Abstract
Imatinib mesylate was the first tyrosine kinase inhibitor (TKI) approved for the management of chronic myeloid leukemia (CML). Imatinib produces acceptable responses in ~ 60% of patients; with ~20% discontinuing therapy due to intolerance and ~20% developing drug resistance. The advent of newer TKIs’ such as, nilotinib, dasatinib, bosutinib and ponatinib have provided multiple options for patients. These agents are more potent, have unique side effect profiles and are more likely to achieve relevant milestones such as, early molecular responses (3-6 months) and optimal molecular responses (12 months). The acquisition of BCR-ABL kinase domain mutations is also reportedly lower with these drugs. Thus far, none of the randomized phase III clinical trials have shown a clinically significant survival difference between frontline imatinib versus newer TKIs’. Cost and safety issues with the newer TKIs’ such as, vascular disease with nilotinib and ponatinib and pulmonary hypertension with dasatinib have dampened the enthusiasm of using these drugs as frontline options. While the utility of new TKIs’ in the setting of imatinib failure or intolerance is clear, their use as frontline agents should factor in the age of the patient, additional comorbidities, risk stratification (Sokal score) and cost. Combination therapies and newer agents with potential to eradicate quiescent CML stem cells offer future hope.
Keywords: Chronic myeloid leukemia, BCR-ABL1, Tyrosine kinase inhibitors
Introduction
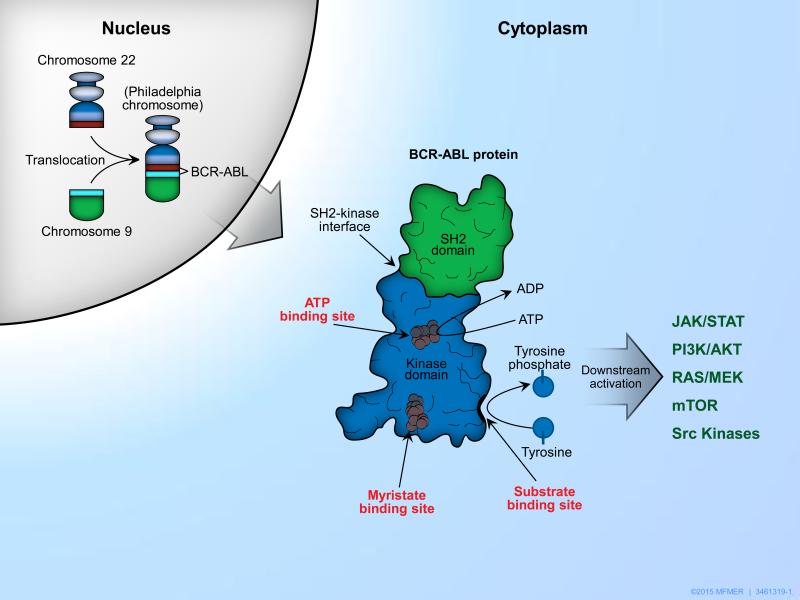
Chronic myeloid leukemia (CML) is a clonal, hematopoietic stem cell disorder, characterized by a reciprocal translocation that involves fusion of the Abelson oncogene (ABL) located on chromosome 9q34 with the breakpoint cluster region (BCR) on chromosome 22q11.2.1 This reciprocal translocation, t (9; 22) (q34; q11.2), results in a shortened chromosome 22 called the Philadelphia (Ph) chromosome, which encodes the BCR-ABL1 fusion oncoprotein [Figure 1]. 2-4 This protein has constitutive tyrosine kinase activity, which stimulates hematopoietic transformation and myeloproliferation.5 The predominant isoform of BCR-ABL is a 210-kDa protein that is present in >90% of patients with CML.6
Figure 1.
The pathogenesis of chronic myeloid leukemia (CML) involves reciprocal translocation t (9; 22) (q34; q11.2) forming an abnormal chromosome 22- the Philadelphia chromosome. Fusion of the Abelson gene (ABL) located on chromosome 9 with the breakpoint cluster region (BCR) on chromosome 22 leads to the formation of an oncogene which encodes the BCR-ABL1 fusion oncoprotein. This protein has constitutively upregulated tyrosine kinase activity and by phosphorylation of substrates causes downstream activation of various molecular pathways such as JAK/STAT, PI3K/AKT, RAS/MEK, mTOR, Src kinases. These in turn dysregulate the adhesion, proliferation, transformation, and apoptotic behavior of hematopoietic cells.5
The BCR-ABL protein has been described as a complex coiled molecular structure with spatial domains: Src-homology-2 (SH2) and SH3 domains which bind adapter proteins; and the kinase domain which has the ATP, substrate and allosteric myristate-binding pockets. These different binding sites as well as downstream pathways are potential therapeutic targets for drug development against CML.87,88
Prior to the advent of tyrosine kinase inhibitors (TKI), treatment options for patients with CML were limited to cytoreductive agents, Interferon alpha (INF-α) and allogeneic stem cell transplantation (HCT).7 The use of cytoreductive agents such as, hydroxyurea, arsenic and busulfan, were largely palliative (symptom control), bearing no impact on the natural course of the disease. INF-α, was the first agent with disease modifying effect, with a favorable impact on over-all survival (OS).7 In prospective, randomized studies, INF-α produced rates of major cytogenetic responses (Mcg) of 11 to 30% and complete cytogenetic responses (CCgR) in ~ 10% of patients.8-10 Treatment with INF-α was however limited by poor tolerability and progressive disease.11 Similarly, although allogeneic stem cell transplant is considered curative, it is associated with high morbidity (acute and chronic graft versus host disease) and mortality (non-relapse mortality), limiting generalized applicability.7
In 2003, the first major breakthrough was documented, with the results of the IRIS study (International Randomized Study of Interferon and STI571), demonstrating superiority of imatinib mesylate in comparison to combination therapy with INF-α and low dose cytarabine, in patients with chronic phase CML (CP-CML) [Table 1].12 At 18 months, the rates of Mcg and CCgR were; 87.1% vs 34.7% and 76.2% vs 14.5% in the imatinib versus combination-therapy arms, respectively.12 The estimated rate of freedom from progression to accelerated or blast phase CML (AP and BP) was 96.7% vs 91.5% in the imatinib vs combination-therapy arm respectively, and importantly imatinib was better tolerated.12 The study was designed as a cross over study and given the success of imatinib, a large proportion of patients [65% (N=359), 26% due to intolerance to INF-α] crossed over to the imatinib arm.13 At the 5-year follow up 87% of patients had achieved a CCgR, with an estimated OS of 89%.13
Table 1. Summary of major TKI trials.
