Abstract
To gain insights into the pathogenicity of Imjin virus (MJNV), a newfound hantavirus isolated from the Ussuri white-toothed shrew (Crocidura lasiura), groups of Syrian hamsters (Mesocricetus auratus) of varying ages (<1, 5, 10, 14, 21, 35 and 56 days) were inoculated by the intraperitoneal route with 1,000 pfu of MJNV strains 04-55 and 05-11. MJNV-infected Syrian hamsters, aged 21 days or less, exhibited reduced activity, weight loss, respiratory distress, hind-limb paralysis and seizures. Death ensued 1 to 6 days after onset of clinical disease. MJNV RNA was detected in brain and other major organs by RT-PCR and real time-PCR. Histopathological examination showed alveolar hemorrhage, interstitial pneumonia and severe pulmonary congestion; focal hepatic necrosis and portal inflammation; and acute meningoencephalitis. By immunohistochemistry, MJNV antigen was detected in pulmonary microvascular endothelial cells and glial cells. Older hamsters (35 and 56 days of age) developed subclinical infection without histopathological changes. Future studies are warranted to determine the pathophysiologic bases for the differential age susceptibility of Syrian hamsters to lethal MJNV disease.
Keywords: Imjin virus, Syrian hamster, hantavirus, meningoencephalitis
1. Introduction
Rodents (order Rodentia), belonging to the Muridae and Cricetidae families, have long been known to serve as reservoir hosts of hantaviruses (family Bunyaviridae, genus Hantavirus), which are enveloped viruses with a negative-sense, single-stranded, tripartite RNA genome, consisting of large (L), medium (M) and small (S) segments, which encode an RNA-dependent RNA polymerase (RdRp), two envelope glycoproteins (Gn, Gc) and a nucleocapsid protein (NP), respectively (Schmaljohn and Dalrymple, 1983; Schmaljohn et al., 1983). Hantaviruses, such as Hantaan virus (HTNV), Seoul virus (SEOV) and Puumala virus (PUUV), which are harbored by murid and arvicolid rodents, cause hemorrhagic fever with renal syndrome (HFRS), an acute febrile disease characterized by varying degrees of hemorrhage and renal insufficiency (Brummer-Korvenkontio et al., 1980; Lee et al., 1978; Lee and Vandergroen, 1989; Yanagihara and Gajdusek, 1988). By contrast, hantaviruses, such as Sin Nombre virus (SNV), Andes virus (ANDV), Black Creek Canal virus (BCCV) and Choclo virus (CHOV), hosted by neotomine and sigmodontine rodents, cause hantavirus pulmonary syndrome (HPS), which is characterized by rapidly progressive respiratory failure with high mortality (Duchin et al., 1994; Lopez et al., 1996; Nelson et al., 2010; Nichol et al., 1993; Ravkov et al., 1995; Zaki et al., 1995).
More recently, attention has been drawn toward genetically distinct hantaviruses detected by reverse transcription polymerase chain reaction (RT-PCR) in multiple species of shrews and moles (order Eulipotyphla, families Soricidae and Talpidae) and insectivorous bats (order Chiroptera) in widely separated geographic regions across Europe, Asia, Africa and North America (Bennett et al., 2014; Yanagihara et al., 2014). However, of the more than 30 non-rodent-borne hantaviruses, only two have been isolated in cell culture. The first is Thottapalayam virus (TPMV), originally isolated from spleen tissue of an Asian house shrew (Suncus murinus) captured in southern India in 1964 (Carey et al., 1971; Song et al., 2007). And the second is Imjin virus (MJNV), isolated from lung tissues of Ussuri white-toothed shrews (Crocidura lasiura), captured near the demilitarized zone in Korea (Song et al., 2009).
Whether or not soricid-borne hantaviruses infect and cause disease in humans is unknown. In this regard, the paucity of hantavirus isolates and the lack of animal models for these newfound hantaviruses, harbored by shrews, moles and bats, has impeded our understanding about their infectivity and pathogenicity in humans. We reasoned that studying the clinical and pathological features of experimental MJNV infection in laboratory animals might indirectly provide insights about MJNV-caused diseases in humans. Moreover, because the Syrian hamster has served as a reliable host for experimental infection with HFRS- and HPS-causing hantaviruses, for both the study of HPS pathogenesis and the testing of hantavirus vaccines (Brocato et al., 2014; Chu et al., 1995; Hooper et al., 1999, 2001, 2013; Safronetz et al., 2011, 2012; Sanada et al., 2011; Schmaljohn et al., 1990; Wahl-Jensen et al., 2007), we selected this cricetid rodent species to conduct a proof-of-concept study of experimental MJNV infection. Our results indicate that infant and juvenile Syrian hamsters develop lethal disease, while adult hamsters develop subclinical infection, following intraperitoneal inoculation with MJNV. Such models might provide valuable insights into the persistence and pathogenicity of MJNV and of other still-orphan non-rodent-borne hantaviruses, which have yet to be isolated.
2. Materials and Methods
2.1. Virus and cell culture
MJNV strains 04-55 and 05-11, isolated in Vero E6 cells (Vero C1008; CRL 1586, American Type Culture Collection, Manassas, VA) from lung tissues of Ussuri white-toothed shrews captured in Yeoncheon county and Paju city, respectively, in Korea (Song et al., 2009), were propagated in Vero E6 cells, maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% heat-inactivated fetal bovine serum (Lonza, Walkersville, MD), 2 mM L-glutamine and antibiotics (penicillin/streptomycin) at 37°C at 5% CO2. As determined by plaque assay (Song et al., 2009), the infectivity titers of the MJNV stocks were 2×105 pfu/mL.
2.2. Ethics statement
All inoculation and handling of rodents, as well as the method of euthanasia and collection of tissues, were performed according to well-established protocols, approved by the Institutional Animal Care and Use Committee of Korea University.
2.3. Animals and virus inoculation
Syrian hamsters (Mesocricetus auratus), designated as “special pathogens free”, were purchased from Daehan Biolink Co., Ltd (Chungcheongbuk-do, Korea). Hamsters were divided into seven groups (with five to 10 hamsters per group) according to their postnatal age (<1, 5, 10, 14, 21, 35 and 56 days), were inoculated with 1,000 pfu of MJNV strains 04-55 and 05-11 by the intraperitoneal route. Hamsters were monitored daily for clinical signs, and were euthanized if moribund or at prescribed time points post-inoculation. Experiments were terminated 42 days after inoculation. Isoflurane anesthesia, followed by cardiac puncture, was used for euthanasia. Sera were tested for immunoglobulin G (IgG) antibodies against MJNV by the indirect immunofluorescent antibody (IFA) test and enzyme-linked immunosorbent assay (ELISA). All animal experiments were performed under ABSL-3 containment, in a facility in which ANDV, SNV and other HPS-causing hantaviruses had never been handled.
