Abstract
Myostatin and GDF11 are highly homologous TGFβ family members implicated in skeletal and cardiac muscle growth. Myostatin promotes muscle wasting. In contrast, GDF11 was identified as a factor that reverses aging-related cardiac hypertrophy, and in separate experiments, GDF11 supplementation was linked to the reversal of aging associated skeletal muscle dysfunction. These initial findings have been challenged by work from others, specifically where injection of GDF11 did not replicate the original findings. Now, a new report in Circulation Research demonstrates that GDF11 delivery did produce a reduction in cardiac mass in both young and old animals.
Keywords: GDF11, GDF8/myostatin, cardiac hypertrophy, skeletal muscle hypertrophy, aging
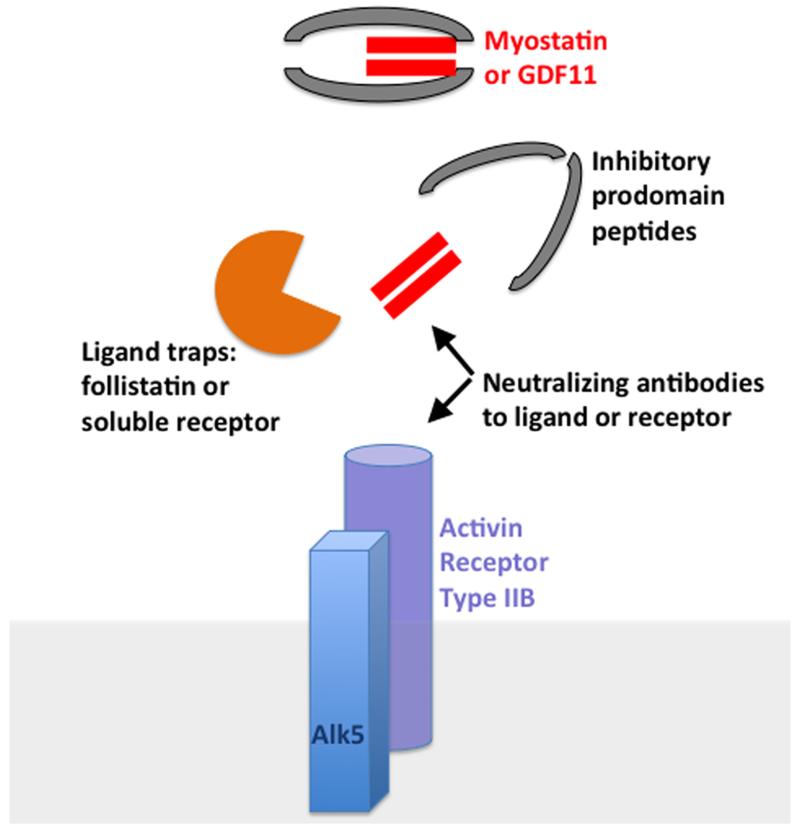
Myostatin, a member of the TGFβ superfamily of proteins, is a negative regulator of muscle mass.1 Both naturally-occurring and genetically engineered mutations that ablate or inhibit myostatin processing lead to a dramatic increase in muscle mass, an observation not lost on body builders and the pharmaceutical industry.2 Intriguingly, deletion of myostatin specifically in the heart has been shown to block muscle wasting that occurs in cardiac cachexia, although others have found that body-wide myostatin deletion renders little change in basal phenotypes.3, 4 GDF11 is the most closely related TGFβ superfamily member to myostatin, and shows near identity in its active domain to myostatin (Figure 1). Inhibiting myostatin activity is expected to be useful to treat a number of muscle wasting conditions, as well as sarcopenia with aging. Myostatin inhibition can be achieved through a variety of mechanisms including blocking peptides or neutralizing antibodies directed towards myostatin’s active domain (Figure 2). Alternative avenues to block myostatin’s effects include ligand traps like soluble receptor or follistatin, or blocking its major receptor, the activin type II receptor. The clinical efficacy of myostatin inhibition remains to be demonstrated in the human setting, although its application may ultimately be useful for treating degenerative muscle diseases in children or reversing sarcopenia in the elderly.
Figure 1.
Myostatin (also known as GDF8) and GDF11 are nearly identical in their active domains. Like other members of the TGFβ superfamily, myostatin and GDF11 are secreted in a small latent complex, which includes the prodomain linked to the active domain. The active domain is typically found as a dimer, linked by cysteine-bonds. On immunoblotting, active dimers migrate at 25 KDa and, if fully reduced to monomers, 12.5 KDa.
Figure 2.
As a negative regulator of muscle mass, multiple methods to inhibit myostatin are in development or clinical testing. These approaches include antibodies that neutralize myostatin itself or its receptor. Alternative approaches include ligand traps like follistatin or soluble receptors. Antibodies and ligand traps typically target the active domain of myostatin, which has high homology to GDF11. Alternative approaches include antibodies that antagonize the myostatin receptor, the activin type II receptor.