| Phase III trial | IRIS 12 | DASISION 32 | ENEST 26 | BELA 35 | ||||||||
| Study design | ||||||||||||
| Number of study arms |
Two | Two | Three: Imatinib 400 mg OD/ Nilotinib 300mg/400mg BID¥ |
Two | ||||||||
| Crossover | Allowed if treatment failure or intolerance |
No | No | No | ||||||||
| Dose modification |
Dose escalation allowed | Interruptions, dose reductions or escalations allowed |
Allowed escalation in Imatinib dose; not Nilotinib |
Allowed escalation for both Bosutinib and Imatinib |
||||||||
| Total number of patients |
1106 | 519 | 846 | 502 | ||||||||
|
Interferon
+low dose cytarabine |
Imatinib |
P-
value |
Dasatinib | Imatinib |
P-
value |
Nilotinib | Imatinib |
P-
value |
Bosutinib | Imatinib |
P-
value |
|
| Study dosage | Interferon: max tolerated. Cytarabine: 20 mg/m2 |
400 mg daily |
100 mg daily |
400 mg daily |
300 mg BID¥ |
400 mg daily |
500 mg daily |
400 mg daily |
||||
|
Follow-up at
initial analysis |
12 mo | 12 mo | 12 mo | 12 mo | ||||||||
| CHR | 56% | 95% | <.001 | NA | NA | 71% | 85% | NS | ||||
| CCR | 9% | 74% | <.001 | 83% | 72% | 0.001 | 80% | 65% | <.001 | 70% | 68% | NS |
| MMR | NA | 39% | 46% | 28% | <.001 | 44% | 22% | <.001 | 41% | 27% | <.001 | |
| Median time to first CHR |
2.5 mo | 1 mo | NA | NA | NA | NA | 4.4 wk | 4.6 wk | NS | |||
| Median time to first CCR |
NA | NA | NA | NA | NA | NA | 12.9 wk | 24.6 wk | <.001 | |||
| Median time to first MMR |
NA | NA | HR 2.0 | <.001 | 8.6 mo | NA | 37.1 wk | 72.3 wk | <.001 | |||
| Progression to AP/BP |
7% | 1% | <.001 | 2% | 4% | <1% | 4% | 0.01 | 2% | 4% | ||
| Event free survival |
NA | NA | NA | NA | NA | NA | 94% | 93% | ||||
| Progression free survival |
80% | 97% | <.001 | 96% | 97% | NS | NA | NA | NA | NA | ||
| Overall survival |
95%* | 97%* | NS* | 97% | 99% | NS | NA | NA | 99% | 97% | ||
|
Follow-up
last reported |
8 year 17 | 5 year 49 | 5 year 27,28 | 2 year 50 | ||||||||
| Patients on study |
NA | 55% | 61% | 63% | 60% | 50% | 63% | 71% | ||||
| CCR | NA | NA | 83% | 78% | NS | NA | NA | 58% | 65% | NS | ||
| MMR | NA | 86% | 76% | 64% | .002 | 77% | 60% | <.001 | 47% | 41% | NS | |
| MR4.5 | NA | NA | 42% | 33% | 0.025 | 54% | 31% | <.001 | NA | NA | ||
| Progression to AP/BP |
NA | 8% | 5% | 7% | 4% | 8% | 0.04 | 2% | 5% | 0.019 | ||
| Event free survival |
NA | 81% | NA | NA | NA | NA | 92% | 88% | ||||
| Progression free survival |
NA | NA | 85% | 86% | NS | NA | NA | NA | NA | |||
| Overall survival |
NA | 85% | 91% | 90% | NS | 94% | 92% | NS | 97% | 95% | ||
Abbreviations: CHR: Complete hematologic response; CCR: Complete cytogenetic response; MMR: Major molecular response BCR-ABL1 PCR ≤ 0.01% on International Scale; MR4.5: BCR-ABL1 PCR ≤ 0.0032% on International Scale; AP: Accelerated phase; BP: Blast phase; NA: Not available; NS: Not significant;
at 18 months.
there was no significant difference in results between the two Nilotinib arms so we include the more commonly used dose of 300mg BID in this table.
With the use of imatinib the survival of patients with CP-CML has dramatically improved. However, with time, 20-30% of patients develop TKI resistance, commonly attributable to BCR-ABL1 kinase domain mutations.14,15,16 Some patients fail therapy despite inhibition of BCR-ABL1, implicating activation of alternative resistance mechanisms.15 Additionally, 5-10% discontinue therapy secondary to poor tolerability.13 This has led to the development of newer TKIs’ such as nilotinib, dasatinib, bosutinib and ponatinib [Tables 1, 2 and 3]. These agents inhibit a spectrum of tyrosine kinases apart from BCR-ABL1 and have differing potencies and side effect profiles. In this review, we attempt to discuss the role of newer TKIs’ in the management of CML.
Table 2. Pharmacological properties of Tyrosine Kinase Inhibitors.
| Imatinib 51 | Dasatinib 52 | Nilotinib 53 | Bosutinib 54 | Ponatinib 55 | |
|---|---|---|---|---|---|
|
Year of FDA/EMA
approval |
2001/2001 | 2006/2006 | 2007/2007 | 2012/2013 | 2012/2013 |
| Kinases inhibited | BCR-ABL, PDGF, SCF, c- kit |
BCR-ABL, SRC family kinases, c-kit, EPHA2, PDGFRβ |
BCR-ABL, c-kit, PDGFR, CSF-1R, DDR1 |
BCR-ABL, SRC family kinases, Minimal activity against c-kit or PDGFR |
Native/mutant BCR- ABL, including T315I, VEGFR, PDGFR, FGFR, EPH receptors, SRC family kinases, KIT,RET, TIE2, FLT3 |
|
Indications and
usage in CML |
First line | First or second line | First or second line | Resistance or intolerance to prior therapy |
T315I-positive or when no other TKI is indicated |
|
Absolute oral
bioavailability |
98% | 14-34% (animal studies) 56 |
50-82% | 23-64% (animal studies) |
Not determined |
|
Time to maximum
concentration (hours) |
2-4 | 0.5- 6 | 3 | 4-6 | 6 |
|
Volume of
distribution (liters) |
435 | 2505 | 273 57 | 6080 | 1223 |
| Half-life (hours) | 18 | 3- 5 | 17 | 22.5 | 24 |
| CNS penetration | 0.5-2% 58 | 5-28% 59 | 0.23-1.5% 60 | ~50% 61 | Not determined |
| Metabolism | Major: CYP3A4 Minor: CYP1A2, CYP2D6, CYP2C9, and CYP2C19 |
Major: CYP3A4 | Major: CYP3A4 | Major: CYP3A4 | Major: CYP3A4 Minor: CYP2C8, CYP2D6, CYP3A5 esterases and/or amidases |
| Mode of elimination | ~81% in feces, mostly as metabolites |
~85% in feces, mostly as metabolites |
~93% in feces, mostly as parent drug |
~91% in feces | ~ 87% in feces |
|
Recommended
dosage |
CP: 400-800 mg/day AP/BP: 600-800 mg/day |
CP: 100 mg OD AP/BP:140 mg OD With/without food |
First line in CP: 300 mg BID Second line in CP/AP: 400 mg BID Without food |
CP/AP/BP: 500-600 mg OD With food |
CP/AP/BP: 45 mg OD With food |
|
Dose adjustment:
Hepatic impairment Renal impairment |
Yes Yes |
No No |
Yes No |
Yes No |
Yes No |
|
Important drug
interactions |
CYP3A4 inducers CYP3A4 inhibitors Warfarin |
CYP3A4 inducers CYP3A4 inhibitors Proton pump inhibitors Antacids H2 Antagonists |
CYP3A4 inducers CYP3A4 inhibitors Proton pump inhibitors Anti-arrhythmics |
CYP3A4 inducers CYP3A4 inhibitors Proton pump inhibitors |
Strong CYP3A inhibitors Strong CYP3A inducers |
| Use in Pregnancy | Category D | Category D | Category D | Category D | Category D |
|
Use in breast
feeding mothers |
Not known | Not known | Not known | Not known | Not known |
| Contraindications | Hypokalemia Hypomagnesemia Long QT syndrome |
Hypersensitivity to Bosutinib* |
|||
|
Approximate cost
for one month supply |
$11053 | $12428 | $12433 | $13078 | $13536 |
| Other indications | Ph+ ALL; MDS/MPD; Aggressive systemic mastocytosis; Hypereosinophilic syndrome and/or chronic eosinophilic leukemia; Dermatofibrosarcoma protuberans; GIST |
Ph+ ALL GIST |
GIST | Ph+ ALL when no other TKI is indicated or T315I positive |
Abbreviations: FDA: US Food and Drug Administration; EMA: European Medicines Agency; CP: Chronic phase; AP: Accelerated phase; BP: Blast phase; OD: Once daily; BID: Twice daily;
In the BOSULIF clinical trials, anaphylactic shock occurred in less than 0.2% of treated patients.
