calling in infant marmosets [3]. ELSA has been implicated in affective expression in nonhuman primates both in infancy and in adulthood. Macaques reared in sub-optimal mother-only conditions show higher fear and anxiety responses to a stress-inducing challenge than those from rearing conditions in which multiple adults and peers are present [1]. These affective responses, also characterized by pronounced behavioral inhibition, in SOR-exposed macaques persist well into adulthood [4]. Some behavioral effects of ELSA may not be long lasting, as macaques that experienced STS as infants displayed normative behavioral reactivity when placed in short-term social isolation as adults [5]. ELSA alters distress behavior and vocalizations in both macaques and marmosets. Adolescent macaques exposed to ELSA exhibit enhanced responses to an acoustic startle test and elevated rates of contact calling during social separation, indicating higher levels of anxiety and fear [6,7]. Similarly, marmoset infants exposed to STS demonstrate altered rates of distress vocalizations and enhanced anxiety behavior in both homecage interactions and during social separations [3,8]. Increased rates of distress calls are also observed in infant macaques that experienced abuse compared to infants who were not abused [9]. Despite the increased anxiety phenotype exhibited by ELSA-exposed animals in social contexts, ELSA may lead to decreased distress and increased novelty seeking in non-social contexts. Macaques exposed to SOR showed lower levels of distress during a developmental assessment compared to control animals [10]. Squirrel monkeys exposed to STS and macaques that experienced early life abuse showed increased exploratory behavior in the context of novel objects and environments [2,11,12]. These findings suggest that some benefits may derive from ELSA, such as increased resistance to the stress of novel nonsocial situations. The increased emotionality observed in social contexts following ELSA suggests that poor early life social experiences modify the perception of social environments, thereby altering behavioral response patterns. Fear, anxiety and distress behaviors follow similar trajectories in primates exposed to ELSA, suggesting a generalized shift in affective processing.
ELSA in the form of extreme maternal abuse alters the expression of affective behavior. Abused infant macaques display deficits in emotional expression such as the use of inappropriate vocalizations in response to contact and non-contact aggression [13]. Abused infants also exhibit high rates of distress vocalizations and anxiety-related behavior during normal social interactions, and in response to stressors and high-fear stimuli (animated toy accompanied by sounds) [11,14]. However, abused macaques show reduced anxiety-related behavior when exposed to low-fear or neutral stimuli (roll of tape) [11]. This differential response to low- and high-fear stimuli suggests that macaques that have experienced SOR have an altered system for threat assessment, with an increased threshold required to elicit a behavioral response relative to infants with normal developmental environments.
Social behavior
ELSA reduces competency in a wide domain of social interactions across developmental stages, beginning with the nature of interactions with caregivers. Both macaque and marmoset infants exposed to ELSA engage in abnormal interactions with caregivers. Infant macaques that experience higher levels of maternal abuse show lower levels of ventral-ventral contact with the mother, yet at the same time exhibit lower rates of breaking contact with their mothers [14]. Females that experienced abuse initiated contact at higher levels than nonabused females; however, this pattern was not observed in males that experienced ELSA [14]. Normal mother-infant social dynamics are altered in macaques exposed to STS – levels of mother-infant contact remain high at the developmental stage when normally-reared infants decrease maternal contact [7]. Marmosets that experienced STS spend more time in contact with their caregivers and more time in a nursing position on mothers compared to controls [8,15]. Despite increased rates of parental contact in the homecage, marmosets exposed to STS received decreased levels of paternal carrying after a social stressor [3].
ELSA has persistent effects on social behavior into adolescence and adulthood. Marmosets that experienced STS displayed decreased rates of social play [8,16]. While ELSA is associated with decreases in play behavior in macaques as well, levels of solitary play are higher as a consequence of ELSA [14]. Despite lower levels of social play, juvenile female macaques that experienced abuse spent more time in contact with infants [17]. In spite of this increased interest as juveniles, approximately 50% of females abused as infants (regardless of whether they were born to, or cross-fostered with, abusive mothers) abused their own infants, while nonabused females showed normal maternal care [18]. Long-term effects of ELSA are also observed in group-wide social dynamics. Macaques that experienced SOR have lower social dominance rankings than controls at both three and five years of age [19]. Though social dominance appears to be mediated by ELSA, rates of aggressive interactions in adolescent macaques appear to be unaffected by variation in early life rearing experience [20]. Thus, lower social dominance in ELSA-exposed macaques may reflect generalized deficits in social skills, such as an inability to form and maintain social alliances.