The aging population, which includes scientists, is drawn to any capacity to reverse the steady decline of function that occurs with time. With this goal in mind, mice were joined by heterochronic parabiosis where the bodies of old and young are sutured together to learn what factors could transmit features of youth to aged animals. In mice, parabiosis entails combining the fascial layers along the flanks of two animals, which then survive side-by-side sharing circulating factors.5 Loffredo and colleagues used heterochronic parabiosis and surgically joined 23 month old C57BL/6 animals with two month old strain-matched animals for four weeks.6 This time frame was sufficient to observe of reduction of heart mass/tibial length (HM/TL) in the older animals and partial improvement towards a “young” phenotype. Using an aptamer-based proteomics screen, which allowed examination of approximately 1000 proteins, 13 different factors were identified that differed between young and old mouse serum. The authors focused on GDF11, a distinct member of the TGFβ family that is highly related, and indeed, nearly identical to myostatin in its active domain (Figure 2). Serum profiling of old, young and parabiosed animals supported a reduction in GDF11 in aged animals, and older animals that had undergone parabiosis had increased serum GDF11 levels. To confirm the role of GDF11, recombinantly-produced GDF11 active domain was injected intraperitoneally into old animals at 0.1 mg/Kg, and this was sufficient to produce a similar magnitude of reduced heart mass as in the parabiosis experiments.6
A recent study by Smith and colleagues did not reproduce the effect on cardiac hypertrophy seen by Loffredo et al., and instead they observed little effect from GDF11 injections.7 Using similar dosing (0.1 mg/Kg) in 24 month old C57Bl/6 male mice, no decrease in heart mass/body mass (HM/BM) was seen after 28 days of treatment. Similarly, when evaluating HL/TL measurements, there were no significant changes. The authors concluded that GDF11 is ineffective to reduce heart mass in older mice. There were several important differences between the two studies including the source of the mice and their sex, male versus female mice, as well as the source of recombinant GDF11 active domain used in the injections.
And yet the plot thickens. A new study, Poggioli et al, now provides support to the notion that GDF11 delivery can reduce heart mass.8 The authors injected recombinant active GDF11 into both young and old mice. They found that GDF11 injection at 0.5 mg/kg and 1 mg/kg daily produced a reduction of HM/TL compared to vehicle treated after only 9 days in both young and old animals. Cardiac mass itself was reduced after 9 days of daily GDF11 in both young and old (Online Table 1 in 8). These results are interesting because it was originally suggested that only older animals with reduced GDF11 were responsive to GDF11 supplementation.7 More complicated to interpret is the effect of GDF11 injections on body mass. In old animals, GDF11 injections resulted in a decrease in body mass at all doses, although this was significant only at the highest doses. In young animals, a decline of body mass was only seen at the highest doses, and other doses were not sufficient to overcome the rise in body mass that occurs in young animals. Because GDF11 injection reduced body mass, the authors focused on evaluating HM/TL as the readout. From these data, it appears that GDF11 injection has a similar effect to myostatin, namely that it decreases body mass. Myostatin inhibition affects not only muscle mass but also body composition, including fat deposition.9 GDF11 has also been linked to anemia and vascularity 10, 11. Thus GDF11’s effect on body composition and size may be through a multi-organ or systemic effect, but it appears to mimic the known effects of myostatin.
Do serum GDF11 levels decrease with age?
The Loffredo and Poggioli papers emphatically take this view.6, 8 These authors suggest that a commercial anti-GDF11 antibody, crossreacts not only to myostatin/GDF8 but also to serum immunoglobulin. They assert that it is this nonspecificity that accounts for observations that GDF11 levels do not decline with age.12 Given the tight homology between these proteins in their active domains, it is unlikely that any immunoreagent can reliably detect myostatin versus GDF11 in a manner than is not concentration dependent.6, 8, 12 The Abcam antibody used in immunoblotting is currently labeled as detecting both GDF11 and myostatin. However, it was an R&D antibody used in immunoassays of human serum that showed no significant decline of GDF11 levels.12 R&D lists this antibody as being specific to GDF11. The R&D anti-GDf11 antibody was raised to the entire active domain, and data is not provided that this antibody specifically recognizes amino acid residues 353-350, as this region may be sufficient to distinguish between GDF11 and myostatin (Figure 1, underlined region). There is a substantial literature with vastly conflicting reports of serum myostatin levels in human. It would appear that GDF11 levels have similar challenges for serum detection.
Perhaps far more relevant than serum levels, it may be the local concentration of these molecules and their relative bioavailability that is most related to function. TGFβ superfamily members undergo strict processing and regulation where they are held inactive by both small and large latent complexes. Only once released from these layers of latent complexes can the active domains engage local cell surface receptors. Myostatin has been considered a chalone, which are proteins secreted by and responsible for growth of specific organs.1 That deletion of myostatin in heart blocks cardiac cachexia implies that these proteins can exert effect beyond the targeted organ.1 Whether serum levels have bearing on local tissue levels and availability is an area that needs more investigation. The greatest sequence differences between GDF11 and GDF8/myostatin reside in the prodomains, and these areas play an essential role in differential regulation and availability.