Strong CYP3A inducers: rifampin, phenytoin, carbamazepine, St. John’s Wort, rifabutin and phenobarbital.
Moderate CYP3A inducers include bosentan, nafcillin, efavirenz, modafinil and etravirine
Strong CYP3A inhibitors include ritonavir, indinavir, nelfinavir, saquinavir, ketoconazole, boceprevir, telaprevir, itraconazole, voriconazole, posaconazole, clarithromycin, telithromycin, nefazodone and conivaptan.
Moderate CYP3A inhibitors include fluconazole, darunavir, erythromycin, diltiazem, atazanavir, aprepitant, amprenavir, fosamprevir, crizotinib, imatinib, verapamil, grapefruit products and ciprofloxacin
Table 3. Adverse effects of Tyrosine Kinase Inhibitors.
| Imatinib | Dasatinib | Nilotinib | Bosutinib | Ponatinib | |
|---|---|---|---|---|---|
| Laboratory: | |||||
| Neutropenia | ++++++ | ++++++ | ++++++ | ++++ | +++++ |
| Thrombocytopenia | +++++ | +++++ | ++++++ | +++++ | ++++++ |
| Anemia | +++ | ++++ | ++++ | ++++ | +++ |
| Elevations in ALT/AST | +++ | + | +++ | ++++ | ++++ |
| Elevated lipase | ++ | ++++ | +++ | ++++++ | |
| Elevated bilirubin | + | ++ | +++ | ++ | ++ |
| Elevated alkaline phosphatase | + | + | ++ | ||
| Elevated Creatinine | ++ | ++ | + | ++ | + |
| Hypophosphatemia | ++++++ | ++++ | ++++ | +++ | +++ |
| Hyperglycemia | + | ++++ | +++ | ||
| Hyperkalemia | ++ | +++ | ++ | ||
| Hyponatremia | + | +++ | +++ | ||
| Hypokalemia | ++ | ++ | ++ | ++ | |
| Hypocalcemia | ++ | ++ | ++ | ++ | |
| Clinical: | |||||
| Sudden death | + | ||||
| Hypersensitivity | + | ||||
| Abdominal pain | ++ | ++ | ++ | ++ | ++++ |
| Clinical pancreatitis | +++ | ||||
| Nausea | ++ | ++ | ++ | ++ | ++ |
| Vomiting | ++ | + | ++ | ++ | |
| Diarrhea | ++ | ++ | ++ | +++ | ++ |
| Constipation | + | ++ | + | ++ | |
| Hemorrhage | ++ | ++ | ++ | +++ | |
| GI bleed | + | ++ | + | ++ | |
| CNS bleed | ++ | ++ | |||
| Hypertension | + | ++ | ++++++ | ||
| Myocardial ischemia | ++ | ++ | +++ | ++++ | |
| Stroke/TIA | + | + | ++ | +++ | |
| Peripheral arterial occlusion | + | ++ | +++ | ||
| Venous thromboembolism | +++ | ||||
| Ocular toxicity | ++ | ||||
| QTc prolongation | + | + | |||
| Tachyarrhythmias | +++ | +++ | +++ | ||
| Bradyarrhythmias | ++ | ||||
| Pulmonary hypertension | ++ | ||||
| Heart failure | ++ | +++ | +++ | ||
| Pericardial effusion | ++ | ++ | |||
| Pleural effusion | ++ | +++ | ++ | ||
| Dyspnea | + | ++ | ++ | ++ | ++ |
| Skin rash | ++ | ++ | + | +++ | +++ |
| Muscle cramps/spasms | ++ | + | ++ | ||
| Musculoskeletal Pain | +++ | ++ | ++ | + | ++ |
| Headache | + | ++ | ++ | ++ | |
| Peripheral neuropathy | ++ | ||||
| Fluid retention/edema | ++ | +++ | + | + | ++ |
| Fatigue | ++ | ++ | ++ | ++ | ++ |
| Fever | ++ | + | + | ++ |
Grade 3/4 toxicity from the clinical trial data available for first/second line use of the TKI in CP-CML.
Bold font indicates clinically important adverse events noted on post-marketing surveillance.
Key Reported frequency of toxicity
+ <1 %
++ 1-5 %
+++ 5-10 %
++++ 10-20 %
+++++ 20-30 %
++++++ >30 %
1. Imatinib mesylate
Imatinib mesylate was the first TKI, U.S. Food and Drug Administration (FDA) approved for the management of CML. Imatinib binds to the BCR-ABL1 kinase domain, which is in an inactive conformation in a pocket reserved for the ATP binding site, thus preventing the transfer of a phosphate group to tyrosine on the protein substrate and the subsequent activation of phosphorylated protein [Figure 1]. The pharmacokinetic properties and drug interactions for imatinib are detailed in Table 2, while Table 3 lists the adverse effect profile. At 8 years of follow-up on the IRIS study, 45% of patients had discontinued treatment due to adverse events/safety (6%), unsatisfactory therapeutic outcomes (16%), allogeneic HCT (3%), death (3%) or other reasons.17 The OS rate was 85%, with the annual rates of progression to AP or BP in years 4 to 8 after imatinib therapy being: 0.9%, 0.5%, 0%, 0%, and 0.4%, respectively. Progression to AP or BC was noted only in 3% of patients who achieved CCgR, and in none of patients who achieved major molecular response (MMR).17 While conventional response criteria for CML have followed the optimal achievement of a complete hematological response (CHR) by 3 months, CCgR by 9-12 months and a MMR by 18 months [Table 4], there is increasing data suggesting that the achievement of BCR-ABL1 transcripts of ≤ 10% at 3 months (EMR- early molecular response) predicts for better OS, progression free survival (PFS), cumulative incidence of CCgR and MMR.18,19 This goal seems to be better achieved by second generation TKI; with a recent study showing that 91.4% of patients receiving dasatinib achieved an EMR.20 In addition, sub-optimal responses to imatinib and intolerance often lead to poor disease control with higher rates of AP or BP.
Table 4. Response criteria.
| Time on TKI therapy |
Current ELN 62/NCCN 63 recommendations | Older criteria | ||||
|---|---|---|---|---|---|---|
| Optimal | Warning | Failure | Optimal | Suboptimal | Failure | |
| Baseline | NA | High risk or CCA/ Ph +, major route |
NA | |||
| 3 months | BCR-ABL1 ≤ 10% and/or Ph+ ≤ 35% |
BCR-ABL1 > 10% and/or Ph+ 36-95% |
Non-CHR and/or Ph+ >95% |
CHR, minor CHR (<65%) |
No CHR | Less than CHR |
| 6 months | BCR-ABL1 ≤ 1% and/or Ph+ 0 |
BCR-ABL1 1- 10% and/or Ph+ 1-35% |
BCR-ABL1 > 10% and/or Ph+ >35% |
PCgR (<35%) | Less than PCgR (>35%) |
No CgR |
| 12 months | BCR-ABL1 ≤ 0.1% |
BCR-ABL1>0.1- 1% |
BCR-ABL1 >1% and/or Ph+ >0 |
CCgR | PCgR (1-35%) | Less than PCgR (>35%) |
| 18 months | MMR | Less than MMR | Less than CCgR |
|||
|
Then, and
at any time |
BCR-ABL1 ≤ 0.1% |
CCA / Ph- (−7, or 7q-) |
Loss of CHR Loss of CCgR Confirmed loss of MMR Mutations CCA/ Ph+ |
Stable or improving MMR |
Loss of MMR | 2 fold increase in transcripts |
ELN: European LeukemiaNet
NCCN: National Comprehensive Cancer Network
CCA: Clonal Chromosome Abnormalities
CHR: Complete Hematological Response defined as WBC < 10 × 109/L, Basophils <5%, No myelocytes, promyelocytes, myeloblasts in the differential, Platelet count <450 × 109/L, Spleen nonpalpable.
