Abstract
PFOS is a chemical of nearly ubiquitous exposure in humans. Recent studies have associated PFOS exposure to adipose tissue-related effects. The present study was to determine whether PFOS alters the process of adipogenesis and regulates insulin-stimulated glucose uptake in mouse and human preadipocytes. In murine-derived 3T3-L1 preadipocytes, PFOS enhanced hormone-induced differentiation to adipocytes and adipogenic gene expression, increased insulin-stimulated glucose uptake at concentrations ranging from 10 to 100 µM, and enhanced Glucose transporter type 4 and Insulin receptor substrate-1 expression. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2), NAD(P)H dehydrogenase, quinone 1 and Glutamate-cysteine ligase, catalytic subunit were significantly induced in 3T3-L1 cells treated with PFOS, along with a robust induction of Antioxidant Response Element (ARE) reporter in mouse embryonic fibroblasts isolated from ARE-hPAP transgenic mice by PFOS treatment. Chromatin immunoprecipitation assays further illustrated that PFOS increased Nrf2 binding to ARE sites in mouse Nqo1 promoter, suggesting that PFOS activated Nrf2 signaling in murine-derived preadipocytes. Additionally, PFOS administration in mice (100 µg/kg/day) induced adipogenic gene expression and activated Nrf2 signaling in epididymal white adipose tissue. Moreover, the treatment on human visceral preadipocytes illustrated that PFOS (5 and 50 µM) promoted adipogenesis and increased cellular lipid accumulation. It was observed that PFOS increased Nrf2 binding to ARE sites in association with Nrf2 signaling activation, induction of Peroxisome proliferator-activated receptor γ and CCAAT/enhancer-binding protein α expression, and increased adipogenesis. This study points to a potential role PFOS in dysregulation of adipose tissue expandability, and warrants further investigations on the adverse effects of persistent pollutants on human health.
Keywords: PFOS, Nrf2, Pparγ, adipogenesis, glucose uptake, ARE
Introduction
Perfluorooctane sulfonate (C8HF17O3S, PFOS) and Perfluorooctanoic acid (C8HF15O2, PFOA) are organic fluoroalkyl chemicals widely used in industrial and consumer applications as powerful surfactants and building material components, as they are stable at high temperature and nonflammable (Olsen et al., 2005). These two chemicals have been detected worldwide in the environment, including drinking water (Skutlarek et al., 2006), atmosphere air (Shoeib et al., 2005), soil, sediments (Boulanger et al., 2005), and even the wildlife in the Antarctic Pole (Giesy and Kannan, 2001). Average human serum PFOS and PFOA content has decreased between 2000 and 2005 due to the phase out of Perfluorooctanesulfonyl-fluoride (POSF, C8F17SO2F)-based materials by the primary global manufacturer, 3M Company, in May 2000 (Olsen et al., 2007b), but the related health effects still remain a concern. Although have been banned in United States and Europe, PFOS and PFOS-related chemicals are still currently produced in China.
Importantly, PFOS and PFOA are readily absorbed and poorly eliminated from humans. Increased renal absorption via transport mechanisms is suspected to be the mechanism that contributes to the relatively long serum half-lives observed in humans (Olsen et al., 2007a), which has been estimated to 5.4 (PFOS) and 3.8 (PFOA) years. Because of its persistence in the body and presence in populations across the world, it is important to assess potential health effects. Liver is the major organ affected by exposure of PFOS in animal models. It is known that PFOS will be deposited in liver, which associated with increased liver weight in rats, mice, and monkeys (Chang et al., 2012; Wan et al., 2012). Exposure of PFOS has been shown to induce further undesirable effects, such as increased hepatic lipid accumulation along with significant induction of Cluster of differentiation 36 (FAT/CD36) and Lipoprotein lipase (Lpl) expression, which resulted in disturbance of lipid metabolism and excessive fatty liver (Wan et al., 2012). In addition, dietary administration of PFOS in mice (0.005%, w/w) for 10 days reduced serum cholesterol and triglycerides levels, and induced a moderate hepatomegaly (Qazi et al., 2010). More recent publications reported that exposure of PFOS altered expression of genes mainly involved in lipid modulation, energy metabolism, reproduction, hormone regulation, suggesting a role for PFOS in regulating lipid metabolism and development (Hu et al., 2005; Hagenaars et al., 2008). The observed hepatomegaly that results from PFOS administration is due to Peroxisome proliferator-activated receptor (Ppar) activation (Takacs and Abbott, 2007; Bjork and Wallace, 2009). Rosen et al. also reported that PFOS may modulate various gene expression related to lipid metabolism, inflammation, and xenobiotic metabolism via Pparα-independent mechanisms, such as the modest activation of Constitutive Androstane Receptor (CAR) and Pparγ signaling pathway (Rosen et al., 2010). And PFOA was reported to induce various xenobiotic metabolism genes, which were under the control of CAR and transcription factor of Nuclear factor erythoid 2-related factor 2 (Nrf2) (Rosen et al., 2008). However, PFOS effects on adipogenesis and white adipose tissue (WAT) expansion were still unknown.
Adipogenesis is a process involving sequential coordinated gene induction (Rosen and Spiegelman, 2000). CCAAT/enhancer-binding protein (Cebp) δ and Cebpβ induce the expression of Pparγ, which activates Cebpα expression, and these two transcriptional factors of Pparγ and Cebpα can work in concert to maintain the differentiated status. Subsequently, Pparγ and Cebpα activation induce the subsequent steps of adipogenesis and lipogenesis. Gain-of-function experiments that forced Pparγ and/or in combination with potential agonists demonstrated that non-adipogenic, fibroblast cells can be transdifferentiated to adipocytes (Wu et al., 1995). Emerging data points to new regulators of Ppar signaling pathway, such as transcription factor of Nrf2, regulated lipid metabolism and the process of adipogenesis (Shin et al., 2007; Pi et al., 2010). Classically, Nrf2 binds to Antioxidant-Responsive Elements (ARE), induces expression of a battery of detoxification and antioxidant genes to counter cellular electrophilic and oxidative stress (Kensler et al., 2007; Klaassen and Reisman, 2010). Pi et al. reported that an ARE element exists in mouse Pparγ promoter, and loss of Nrf2 reduced Pparγ expression and prevented the process of adipogenesis in 3T3-L1 preadipocytes (Pi et al., 2010). This work provided a link between Nrf2 and adipogenesis. Other studies have demonstrated that too much Nrf2 activation can also perturb adipogeneis. An early study by Kensler et al. described Nrf2 prevented adipogenesis via modulating Aryl hydrocarbon receptor Signaling (Shin et al., 2007). Our previous results demonstrated that enhanced Nrf2 activity via Kelch-like ECH-associated protein 1 - knockdown (Keap1-KD) inhibited WAT expansion and the adipocyte differentiation, suggesting the Nrf2-independent mechanism involved in Keap1-KD system (Xu et al., 2012). The relationship of PFOS and Nrf2 has been reported in zebrafish in 2010. In the publication, the authors reported that PFOS increased cellular ROS content. Activation of Nrf2 signaling by sulforaphane reduced ROS content, further reduced MAPK activation of JNK and p38 signaling, suggesting that Nrf2 signaling regulated PFOS-induced oxidative stress (Shi and Zhou, 2010).