Because immunoreagents cannot reliably distinguish between active GDF11 and GDF8/myostatin, most investigators have relied on measuring mRNA levels, which may only partly reflect protein levels. Examining 3 month-old mice, Loffredo reported that GDF11 mRNA levels were greatest in spleen with lower levels in muscle and even lower in heart 6. How GDF11 levels changes with age and disease status will need careful study. Egerman reported an increase in GDF11 and a decrease in myostatin mRNA levels with age in rat muscle, whereas Poggioli found a decrease in spleen and kidney GDF11 with age in mice.8, 12
Answer: Given the high homology between the active domains of GDF11 and myostatin, most immunoreagents can be expected to detect both GDF11 and myostatin. Mass spectrometry methods targeting peptides where myostatin and GDF11 differ should be a more reliable means to achieve specificity.
Does injection of GDF11’s active domain affect body mass?
Smith et al. did not observe a reduction of in body mass after 28 days of intraperitoneal injections of GDF117 while the Poggioli study did see trends or significantly reduced body mass in older animals after only 9 days of dosing.8 Although both studies used C57Bl/6 mice, the mice were derived from different colonies and perhaps from different substrains. Genetic drift is known to occur in all mice including C57Bl/6 strains, and this genetic drift can alter genes important for metabolism like the Nnt gene.13 The Smith study, where no decrease in mass was seen after GDF11, used mice that were considerably leaner than in the Poggioli study (34.19 ± 3.73 gm compared to 39.42 ± 5.63 gm). Moreover, animals were housed in distinct caging and environments. It is fair to assume that the diets fed to these animals during aging and even during the course of GDF11 injections were not identical.
Answer: Injection of GDF11 active domain appears to reduce body mass in mice, but this result depends on initial body composition, age, genetic modifiers, sex, and diet.
Does cardiac pathological hypertrophy occur with age?
The Smith paper also raised a series of important points regarding the nature of pathological cardiac hypertrophy as it occurs in aging.7 These authors compared HM/BM in young and old male mice and did not find any significant increase in this ratio with age (4.75 ± 0.21 young vs 4.70 ± 0.38 old). However, when the authors calculated heart mass relative to tibial length (HM/TL), they did observe an increase with age (6.64 ± 0.43 vs 8.87 ± 0.75 comparing 8 week male mice to 24 month male mice.) Interestingly, the Loffredo group reported the basal HM/TL measurements in aged animals as very similar to those reported by Smith (~9 vs 8.87).
Answer: Heart mass increases relative to tibial length with age in mice, but heart mass to body mass appears not to change. Body mass may better reflect the physiological demands on the heart.
What is the potential for GDF11 as a fountain of youth?
The ability to reverse any aspect of aging and finding the fountain (or infusion) of youth has drawn Juan Ponce de León and many others to Florida. Yet the fountain of youth remains as elusive as sunshine in the Chicago winter. Whether infusion of GDF11 active domain is useful requires further study. It is known that there is a remarkably high degree of identity between myostatin/GDF8 and GDF11 active domains, and that these two proteins utilize the same intracellular signaling pathways and likely the same receptors.
Answer: Dosing and especially local concentration and regulation may be keys to unlocking the secrets of these GDFs, and systemic dosing of these agents may not be ideal to achieve this local specificity.
ACKNOWLEDGEMENTS
The author would like to thank Jeffery Molkentin and Se-Jin Lee for helpful comments.
Footnotes
Author disclosure: The author consults for Novartis.
REFERENCES
- 1.Lee SJ, McPherron AC. Myostatin and the control of skeletal muscle mass. Current opinion in genetics & development. 1999;9:604–607. doi: 10.1016/s0959-437x(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 2.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. The FEBS journal. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 3.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn RD, Liang HY, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscular disorders : NMD. 2007;17:290–296. doi: 10.1016/j.nmd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: Historical perspective and methodological considerations for studies of aging and longevity. Aging cell. 2013;12:525–530. doi: 10.1111/acel.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR. Gdf11 does not rescue aging-related pathological hypertrophy. Circulation research. 2015;117:926–932. doi: 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poggioli T, Vujic A, Yang P, Macias-Trevino C, Uygur AN, Loffredo FS, Pancoast JR, Cho M, Goldstein J, Tandias RM, Gonzalez E, Walker RG, Thompson TB, Wagers AJ, Fong YW, Lee RT. Circulating growth differentiation factor 11/8 levels decline with age. Circulation research. 2016;118:xxx–xxx. doi: 10.1161/CIRCRESAHA.115.307521. in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. The Journal of clinical investigation. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulson RF. Targeting a new regulator of erythropoiesis to alleviate anemia. Nature medicine. 2014;20:334–335. doi: 10.1038/nm.3524. [DOI] [PubMed] [Google Scholar]
- 11.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science (New York, N.Y.) 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg AU, Brack AS, Glass DJ. Gdf11 increases with age and inhibits skeletal muscle regeneration. Cell metabolism. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almodovar AJ, Luther RJ, Stonebrook CL, Wood PA. Genomic structure and genetic drift in c57bl/6 congenic metabolic mutant mice. Molecular genetics and metabolism. 2013;110:396–400. doi: 10.1016/j.ymgme.2013.06.019. [DOI] [PubMed] [Google Scholar]