CCgR: Complete Cytogenetic Response defined as no Ph+ metaphases on chromosome banding analysis (at least 20 bone marrow cell metaphases) or <1% BCR-ABL1 positive nuclei of at least 200 nuclei on fluorescence in situ hybridization.
PCgR: Partial Cytogenetic Response defined as 1 to 35% Ph+ metaphases
MMR: Major Molecular Response defined as BCR-ABL1 expression of ≤0.1% on the international scale
Imatinib is a highly effective TKI for ~60% of CML patients.21 It is however, less potent than the second generation TKIs’, an issue that can result in sub-optimal kinase inhibition leading to resistance and loss of responses.22 Nearly all patients experience some impairment in quality of life, such as fluid retention (periorbital and peripheral), muscle cramps, or gastrointestinal disturbances (nausea, vomiting and diarrhea).12,13,23 However, there are no worrying organ toxicity signals emerging after prolonged therapy with imatinib. The early concerns about an increase in cancer susceptibility and cardiac dysfunction have also not borne out.24
2. Nilotinib
Nilotinib is a more potent analogue of imatinib and was approved by the U.S. FDA in 2007 for the treatment of patients with CP or AP CML that were resistant to, or intolerant to imatinib. In vitro profiling has demonstrated that nilotinib is effective against most imatinib-resistant Abl kinase domain mutations. Clinically, five kinase domain mutations remain of major concern; T315I (gate keeper mutation), F359V, E255K/V, and Y253H [Table 4].25 These mutations most frequently emerge on frontline or second-line nilotinib therapy and their presence is a contraindication to the use of nilotinib.22 The Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd) study was a phase 3, randomized, open-label, multicenter study that assigned 846 patients with CP-CML in a 1:1:1 ratio to receive nilotinib (at a dose of either 300 mg or 400 mg twice daily) or imatinib (at a dose of 400 mg once daily) [Table 1].26 At 12 months, the rates of MMR for nilotinib (44% for the 300-mg dose and 43% for the 400-mg dose) were nearly twice that for imatinib (22%) (P<0.001).26 The rates of CCgR by 12 months were significantly higher for nilotinib (80% for the 300-mg dose and 78% for the 400-mg dose) than for imatinib (65%).26 Patients receiving either the 300-mg dose or the 400-mg dose of nilotinib twice daily had a significant improvement in the time to progression to AP or BP. Gastrointestinal and fluid-retention events were more frequent among patients receiving imatinib, whereas dermatologic events and headache were more frequent in those receiving nilotinib.26 This study led to the frontline approval of nilotinib in the management of patients with newly diagnosed CML. At the 5-year follow up of the ENESTnd study, 60%, 62%, and 50% of pts in the nilotinib 300 mg BID, 400 mg BID, and imatinib arms, respectively, remained on core treatment.27,28 Over half of pts in the nilotinib arms achieved MR4.5 (BCR-ABLIS ≤ 0.0032%) by 5 years. Rates of MMR, freedom from progression to AP and BP, and OS were higher with nilotinib in comparison to imatinib. An emerging concern related to the use of nilotinib is the occurrence of vascular events including peripheral arterial occlusive disease (PAOD), coronary artery disease (CAD), cerebrovascular disease (CVA), hyperglycemia and hypercholesterolemia.27,28 In the ENESTnd trial, 20% of patients on the nilotinib 300 mg arm who were not diabetic at baseline were diabetic by 3 years, in comparison to 9% on the imatinib arm.29
3. Dasatinib
Dasatinib is a second-generation BCR-ABL1 inhibitor that has a 325-fold higher potency in vitro in comparison to imatinib. Dasatinib is active against majority of the kinase domain mutations, with the exception of the T315I gatekeeper mutation.30 In the CA180-034 trial of patients with imatinib-resistant or imatinib-intolerant CP-CML, dasatinib 100 mg once daily showed durable efficacy and safety, including a 6-year PFS of 49%, OS of 71%, and cumulative MMR rate of 43%.31 In the phase 3 DASISION (DASatinib versus Imatinib Study In treatment-Naive CML patients) trial, patients with newly diagnosed CP-CML were randomized to receive dasatinib 100 mg (n = 259) or imatinib 400 mg (n = 260) once daily [Table 1]. 32 Cumulative response rates by 24 months in dasatinib and imatinib arms were: CCgR in 86% versus 82%, MMR in 64% versus 46%, and MR4.5 in 17% versus 8%.33 Transformation to AP/BP CML on study occurred in 2.3% with dasatinib versus 5.0% with imatinib. In safety analyses, fluid retention, superficial edema, myalgia, vomiting, and rash were less frequent with dasatinib compared with imatinib, whereas pleural effusion and grade 3/4 thrombocytopenia were more frequent with dasatinib.33 This trial led to the frontline approval of dasatinib for newly diagnosed patients with CP-CML. At four years, 76% of patients on the dasatinib arm and 63% of patients on the imatinib arm had achieved a MMR.34 In patients who achieved MMR, the median time to MMR for dasatinib and imatinib patients was 9.2 and 15.0 months, respectively. Through four years, transformation to AP and BP occurred in 12 patients receiving dasatinib and 18 patients receiving imatinib. PFS at four years was 90% in both arms and OS was 93% and 92% for dasatinib and imatinib respectively.34 At three months, 84% of dasatinib treated patients vs. 64% of imatinib treated patients achieved an EMR. In the dasatinib arm, PFS and OS rates at four years for patients who had BCR-ABL ≤10% at three months vs. those who did not were 92% vs. 67% (p=0.0004) and 95% vs. 83% (p=0.0092), respectively. In the imatinib arm, PFS and OS rates at four years for patients who had BCR-ABL ≤10% at three months vs. those who did not were 95% vs. 70% (p<0.0001) and 96% vs. 84% (p=0.0021), respectively. Most drug-related adverse events occurred within the first year of treatment, and included myelosuppression, fluid retention (pleural effusion and superficial localized edema), diarrhea, headache, musculoskeletal pain, rash, and nausea. Of more concern are reports of pulmonary arterial hypertension, which by and large seems to resolve in most patients after discontinuing the medication. 34
4. Bosutinib
Bosutinib is an oral Src/Abl tyrosine kinase inhibitor. The phase III Bosutinib Efficacy and Safety in Newly Diagnosed CML (BELA) trial compared bosutinib with imatinib in newly diagnosed, CP-CML [Table 1].35 A total of 502 patients were randomly assigned 1:1 to bosutinib 500 mg per day or imatinib 400 mg per day. The CCgR rate at 12 months was not different for bosutinib (70%; 95% CI, 64% to 76%) versus imatinib (68%; 95% CI, 62% to 74%; two-sided P = .601); hence, the study did not achieve its primary end point.35 The MMR rate at 12 months was higher with bosutinib (41%; 95% CI, 35% to 47%) compared with imatinib (27%; 95% CI, 22% to 33%; two-sided P < .001). Time to CCgR and MMR was faster with bosutinib compared with imatinib (two-sided P < .001 for both). On-treatment transformation to AP/BP occurred in four patients (2%) on bosutinib compared with 10 patients (4%) on imatinib. The safety profiles of bosutinib and imatinib were distinct; GI and liver-related events were more frequent with bosutinib, whereas neutropenia, musculoskeletal disorders, and edema were more frequent with imatinib. Bosutinib, compared with imatinib, was associated with higher incidences of diarrhea (68% v 21%, respectively), vomiting (32% v 13%, respectively), and abdominal pain (11% v 5%, respectively).35 Conversely, bosutinib, compared with imatinib, was associated with lower incidences of edema (11% v 38%, respectively), bone pain (4% v 10%, respectively), and muscle spasms (2% v 20%, respectively). The aggregate incidence of grade 3 or 4 AEs was 64% in the bosutinib arm and 48% in the imatinib arm (P < .001).35