To evaluate the effect of PFOS on adipogenesis, and further explore its effects on regulating WAT expansion and the development of obesity, 3T3-L1 preadipocytes were induced to differentiation to adipocytes with the presence of PFOS with the dose of environmental exposure-relevant concentrations. The cellular lipid content and adipogenic gene expression were evaluated. Additionally, glucose uptake was determined in the mature adipocytes with PFOS administration. Lastly, PFOS-induced adipogenic effects were also evaluated in a whole animal model with daily PFOS administration and in human visceral preadipocytes. The current study demonstrates that PFOS induces Nrf2 activation in association with promoting Pparγ and Cebpα signaling, and induction of adipogenesis that increases lipid accumulation in 3T3-L1 preadipocytes. Moreover, pro-adipogenic effects were observed in human visceral preadipocytes exposed to PFOS.
Material and Methods
Chemicals
Heptadecaperfluorooctanesulfonic acid potassium salt (#77282, PFOS), insulin (#I6634), dexamethasone (#D4902, DEX) isobutylmethylxanthine (#I5879, IBMX), and Oil Red O (#O0625) were got from Sigma-Aldrich (St. Louis, MO). Ethanol, methanol, isopropanol, MTT solution and other chemicals without specific illustration were got from Thermo Fisher Scientific Inc. (Waltham, MA)
Animals and PFOS administration
10-week-old male C57BL/6 mice weighing approximately 30 grams were purchased from Charles River Laboratories (Wilmington, MA). The mice were housed under a controlled temperature (22–25 °C) with relative humidity (30–70%), lighting (12 hrs, light-dark cycles) environment and acclimated for 5 weeks on the standard rodent chow to allow for additional weight gain. At 15 weeks of age, the mice were then fed a purified rodent chow (AIN-93G Growth Purified Diet, TestDiet, St. Louis, MO). At 21 weeks of age, mice (n=8) were administered water as vehicle via oral gavage (5 mL/kg) or PFOS (100 µg/kg, 5 mL/kg) for 36 days. Body weight and food intake were monitored daily and recorded. Epididymal WAT was collected, snap frozen with liquid nitrogen, and stored at −70 °C until analysis. All procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Rhode Island Animal Care and Use Committee.
Acute cytotoxicity assay
A minimum of 5 replicates of 7,500 3T3-L1 preadipocytes per well were plated in 96-well plates and allowed to adhere to the plate for approximately 12 hrs, at which time the media was removed and replaced with fresh media containing varying concentrations of PFOS in DMSO (1 nM, 5 nM, 10 nM, 100 nM, 500 nM, 1 µM, 5 µM, 10 µM, 50 µM, 100 µM). Cells were subsequently incubated for an additional 48 hrs. Then 20 µL MTT solution (5 mg/mL in PBS) was added and the plate was incubated for another 3 hrs. The supernatant was removed carefully and 150 µL MTT solvent (4 mM HCl, 0.1% NP-40 in isopropanol) was added to each well. The plate was covered with foil and agitated on an orbital shaker for 15 mins. The cell viability was determined by measuring the absorbance at 590 nm with 620 nm as reference filter. Relative cell viability (%) was displayed using vehicle (0.1% DMSO)-treated samples as a standard.
Cell culture and 3T3-L1 pre-adipocyte differentiation
Mouse 3T3-L1 (ATCC® CL-173™) preadipocytes were obtained from ATCC (American Type Culture Collection, Manassas, VA, USA) and maintained in high-glucose DMEM medium supplemented with 10% fetal bovine serum (FBS). 3T3-L1 preadipocyte differentiation was induced according to the protocol described previously (Shin et al., 2007; Xu et al., 2012). Briefly, 2 days post 100% confluence, 3T3-L1 preadipocytes were stimulated to differentiation to adipocytes in a standard adipogenic differentiation medium (DMEM containing 10% FBS, 10 µg/mL insulin, 1 µM DEX, 0.5 mM IBMX), which was considered Day0. Cells were cultured in the latter media for Days 0–3 and then cultured in DMEM containing 10% FBS and 10 µg/mL insulin for the remaining days. Media was refreshed every 2 days. Cell treatment and corresponding assay were described as Figure 1A.
Figure 1. MTT assay of PFOS on 3T3-L1 preadipocytes.
The illustration of study design on (A) 3T3-L1 preadipocytes, (B) human visceral preadipocytes, and (C) mouse embryonic fibroblasts. (D) 3T3-L1 preadipocytes were exposed to PFOS at concentration of 1 nM, 5 nM, 10 nM, 100 nM, 500 nM, 1 µM, 5 µM, 10 µM, 50 µM, 100 µM or DMSO in DMEM with 10% FBS for 48 hrs. PFOS toxicity at different dosage was determined by MTT assay. Data for vehicle-treated group was considered as 100%. N=5. *, P<0.05, PFOS-treated vs. vehicle (Veh).