2.4. RNA extraction and RT-PCR
Total RNA was extracted from 100 mg of tissues (heart, lung, liver, kidney, spleen, intestine and brain), using RNA-Bee™ (Tel-Test, Inc., Friendswood, TX). Each 20-μL reaction contained 10 μL total RNA, 2 μL random primer (10 μM), 1 μL dNTP (Finnzymes, Vantaa, Finland), 0.5 μL RNase inhibitor (Thermo Fisher Scientific. Waltham, MA), 0.5 μL Moloney murine leukemia virus (M-MLV) Reverse Transcriptase (Promega, Madison, WI) and 4 μL M-MLV 5X reaction buffer. The reaction was performed at 37°C for 1 hr and 94°C for 3 min for terminal reaction. Gene-amplification reactions were performed in 50-μL reaction mixtures, containing 200 μM dNTP 1.5 mM MgCl2, 0.5 U of Super-Therm Taq polymerase (JMR Holdings, London, UK) and 0.2 μM of each primer (Song et al., 2007). Oligonucleotide primer sequences for hemi-nested PCR were MJN-M2235F: 5′–CATGGAAGAGTGCAACATGT–3′, MJN-M2855R: 5′–TATGGTCCCTAGATGTACT–3′, then MJN-M2235F and MJN-M2805R: 5′–TCTATAATAGGATCAGTCAT–3′. Initial denaturation was at 94°C for 5 min, followed by 15 cycles of denaturation at 94°C for 40 sec, annealing at 50°C for 40 sec, elongation at 72°C for 1 min, then 25 cycles of denaturation at 94°C for 40 sec, annealing at 52°C for 40 sec and elongation at 72°C for 1 min, in a Mastercycler ep gradient S (Eppendorf, Hamburg, Germany).
2.5. Construction of standard MJNV RNA
MJNV RNA was constructed from pSTBlue I vector (Novagen, Darmstadt, Germany) with a cloned 570-bp fragment of the M segment. The plasmid was linearized with the restriction enzyme EcoR I and served as a template for RNA transcription using the MEGAscript High Yield Transcription Kit (Ambion, Austin, TX), according to the manufacturer’s instructions. The synthesized RNA was incubated with DNase I (Roche Applied Science, Basel, Switzerland) at 37°C for 15 min and purified using RNA-Bee™ (Tel-Test, Inc.). RNA concentration was measured using a NanoDrop spectrophotometer. After RT-PCR using random primers (10 μM), 10-fold serial dilutions of the cDNA was used to generate a real-time PCR standard curve.
2.6. Quantitative Taqman real-time PCR
The primers and probe targeting the MJNV M segment were designed using the Primer Express® software version 3.0 (Applied Biosystems, Foster City, CA). The M-segment primer sequences for real-time PCR were MJN-RTM2439F: 5′–GTGAATGTAAGAAAATAACTGGAAATGACT–3′ and MJN-RTM2515R: 5′–GACTGTTGTGCTAAGTAGACATACCTTGA–3′. The probe (MJN-probe 2471: 5′–CTCAGCACACATGGG–3′) was labeled with the reporter dye FAM at the 5′-end and quencher dye MGB/non fluorescent at the 3′-end, respectively. Each 20-μL reaction contained 1 μL cDNA, 10 μL 2X TaqMan Gene Expression master mix (Applied Biosystems), 0.5 μL forward and reverse primers (36 μM), 0.5 μL fluorescent probe (10 μM), and 7.5 μL double deionized water. The reaction was performed at 50°C for 2 min and 90°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min, in a StepOne Real-Time PCR system (Applied Biosystems).
2.7. IFA test
Sera from infected hamsters, diluted 1:32, were placed on duplicate wells of MJNV-infected Vero E6 cells, spotted onto 10-well slides, fixed with cold acetone for 10 min, and incubated at 37°C for 30 min. After incubation, slides were washed and 25 μL of fluorescein isothiocyanate (FITC)-labeled anti-hamster IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD) was added to each well and incubated at 37°C for 30 min. Following washes, the slides were examined for virus-specific fluorescence, using an Axioscope fluorescent microscope (Carl Zeiss AG, Oberkochen, Germany). Sera, which are screen-test positive (as defined by the intensity of intracytoplasmic granular fluorescence of ++ or +++), were endpoint titered by testing serial twofold dilutions.
2.8. Histopathology and immunohistochemistry
Lung, liver, kidney, spleen, heart and brain tissues were fixed in 10% buffered formaldehyde and embedded in paraffin. Thin sections (4 μm) of each tissue, stained with hematoxylin and eosin (H&E), were examined by light microscopy. For immunolocalization of MJNV NP, hyperimmune monoclonal mouse ascitic fluid directed against the recombinant nucleocapsid protein (rNP) of MJNV 05-11 was employed as the primary antibody and goat anti-mouse IgG (DAKO Cytomation, Carpinteria, CA) as the secondary antibody, according to the manufacturer’s instructions. Tissue sections were initially deparaffinized in xylene and absolute ethanol, then treated with DAKO Target Retrieval Solution (DAKO Cytomation) at 90°C for 20 min. Endogenous peroxidase activity was minimized by treating tissue sections with 3% hydrogen peroxide. The chromogen and counterstain were diaminobenzidine and hematoxylin, respectively. The slides were mounted with aqueous mounting media for viewing.
3. Results
3.1. Experimental MJNV infection
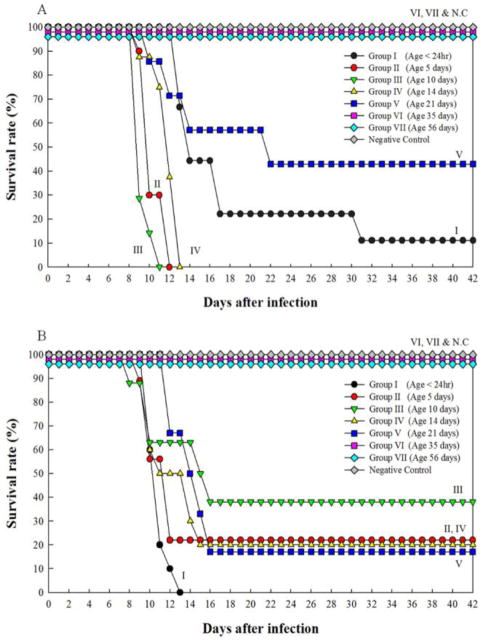
All Syrian hamsters, aged <1 to 21 days (Groups I to V), developed clinical disease after intraperitoneal inoculation with 1,000 pfu of MJNV strains 04-55 and 05-11. The mortality rates in these infant and juvenile hamsters ranged from 62.5–100% (Fig. 1). Death usually occurred one to six days after the onset of clinical signs, which included weight loss, reduced activity, tachypnea, respiratory distress, hind-limb paralysis, seizures and lethargy. Surviving hamsters had profound neurological sequelae, when euthanized at the end of the 42-day observation period. By contrast, hamsters aged 35 (Group VI) and 56 days (Groups VII) developed subclinical infection, showing no evidence of disease and no mortality following MJNV inoculation (Fig. 1). Uninoculated, control hamsters also exhibited no signs of disease.