5. Ponatinib
Ponatinib, a highly potent third generation TKI was granted accelerated approval by the U.S FDA in December 2012 for the treatment of patients with CML that were resistant to, or intolerant of prior TKI therapy. Ponatinib is effective against a vast spectrum of kinase domain mutations, including the T315I gatekeeper mutation.36 In a phase II study (PACE-Ponatinib in patients with CML or Ph+ ALL), among 267 patients with CP-CML, 56% had a MCgR (including 70% of patients with the T315I mutation), 46% had a CCgR (66% with the T315I mutation), and 34% had a MMR (56% of patients with the T315I mutation).37 Responses were observed regardless of the baseline kinase domain mutation status. No single BCR-ABL1 mutation conferring resistance to ponatinib was detected. Among 83 patients with AP-CML and 62 with BP-CML, 55% and 23% had a MCgR. Common adverse events reported were thrombocytopenia (37%), skin rash (34%), dry skin (32%), and abdominal pain (22%). Serious arterial thrombotic events were observed in 9% of patients; these events were considered to be treatment-related in 3%.37 Additional follow-up from these ponatinib trials has since revealed a much higher frequency of serious adverse vascular events (48% and 24% in the phase I and II trials, respectively). 38This concern led the U.S. FDA and Ariad Pharmaceuticals to abruptly withdraw ponatinib from the market in October 2013. Importantly, an ongoing phase III trial (EPIC- Exploring Ponatinib In untreated patients with CML) comparing ponatinib to imatinib for the first-line treatment of CML was also closed, patients were crossed over to imatinib, and their follow-up was terminated.38
In January 2014, the US FDA allowed Ariad Pharmaceuticals to resume the marketing of ponatinib with new safety measures. The current indications for ponatinib include; treatment of adult patients with T315I-positive CML (CP, AP and BP), or T315I-positive Ph+ ALL, and the treatment of adult patients with CP, AP or BP CML or Ph+ ALL for whom no other TKI therapy is indicated. The warnings and precautions in the label were revised to describe the vascular occlusion events, including venous thromboembolism, PAOD, CAD and CVA. The optimal starting dose for ponatinib was decreased to 30 mg daily. Additional side effects include, pancreatitis (biochemical and clinical), hepatic transaminitis and treatment emergent hypertension [Table 3].
Choosing the optimal TKI
Imatinib was the first TKI approved for the management of CML and has >15 years of safety and efficacy data associated with it.24 It is generally well tolerated and all the randomized studies comparing imatinib with second generation TKIs’ have not demonstrated a clinically significant survival difference (both for OS and PFS). Therefore, while considering frontline therapy in patients in whom survival is the predominant goal, imatinib still remains an excellent option. This is especially relevant if patients have a low Sokal score or a low EUTOS (European Treatment and Outcome Study Score) score.39 One strategy designed to maximize the use of imatinib and only use more potent TKIs where there is evidence of a high risk of progression is to use frontline imatinib and rely on the molecular responses at 3 and/or 6 months to identify the high-risk patients and switch them to a second generation TKI (lack of EMR at 3-6 months or lack of an optimal molecular response at 12 months). This concept is supported in part by the recent TIDEL-II (Therapeutic Intensification in De Novo Leukemia-II) study. Two-hundred and ten patients with CP-CML were enrolled in two equal, sequential cohorts.40 All patients started treatment with imatinib 600 mg/day. Imatinib plasma trough level was performed at day 22 and if <1000 ng/mL, the dose of imatinib was escalated to 800 mg/day. Patients were then assessed against molecular targets: BCR-ABL1 ≤10%, ≤1%, and ≤0.1% at 3, 6, and 12 months, respectively. Cohort 1 patients failing any target escalated to imatinib 800 mg/day, and subsequently switched to nilotinib 400 mg twice daily for failing the same target 3 months later. Cohort 2 patients failing any target were switched to nilotinib directly, as did patients with intolerance or loss of response in either cohort. At 2 years, 55% of patients remained on imatinib, and 30% on nilotinib. Only 12% had >10% BCR-ABL1 at 3 months. Confirmed MMR was achieved in 64% at 12 months and 73% at 24 months. At 3 years, OS was 96% and transformation-free survival was 95%. 40
In contrast to imatinib, the front line use of second-generation TKIs result in faster and deeper molecular responses (MMR/MR3 and MR4.5), 26,33,41 lower risks of AP/BC transformation,33 and in the case of nilotinib, lower risk of acquired kinase domain mutations.22 Hence these agents can be used preferentially in younger patients and in those with high Sokal or EUTOS scores (studies need further validation). However, outstanding questions remain over the long-term safety profile of these drugs. The development of vascular side effects such as PAOD, CVA, CAD and VTE with nilotinib and ponatinib and cardiopulmonary toxicities such as pleural effusions, interstitial pneumonitis and pulmonary hypertension with dasatinib warrant caution while prescribing these agents for frontline therapy. These toxicities may, in part, contribute to the lack of significant differences in OS between patients treated with imatinib and those receiving second generation TKIs’.
Newer TKIs’ have a distinct role in patients who have known mutations precluding the use of imatinib or other second generation TKIs’ [Table 5] and in the setting of intolerance to imatinib therapy. Co-morbidities of the patient and side effect profile of the TKI of interest should be an important consideration in decision making. For example, nilotinib and ponatinib should be avoided in someone who has preexisting peripheral vascular disease or cardiovascular disease, while dasatinib should be avoided in someone with pre-existing pleural effusions or pulmonary hypertension. At present, the cost of second generation TKIs’ is not remarkably different from imatinib. However, the patent for imatinib is expected to expire soon, and it will be available as a generic product. Clinicians, then, need to weigh the advantages some patients gain with nilotinib or dasatinib in the frontline setting against the difference in cost.42
Table 5. BCR-ABL mutations and therapeutic options 64-70*.
| Mutation | Drug resistance |
Therapeutic options |
|---|---|---|
| Wild type M244V Q252H M315T F311L L387M H396P |
- | Imatinib Dasatinib Nilotinib Bosutinib |
| G250E F311I H396R |
Imatinib | Dasatinib Nilotinib Bosutinib |
| V299L T315A F317L/V/I/C |
Dasatinib Bosutinib |
Nilotinib Imatinib |
| Y253H/F E255K/V E355G V379I F359V/C/I |
Imatinib Nilotinib |
Dasatinib |
| T315I | Imatinib Dasatinib Nilotinib Bosutinib |
Ponatinib |
| T315M | All TKIs | - |
This table compiles the mutations and sensitivities from studies reporting IC50 values derived using a cell line model. It suggests sensitivity to TKIs based on mutations but firm clinical data is not currently available.