Human visceral preadipocytes culture and induction to adipocytes
Poietics™ Human visceral preadipocytes (Donor #: 24711; Lot #:0000313366; Cat #: PT-5005) were obtained from Lonza (Lonza Walkersville, Inc., Walkersville, MD) and maintained in PBM-2 media according to the manufacturer’s instructions. The preadipocytes were plated at 8,700 cells per well. Cells were induced to differentiation to adipocytes by switching with the differentiated media of PBM-2 supplementing with dexamethasone, isobutylmethylxanthine and insulinSingleQuots™ 24 hrs post 100% confluence and keep in the same media for the next 11 days. Cells were treated with DMSO (0.1%) or PFOS (5 or 100 µM) in quadruplicates. Oil red O was used to image the lipid droplets. The cellular lipid was purified via isopropanol extraction and the lipid content was quantified spectrophotometrically at what 520 nm. Cell treatment and corresponding assay were described as Figure 1B.
RNA isolation and quantitative real-time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, CA) according to the manufacturer’s instructions. One microgram of total RNA was converted to cDNA and mRNA levels were quantified by quantitative real-time PCR using a Roche LightCycler 480 System (Roche Applied Science, Mannheim, Germany). SYBR green chemistry was used and relative target gene expression was normalized to 18S rRNA. The primers used are listed in Supplementary Table 1.
Oil Red O staining
3T3-L1 preadipocytes or human visceral preadipocytes were induced to differentiation to adipocytes. The supernatant was removed and cell layer was washed twice with 2 mL ice-cold PBS. Cells were fixed with 10% formalin at 4 °C for 30 mins, then stained with Oil Red O solution (six parts Oil Red O stock solution [0.5% Oil Red O in 100% isopropanol] and four parts H2O) for 60 mins. Cells were counterstained with hematoxylin and mounted in glycerin jelly (Carolina Biological Supply Company, Burlington, NC).
Measurement of triglycerides in 3T3-L1 preadipocytes
3T3-L1 preadipocytes plated on 60-mm dishes were induced to adipocytes for 8 days. Lipids were extracted according to a previous protocol (Xu et al., 2012). Triglycerides content were determined with reagent kits (Pointe Scientific, Inc, MI), and the absorbance was measured spectrophotometrically at 520 nm. Relative triglycerides content (%) was displayed using differentiation medium containing 0.1% DMSO - treated cells as a standard.
Glucose uptake assay
Glucose uptake in 3T3-L1 mature adipocytes was measured by using 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose (2-NBDG) according to the manufacturer’s instructions (Cayman Chemical Company, MI). In brief, 3T3-L1 preadipocytes plated on 96-well fluorescent plates were induced to adipocytes as described above. At Day11, cells were washed with PBS and then treated with serum-free DMEM containing PFOS (0 – 100 µM) or DMSO (0.1%) for 5 hrs. Then cells were washed with sterilized PBS for 3 times, and stimulated with 100 nM insulin for 20 mins in KRPH buffer (20 mM HEPES, 5 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2, 136 mM NaCl, and 4.7 mM KCl, pH 7.4) with the presence of PFOS or DMSO, respectively. Glucose uptake was initiated by the addition of 100 µg/mL 2-NBDG to each well. The fluorescence activity was monitored at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. At least eight replicates for each dosage was performed. Relative glucose uptake was displayed using vehicle-treated group as a standard.
hPAP induction assay
Mouse embryonic fibroblast (MEF) differentiation to adipocytes was performed as described elsewhere (Shin et al., 2007; Xu et al., 2012). MEFs were isolated from 13.5- to 15.5-d post coital mouse embryos from ARE-hPAP transgenic mice, then cells were cultured in DMEM supplemented with 10% FBS in 10 cm culture dishes (Johnson et al., 2002). MEFs were collected and cultured in 6-well plate, 2 days post of 100% confluence, cells were switched to differentiated media with DMEM containing 10% FBS, 10 µg/mL insulin, 1 µM DEX, 0.5 mM IBMX (Day0). Three days later, cells were switched to media only containing 10% FBS and 10 µg/mL insulin for the remaining days. And four days post differentiation, total RNA was extracted and the relative mRNA levels of hPAP were measured using quantitative real-time PCR. The detailed design was described as Figure 1C.
Chromatin Immunoprecipitation (ChIP) assay
3T3-L1 cells were induced to adipocytes as described above. Two days post differentiation to adipocytes, cells were harvested and the Chip assay was performed according to the manufacturer’s instructions (Active Motif, Carlsbad, CA). Equal amount of sheared chromatin DNA (15 µg) was incubated with anti-Nrf2 antibody (Cell Signaling, Danvers, MA) or IgG as negative control overnight at 4 °C. A portion of sheared chromatin DNA was preserved as input (10 µL). Purified DNA was PCR-amplified for 35 cycles (30 s at 94 °C, 30 s at 59 °C, and 30 s at 72 °C) with the primers that cover putative AREs sequences in mouse Nqo-1 promoters (Forward: 5’-GCAGTTTCTAAGAGCAGAACG-3’; Reverse: 5’-GTAGATTAGTCCTCACTCAGCCG-3’).
Statistical Analysis
Quantitative data were presented as average ± SE. Statistic differences were determined by a one-way ANOVA followed by a Duncan’s Multiple Range post hoc test. All statistical tests with P<0.05 were considered significant.
Results
PFOS induces adipogenesis in 3T3-L1 preadipocytes
In order to explore the association of PFOS exposure and adipocyte differentiation, we determined the effect of PFOS concentrations on 3T3-L1 pre-adipocyte viability. No overt toxicity was observed at <50 µM in the current study (Figure 1D). 3T3-L1 preadipocytes were differentiated to adipocytes in the presence of differentiated cocktail with or without PFOS. Oil Red O staining of mature lipid-containing adipocytes at Day8 was performed to evaluate PFOS effects on adipogenesis. Figure 2A illustrates that high concentrations of PFOS (1–100 µM) increased lipid accumulation in 3T3-L1 adipocytes compared to vehicle-treated group (Figure 2A). However, staining was similar between vehicle- and PFOS-treated groups treated with concentrations less than 1 µM (1–500 nM), except there was lower lipid content at the dosage of 5 nM (Figure, 2B, Figure, S1). Similar to the observed staining, higher PFOS concentrations (1–50 µM) increased triglycerides content in 3T3-L1 adipocytes by more than 20% above control, but this effect was not observed with the relatively lower PFOS concentrations (1–100 nM) (Figure 2B). The data suggest that PFOS has the potential to potentiate induction of mouse preadipocyte differentiation to mature adipocytes and promote lipid accumulation.
Figure 2. Non-cytotoxic levels of PFOS enhances lipid content in differentiated 3T3-L1 preadipocytes.