Fig. 1.
Survival rates of Syrian hamsters inoculated at different ages with 1,000 pfu of MJNV strains 04-55 (A) and 05-11 (B). NC denotes negative control.
3.2. Anti-MJNV antibody response
IgG antibodies against MJNV, ranging in titer from 512 to 4,096 by the IFA test, were found in sera from hamsters in Groups I to V with clinical disease (Table 1). High-titers of IgG antibodies were detectable at the time of death between 8 to 16 days post-inoculation. Terminal sera from surviving hamsters in Groups I to V, collected at 42 days post-inoculation, had similarly high titers of anti-MJNV IgG antibodies. Although without clinical disease, hamsters in Group VI (35 days old) and Group VII (56 days old) exhibited anti-MJNV antibodies by the IFA test, at the end of the 42-day observation period (Table 1).
Table 1.
Serum anti-MJNV IgG antibodies by IFA in hamsters experimentally infected with MJNV strains 04-55 and 05-11.
| MJNV strain 04-55 | MJNV strain 05-11 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Group | No. | Clinical Outcome | Days Post-inoculation | IFA Titer | Age Group | No. | Clinical Outcome | Days Post-inoculation | IFA Titer |
| I (<1 day) | 1 | sd | 13 | 512 | I (<1 day) | 1 | sd | 10 | 4,096 |
| 2 | sd | 13 | 512 | 2 | sd | 10 | 4,096 | ||
| 3 | sd | 14 | 2,048 | 3 | sd | 10 | 2,048 | ||
| 4 | sd | 14 | 4,096 | 4 | sd | 11 | 4,096 | ||
| 5 | ss | 42 | 4,096 | 5 | sd | 11 | 4,096 | ||
| 6 | sd | 11 | 4,096 | ||||||
| 7 | sd | 11 | 4,096 | ||||||
| 8 | sd | 12 | 4,096 | ||||||
| 9 | sd | 13 | 4,096 | ||||||
| II (5 days) | 1 | sd | 10 | 4,096 | II (5 days) | 1 | sd | 10 | 4,096 |
| 2 | sd | 10 | 2,048 | 2 | sd | 10 | 2,048 | ||
| 3 | sd | 10 | 512 | 3 | sd | 10 | 2,048 | ||
| 4 | sd | 10 | 1,024 | 4 | sd | 12 | 4,096 | ||
| 5 | sd | 10 | 4,096 | 5 | ss | 42 | 2,048 | ||
| 6 | sd | 12 | 1,024 | 6 | ss | 42 | 2,048 | ||
| 7 | sd | 12 | 512 | ||||||
| 8 | sd | 12 | 512 | ||||||
| III (10 days) | 1 | sd | 9 | 512 | III (10 days) | 1 | sd | 8 | 4,096 |
| 2 | sd | 9 | 1,024 | 2 | sd | 10 | 4,096 | ||
| 3 | sd | 9 | 4,096 | 3 | sd | 10 | 4,096 | ||
| 4 | sd | 9 | 2,048 | 4 | sd | 15 | 4,096 | ||
| 5 | sd | 9 | 4,096 | 5 | sd | 16 | 2,048 | ||
| 6 | sd | 10 | 4,096 | 6 | ss | 42 | 2,048 | ||
| 7 | sd | 11 | 4,096 | 7 | ss | 42 | 2,048 | ||
| 8 | ss | 42 | 2,048 | ||||||
| IV (14 days) | 1 | sd | 9 | 4,096 | IV (14 days) | 1 | sd | 10 | 4,096 |
| 2 | sd | 11 | 4,096 | 2 | sd | 10 | 4,096 | ||
| 3 | sd | 12 | 4,096 | 3 | sd | 10 | 4,096 | ||
| 4 | sd | 12 | 4,096 | 4 | sd | 10 | 4,096 | ||
| 5 | sd | 12 | 4,096 | 5 | sd | 11 | 4,096 | ||
| 6 | sd | 13 | 4,096 | 6 | sd | 14 | >4,096 | ||
| 7 | sd | 13 | 4,096 | 7 | sd | 14 | >4,096 | ||
| 8 | sd | 13 | 4,096 | 8 | sd | 15 | >4,096 | ||
| 9 | ss | 42 | 512 | ||||||
| 10 | ss | 42 | 1,024 | ||||||
| V (21 days) | 1 | sd | 10 | 4,096 | V (21 days) | 1 | sd | 12 | 4,096 |
| 2 | sd | 12 | 2,048 | 2 | sd | 12 | 4,096 | ||
| 3 | sd | 14 | 4,096 | 3 | sd | 14 | 4,096 | ||
| 4 | ss | 42 | 4,096 | 4 | sd | 15 | 4,096 | ||
| 5 | ss | 42 | 4,096 | 5 | sd | 16 | 4,096 | ||
| 6 | ss | 42 | 4,096 | 6 | ss | 42 | 1,024 | ||
| VI (35 days) | 1 | hs | 42 | 4,096 | VI (35 days) | 1 | hs | 42 | 2,048 |
| 2 | hs | 42 | 4,096 | 2 | hs | 42 | 4,096 | ||
| 3 | hs | 42 | 4,096 | 3 | hs | 42 | 4,096 | ||
| 4 | hs | 42 | 4,096 | 4 | hs | 42 | 4,096 | ||
| 5 | hs | 42 | 4,096 | 5 | hs | 42 | 4,096 | ||
| 6 | hs | 42 | 4,096 | 6 | hs | 42 | 4,096 | ||
| 7 | hs | 42 | 4,096 | 7 | hs | 42 | 4,096 | ||
| 8 | hs | 42 | 4,096 | ||||||
| VII (56 days) | 1 | hs | 42 | 2,048 | VII (56 days) | 1 | hs | 42 | 256 |
| 2 | hs | 42 | 2,048 | 2 | hs | 42 | 2,048 | ||
| 3 | hs | 42 | 2,048 | 3 | hs | 42 | 256 | ||
| 4 | hs | 42 | 512 | 4 | hs | 42 | 512 | ||
| 5 | hs | 42 | 2,048 | 5 | hs | 42 | 512 | ||
| 6 | hs | 42 | 2,048 | 6 | hs | 42 | 1,024 | ||
| 7 | hs | 42 | 1,024 | 7 | hs | 42 | 1,024 | ||
| 8 | hs | 42 | 1,024 | ||||||
Abbreviations for clinical outcomes: sd, sick and dead; ss, sick but surviving at 42 days post-inoculation; hs, healthy and surviving at 42 days post-inoculation
Uninoculated control hamsters in each age group, numbering between 5 and 10, remained healthy during the 42-day study period.
