Abstract
Tamoxifen, a selective estrogen receptor modulator, is a commonly prescribed adjuvant therapy for estrogen receptor-α (ERα)-positive breast cancer patients. To determine if extracellular factors contribute to the modulation of IGF-1 signaling after tamoxifen treatment, MCF-7 cells were treated with IGF-1 in conditioned medium (CM) obtained from 4-OHT-treated MCF-7 cells and the accumulation of phospho-Akt (S473) was measured. CM inhibited IGF-1-dependent cell signaling and suggesting the involvement of extracellular factors (ie. IGFBPs). A significant increase in IGFBP-1 mRNA and extracellular IGFBP-1 protein was observed in 4-OHT-treated MCF-7 cells. Knockdown experiments demonstrated that both GPER1 and CREB mediate IGFBP-1 induction. Furthermore, experiments showed that 4-OHT-dependent IGFBP-1 transcription is downstream of GPER1-activation in breast cancer cells. Additionally, neutralization and knockdown experiments demonstrated a role for IGFBP-1 in the observed inhibition of IGF-1 signaling. These results suggested that 4-OHT inhibits IGF-1 signaling via GPER1 and CREB mediated extracellular IGFBP-1 accumulation in breast cancer cells.
Keywords: Tamoxifen, GPER1, IGF-1, IGFBP-1, breast cancer
1. Introduction
Breast cancer is the second leading cause of cancer-related deaths for women in the United States and one of the leading causes of cancer-related deaths in the world (Jemal et al., 2009). Tamoxifen, an adjuvant hormone therapy, is commonly prescribed to women with estrogen receptor alpha (ERα)-positive breast cancer. The active metabolite of tamoxifen, 4-hydroxytamoxifen (4-OHT), is a selective estrogen receptor modulator (SERM) that functions as an ERα antagonist in breast tissue and breast cancer cells. As an ERα antagonist, 4-OHT inhibits the expression of genes that induce cell cycle progression thus reducing breast cancer cell proliferation rates (Osborne et al., 1983). Experiments in vitro show that breast cancer cells treated with tamoxifen for 72 hours have decreased IGF-1-dependent IGF-1 receptor (IGF-1R) phosphorylation (Guvakova and Surmacz, 1997). One potential hypothesis for this observation is 4-OHT-induced accumulation of an extracellular factor that inhibits IGF-1 stimulation in this cell type.
The insulin-like growth factor-1 (IGF-1)-stimulated signal transduction pathway induces breast cancer cell proliferation and survival via activation of the IGF-1R (Arteaga, 1992; Arteaga et al., 1989; Burgaud et al., 1995; Sachdev and Yee, 2001; Yee et al., 1989). Inhibitors of IGF-1R decrease breast cancer cell proliferation rates, therefore strategies that target this signal transduction pathway have been suggested as a potential therapeutic approach (Li et al., 2009). IGFBPs are secreted proteins that have been shown to modulate both IGF-dependent and IGF-independent cell signaling (Baxter, 2013; Firth and Baxter, 2002; Galiano et al., 1996; Jones et al., 1995; Oh et al., 1993). IGFBPs can modulate IGF-dependent signaling by sequestering IGF from IGF-receptors and reducing receptor activation (Butt et al., 1999; Cullen et al., 1990; Figueroa et al., 1993; Firth and Baxter, 2002; Jones and Clemmons, 1995; Yee et al., 1994). IGF-independent modulation can occur via interaction with other cell surface receptors or can require intracellular mechanisms (Firth and Baxter, 2002; Galiano et al., 1996; Jones et al., 1995). In MCF-7 breast cancer cells, exogenously expressed IGFBP-1 inhibits IGF-1-induced cell proliferation (Figueroa et al., 1993; Yee et al., 1994), however it is not clear if breast cancer cells express and secrete IGFBP-1.