Cognition
A hallmark of primate biology is cognitive complexity and flexibility, and ELSA can lead to differential effects on these processes, depending on the timing of the manipulation. In adult marmosets, simple visual discrimination performance is not affected by early adversity, but reversal learning, which requires suppressing or inhibiting previously rewarded responses, is impaired by STS [21,22]. Further, motivational components of learning and cognition are reduced by ELSA: marmosets reared under early STS give up sooner and receive fewer rewards in an operant task requiring increasing response:reinforcement ratios [21]. STS experienced later in postnatal life enhances behavioral inhibition in learning tasks in squirrel monkeys. Adults exposed to STS as infants performed better than controls on a food-reward task that required a shift from a well-learned response [23,24]. The inhibition of prepotent responses is mediated, in part, by activity in the prefrontal cortex (PFC), and ELSA exposure at varying stages of development may produce functional differences in the PFC (see Neuroanatomy and function below). Increased competency at tasks involving behavioral inhibition highlights a potential benefit of ELSA. Animals who have experienced early life stress may be better equipped to handle unpredictability or change later in life, since mild exposure to stress early in development increases both cognitive and social capability later in life [2,23].
Gene regulation and expression
ELSA certainly interacts with genomes and induces modifications in the epigenome to influence developmental outcomes [25]. Macaques that experience SOR and also carry a low-activity monoamine oxidase A polymorphism display increased anxiety responses relative to carriers of this allele that experience normative social environments [1]. ELSA has effects on the serotonergic system both through receptor expression and function. STS has differential regional effects on serotonin receptor mRNA expression in the ACC of marmosets, increasing expression in superficial laminae but decreasing expression in deep laminae [16]. Exposure to social adversity modifies two important signaling pathways that regulate SERT function in macaques. Macaques experiencing ELSA have lower hippocampal h3k4me3 expression (a marker of transcriptionally active genes) [26], and enhanced p38MAPK activity (a signaling molecule increasing SERT function), resulting in altered central serotonergic tone [27]. ELSA therefore produces long-term, perhaps lifetime changes in serotonin function.
ELSA in nonhuman primates has also been associated with DNA methylation profiles in neural and peripheral tissues that have important implications for behavioral phenotypes. There is an interaction between rearing condition and methylation in the promoter region (5-HTTLPR) of the serotonin transporter gene: macaques that are exposed to SOR and have high levels of methylation in this region show increased stress reactivity [28]. SOR in macaques produces differential DNA methylation in promoter regions for more than 1,000 genes expressed in the PFC, including those regulating CNS development, immune function, axonogenesis, and cellular responses to stress [29]. A recent comparative study examined ELSA-induced changes in genome-wide DNA methylation in peripheral lymphocytes in humans and macaques, and in rat PFC tissue. Common epigenetic changes among species were identified in the promoter region for five genes, one of which (MORC1) is strongly associated with major depressive disorder in humans [30]. Differential behavioral outcomes among individuals as a consequence of ELSA must therefore take into account genetic and epigenetic variation [9,10,20,31].
Neuroendocrinology and neurochemistry
Modification of HPA axis function is a common outcome of ELSA, and these changes can be broadly classified into (i) differences in baseline function and (ii) differential responses to stressors. Decreased baseline levels of cortisol (CORT) and/or adrenocorticotrophic hormone (ACTH) after exposure to ELSA have been observed in macaques [32,33], marmosets [3,8], and squirrel monkeys [2]. However, other studies have failed to find these differences [12,17,34,35]. Decreased baseline CORT levels may indicate a lower set point of the HPA stress axis in animals exposed to ELSA. STS has also been implicated in increased baseline norepinephrine levels [21], suggesting dual effects of ELSA on both the HPA axis and the sympathetic nervous system.