3.3. Conventional RT-PCR
Using conventional RT-PCR, MJNV RNA was detected in heart, lung, kidney, spleen and brain from all or nearly all experimentally infected hamsters in each group less than 14 days of age (Groups I to IV) (Table 2). MJNV strain 04-55 was more hepatotropic than MJNV strain 05-11. In Group V (21 days of age), moribund and surviving hamsters at 42 days post-inoculation also had widespread distribution of MJNV RNA, particularly in lung and brain. Similarly, in hamsters inoculated at 35 days of age (Groups VI), MJNV RNA was absent in visceral organs, but was regularly detectable in brain. Interestingly, MJNV RNA was even found in brains of two hamsters inoculated with either MJNV strain 04-55 or 05-11 at 56 days of age, despite showing no clinical signs of disease, suggesting that MJNV may be neurotropic in hamsters.
Table 2.
Viral RNA in tissues of hamsters inoculated intraperitoneally with MJNV strains 04-55 and 05-11, as detected by conventional RT-PCR.
| Age Group | Tissues (No. viral RNA/no. tested)
|
||||||
|---|---|---|---|---|---|---|---|
| Heart | Lung | Liver | Kidney | Spleen | Intestine | Brain | |
| MJNV 04-55 | |||||||
| I (<1 day) | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 5/5 |
| II (5 days) | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 |
| III (10 days) | 7/7 | 7/7 | 7/7 | 7/7 | 7/7 | 7/7 | 7/7 |
| IV (14 days) | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 5/8 | 8/8 |
| V (21 days) | 3/6 | 4/6 | 3/6 | 3/6 | 3/6 | 2/6 | 6/6 |
| VI (35 days) | 2/7 | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 6/7 |
| VII (56 days) | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 2/8 |
| Negative control* | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| MJNV 05-11 | |||||||
| I (<1 day) | 9/9 | 9/9 | 8/9 | 9/9 | 9/9 | 9/9 | 9/9 |
| II (5 days) | 5/6 | 5/6 | 1/6 | 5/6 | 5/6 | 5/6 | 6/6 |
| III (10 days) | 7/8 | 8/8 | 0/8 | 8/8 | 8/8 | 5/8 | 8/8 |
| IV (14 days) | 9/10 | 10/10 | 2/10 | 10/10 | 7/10 | 0/10 | 10/10 |
| V (21days) | 6/6 | 5/6 | 0/6 | 6/6 | 3/6 | 1/6 | 6/6 |
| VI (35 days) | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 7/8 |
| VII (A56 days) | 0/7 | 1/7 | 0/7 | 0/7 | 0/7 | 0/7 | 2/7 |
| Negative control* | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
Negative controls were not inoculated.
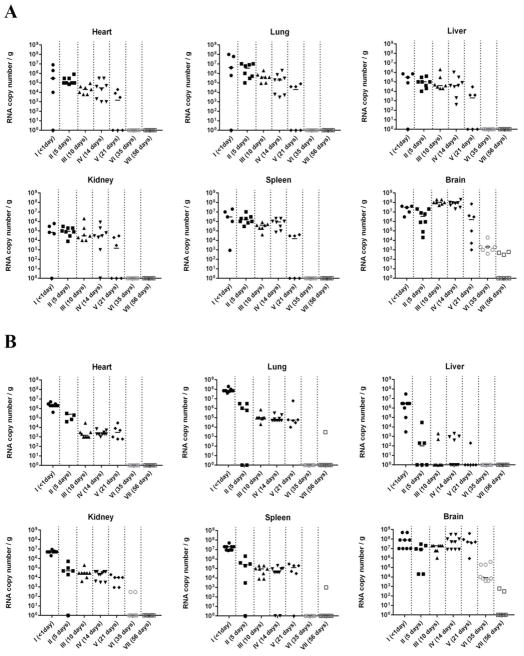
3.4. Quantitative real-time PCR
As determined by quantitative real-time PCR, viral RNA copies in the brain of MJNV-infected hamsters usually exceeded that in other organs (Fig. 2). This was particularly true for hamsters in Groups III, IV and V, inoculated at 10, 14 and 21 days of age, in which MJNV RNA copies in brain were 50 to 1,000 times higher than in heart, lung, liver, kidney, spleen and small intestine. In hamsters infected with MJNV strain 04-55, viral RNA load decreased in an age-dependent fashion in the heart, lung, spleen and small intestine but not in the liver, kidney and brain (Fig. 2A). A less clear organ-specific pattern was found for hamsters infected with MJNV strain 05-11, but in general an age-dependent decrease in MJNV RNA load was also evident, except in brain, which exhibited 104 to 105 MJNV RNA copies/g (Fig. 2B). Moreover, Syrian hamsters inoculated at 35 and 56 days of age exhibited MJNV RNA in brain at 42 days following inoculation, despite showing no neurological signs (Table 2 and Fig. 2).
Fig. 2.
Real-time RT-PCR quantification of viral RNA in heart, lung, liver, kidney, spleen and brain of Syrian hamsters inoculated with MJNV strains 04-55 (A) and 05-11 (B). MJNV RNA copies per gram of tissues from individual hamsters are shown for each age group. Terminal tissues from surviving hamsters were collected at 42 days following inoculation. MJNV RNA copies in small intestine were similar to that in other tissues (data not shown).