Cells were differentiated 2 days post 100% confluences (Day0) by switching with differentiated media containing 10 µg/mL insulin, 1 µM dexamethasone, 0.5 mM isobutylmethylxanthine in DMEM with 10% FBS for the first 3 days; then switch to media only containing 10 µg/mL insulin in DMEM with 10% FBS for the additional 5 days. Indicated concentration of PFOS or vehicle was included in media from Day0 to Day8. (A) Representative images of Oil red O staining of 3T3-L1 preadipocytes at indicated concentration of PFOS. (B) Lipids were extracted from differentiated 3T3-L1 adipocytes by using chloroform/ methanol mixture, and triglycerides (TG) content was determined spectrophotometrically. Relative triglycerides content (%) was displayed using differentiated media containing DMSO (0.1%) - treated cells as a standard (Veh). *, P<0.05, PFOS-treated vs. vehicle (Veh).
PFOS increases adipogenic gene expression in 3T3-L1 preadipocytes
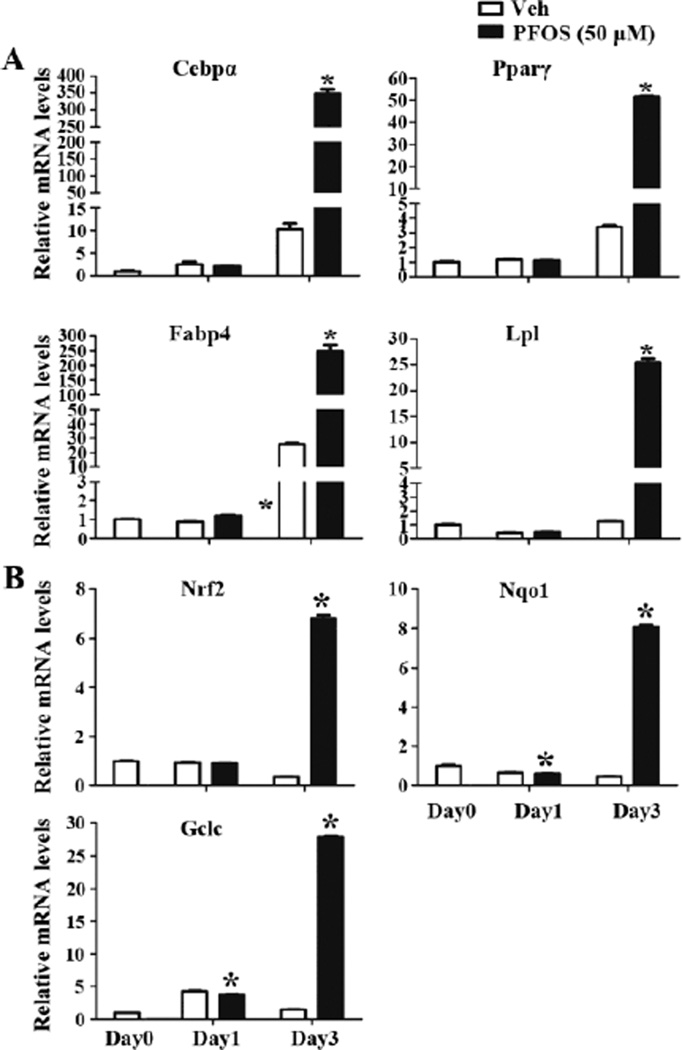
The underlying molecular mechanisms for PFOS function on adipogenesis were evaluated. 3T3-L1 preadipocytes were induced to adipocytes with PFOS administration for continuous 3 days, total RNA was extracted and the relative mRNA levels of genes related with adipogenesis of Cebpα, Pparγ, Fatty acid-binding protein 4 (Fabp4) and Lpl were determined. There is no significant difference for these four genes expression between vehicle- and PFOS-treated groups at Day1. After induction to adipocytes for 3 days (Day3), Cebpα, Pparγ, Fabp4 and Lpl were significantly induced in both groups; with induction being significantly higher in PFOS-treated adipocytes than vehicle-treated group (increased by 32.2-, 14.2-, 8.6-, and 19.7-fold, respectively), suggesting PFOS increased adipogenic gene expression, which may contribute to the increased adipogenesis (Figure 3A). Additionally, the mRNA levels of Nrf2 and two target genes, NAD(P)H dehydrogenase, quinone 1 (Nqo1) and Glutamate-cysteine ligase, catalytic subunit (Gclc) were determined. At Day1, PFOS-treatment slightly decreased Nqo1 and Gclc mRNA levels compared to vehicle-treated group. After 3 days of induction to adipocytes (Day3), PFOS significantly increased Nrf2, Nqo1 and Gclc mRNA levels in 3T3-L1 adipocytes than vehicle-treated group by more than 15-fold, suggesting that PFOS has the potential to activate Nrf2 signaling in preadipocytes (Figure 3B).
Figure 3. PFOS increases adipogenic gene expression and induced Nrf2 signaling in 3T3-L1 preadipocytes.
3T3-L1 preadipocytes were induced to differentiation to adipocytes with or without PFOS (50 µM) for 3 days. Total RNA was extracted at the indicated time. Relative mRNA levels were quantified by quantitative real-time PCR. PFOS increased adipogenic gene expression of Cebpα, Pparγ, Fabp4, Lpl (A), and increased Nrf2 signaling of Nrf2, Nqo1, Gclc (B) mRNA levels in 3T3-L1 preadipocytes All data were normalized to 18S rRNA levels. *, P<0.05, PFOS-treated vs. vehicle (Veh)..