Newer Agents for CML
Although TKIs’ have offered much in terms of OS and quality of life for patients with CML, the ability of these agents to cure CML is limited. In the prospective, multicentre, Stop Imatinib (STIM) study, imatinib treatment (of >2 years duration) was discontinued in patients with CML who had molecularly undetectable leukemia (CMR= >5-log reduction in BCR-ABL). 100 patients were enrolled and 69 patients had at least 12 months follow-up. Forty-two (61%) of these 69 patients relapsed.43 At 12 months, the probability of persistent CMR for these 69 patients was 41% (95% CI 29-52). All patients who relapsed responded to reintroduction of imatinib.43 This failure results from the inability of TKIs’ to eradicate quiescent CML stem cells. In a recent study, the activation of peroxisome proliferator-activated receptor-gamma (PPAR-γ) by glitazones (anti-diabetic drugs), decreased the expression of STAT5 and its downstream targets HIF2α and CITED2, which are key guardians of quiescence in stem cells.44 When pioglitazone was given temporarily to three patients with CML with chronic residual disease in spite of imatinib therapy, all of them achieved sustained CMRs’ up to 4.7 years after withdrawal of pioglitazone.44 These exciting results need validation in larger prospective studies.
Newer TKIs’ with higher potencies and activity against the gatekeeper mutation, such as danusertib (PHA-739358)45-47 and rebastinib (DCC-2036) are undergoing clinical development [Table 6]. ABL001 is a potent, specific BCR-ABL inhibitor with a distinct, allosteric mechanism of action that recently entered phase I development.48 It has been developed for use in combination with nilotinib to provide greater coverage of BCR-ABL and in order to avoid the development of resistance. In contrast to TKIs that bind to the ATP-site of the ABL1 kinase domain, ABL001 binds to a pocket on the BCR-ABL kinase domain that is normally occupied by the myristoylated N-terminus of ABL1 [Figure 1]. ABL001 functionally mimics the role of the myristoylated N-terminus by occupying its vacant binding site and restores the negative regulation of the kinase activity.48 Given that it does not act on the ATP binding site of the kinase domain; it has demonstrable in vitro activity against the T315I gatekeeper mutation.
Table 6. Newer TKIs & non-TKIs under study for CML.
| Agent | Mode of action | Activity in the presence of mutations/ Preliminary data |
Phase of development |
References |
|---|---|---|---|---|
| Omacetaxine mepesuccinate (Synribo) |
Semisynthetic formulation of homoharringtonine. The mechanism of action includes inhibition of protein synthesis leading to cell death. |
Not known/ CML-CP 18% MCR. |
Phase 3 | 71-74 |
| MK 0457 | Aurora kinase inhibitor | T315I/ Minimal response in clinical trials |
Phase 2 | 75 |
| PHA-739358 (Danusertib) |
Pan aurora kinase inhibitor and third generation TKI | T315I | Phase 2 | 45 |
| ABL001 | Allosteric inhibitor of BCR-ABL prevents emergence of resistant disease when administered in combination with nilotinib |
Yes | Phase 1 | 48 |
| DCC-2036 (Rebastinib) |
TIE2, VEGFR1, BCR-ABL kinase inhibitor | T315I | Phase 1 | 46,47 |
| AT9283 | Aurora kinase inhibitor | Yes | Phase 1 | 76 |
| BP5-087 | STAT3 SH2 domain inhibitor combines with bcr-abl1 inhibition to overcome kinase-independent resistance in chronic myeloid leukemia |
Yes | Pre-clinical | 77,78 |
| ON012380 | Non-ATP competitive inhibitor of BCR-ABL | Unknown | Pre-clinical | 79 |
| SGX70393 | Azapyridine-based inhibitor of native and T315I-mutant BCR-ABL kinase |
T315I | Pre-clinical | 80 |
| TG101223 | Small molecule BCR-ABL inhibitor | T315I | Pre-clinical | 81 |
| GNF-2/ GNF-5 | Allosteric inhibitors of BCR-ABL | T315I | Pre-clinical | 82 |
| ESKM | Human IgG1 T-cell receptor mimic monoclonal antibody used alone or in combination with TKIs |
T315I | Pre-clinical | 83 |
| U0126 | MEK1/2 inhibitor reverses imatinib resistance through down- regulating activation of Lyn/ERK signaling pathway in imatinib-resistant K562R leukemia cells |
Yes | Pre-clinical | 84 |
| KW-2449 | Dual BCR-ABL Aurora kinase inhibitor | Yes | Pre-clinical | 85,86 |
Summary
TKIs’ have revolutionized the management of patients with CML and have markedly improved their OS. Imatinib was the first TKI approved and is currently considered a very effective front line option for most patients. With more than a decade of safety data, the long term use of imatinib is generally safe and no major safety concerns have emerged. With time, a fair number of patients discontinue imatinib, either due to disease progression/resistance or secondary to intolerance. For this group of patients newer TKIs’ have revolutionized care. The newer TKIs’ are more potent than imatinib and have unique side effect profiles. These agents are more likely than imatinib to achieve optimal molecular responses, especially EMR, leading to a considerable debate on which TKI is the best frontline option. While there is no doubting their efficacy, especially in patients with kinase domain mutations, their safety profiles are somewhat controversial and continue to evolve. Serious vascular side effects such as PAOD, CVA and CAD with nilotinib and ponatinib and the development of pulmonary hypertension with dasatinib, currently continue to dampen the enthusiasm for using these drugs as frontline agents. Newer TKIs’ with enhanced potencies and safety profiles and drugs acting at alternate sites optimizing responses are exciting prospects.
Acknowledgments
Current publication is supported in part by grants from the “The Henry J. Predolin Foundation for Research in Leukemia, Mayo Clinic, Rochester, MN, USA”.
This publication was supported by CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement:
None of the authors have any conflict of interest to disclose in regards to the current manuscript.