3.5. Histopathology
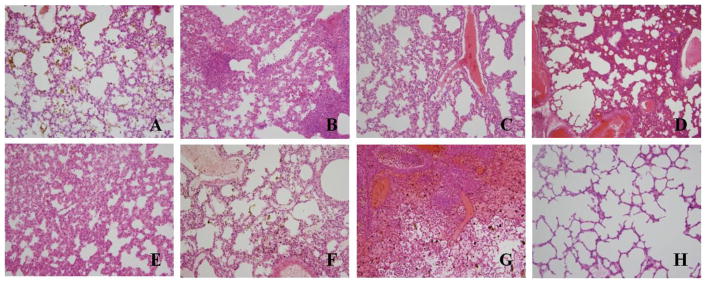
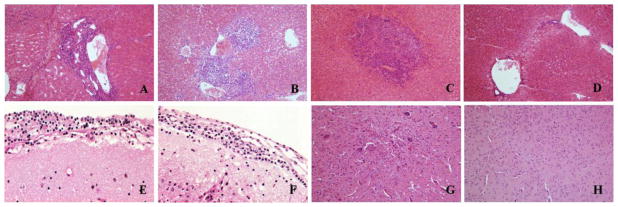
Light-microscopic examination of H&E-stained tissues from hamsters succumbing to experimental infection with MJNV strains 04-55 and 05-11 revealed widespread abnormalities in the lung, liver and brain (Fig. 3 and 4). In particular, alveolar hemorrhage, severe vascular congestion, interstitial thickening and severe pneumonia were observed in lung tissues of hamsters infected with MJNV 04-55 (Fig. 3A to 3E) and MJNV 05-11 (Fig. 3F and 3G). Liver tissues exhibited portal tract and hepatic inflammation (Fig. 4A and 4B), as well as acute inflammation with necrosis of hepatocytes (Fig. 4C). Severe inflammation of the meninges and pons (Fig. 4E and 4F) and acute inflammation of the brain parenchyma with vascular proliferation (Fig. 4G) were also observed in MJNV-infected hamsters. No significant pathologic changes were found in kidney, spleen and intestine (data not shown).
Fig. 3.
Histopathology of lung tissues obtained from moribund hamsters infected with MJNV. (A) alveolar hemorrhage in MJNV 04-55 infected hamster, group I (Age<24 hr), No. 2; (B) interstitial pneumonia in MJNV 04-55 infected hamster, group I (Age<24 hr), No. 5; (C) severe congestion in MJNV 04-55 infected hamster, group III (Age 10 days), No. 4; (D) interstitial pneumonia and congestion in MJNV 04-55 infected hamster, group IV (Age 14 days), No. 8; (E) interstitial thickening in MJNV 04-55 infected hamster, group VII (Age 56 days), No. 6; (F) alveolar old hemorrhage and congestion in MJNV 05-11 infected hamster, group III (Age 10 days), No. 2; (G) interstitial pneumonia, severe alveolar hemorrhage and congestion in MJNV 05-11 infected hamster, group V (Age 21 days), No. 2; (H) lung from control uninfected hamster. H&E stain; Original magnifications, X200.
Fig. 4.
Histopathology of liver and brain tissues obtained from hamsters infected with MJNV. (A) portal inflammation in liver of MJNV-infected hamster, group I (Age<24 hr), No. 5; (B) portal inflammation and hepatic perivenulitis in liver of MJNV 04-55 infected hamster, group IV (Age 14 days), No. 8; (C) acute inflammation with necroses of hepatocytes in liver of MJNV 05-11 infected hamster, group IV (Age 14 days), No. 10; (D) liver from control uninfected hamster. (E) inflammation in brain of MJNV 04-55 infected hamster, group I (<24 hr), No. 5; (F) inflammation in brain of MJNV 04-55 infected hamster, group III (Age 10 days), No. 5; (G) acute inflammation in brain of MJNV 04-55 infected hamster, group IV, No. 8; (H) brain from control uninfected hamster. H&E stain; Original magnifications, X200.
3.6. Immunohistochemistry
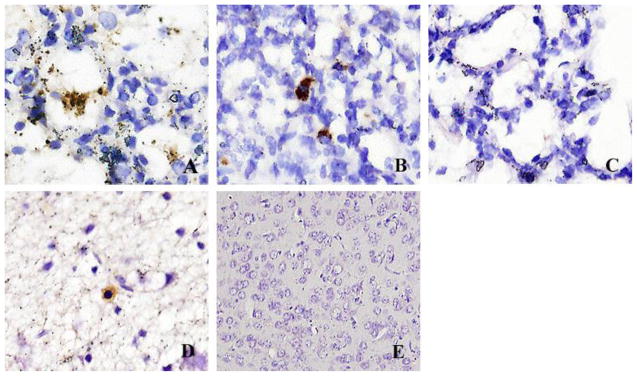
By immunostaining, MJNV NP antigen was detected in the alveoli and interstitium of lung tissues from hamsters infected with MJNV 04-55 and MJNV 05-11 (Fig. 5A and 5B). Viral antigens were also detected in the cytoplasm of glial cells in brain tissue from MJNV 04-55 infected hamsters (Fig. 5D). Interestingly, viral antigens were widely distributed in cortical neurons, including dendrites without inflammatory cell infiltrations, in some areas of the meninges and in Purkinje cells and granular layers of the cerebellum. MJNV NP antigen was not detected in other tissues (data not shown).
Fig. 5.
Immunohistochemical localization of MJNV NP in lung and brain tissue of hamsters inoculated with MJNV. (A) MJNV 04-55 infected hamster group IV (Age 14 days), No. 8. (B) MJNV 05-11 infected hamster group V (Age 21 days), No. 2. (C) lung from control uninfected hamster. (D) MJNV 04-55 infected hamster group IV (Age 14 days), No. 8. (E) brain from control uninfected hamster. Original magnifications, X400.
4. Discussion
Developing animal models of hantavirus infection is vital to gain a better understanding about hantavirus persistence. Such models have used the natural reservoir host species, such as wild-caught striped field mice (Apodemus agrarius) for HTNV (Lee et al., 1981), laboratory-bred bank voles (Myodes glareolus) for PUUV (Yanagihara et al., 1985a), and free-ranging deer mice (Peromyscus maniculatus) for SNV (Botten et al., 2000, 2003). More readily available small laboratory rodent species have also been employed to study hantavirus persistence, such as brown rats (Rattus norvegicus) for SEOV infection (Easterbrook et al., 2007), and Syrian hamsters and Mongolian gerbils (Meriones unguiculatus) for PUUV infection (Sanada et al., 2011; Yanagihara et al., 1985b).
Animal models for hantavirus infection and disease would also facilitate the testing of candidate vaccines and new therapies. Attempts at developing models of HFRS and HPS in nonhuman primates have had mixed results (Groen et al., 1995; Klingström et al., 2002; McElroy et al., 2002; Sironen et al., 2008; Yanagihara et al., 1988). For example, following intratracheal inoculation with PUUV, cynomolgus monkeys (Macaca fascicularis) exhibited mild proteinuria and/or microhematuria (Groen et al., 1995; Klingström et al., 2002). Detection of PUUV RNA by in situ hybridization and localization of PUUV NP by immunohistochemistry in kidney, spleen and liver tissues, as well as elevated cytokine levels, are consistent with those observed in patients with HFRS (Klingström et al., 2002; Sironen et al., 2008). At the same time, mild, transient proteinuria and azotemia were produced in cynomolgus monkeys and a chimpanzee (Pan troglodytes) following intravenous inoculation with Prospect Hill virus, a putative nonpathogenic hantavirus harbored by meadow voles (Yanagihara et al., 1988). By contrast, cynomolgus macaques inoculated by the intravenous or aerosol route with the highly lethal HPS-causing ANDV did not develop clinical disease (McElroy et al., 2002).