PFOS promotes insulin-stimulated glucose uptake in 3T3-L1 adipocytes
In order to assess the metabolic consequence of PFOS treatment in 3T3-L1 preadipocytes, insulin-stimulated glucose uptake were monitored in vehicle- and PFOS-treated adipocytes. After 3T3-L1 preadipocytes were differentiated to adipocytes for 10 days, cells were exposed to PFOS for 5 hrs, then 2-NBDG was added, and the fluorescence activity was monitored. There was no significant difference between the low dose PFOS treatments (1 and 5 µM) and vehicle-treated group. However, insulin-stimulated glucose uptake was significantly higher in PFOS-treated at the concentration of 10 µM than vehicle-treated group (by 26%). And a dose-dependent increase of glucose uptake by PFOS-treatment was observed, with the highest induction being at the dosage of 100 µM (61.0% higher than vehicle) (Figure 4A). The gene expression related to insulin signaling and glucose metabolism was monitored. Low dose PFOS treatment (1 µM) did not affect Pparγ, Sterol regulatory element-binding protein 1 (Srebp1c), Glucose transporter type 4 (Glut4), Insulin receptor substrate 1 (Irs-1), and Insulin Receptor (Insr) gene expression. However, treatment with 10 or 50 µM PFOS increased Pparγ, Srebp1c, Glut4 and Irs-1 expression compared to vehicle-treated group (by 1.3-, 1.8-, 1.7-, 1.4-and 1.2-, 1.3-, 1.2-, 1.2-fold, respectively), but with similar Insr expression between vehicle- and PFOS-treated groups (Figure 4B). It was hypothesized that in the early induction process, PFOS might induce the antioxidant response, then leading to Nrf2 activation that augmented Pparγ induction (Shi and Zhou, 2010). PFOS (1 µM) did not induce Nrf2 or Nqo1 expression, but expression was induced by about 30% in cells treated with 10 and 50 µM PFOS, suggesting the potential role of PFOS activating Nrf2 signaling in mature adipocytes (Figure 4C).
Figure 4. PFOS promotes insulin-stimulated glucose uptake in 3T3-L1 adipocytes.
(A) 3T3-L1 preadipocytes were induced to differentiation to adipocytes for 10 days, then treated with PFOS (1, 5, 10, 50, 100 µM) for 5 hrs. Glucose uptake of differentiated 3T3-L1 preadipocytes was determined by using 2-NBDG according to the manufactory instruction. The fluorescence activity was monitored at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. Relative glucose uptake was displayed using vehicle-treated group as a standard. Total RNA was extracted from 3T3-L1 adipocytes after treated with PFOS (10 or 50 µM) for 5 hrs. Relative mRNA levels of (B) Pparγ, Srebp1c, Glut4, Irs-1, Insr and (C) Nrf2, Nqo1 were quantified by quantitative real-time PCR. All data were normalized to 18S rRNA levels. *, P<0.05, PFOS-treated vs. vehicle (Veh).
Adipogenic gene expression and Nrf2 signaling is increased in white adipose tissue from mice administered PFOS
C57BL/6 male mice were administered PFOS for 36 days (100 µg/kg/day) and the transcript levels corresponding to adipogenesis and Nrf2 signaling were measured in epididymal WAT. PFOS significantly increased the expression of adipogenic genes, including Cebpα, Pparγ, Fabp4 and Lpl in WAT (by 66.4%, 93.2%, 73.3%, and 67.6%). Srebp1c and Insr, related with insulin signaling, were significantly induced after PFOS administration (by 67.2% and 59.7%), along with the increasing tread of Glut4 and Irs-1 expression (by 44.1% and 25.9%) (Figure 5A). Additionally, PFOS significantly induced Nrf2, Nqo1 and Gclc mRNA levels (by 97.2%, 173.0% and 87.3%), suggesting enhanced Nrf2 signaling in WAT of mice administrated with PFOS. Expression of Heme oxygenase 1 (Ho-1), Multidrug resistance-associated protein (Mrp) 2, Mrp4, UDP-glucuronosyltransferase (Ugt) 1a6, and Superoxide dismutase (Sod) 1, which are highly associated with Nrf2 signaling (Thimmulappa et al., 2002; Dreger et al., 2009), were increased in the current study (37.6%, 26.0%, 334%, 65.2%, and 48.1% higher than vehicle-treated group). PFOS decreased Cytochrome P450, family 7, subfamily A, polypeptide 1 (Cyp7a1) by 78.5%, which is consistent with the previous study (Chang et al., 2009).
Figure 5. Adipogenic gene expression and Nrf2 signaling of Nrf2 and Nqo1 expression increase in white adipose tissue from mice administered PFOS.
Mice were administrated with PFOS (100 µg/kg/day) for 36 days. Epididymal white adipose tissue was collected and total RNA was extracted. (A, B) Relative mRNA levels of the indicated gene were quantified by quantitative real-time PCR. All data were normalized to 18S rRNA levels. *, P<0.05, PFOS-treated vs. vehicle (Veh).
PFOS increased lipid accumulation in differentiated human visceral preadipocytes
Human visceral preadipocytes were obtained and differentiated to adipocytes, and cellular lipid content was evaluated by Oil red O staining. Eleven days post-differentiation, PFOS (5 and 50 µM) significantly increased staining in mature adipocytes (Figure 6A). Furthermore, the stain was extracted and quantified spectrophotometrically. Figure 6B illustrates that PFOS increased staining in adipocytes by 48% at 5 µM and 40% at 50 µM, suggesting PFOS may increase adipogenesis in human visceral preadipocytes, contribute to enhance lipid accumulation in PFOS-treated group (Figure 6B).
Figure 6. PFOS increased lipid accumulation in differentiated human visceral preadipocytes.
Human visceral preadipocytes obtained from Lonza were induced to differentiation to adipocytes by switch with the differentiated media contain dexamethasone, isobutylmethylxanthine and insulinSingleQuots™ according to the manufacturer’s instructions, with PFOS (5 or 50 µM) or not (0.1% DMSO) for 11 days. (A) Representative images of Oil red O staining of differentiated human visceral preadipocytes treated with PFOS at indicated concentration. (B) Staining of lipids was extracted from differentiated human visceral preadipocytes via isopropanol isolation, and the lipid content was determined spectrophotometrically. Relative lipid content (%) was displayed using differentiated media containing DMSO (0.1%) -treated cells as a standard (Veh). *, P<0.05, PFOS-treated vs. vehicle (Veh).