There are mixed findings regarding HPA responses to stressors in monkeys exposed to ELSA. Lower quality parental care has been associated with increased CORT levels after a stressor in marmosets [34] and female macaques [35], suggesting increased reactivity to a stressor. In contrast, higher quality parental behavior such as grooming and carrying by caregivers does not predict CORT responses in marmosets [34]. These findings imply that exposure to poor parental care alters HPA axis dynamics, while variation in more positive parent-infant interactions have little or no effect on this system. Increased rates of social play as juveniles may have a protective effect in stress-prone marmosets, since they exhibit decreased stress-induced CORT [36]. Interestingly, there may be sex differences in HPA response to stressors, as male macaques exposed to abuse had a lower CORT response to a stressor compared to non-abused males, while the opposite pattern (i.e., increased CORT response) was observed in abused females [35]. Female macaques [7] that experienced STS had higher CORT responses to social isolation than did control females or males exposed to either rearing condition, again suggesting that ELSA may have differential sex effects. In some model systems, ELSA has also been associated with reduced HPA responses to stressors: infant macaques exposed to SOR display lower CORT responses to standard stressors than controls [33]. Further, squirrel monkeys exposed to STS in infancy also have decreased stress-induced CORT responses after exposure to novel stimuli [2]; cf. [24]. However, marmosets and male macaques showed no STS-dependent difference in CORT levels after social separation [3,7,37] or exposure to a novel stimulus [37]. These differential effects of ELSA on hormonal responses to stressors may in part be related to differences in the nature of the stressor (acute or chronic), or to the degree and timing of early life adversity (length of separations, developmental timing). SOR reduces the responsiveness of the HPA axis in macaques to both dexamethasone-suppression and ACTH stimulation tests [33] and these neuroendocrine effects may be mediated by changes in glucocorticoid and mineralocorticoid receptor mRNA expression [38]. Together, these findings suggest ELSA impacts HPA axis function by altering both central and peripheral regulatory processes [34,37].
Finally, ELSA alters long-term profiles of neurotransmitter metabolites in cerebrospinal fluid (CSF) throughout adolescence and adulthood. Differential early social environments particularly affect CSF levels of the 5-HT metabolite, 5-hydroxyindoleacetic acid (5-HIAA). Adolescent and adult macaques exposed to ELSA have lower 5-HIAA titers, implying reduced serotonergic tone [26,39,40], cf. [41]. Modification of CSF levels as a consequence of ELSA for other monoamine metabolites and corticotropin-releasing hormone has also been documented [12,22,40,42]. As is the case for behavior, though, the effects of ELSA on neurotransmitter levels may depend upon allelic status in 5-HT relevant genes [42].
Neuroanatomy and function
ELSA can produce long-term changes in neural circuits in regions that are relevant for the behavioral, cognitive and neuroendocrine differences. ELSA in macaques is associated with significant structural changes in the brain, including larger volumes of the anterior cingulate cortex (ACC) and dorsomedial prefrontal cortex (dmPFC) [41], reduced hippocampal and temporal gyri volume [43], and larger amygdala volume [11]. ELSA also selectively alters regional metabolic activity in the brain of macaques. Adults that experienced STS as infants showed an increase in glucose uptake in the orbitofrontal cortex (OFC) and superior temporal sulci, regions that are critical for decision-making, emotional regulation, and social behavior. STS was also associated with reduced glucose uptake in the hippocampus, a region associated with inhibitory control over the stress response [5].
Receptor distribution and gene expression in the brain, particularly those components involved in serotonin (5-HT) function, are altered by exposure to ELSA. The distribution of 5-HT binding, serotonin transporter (SERT) expression, and 5-HT1a receptor binding in multiple brain regions are reduced in macaques for months to years as a consequence of SOR [44,45]. In females, however, SOR is associated with enhanced 5-HT1a receptor density in the PFC [45].
Conclusions
Multiple models of ELSA in nonhuman primates have identified important and long-lasting modifications in behavioral, neuroendocrine, and neural endpoints, and these effects are summarized in Fig. 1. Interestingly, though rodent research has found many sex differences in response to ELSA, much of the primate literature fails to find these differential effects of sex. Furthermore, many studies have been conducted only on one sex [Males only: 4,6,20,26,29,42–44; Females only: 13,17,18,31,39]. Given the recent emphasis on the importance of sex as a biological variable in translational and clinical research at National Institutes of Health (NOT-OD-15-102; URL: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html), future studies should explicitly assess the differential effect of ELSA on males and females.