Attempts to replicate HFRS in rodents have been largely unsuccessful. Generally, experimental hantavirus infection in rodents are either asymptomatic (Wahl-Jensen et al., 2007; Yanagihara et al., 1985b) or characterized by differential age-dependent susceptibility and fatal meningoencephalitis (Kim and McKee, 1985; Kurata et al., 1983; McKee et al., 1985; Nakamura et al., 1985; Wichmann et al., 2002; Yamanouchi et al., 1984; Yoshimatsu et al., 1997). Recently, the age-dependent susceptibility of mice to lethal hantavirus meningoencephalitis has been attributed to the mouse strain. That is, while adult mice are usually resistant, several strains of adult Mus musculus (C57BL/6, BALB/c, AKR/J, and SJL/J) develop fatal neuroinvasive disease following intraperitoneal inoculation with HTNV (Wichmann et al., 2002). Although experimental meningoencephalitis in mice does not provide a suitable HFRS disease model, it has nevertheless served as an acceptable infection model in which to test the efficacy of the antiviral drug, ribavirin (Huggins et al., 1986). Also, a PUUV infection model in bank voles has been used to test a chimeric HBV core-PUUV NP vaccine (Ulrich et al., 1998) and protection against SEOV infection was achieved in Syrian hamsters using a DNA vaccine construct containing the SEOV M segment (Hooper et al., 1999).
A major breakthrough in hantavirology has been the development of a lethal HPS model in Syrian hamsters inoculated with ANDV (Hooper et al., 2001). Experimental ANDV infection in adult Syrian hamsters is characterized by acute respiratory failure and histopathological findings in the lung that are essentially indistinguishable from that in HPS in humans (Hooper et al., 2001; Safronetz et al., 2011). However, not all HPS-causing hantaviruses uniformly produce HPS-like diseases in Syrian hamsters. For example, SNV and CHOV, two hantaviruses known to cause HPS in North and South America, produce subclinical infection in hamsters (Eyzaguirre et al., 2008; Wahl-Jensen et al., 2007). Recently, transient immunosuppression with dexamethasone and cyclophosphamide monohydrate has rendered hamsters susceptible to HPS with SNV (Brocato et al., 2014).
In this exploratory study, we attempted to ascertain the pathogenic potential of a newly isolated shrew-borne hantavirus by inoculating Syrian hamsters of different ages. Despite differences in experimental design from previous studies, including age, route of inoculation and inoculum dose, MJNV-infected Syrian hamsters shared clinical and pathological features reported in hamsters experimentally infected with ANDV and SNV (Brocato et al., 2014; Hooper et al., 2001). Similarities included respiratory distress, clinical time course, presence of virus-specific IgG at the onset of clinical disease, widespread inflammation in lung and liver, distribution of hantaviral antigen in microvascular endothelial cells, and overall high mortality. However, distinct differences were also evident. MJNV-infected infant and juvenile hamsters developed neurologic disease, characterized by paralysis and seizures, whereas these features are absent in ANDV- and SNV-infected adult hamsters. Also, MJNV caused hepatic necrosis, whereas ANDV and SNV do not. Also, in stark contrast to experimental ANDV and SNV infection in hamsters, pulmonary edema was not evident in MJNV-infected hamsters. This is reminiscent of what has been reported for CHOV, an HPS-causing hantavirus. That is, despite the widespread distribution of viral antigen in pulmonary microvascular endothelial cells, HPS-like disease and pulmonary edema were not observed in CHOV-infected Syrian hamsters (Eyzaguirre et al., 2008).
Importantly, experimental MJNV infection in infant and juvenile hamsters exhibited an age-dependent susceptibility to lethal disease, not unlike the meningoencephalitis described previously in mice and rats experimentally infected with HTNV (Kurata et al., 1983; McKee et al., 1985; Nakamura et al., 1985; Yamanouchi et al., 1984). That is, MJNV caused disease only in hamsters aged three weeks or younger, and not in hamsters inoculated at 35 and 56 days of age. On the other hand, ANDV and SNV cause lethal HPS-like disease in adult hamsters.
The development of animal models of human diseases, or the use of animals to predict human diseases, is fraught with uncertainties about relevance. For example, Maporal virus, which causes an HPS-like disease in hamsters (Milazzo et al., 2002), is not associated with HPS in humans, and conversely, CHOV, which causes HPS in humans (Nelson et al., 2010), does not cause HPS in hamsters (Eyzaguirre et al., 2008). Thus, despite the MJNV-associated pathology in hamsters, this does not prove that MJNV is pathogenic in humans. Nevertheless, the findings reported here suggest that hamsters provide an acute MJNV disease model, and may be a convenient experimental host for ascertaining the infectivity and pathogenicity of newfound non-rodent-borne hantaviruses, as well as provide helpful clues about the type(s) of disease MJNV might cause in humans. That is, while neurological manifestations have only rarely been a feature of rodent-borne hantavirus infection in humans (Cerar et al., 2007; Chan et al., 1996), the neurotropism and neuropathology, with high viral load in brains of MJNV-infected hamsters, warrant targeted investigations for disease correlates in humans.
Highlights.