PFOS increases Antioxidant Response Element activity and enhances Nrf2 enrichment at the ARE element in mouse Nqo1 promoter
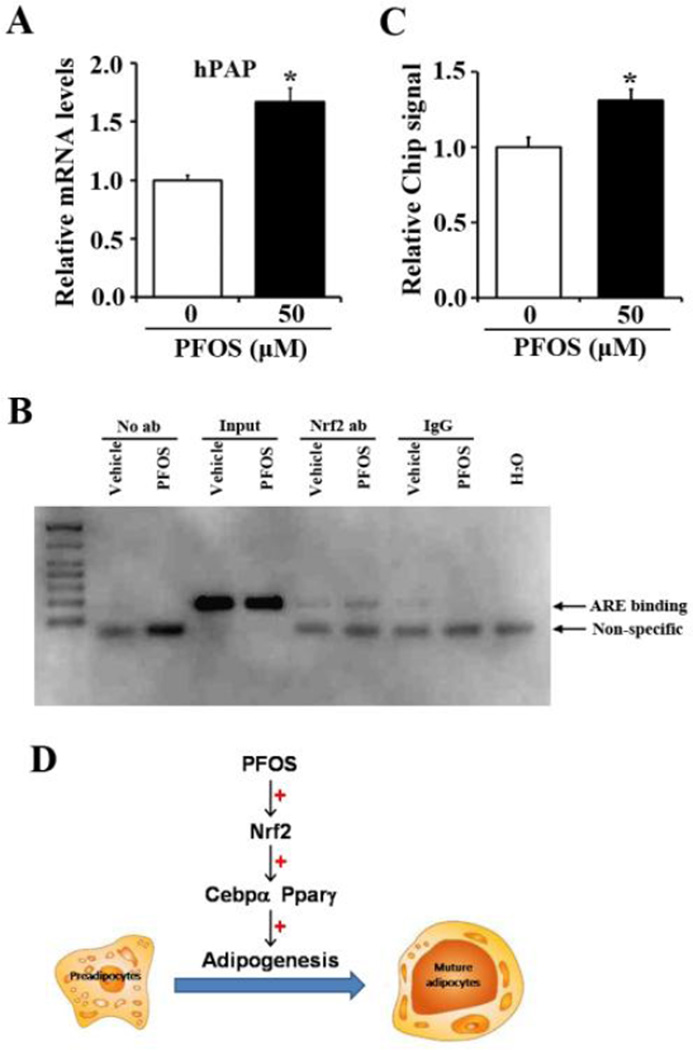
Some recent work indicates that the Nrf2 pathway is inducible in MEFs and adipose tissue (Shin et al., 2007; Xu et al., 2012). In order to determine whether PFOS induces Nrf2 signaling and might increase adipogenesis via the Nrf2 signaling pathway, MEFs from ARE-hPAP transgenic mice (Johnson et al., 2002) were isolated and differentiated to adipocytes with or without PFOS for 4 days. Next, hPAP mRNA levels were measured by quantitative real-time PCR. PFOS treatment (50 µM) doubled hPAP mRNA levels compared to vehicle controls (Figure 7), highly suggesting PFOS increased ARE binding activity (Figure 7A). Furthermore, to determine whether the increased ARE binding was via the increased Nrf2 binding in PFOS treatment, Chip assay was carried out. Figure 7B and 7C illustrate that two days into differentiation, PFOS increased Nrf2 binding to ARE sites in mouse Nqo1 promoter (by 31%), suggesting that Nrf2 binding was increased after PFOS treatment during the differentiation process (Figure 7B, 7C).
Figure 7. FFOS increases hPAP expression and enhances Nrf2 enrichment at ARE sites in mouse Nqo1 promoter.
(A) Mouse embryonic fibroblasts isolated from ARE-hPAP transgenic mice were induced to differentiation to adipocytes for 4 days, then incubated with PFOS (50 µM) for additional 4 days. Total RNA was extracted and hPAP mRNA levels were quantified by quantitative real-time PCR. All data were normalized to 18S rRNA levels. *, P<0.05, PFOS-treated vs. vehicle (Veh). (B) 3T3-L1 cells were induced to differentiation for 2 days. Cells were collected for Chromatin immunoprecipitation assays using either Nrf2 antibody or IgG as the negative control. A primer targeted for antioxidant response element in mouse Nqo1 promoter was used for PCR amplification. Non-immunoprecipitated chromation (1%) was used as an input control. (C) Relative chip signal for PFOS function on Nrf2 enrichment to ARE element of mouse Nqo1 promoter. (D) The work flow for PFOS increased adipogenesis via activating Nrf2 signaling and inducing Cebpα, Pparγ expression in preadipocytes.
Discussion
PFOS has been manufactured for over 60 years. Epidemiological studies and recent research with PFOS primarily focuses on widespread exposure (Giesy and Kannan, 2001; Olsen et al., 2005), hepatic effects in humans (Nelson et al., 2010), primates (Chang et al., 2012) and rodents (Wan et al., 2012), and metabolic perturbations (Wan et al., 2012). However, PFOS effects on adipogenesis or adipocyte health is largely undescribed, yet is of growing concern because of the growing population of obese people worldwide. In the current study, we demonstrated that PFOS augmented adipocyte differentiation, increased the expression of key transcription factors of Pparγ and Cebpα involved in adipogenesis, and increased ARE binding activity and activation of Nrf2 signaling, which increase the binding to promoters for oxidative stress-related and adipogenic genes, suggesting the potential roles of PFOS regulating white adipose tissue expansion and the related obesity.
The activation of Pparγ by PFOS suggested that PFOS has Pparα-independent mechanism, which is in agreement with the previous study (Rosen et al., 2010). PFOS increased liver weight and caused modest hepatomegaly, which related with the activation of peroxisome proliferator, consistent with the effects of Pparα activator WY14,643. Also, PFOS induced both mouse and human Pparα activation, but exerted a greater level of induction of mouse Pparα than human Pparα at similar concentrations (Takacs and Abbott, 2007). It has been reported that PFOS increased Pparγ-luciferance reporter plasmid activity at the concentration from 1 to 100 µM, with little toxicity at the concentration of 250 µM (Takacs and Abbott, 2007), illustrated that the dosage of 50 µM used in the current study is the optimal concentration for PFOS treatment in vitro.