Figure 1.
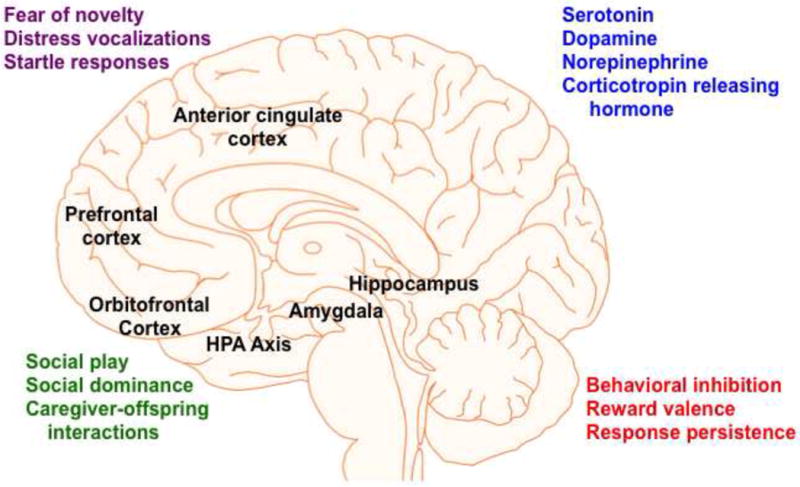
Early-life social adversity (ELSA) in nonhuman primates has effects on multiple behavioral systems, neurotransmitter/neuroendocrine measures, and brain structure and function. Brain regions labeled with a indicate neuroanatomical volume differences between individuals exposed to ELSA and controls; b indicates differential functional effects between individuals exposed to ELSA and controls. Please refer to the text for details on the relationship between ELSA and endocrine and neurotransmitter function (blue). For social (green), affective (purple), and cognitive (red) outcomes, arrows characterize general patterns of ELSA on these measures.
The more extreme manipulations (SOR, STS) are clearly relevant for the etiology of psychiatric and behavioral disorders in humans, including depression, affective disorders, and social dysfunction. A recent meta-analysis [46] of the lifelong effects of SOR in macaques highlights the persistence and penetrance of early social adversity, including increased lifetime frequency and severity of illnesses, a higher probability of developing atypical stereotypies, and an increased risk of being wounded in aggressive interaction. Models that utilize normative variation in early social experiences are relevant for identifying the origins and limits of phenotypic plasticity in response to differential early experience, and can explore the potential adaptive scenarios associated with the resulting phenotypic variants. Differences in the quality and quantity of early social interactions with caregivers may represent an important mechanism for programming brain and behavior for the social world that each organism will likely experience throughout their lifetime.
Highlights.
Nonhuman primates are excellent models for the impact of social adversity on development
Early social adversity enhances anxiety and impairs social function
Multiple neurotransmitter systems are modulated as a consequence of early experience
Social adversity produces lifetime changes in brain structure and function
Acknowledgments
The authors thank J. Cavanaugh, A. Mustoe, and J. Taylor for critiques of this paper, and acknowledge grant support from the National Institutes of Health (HD042882) to JAF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol Psychiatry. 2009;65:770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- 3.Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol Biochem Behav. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- 4.Corcoran CA, Pierre PJ, Haddad T, Bice C, Suomi SJ, Grant KA, Friedman DP, Bennett AJ. Long-term effects of differential early rearing in rhesus macaques: Behavioral reactivity in adulthood. Dev Psychobiol. 2012;54:546–555. doi: 10.1002/dev.20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parr LA, Boudreau M, Hecht E, Winslow JT, Nemeroff CB, Sánchez MM. Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta) Dev Cogn Neurosci. 2012;2:181–193. doi: 10.1016/j.dcn.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Nelson EE, Herman KN, Barrett CE, Noble PL, Wojteczko K, Chisholm K, Delaney D, Ernst M, Fox NA, Suomi SJ, et al. Adverse rearing experiences enhance responding to both aversive and rewarding stimuli in juvenile rhesus monkeys. Biol Psychiatry. 2009;66:702–704. doi: 10.1016/j.biopsych.2009.04.007. The authors demonstrate that the effects of SOR expand across a wide range of behaviors from food-motivated reward to threat response. The increased consumption of sweetened water suggests early adversity can change behavioral patterns that might be relevant for human response to ELSA (i.e. addiction). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus, primates) and analysis of its effects on early development. Biol Psychiatry. 2002;52:1037–46. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- 9.McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- 11.Howell BR, Grand AP, McCormack KM, Shi Y, LaPrarie JL, Maestripieri D, Styner MA, Sanchez MM. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: Relation to amygdala volume. Dev Psychobiol. 2014;56:1735–1746. doi: 10.1002/dev.21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker KJ, Rainwater KL, Buckmaster CL, Schatzberg AF, Lindley SE, Lyons DM. Early life stress and novelty seeking behavior in adolescent monkeys. Psychoneuroendocrinology. 2007;32:785–792. doi: 10.1016/j.psyneuen.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovanovic T, Maestripieri D. Effects of early traumatic experience on vocal expression of emotion in young female rhesus macaques. Dev Psychobiol. 2010;52:794–801. doi: 10.1002/dev.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack K, Sanchez MM, Bardi M, Maestripieri D. Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Dev Psychobiol. 2006;48:537–550. doi: 10.1002/dev.20157. [DOI] [PubMed] [Google Scholar]