Imjin virus, a shrew-borne hantavirus, causes lethal disease in infant and juvenile Syrian hamsters
Lethal disease is characterized by high viral load and histopathology in lung and brain
Adult Syrian hamsters are resistant to Imjin virus-associated disease
Acknowledgments
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases (R01AI075057) and the National Institute of General Medical Sciences (P20GM103516) of the National Institutes of Health, and the Global Emerging Infections Surveillance and Response System (GEIS), U.S. Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett SN, Gu SH, Kang HJ, Arai S, Yanagihara R. Reconstructing the evolutionary origins and phylogeography of hantaviruses. Trends Microbiol. 2014;22:473–482. doi: 10.1016/j.tim.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten J, Mirowsky K, Kusewitt D, Bharadwaj M, Yee J, Ricci R, Feddersen RM, Hjelle B. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus) Proc Natl Acad Sci U S A. 2000;97:10578–10583. doi: 10.1073/pnas.180197197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botten J, Mirowsky K, Kusewitt D, Ye CY, Gottlieb K, Prescott J, Hjelle B. Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: sites of replication and strand-specific expression. J Virol. 2003;77:1540–1550. doi: 10.1128/JVI.77.2.1540-1550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocato RL, Hammerbeck CD, Bell TM, Wells JB, Queen LA, Hooper JW. A lethal disease model for hantavirus pulmonary syndrome in immunosuppressed Syrian hamsters infected with Sin Nombre virus. J Virol. 2014;88:811–819. doi: 10.1128/JVI.02906-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer-Korvenkontio M, Vaheri A, Hovi T, von Bonsdorff CH, Vuorimies J, Manni T, Penttinen K, Oker-Blom N, Lähdevirta J. Nephropathia epidemica: detection of antigen in bank voles and serologic diagnosis of human infection. J Infect Dis. 1980;141:131–134. doi: 10.1093/infdis/141.2.131. [DOI] [PubMed] [Google Scholar]
- Carey DE, Reuben R, Panicker KN, Shope RE, Myers RM. Thottapalayam virus: a presumptive arbovirus isolated from a shrew in India. Indian J Med Res. 1971;59:1758–1760. [PubMed] [Google Scholar]
- Cerar D, Avsic-Zupanc T, Jereb M, Strle F. Severe neurological manifestation of Dobrava hantavirus infection. J Med Virol. 2007;79:1841–1843. doi: 10.1002/jmv.21021. [DOI] [PubMed] [Google Scholar]
- Chan KP, Chan YC, Doraisingham S. A severe case of hemorrhagic fever with renal syndrome in Singapore. Southeast Asian J Trop Med Public Health. 1996;27:408–410. [PubMed] [Google Scholar]
- Chu YK, Jennings GB, Schmaljohn CS. A vaccinia virus-vectored Hantaan virus vaccine protects hamsters from challenge with Hantaan and Seoul viruses but not Puumala virus. J Virol. 1995;69:6417–6423. doi: 10.1128/jvi.69.10.6417-6423.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, Ksiazek TG, Rollin PE, Nichol S, Umland ET, Moolenaar RL, Reef SE, Nolte KB, Gallaher MM, Butler JC, Breiman RF for the Hantavirus Study Group. Hantavirus pulmonary syndrome - a clinical description of 17 patients with a newly recognized disease. N Engl J Med. 1994;330:949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- Easterbrook JD, Zink MC, Klein SL. Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host. Proc Natl Acad Sci U S A. 2007;104:15502–15507. doi: 10.1073/pnas.0707453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyzaguirre EJ, Milazzo ML, Koster FT, Fulhorst CF. Choclo virus infection in the Syrian golden hamster. Am J Trop Med Hyg. 2008;78:669–674. [PMC free article] [PubMed] [Google Scholar]
- Groen J, Gerding M, Koeman JP, Roholl PJM, Vanamerongen G, Jordans HGM, Niesters HGM, Osterhaus ADME. A macaque model for hantavirus infection. J Infect Dis. 1995;172:38–44. doi: 10.1093/infdis/172.1.38. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Kamrud KI, Elgh F, Custer D, Schmaljohn CS. DNA vaccination with hantavirus M segment elicits neutralizing antibodies and protects against Seoul virus infection. Virology. 1999;255:269–278. doi: 10.1006/viro.1998.9586. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Larsen T, Custer DM, Schmaljohn CS. A lethal disease model for hantavirus pulmonary syndrome. Virology. 2001;289:6–14. doi: 10.1006/viro.2001.1133. [DOI] [PubMed] [Google Scholar]
- Hooper JW, Josleyn M, Ballantyne J, Brocato R. A novel Sin Nombre virus DNA vaccine and its inclusion in a candidate pan-hantavirus vaccine against hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS) Vaccine. 2013;31:4314–4321. doi: 10.1016/j.vaccine.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins JW, Kim GR, Brand OM, McKee KT. Ribavirin therapy for Hantaan virus infection in suckling mice. J Infect Dis. 1986;153:489–497. doi: 10.1093/infdis/153.3.489. [DOI] [PubMed] [Google Scholar]
- Kim GR, McKee KT., Jr Pathogenesis of Hantaan virus infection in suckling mice: clinical, virologic, and serologic observations. Am J Trop Med Hyg. 1985;34:388–395. doi: 10.4269/ajtmh.1985.34.388. [DOI] [PubMed] [Google Scholar]
- Klingström J, Plyusnin A, Vaheri A, Lundkvist A. Wild-type Puumala hantavirus infection induces cytokines, C-reactive protein, creatinine, and nitric oxide in cynomolgus macaques. J Virol. 2002;76:444–449. doi: 10.1128/JVI.76.1.444-449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata T, Tsai TF, Bauer SP, McCormick JB. Immunofluorescence studies of disseminated Hantaan virus infection of suckling mice. Infect Immun. 1983;41:391–398. doi: 10.1128/iai.41.1.391-398.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, French GR, Lee PW, Baek LJ, Tsuchiya K, Foulke RS. Observations on natural and laboratory infection of rodents with the etiologic agent of Korean hemorrhagic fever. Am J Trop Med Hyg. 1981;30:477–482. doi: 10.4269/ajtmh.1981.30.477. [DOI] [PubMed] [Google Scholar]
- Lee HW, Lee PW, Johnson KM. Isolation of the etiologic agent of Korean hemorrhagic fever. J Infect Dis. 1978;137:298–308. doi: 10.1093/infdis/137.3.298. [DOI] [PubMed] [Google Scholar]
- Lee HW, Vandergroen G. Hemorrhagic fever with renal syndrome. Prog Med Virol. 1989;36:62–102. [PubMed] [Google Scholar]
- Lopez N, Padula P, Rossi C, Lazaro ME, FranzeFernandez MT. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology. 1996;220:223–226. doi: 10.1006/viro.1996.0305. [DOI] [PubMed] [Google Scholar]
- McElroy AK, Bray M, Reed DS, Schmaljohn CS. Andes virus infection of cynomolgus macaques. J Infect Dis. 2002;186:1706–1712. doi: 10.1086/345768. [DOI] [PubMed] [Google Scholar]