Pparγ activation is the key process for adipocyte differentiation in 3T3-L1 preadipocytes. Pparγ is needed for adipose tissue formation in mice, and in a loss-of-function experiment, adipocyte differentiation and WAT expansion was impaired, and did not develop glucose intolerance and insulin resistance, suggesting the central role for adipogenesis (Jones et al., 2005). We failed to observe WAT expansion in mice administered with PFOS (100 µg/kg/day) for 36 days (Figure S2). This lack of effect could be explained by the relatively low dose of PFOS has been used in the current study, but is consistent with other reports. Qazi et al. have used 0.005% (W/W) dietary treatment of PFOS for 10 days with male mice, resulted in significant reductions for serum cholesterol and triglycerides content (Qazi et al., 2010). And male BALB/c mice fed with high-fat diet (HFD) with the exposure of PFOS at the dose of 5 or 20 mg/kg/day for 14 days, exhibited reduced serum lipid and lipoprotein content, but significantly increased hepatic lipid accumulation, probably via Pparα-independent pathway (Wang et al., 2014). Also, compared to liver, PFOS deposits to a much lower concentration in WAT (Maestri et al., 2006). So perhaps the low dose of PFOS administered might not have a positive induction in adipose tissue expansion in vivo, despite this treatment inducing adipogenic gene expression at transcriptional levels. It was estimated that the possible range of environmental exposure dosage for human in European countries such as Italy, the Netherlands, Sweden and the UK, was between 45 and 58 µg/kg/day based on the mean consumption (Saikat et al., 2013). Dose of 100 µg/kg/day was used in the current study, as planning to mimic the effects of environmental levels exposure of PFOS on WAT expansion and the possible function on obesity in animal model of mice. The second possible reason is that PFOS has a potential function to induce Pparα activation (Shipley et al., 2004). Increased Pparα in adipose tissue could induce lipolysis and promote β-oxidation (Reddy and Hashimoto, 2001; Goto et al., 2011), which is beneficial to prevent the lipid accumulation to adipose tissue and decrease white adipose tissue mass. It was noted that contradict results have been reported that PFOS administration reduced WAT mass, the ventral fat was significantly reduced when mice treated with PFOS (20 mg/kg/day) for 14 days, even in normal diet and HFD-group, which related with the reduced secretion and impaired function of low density lipoproteins (Wang et al., 2014).
It is interesting to note that PFOS increased insulin-stimulated glucose uptake in differentiated adipocytes. A previous study reported that higher serum PFOS concentration was positively associated with increased serum insulin levels and insulin resistance (Lin et al., 2009), which contrasts with the current study. And Nelson et al. reported that higher serum PFOA and PFOS concentration was positively associated with serum cholesterol levels, but displayed a weak association with body weight and insulin resistance (Nelson et al., 2010). Another in vivo study performed on rats, which the pregnant rats were given with PFOS (0.5 or 1.5 mg/kg/day) from gestation day 0 to postnatal day 21. The pups displayed impaired glucose tolerance and enhanced insulin resistance index, suggesting PFOS disrupted insulin signaling in integrate animal study (Lv et al., 2013). In the current in vitro study, we demonstrated that PFOS increased insulin-induced glucose uptake and increased gene expression related to insulin signaling. One possible reason is that PFOS increased adipogenesis in 3T3-L1 preadipocytes and enhanced adipogenesis increases capacity for glucose uptake (Nugent et al., 2001). Also, enhanced Pparγ and Glut4 expression will help to promote glucose uptake, as well as improve insulin signaling and insulin-response activity. Our previous study reported that enhanced Nrf2 activity by Keap1-KD increased glucose tolerance, and increased insulin-stimulated Akt phosphorylation without Glut4 expression change (Xu et al., 2013), suggesting activation Nrf2 can promote insulin signaling, contribute to positively regulate glucose uptake in differentiated 3T3-L1 preadipocytes.
The study herein reported that PFOS could induce Nrf2 activation in preadipocytes. PFOS administration has been shown to increase ROS production, which induces oxidative stress and activate Nrf2 signaling (Qian et al., 2010). ARE consensus elements are the typical transcriptional factor binding sites, which are described to induce the antioxidant gene expression for Nrf2 target genes, such as Ho1, Gclc, Nqo1, and Mrps to provide a protective role against oxidative and cytochemical stress (Nguyen et al., 2009). Also, ARE sites have been reported in the promoter of Pparγ and Cebpα, which are responsible for 3T3-L1 preadipocytes adipogenesis (Pi et al., 2010). ARE binding was enhanced after PFOS treatment in MEFs isolated from transgenic mice that harbor a ARE sequence coupled to a hPAP reporter, suggesting activation of genes via ARE sites is a process that occurs via PFOS-induced adipogenesis. Chip assay revealed that PFOS treatment increased Nrf2 binding to ARE sites in the mouse Nqo1 promoter in 3T3-L1 cells cultured in induction media for 4 days, further confirming that PFOS administration can induce Nrf2 signaling via ARE binding during adipogenesis.
Overall, this study reported that novel effects of PFOS in inducing Pparγ and Cebpα expression and adipogenesis, via enhancing ARE binding activity and Nrf2 signaling in preadipocytes (Figure 7D). Additionally, PFOS increased insulin-stimulated glucose uptake and increased gene expression related with insulin signaling. This study points out the potential roles of PFOS promoting adipose tissue differentiation and the related metabolic conditions of obesity consequentially.
Supplementary Material
Highlights.