- 15.Dettling AC, Schnell CR, Maier C, Feldon J, Pryce CR. Behavioral and physiological effects of an infant-neglect manipulation in a bi-parental, twinning primate: impact is dependent on familial factors. Psychoneuroendocrinology. 2007;32:331–349. doi: 10.1016/j.psyneuen.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Law AJ, Pei Q, Feldon J, Pryce CR, Harrison PJ. Gene expression in the anterior cingulate cortex and amygdala of adolescent marmoset monkeys following parental separations in infancy. Int J Neuropsychopharmacol. 2009;12:761–772. doi: 10.1017/S1461145708009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maestripieri D. Effects of early experience on female behavioural and reproductive development in rhesus macaques. Proc R Soc Lond B Biol Sci. 2005;272:1243–1248. doi: 10.1098/rspb.2005.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc Natl Acad Sci U S A. 2005;102:9726–9729. doi: 10.1073/pnas.0504122102. Female infants reared with abusive biological or foster mothers were more likely to abuse their own infants than females reared with nonabusive biological or foster mothers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastian ML, Sponberg AC, Suomi SJ, Higley JD. Long-term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta) Dev Psychobiol. 2003;42:44–51. doi: 10.1002/dev.10091. [DOI] [PubMed] [Google Scholar]
- 20.Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch K-P. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry. 2004;56:72–79. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Pryce CR, Dettling A, Spengler M, Spaete C, Feldon J. Evidence for altered monoamine activity and emotional and cognitive disturbance in marmoset monkeys exposed to early life stress. Ann N Acad Sci. 2004;1032:245–9. doi: 10.1196/annals.1314.030. [DOI] [PubMed] [Google Scholar]
- 23*.Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. This paper evaluates the notion that mild early-life stress serves as a means to buffer stress responses later in life, highlighting domains in which animals exposed to STS perform better on tasks that require cognitive control and behavioral inhibition. [DOI] [PubMed] [Google Scholar]
- 24.Parker KJ, Buckmaster CL, Lindley SE, Schatzberg AF, Lyons DM. Hypothalamic-pituitary-adrenal axis physiology and cognitive control of behavior in stress inoculated monkeys. Int J Behav Dev. 2012;36:45–52. doi: 10.1177/0165025411406864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, De Kloet ER. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. This paper reviews the ’three-hit’ hypothesis of stress vulnerability that takes into account genetic predisposition, early life experience and later life environments in predicting behavioral outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindell SG, Yuan Q, Zhou Z, Goldman D, Thompson RC, Lopez JF, Suomi SJ, Higley JD, Barr CS. The serotonin transporter gene is a substrate for age and stress dependent epigenetic regulation in rhesus macaque brain: potential roles in genetic selection and gene × environment interactions. Dev Psychopathol. 2012;24:1391–1400. doi: 10.1017/S0954579412000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, Maestripieri D, Miller AH. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry. 2007;12:895–897. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- 28.Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, Mann JJ. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav. 2010;9:575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Provençal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, Bennett AJ, Pierre PJ, Friedman DP, Côté SM, et al. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J Neurosci. 