References
- 1.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Neriah Y, Daley GQ, Mes-Masson AM, et al. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986 Jul 11;233(4760):212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- 3.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 4.Shtivelman E, Lifshitz B, Gale RP, et al. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13-19;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- 5.Bedi A, Zehnbauer BA, Barber JP, et al. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood. 1994 Apr 15;83(8):2038–2044. [PubMed] [Google Scholar]
- 6.Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996 Oct 1;88(7):2375–2384. [PubMed] [Google Scholar]
- 7.Goldman JM. Chronic myeloid leukemia: a historical perspective. Semin Hematol. 2010 Oct;47(4):302–311. doi: 10.1053/j.seminhematol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Talpaz M, Kantarjian HM, McCredie KB, et al. Clinical investigation of human alpha interferon in chronic myelogenous leukemia. Blood. 1987 May;69(5):1280–1288. [PubMed] [Google Scholar]
- 9.Interferon alfa versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials: Chronic Myeloid Leukemia Trialists’ Collaborative Group. J Natl Cancer Inst. 1997 Nov 5;89(21):1616–1620. [PubMed] [Google Scholar]
- 10.Bonifazi F, de Vivo A, Rosti G, et al. Chronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic responders. Blood. 2001 Nov 15;98(10):3074–3081. doi: 10.1182/blood.v98.10.3074. [DOI] [PubMed] [Google Scholar]
- 11.Guilhot F, Chastang C, Michallet M, et al. French Chronic Myeloid Leukemia Study Group Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. N Engl J Med. 1997 Jul 24;337(4):223–229. doi: 10.1056/NEJM199707243370402. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003 Mar 13;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 13.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006 Dec 7;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 14.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001 Aug 3;293(5531):876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 15.Shah NP, Sawyers CL. Mechanisms of resistance to STI571 in Philadelphia chromosome-associated leukemias. Oncogene. 2003 Oct 20;22(47):7389–7395. doi: 10.1038/sj.onc.1206942. [DOI] [PubMed] [Google Scholar]
- 16.Jabbour E, Parikh SA, Kantarjian H, et al. Chronic myeloid leukemia: mechanisms of resistance and treatment. Hematol Oncol Clin North Am. 2011 Oct;25(5):981–995. v. doi: 10.1016/j.hoc.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deininger M, O’Brien SG, Guilhot F, et al. International Randomized Study of Interferon Vs STI571 (IRIS) 8-Year Follow up: Sustained Survival and Low Risk for Progression or Events in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Treated with Imatinib; Abstract presented at ASH; 2009. [Google Scholar]
- 18.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014 Jan 23;123(4):494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014 Feb 27;123(9):1353–1360. doi: 10.1182/blood-2013-06-510396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin D, Hedgley C, Clark RE, et al. Predictive value of early molecular response in patients with chronic myeloid leukemia treated with first-line dasatinib. Blood. 2012 Jul 12;120(2):291–294. doi: 10.1182/blood-2012-01-407486. [DOI] [PubMed] [Google Scholar]
- 21.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009 Jun;23(6):1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 22.Hochhaus A, Saglio G, Larson RA, et al. Nilotinib is associated with a reduced incidence of BCR-ABL mutations vs imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood. 2013 May 2;121(18):3703–3708. doi: 10.1182/blood-2012-04-423418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalmanti L, Saussele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-study IV. Leukemia. 2015 May;29(5):1123–1132. doi: 10.1038/leu.2015.36. [DOI] [PubMed] [Google Scholar]
- 24.Castagnetti F, Gugliotta G, Breccia M, et al. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015 Sep;29(9):1823–1831. doi: 10.1038/leu.2015.152. [DOI] [PubMed] [Google Scholar]
- 25.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009 Sep 1;27(25):4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010 Jun 17;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 27.Larson RA, Kim DW, Issaragrilsil S, et al. Efficacy and Safety of Nilotinib (NIL) vs Imatinib (IM) in Patients (pts) With Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP): Long-Term Follow-Up (f/u) of ENESTnd; Abstract presented at ASH; 2014. [Google Scholar]
- 28.Larson RA, Kim DW, Jootar S, et al. ENESTnd 5-year (y) update: Long-term outcomes of patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib (NIL) versus imatinib (IM); Abstarct presented at ASCO; 2014. [Google Scholar]
- 29.Delphine R, Gautier J, Breccia M, et al. Incidence of Hyperglycemia by 3 Years in Patients (Pts) with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Treated with Nilotinib (NIL) or Imatinib (IM) in ENESTnd; Abstract presented at ASH; 2012. [Google Scholar]
- 30.Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009 Dec 3;114(24):4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah NP, Guilhot F, Cortes JE, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014 Apr 10;123(15):2317–2324. doi: 10.1182/blood-2013-10-532341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010 Jun 17;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 33.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012 Feb 2;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortes JE, Hochhaus A, Kim DW, et al. Four-Year (Yr) Follow-Up Of Patients (Pts) With Newly Diagnosed Chronic Myeloid Leukemia In Chronic Phase (CML-CP) Receiving Dasatinib Or Imatinib: Efficacy Based On Early Response; Abstract presented at ASH; 2013. [Google Scholar]
- 35.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012 Oct 1;30(28):3486–3492. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012 Nov 29;367(22):2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013 Nov 7;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gainor JF, Chabner BA. Ponatinib: Accelerated Disapproval. Oncologist. 2015 Aug;20(8):847–848. doi: 10.1634/theoncologist.2015-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marin D, Ibrahim AR, Goldman JM. European Treatment and Outcome Study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol. 2011 Oct 10;29(29):3944–3945. doi: 10.1200/JCO.2011.37.6962. [DOI] [PubMed] [Google Scholar]
- 40.Yeung DT, Osborn MP, White DL, et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015 Feb 5;125(6):915–923. doi: 10.1182/blood-2014-07-590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011 Sep;12(9):841–851. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 42.Tefferi A, Kantarjian H, Rajkumar SV, et al. In Support of a Patient-Driven Initiative and Petition to Lower the High Price of Cancer Drugs. Mayo Clin Proc. 2015 Aug;90(8):996–1000. doi: 10.1016/j.mayocp.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010 Nov;11(11):1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 44.Prost S, Relouzat F, Spentchian M, et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARgamma agonists. Nature. 2015 Sep 17;525(7569):380–383. doi: 10.1038/nature15248. [DOI] [PubMed] [Google Scholar]
- 45.Paquette RL, Shah NP, Sawyers CL, et al. PHA-739358, an Aurora Kinase Inhibitor, Induces Clinical Responses in Chronic Myeloid Leukemia Harboring T315I Mutations of BCR-ABL; Abstract presented at ASH; 2007. [Google Scholar]
- 46.Eide CA, Adrian LT, Tyner JW, et al. The ABL switch control inhibitor DCC-2036 is active against the chronic myeloid leukemia mutant BCR-ABLT315I and exhibits a narrow resistance profile. Cancer Res. 2011 May 1;71(9):3189–3195. doi: 10.1158/0008-5472.CAN-10-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen Y, Shi X, Pan J. The conformational control inhibitor of tyrosine kinases DCC-2036 is effective for imatinib-resistant cells expressing T674I FIP1L1-PDGFRalpha. PLoS One. 2013;8(8):e73059. doi: 10.1371/journal.pone.0073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wylie ASJ, Berellini G, et al. ABL001, a Potent Allosteric Inhibitor of BCR-ABL, Prevents Emergence of Resistant Disease When Administered in Combination with Nilotinib in an in Vivo Murine Model of Chronic Myeloid Leukemia; Abstract presented at ASH; 2014. [Google Scholar]
- 49.Cortes JSG, Baccarani M, et al. Final Study Results of the Phase 3 Dasatinib Versus Imatinib in Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Trial (DASISION, CA180-056); Abstract presented at ASH; 2014. [Google Scholar]
- 50.Brummendorf TH, Cortes JE, de Souza CA, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. Br J Haematol. 2015 Jan;168(1):69–81. doi: 10.1111/bjh.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Novartis Gleevac: Prescribing information. 2015 Retreived from http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021588s009lbl.pdf.
- 52.Squibb BM. Sprycel: Full Prescribing Information. 2015 Retreived from http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021986s016s017lbledt.pdf.
- 53.Novartis Tasigna: Full prescribing information. 2015 Retreived from http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022068s020lbl.pdf.
- 54.Pfizer Laboratories Div Pfizer Inc. Bosulif: Full Prescribing Information. 2014 Retreived from http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/203341s002lbl.pdf.
- 55.Ariad Iclusig: Full Prescribing Information. 2014 Retreived from http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/203469s009lbl.pdf.