-
●
PFOS induces adipogenesis in association with increased Pparγ and Cebpα mRNA expression
-
●
PFOS increases ARE bindingactivity and activates Nrf2 signaling
-
●
PFOS increases insulin-stimulated glucose uptake
Acknowledgments
This work was supported by National Institute of Health [5R01ES016042] to AS, and in part, by Rhode Island IDeA Network of Biomedical Research Excellence [P20RR016457-10] from the National Center for Research Resources, National Institute of Health [5K22ES013782]. JX was also supported by the National Science Foundation of China [81570788 and 81341102], and Fundamental Research Funds for Northeastern University [N130220001].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Bjork JA, Wallace KB. Structure-activity relationships and human relevance for perfluoroalkyl acid-induced transcriptional activation of peroxisome proliferation in liver cell cultures. Toxicol Sci. 2009;111:89–99. doi: 10.1093/toxsci/kfp093. [DOI] [PubMed] [Google Scholar]
- Boulanger B, Vargo JD, Schnoor JL, Hornbuckle KC. Evaluation of perfluorooctane surfactants in a wastewater treatment system and in a commercial surface protection product. Environ Sci Technol. 2005;39:5524–5530. doi: 10.1021/es050213u. [DOI] [PubMed] [Google Scholar]
- Chang SC, Ehresman DJ, Bjork JA, Wallace KB, Parker GA, Stump DG, Butenhoff JL. Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: toxicokinetics, thyroid hormone status, and related gene expression. Reprod Toxicol. 2009;27:387–399. doi: 10.1016/j.reprotox.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Chang SC, Noker PE, Gorman GS, Gibson SJ, Hart JA, Ehresman DJ, Butenhoff JL. Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reprod Toxicol. 2012;33:428–440. doi: 10.1016/j.reprotox.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Dreger H, Westphal K, Weller A, Baumann G, Stangl V, Meiners S, Stangl K. Nrf2-dependent upregulation of antioxidative enzymes: a novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc Res. 2009;83:354–361. doi: 10.1093/cvr/cvp107. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Goto T, Lee JY, Teraminami A, Kim YI, Hirai S, Uemura T, Inoue H, Takahashi N, Kawada T. Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J Lipid Res. 2011;52:873–884. doi: 10.1194/jlr.M011320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars A, Knapen D, Meyer IJ, van der Ven K, Hoff P, De Coen W. Toxicity evaluation of perfluorooctane sulfonate (PFOS) in the liver of common carp (Cyprinus carpio) Aquat Toxicol. 2008;88:155–163. doi: 10.1016/j.aquatox.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Hu W, Jones PD, Celius T, Giesy JP. Identification of genes responsive to PFOS using gene expression profiling. Environ Toxicol Pharmacol. 2005;19:57–70. doi: 10.1016/j.etap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2009;32:702–707. doi: 10.2337/dc08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Li G, Li Y, Ying C, Chen J, Chen T, Wei J, Lin Y, Jiang Y, Wang Y, Shu B, Xu B, Xu S. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol. 2013;28:532–542. doi: 10.1002/tox.20747. [DOI] [PubMed] [Google Scholar]
- Maestri L, Negri S, Ferrari M, Ghittori S, Fabris F, Danesino P, Imbriani M. Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2728–2734. doi: 10.1002/rcm.2661. [DOI] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010;118:197–202. doi: 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent C, Prins JB, Whitehead JP, Savage D, Wentworth JM, Chatterjee VK, O’Rahilly S. Potentiation of glucose uptake in 3T3-L1 adipocytes by PPAR gamma agonists is maintained in cells expressing a PPAR gamma dominant-negative mutant: evidence for selectivity in the downstream responses to PPAR gamma activation. Mol Endocrinol. 2001;15:1729–1738. doi: 10.1210/mend.15.10.0715. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007a;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Huang HY, Helzlsouer KJ, Hansen KJ, Butenhoff JL, Mandel JH. Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood. Environ Health Perspect. 2005;113:539–545. doi: 10.1289/ehp.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Reagen WK, Ellefson ME, Ehresman DJ, Butenhoff JL, Zobel LR. Preliminary evidence of a decline in perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations in American Red Cross blood donors. Chemosphere. 2007b;68:105–111. doi: 10.1016/j.chemosphere.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-Shnaidman E, Lee C, Lau J, Kurtz TW, Chan JY. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem. 2010;285:9292–9300. doi: 10.1074/jbc.M109.093955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi MR, Abedi MR, Nelson BD, DePierre JW, Abedi-Valugerdi M. Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice. Int Immunopharmacol. 2010;10:1420–1427. doi: 10.1016/j.intimp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Qian Y, Ducatman A, Ward R, Leonard S, Bukowski V, Lan Guo N, Shi X, Vallyathan V, Castranova V. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: role in endothelial permeability. J Toxicol Environ Health A. 2010;73:819–836. doi: 10.1080/15287391003689317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193–230. doi: 10.1146/annurev.nutr.21.1.193. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C, Corton JC. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol Sci. 2008;103:46–56. doi: 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Schmid JR, Corton JC, Zehr RD, Das KP, Abbott BD, Lau C. Gene Expression Profiling in Wild-Type and PPARalpha-Null Mice Exposed to Perfluorooctane Sulfonate Reveals PPARalpha-Independent Effects. PPAR Res. 2010 doi: 10.1155/2010/794739. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikat S, Kreis I, Davies B, Bridgman S, Kamanyire R. The impact of PFOS on health in the general population: a review. Environ Sci Process Impacts. 2013;15:329–335. doi: 10.1039/c2em30698k. [DOI] [PubMed] [Google Scholar]
- Shi X, Zhou B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol Sci. 2010;115:391–400. doi: 10.1093/toxsci/kfq066. [DOI] [PubMed] [Google Scholar]
- Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley JM, Hurst CH, Tanaka SS, DeRoos FL, Butenhoff JL, Seacat AM, Waxman DJ. trans-activation of PPARalpha and induction of PPARalpha target genes by perfluorooctane-based chemicals. Toxicol Sci. 2004;80:151–160. doi: 10.1093/toxsci/kfh130. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Wilford BH, Jones KC, Zhu J. Perfluorinated sulfonamides in indoor and outdoor air and indoor dust: occurrence, partitioning, and human exposure. Environ Sci Technol. 2005;39:6599–6606. doi: 10.1021/es048340y. [DOI] [PubMed] [Google Scholar]
- Skutlarek D, Exner M, Farber H. Perfluorinated surfactants in surface and drinking waters. Environ Sci Pollut Res Int. 2006;13:299–307. doi: 10.1065/espr2006.07.326. [DOI] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci. 2007;95:108–117. doi: 10.1093/toxsci/kfl135. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wei X, Hui KY, Giesy JP, Wong CK. PFOS-induced hepatic steatosis, the mechanistic actions on beta-oxidation and lipid transport. Biochim Biophys Acta. 2012;1820:1092–1101. doi: 10.1016/j.bbagen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J, Zhang A, Fu J, Jiang G. PFOS induced lipid metabolism disturbances in BALB/c mice through inhibition of low density lipoproteins excretion. Sci Rep. 2014;4:4582. doi: 10.1038/srep04582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Xie Y, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes & development. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- Xu J, Donepudi AC, Moscovitz JE, Slitt AL. Keap1-knockdown decreases fasting-induced fatty liver via altered lipid metabolism and decreased fatty acid mobilization from adipose tissue. PLoS One. 2013;8:e79841. doi: 10.1371/journal.pone.0079841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kulkarni SR, Donepudi AC, More VR, Slitt AL. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes. 2012;61:3208–3218. doi: 10.2337/db11-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.