- McKee KT, Kim GR, Green DE, Peters CJ. Hantaan virus infection in suckling mice - virologic and pathologic correlates. J Med Virol. 1985;17:107–117. doi: 10.1002/jmv.1890170203. [DOI] [PubMed] [Google Scholar]
- Milazzo ML, Eyzaguirre EJ, Molina CP, Fulhorst CF. Maporal viral infection in the Syrian golden hamster: a model of hantavirus pulmonary syndrome. J Infect Dis. 2002;186:1390–1395. doi: 10.1086/344735. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yanagihara R, Gibbs CJ, Jr, Amyx HL, Gajdusek DC. Differential susceptibility and resistance of immunocompetent and immunodeficient mice to fatal Hantaan virus infection. Arch Virol. 1985;86:109–120. doi: 10.1007/BF01314117. [DOI] [PubMed] [Google Scholar]
- Nelson R, Canate R, Pascale JM, Dragoo JW, Armien B, Armien AG, Koster F. Confirmation of Choclo virus as the cause of hantavirus cardiopulmonary syndrome and high serum antibody prevalence in Panama. J Med Virol. 2010;82:1586–1593. doi: 10.1002/jmv.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Ravkov EV, Rollin PE, Ksiazek TG, Peters CJ, Nichol ST. Genetic and serologic analysis of Black Creek Canal virus and its association with human disease and Sigmodon hispidus infection. Virology. 1995;210:482–489. doi: 10.1006/viro.1995.1366. [DOI] [PubMed] [Google Scholar]
- Safronetz D, Zivcec M, Lacasse R, Feldmann F, Rosenke R, Long D, Haddock E, Brining D, Gardner D, Feldmann H, Ebihara H. Pathogenesis and host response in Syrian hamsters following intranasal infection with Andes virus. PLoS Pathog. 2011;7:e1002426. doi: 10.1371/journal.ppat.1002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronetz D, Ebihara H, Feldmann H, Hooper JW. The Syrian hamster model of hantavirus pulmonary syndrome. Antiviral Res. 2012;95:282–292. doi: 10.1016/j.antiviral.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada T, Kariwa H, Nagata N, Tanikawa Y, Seto T, Yoshimatsu K, Arikawa J, Yoshii K, Takashima I. Puumala virus infection in Syrian hamsters (Mesocricetus auratus) resembling hantavirus infection in natural rodent hosts. Virus Res. 2011;160:108–119. doi: 10.1016/j.virusres.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Schmaljohn CS, Dalrymple JM. Analysis of Hantaan virus RNA: evidence for a new genus of Bunyaviridae. Virology. 1983;131:482–491. doi: 10.1016/0042-6822(83)90514-7. [DOI] [PubMed] [Google Scholar]
- Schmaljohn CS, Hasty SE, Harrison SA, Dalrymple JM. Characterization of Hantaan virions, the prototype virus of hemorrhagic fever with renal syndrome. J Infect Dis. 1983;148:1005–1012. doi: 10.1093/infdis/148.6.1005. [DOI] [PubMed] [Google Scholar]
- Schmaljohn CS, Chu YK, Schmaljohn AL, Dalrymple JM. Antigenic subunits of Hantaan virus expressed by baculovirus and vaccinia virus recombinants. J Virol. 1990;64:3162–3170. doi: 10.1128/jvi.64.7.3162-3170.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironen T, Klingström J, Vaheri A, Andersson LC, Lundkvist A, Plyusnin A. Pathology of Puumala hantavirus infection in macaques. PLoS One. 2008;3:e3035. doi: 10.1371/journal.pone.0003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JW, Baek LJ, Schmaljohn CS, Yanagihara R. Thottapalayam virus, a prototype shrewborne hantavirus. Emerg Infect Dis. 2007;13:980–985. doi: 10.3201/eid1307.070031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JW, Kang HJ, Gu SH, Moon SS, Bennett SN, Song KJ, Baek LJ, Kim HC, O’Guinn ML, Chong ST, Klein TA, Yanagihara R. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura) J Virol. 2009;83:6184–6191. doi: 10.1128/JVI.00371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R, Lundkvist A, Meisel H, Koletzki D, Sjolander KB, Gelderblom HR, Borisova G, Schnitzler P, Darai G, Krüger DH. Chimaeric HBV core particles carrying a defined segment of Puumala hantavirus nucleocapsid protein evoke protective immunity in an animal model. Vaccine. 1998;16:272–280. doi: 10.1016/s0264-410x(97)00172-2. [DOI] [PubMed] [Google Scholar]
- Wahl-Jensen V, Chapman J, Asher L, Fisher R, Zimmerman M, Larsen T, Hooper JW. Temporal analysis of Andes virus and Sin Nombre virus infections of Syrian hamsters. J Virol. 2007;81:7449–7462. doi: 10.1128/JVI.00238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann D, Grone HJ, Frese M, Pavlovic J, Anheier B, Haller O, Klenk HD, Feldmann H. Hantaan virus infection causes an acute neurological disease that is fatal in adult laboratory mice. J Virol. 2002;76:8890–8899. doi: 10.1128/JVI.76.17.8890-8899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi T, Domae K, Tanishita O, Takahashi Y, Yamanishi K, Takahashi M, Kurata T. Experimental infection in newborn mice and rats by hemorrhagic fever with renal syndrome (HFRS) virus. Microbiol Immunol. 1984;28:1345–1353. doi: 10.1111/j.1348-0421.1984.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Yanagihara R, Amyx HL, Gajdusek DC. Experimental infection with Puumala virus, the etiologic agent of nephropathia epidemica, in bank voles (Clethrionomys glareolus) J Virol. 1985a;55:34–38. doi: 10.1128/jvi.55.1.34-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara R, Amyx HL, Lee PW, Asher DM, Gibbs CJ, Jr, Gajdusek DC. Experimental hantavirus infection in nonhuman primates. Arch Virol. 1988;101:125–130. doi: 10.1007/BF01314657. [DOI] [PubMed] [Google Scholar]
- Yanagihara R, Gu SH, Arai S, Kang HJ, Song JW. Hantaviruses: rediscovery and new beginnings. Virus Res. 2014;187:6–14. doi: 10.1016/j.virusres.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara R, Gajdusek DC. Hemorrhagic fever with renal syndrome: a historical perspective and review of recent advances. In: Gear JHS, editor. CRC handbook of viral and rickettsial hemorrhagic fevers. CRC press, Inc; Boca Raton, FL: 1988. pp. 151–188. [Google Scholar]
- Yanagihara R, Goldgaber D, Gajdusek DC. Propagation of nephropathia epidemica virus in Mongolian gerbils. J Virol. 1985b;53:973–975. doi: 10.1128/jvi.53.3.973-975.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu K, Arikawa J, Ohbora S, Itakura C. Hantavirus infection in SCID mice. J Vet Med Sci. 1997;59:863–868. doi: 10.1292/jvms.59.863. [DOI] [PubMed] [Google Scholar]
- Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, Feddersen RM, Zumwalt RE, Miller GL, Khan AS, Rollin PE, Ksiazek TG, Nichol ST, Mahy BWJ, Peters CJ. Hantavirus pulmonary syndrome - Pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]