- 56.Kamath AV, Wang J, Lee FY, et al. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): a potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother Pharmacol. 2008 Mar;61(3):365–376. doi: 10.1007/s00280-007-0478-8. [DOI] [PubMed] [Google Scholar]
- 57.Xia B, Heimbach T, He H, Lin TH. Nilotinib preclinical pharmacokinetics and practical application toward clinical projections of oral absorption and systemic availability. Biopharm Drug Dispos. 2012 Dec;33(9):536–549. doi: 10.1002/bdd.1821. [DOI] [PubMed] [Google Scholar]
- 58.Neville K, Parise RA, Thompson P, et al. Plasma and cerebrospinal fluid pharmacokinetics of imatinib after administration to nonhuman primates. Clin Cancer Res. 2004 Apr 1;10(7):2525–2529. doi: 10.1158/1078-0432.ccr-03-0155. [DOI] [PubMed] [Google Scholar]
- 59.Porkka K, Koskenvesa P, Lundan T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008 Aug 15;112(4):1005–1012. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- 60.Reinwald M, Schleyer E, Kiewe P, et al. Efficacy and pharmacologic data of second-generation tyrosine kinase inhibitor nilotinib in BCR-ABL-positive leukemia patients with central nervous system relapse after allogeneic stem cell transplantation. Biomed Res Int. 2014;2014:637059. doi: 10.1155/2014/637059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liang S, Pong K, Gonzales C, et al. Neuroprotective profile of novel SRC kinase inhibitors in rodent models of cerebral ischemia. J Pharmacol Exp Ther. 2009 Dec;331(3):827–835. doi: 10.1124/jpet.109.156562. [DOI] [PubMed] [Google Scholar]
- 62.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013 Aug 8;122(6):872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.NCCN NCCN Clinical Practice Guidelines in Oncology: Chronic Myelogenous Leukemia. Version I.2016. 2016 doi: 10.6004/jnccn.2009.0065. Retrieved from http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. [DOI] [PubMed]
- 64.O’Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007 Oct 1;110(7):2242–2249. doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 65.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009 Jan 20;27(3):469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 66.Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood. 2009 Dec 24;114(27):5426–5435. doi: 10.1182/blood-2009-08-215939. [DOI] [PubMed] [Google Scholar]
- 67.Jabbour E, Branford S, Saglio G, et al. Practical advice for determining the role of BCR-ABL mutations in guiding tyrosine kinase inhibitor therapy in patients with chronic myeloid leukemia. Cancer. 2011 May 1;117(9):1800–1811. doi: 10.1002/cncr.25717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011 Aug 4;118(5):1208–1215. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- 69.O’Brien S, Berman E, Moore JO, et al. NCCN Task Force report: tyrosine kinase inhibitor therapy selection in the management of patients with chronic myelogenous leukemia. J Natl Compr Canc Netw. 2011 Feb;9(Suppl 2):S1–25. doi: 10.6004/jnccn.2011.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cortes JE, D K, Pinilla-Ibarz J, et al. Ponatinib In Patients (pts) With Chronic Myeloid Leukemia (CML) and Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL) Resistant Or Intolerant To Dasatinib Or Nilotinib, Or With The T315I BCR-ABL Mutation: 2-Year Follow-Up Of The PACE Trial. Abstract. Blood. 2013 Nov 15;122(21) 2013. [Google Scholar]
- 71.Cortes J, Lipton JH, Rea D, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012 Sep 27;120(13):2573–2580. doi: 10.1182/blood-2012-03-415307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cortes J, Digumarti R, Parikh PM, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate for chronic-phase chronic myeloid leukemia patients resistant to or intolerant of tyrosine kinase inhibitors. Am J Hematol. 2013 May;88(5):350–354. doi: 10.1002/ajh.23408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alvandi F, Kwitkowski VE, Ko CW, et al. U.S. Food and Drug Administration approval summary: omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. Oncologist. 2014 Jan;19(1):94–99. doi: 10.1634/theoncologist.2013-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cortes JE, Kantarjian HM, Rea D, et al. Final analysis of the efficacy and safety of omacetaxine mepesuccinate in patients with chronic- or accelerated-phase chronic myeloid leukemia: Results with 24 months of follow-up. Cancer. 2015 May 15;121(10):1637–1644. doi: 10.1002/cncr.29240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seymour JF, Kim DW, Rubin E, et al. A phase 2 study of MK-0457 in patients with BCR-ABL T315I mutant chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Cancer J. 2014;4:e238. doi: 10.1038/bcj.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka R, Squires MS, Kimura S, et al. Activity of the multitargeted kinase inhibitor, AT9283, in imatinib-resistant BCR-ABL-positive leukemic cells. Blood. 2010 Sep 23;116(12):2089–2095. doi: 10.1182/blood-2009-03-211466. [DOI] [PubMed] [Google Scholar]
- 77.Eiring Anna M., Kraft Ira L., Page Brent D.G., et al. BP5-087, a Novel STAT3 Inhibitor, Combines With BCR-ABL1 Inhibition To Overcome Kinase-Independent Resistance In Chronic Myeloid Leukemia. Abstract. Blood. 2013;122(21) [Google Scholar]
- 78.Eiring AM, Page BD, Kraft IL, et al. Combined STAT3 and BCR-ABL1 inhibition induces synthetic lethality in therapy-resistant chronic myeloid leukemia. Leukemia. 2015 Mar;29(3):586–597. doi: 10.1038/leu.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu J, Meng F, Ying Y, et al. ON012380, a putative BCR-ABL kinase inhibitor with a unique mechanism of action in imatinib-resistant cells. Leukemia. 2010;24(4):869–872. doi: 10.1038/leu.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Hare T, Eide C, Tyner J, et al. SGX70393 inhibits the T315I mutant of Bcr-Abl kinase and pre-empts drug resistance when combined with nilotinib or dasatinib. Molecular Cancer Therapeutics. 2007 Nov 1;6(11 Supplement):A259. 2007. [Google Scholar]
- 81.Zhu H, Hanna E, Lohse D, et al. In Vitro and In Vivo Inhibition of the T315I Mutant BCR/ABL Kinase; Abstract presented at ASH; 2006. [Google Scholar]
- 82.Zhang J, Adrian FJ, Jahnke W, et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010 Jan 28;463(7280):501–506. doi: 10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dubrovsky L, Pankov D, Brea EJ, et al. A TCR-mimic antibody to WT1 bypasses tyrosine kinase inhibitor resistance in human BCR-ABL+ leukemias. Blood. 2014 May 22;123(21):3296–3304. doi: 10.1182/blood-2014-01-549022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi R, Lin J, Guo Y, Gong YP. The MEK1/2 inhibitor U0126 reverses imatinib resistance through down-regulating activation of Lyn/ERK signaling pathway in imatinib-resistant K562R leukemia cells. Pharmazie. 2014 May;69(5):346–352. [PubMed] [Google Scholar]
- 85.Shiotsu Y, Kiyoi H, Ishikawa Y, et al. KW-2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315I-mutated BCR/ABL translocation. Blood. 2009 Aug 20;114(8):1607–1617. doi: 10.1182/blood-2009-01-199307. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen T, Dai Y, Attkisson E, et al. HDAC inhibitors potentiate the activity of the BCR/ABL kinase inhibitor KW-2449 in imatinib-sensitive or -resistant BCR/ABL+ leukemia cells in vitro and in vivo. Clin Cancer Res. 2011 May 15;17(10):3219–3232. doi: 10.1158/1078-0432.CCR-11-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009 Feb 19;113(8):1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hantschel O, Grebien F, Superti-Furga G. The growing arsenal of ATP-competitive and allosteric inhibitors of BCR-ABL. Cancer Res. 2012 Oct 1;72(19):4890–4895. doi: 10.1158/0008-5472.CAN-12-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]