2012;32:15626–15642. doi: 10.1523/JNEUROSCI.1470-12.2012. The authors demonstrate that early-life adversity produces genome-wide changes in methylation profiles, and specifically document epigenetic changes in brain and immune cells that persist into adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieratschker V, Massart R, Gilles M, Luoni A, Suderman MJ, Krumm B, Meier S, Witt SH, Nöthen MM, Suomi SJ, et al. MORC1 exhibits cross-species differential methylation in association with early life stress as well as genome-wide association with MDD. Transl Psychiatry. 2014;4:e429. doi: 10.1038/tp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinnally EL, Feinberg C, Kim D, Ferguson K, Leibel R, Coplan JD, Mann JJ. DNA methylation as a risk factor in the effects of early life stress. Brain Behav Immun. 2011;25:1548–1553. doi: 10.1016/j.bbi.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–8. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- 34**.Birnie AK, Taylor JH, Cavanaugh J, French JA. Quality of maternal and paternal care predicts later stress reactivity in the cooperatively-breeding marmoset (Callithrix geoffroyi) Psychoneuroendocrinology. 2013;38 doi: 10.1016/j.psyneuen.2013.08.011. The authors assessed whether normative variation in caregiver-offspring interactions altered later stress response styles in the family-living marmoset. Positive social interactions did not alter cortisol responses to stressor, but caregiver rejection/removal early in life was associated with elevated stress responses through adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch H, McCormack K, Sanchez MM, Maestripieri D. The development of the hypothalamic–pituitary–adrenal axis in rhesus monkeys: Effects of age, sex, and early experience. Dev Psychobiol. 2014;56:86–95. doi: 10.1002/dev.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mustoe AC, Taylor JH, Birnie AK, Huffman MC, French JA. Gestational cortisol and social play shape development of marmosets’ HPA functioning and behavioral responses to stressors. Dev Psychobiol. 2014;56:1229–1243. doi: 10.1002/dev.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology (Berl) 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, Feldon J, Law AJ, Harrison PJ, Pryce CR. Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biol Psychiatry. 2010;67:1106–1109. doi: 10.1016/j.biopsych.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Maestripieri D, Lindell SG, Higley JD. Intergenerational transmission of maternal behavior in rhesus macaques and its underlying mechanisms. Dev Psychobiol. 2007;49:165–171. doi: 10.1002/dev.20200. [DOI] [PubMed] [Google Scholar]
- 40.Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring’s behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res. 2006;175:90–95. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry. 2009;66:658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coplan JD, Abdallah CG, Kaufman J, Gelernter J, Smith EL, Perera TD, Dwork AJ, Kaffman A, Gorman JM, Rosenblum LA, et al. Early-life stress, corticotropin-releasing factor, and serotonin transporter gene: a pilot study. Psychoneuroendocrinology. 2011;36:289–293. doi: 10.1016/j.psyneuen.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackowski A, Perera TD, Abdallah CG, Garrido G, Tang CY, Martinez J, Mathew SJ, Gorman JM, Rosenblum LA, Smith EL, et al. Early-life stress, corpus callosum development, hippocampal volumetrics, and anxious behavior in male nonhuman primates. Psychiatry Res Neuroimaging. 2011;192:37–44. doi: 10.1016/j.pscychresns.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. Effects of early life stress on [11C] DASB positron emission tomography imaging of serotonin transporters in adolescent peer-and mother-reared rhesus monkeys. J Neurosci. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, Barr CS, Suomi SJ, Higley JD, Stein EA. Effects of early-life stress on serotonin 1A receptors in juvenile rhesus monkeys measured by positron emission tomography. Biol Psychiatry. 2010;67:1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Conti G, Hansman C, Heckman JJ, Novak MF, Ruggiero A, Suomi SJ. Primate evidence on the late health effects of early-life adversity. Proc Natl Acad Sci. 2012;109:8866–8871. doi: 10.1073/pnas.1205340109. This meta-analysis summarizes long-term health outcomes for over 200 macaque monkeys exposed to ELSA. Early social adversity leads to enhanced lifetime risk for physical and mental health. The paper highlights the persistence of ELSA, and the difficulty of reversing the risk associated with early adversity with later interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]