A role for cAMP and the cAMP-response element-binding protein (CREB) in IGFBP-1 expression has been demonstrated in hepatocytes (Frost et al., 2000; Sugawara et al., 2000). cAMP activates protein kinase A (PKA) to phosphorylate the CREB transcription factor at serine 133 (Mayr and Montminy, 2001). This phosphorylation is required for association with the coactivators CBP and p300 and leads to the activation of promoters containing cAMP response elements (CRE) (Chriviaet al., 1993; Kwok et al., 1994; Mayr and Montminy, 2001). The IGFBP-1 has been previously analyzed and, among other response elements, this promoter contains a CRE (Frost et al., 2000; Sugawara et al., 2000).
GPER1 is activated in cells treated with 17β-estradiol (E2) and mediates rapid cell signaling events (Prossnitz and Maggiolini, 2009; Prossnitz et al., 2008; Revankar et al., 2005; Tang et al., 2014). This receptor is also activated by the GPER1-selective agonist G-1, the pure antiestrogen fulvestrant (ICI-182,780), and 4-OHT (Maggiolini et al., 2004; Revankar et al., 2005; Thomas and Dong, 2006; Thomas et al., 2005; Vivacqua et al., 2006). GPER1 activation in breast cancer cells can induce apoptosis and inhibit proliferation via p53-dependent cell cycle arrest (Ariazi et al., 2010; Wei et al., 2014). Conversely, GPER1 activation has been shown to induce cell proliferation in an epidermal growth factor receptor (EGFR)-dependent manner (Maggiolini et al., 2004; Pandey et al., 2009; Pupo et al., 2012). More recently, GPER1 has been shown to play a potential role in the development of tamoxifen resistance in vitro (Ignatov et al., 2010; Mo et al., 2013).
In this contribution, evidence supporting a role for 4-OHT-dependent extracellular IGFBP-1 accumulation in the modulation of IGF-1R signaling in breast cancer cells is presented. Furthermore, data herein show that CREB and GPER1 mediate the observed IGFBP-1 induction after 4-OHT treatment and this effect is independent of ERα. IGFBP-1 knockdown by siRNA demonstrated that IGFBP-1 is, at least in part, required for the inhibition of IGF-1-dependent cell signaling associated with 4-OHT treatment. Furthermore, antibody neutralization experiments support a role for extracellular IGFBP-1 in the observed inhibition. Taken together, these data suggest that GPER1-mediated CREB activation results in the accumulation of extracellular IGFBP-1 in 4-OHT-treated breast cancer cells thus revealing a previously unidentified mechanism of tamoxifen action.
2. Materials and Methods
2.1. Cell culture and treatment
MCF-7 and SKBr-3 breast cancer cells (ATCC, Manassas, VA) were maintained in DMEM (Life Technologies, Carlsbad, CA) and DMEM/F12 (Life Technologies, Carlsbad, CA), respectively. Maintenance media were supplemented with 10% fetal bovine serum (Life Technologies, Carlsbad, CA). 48 hours prior to treatment, cells were washed with 1X PBS, and phenol red-free DMEM supplemented with 10% charcoal-stripped FBS (Life Technologies, Carlsbad, CA) was added to the cells. After 24 hours, cells were washed with 1X PBS, then serum-starved in phenol red-free DMEM overnight followed by treatment with the indicated dose of G-1, G36 (provided by Jeffrey B. Arterburn, New Mexico State University) or 4-hydroxytamoxifen (4-OHT) (Fluka, St. Louis, MO). G-1, G36 and 4-OHT were dissolved in ethanol (vehicle) and the final concentration of ethanol in medium was 0.1%. For GPER1 inhibition in the cell viability assay, cells were pre-incubated with 1μM G36 for 30 min prior to 4-OHT treatment.
2.2. siRNA transfection
The knockdown experiment was performed as described previously (Ariazi et al., 2010). Briefly, all cell transfections were performed using Lipofectamine 2000 reagent for 4–6h in serum-free Opti-MEM (Life Technology, Carlsbad, CA). For siRNA knockdown, cells were transfected with GPER1 siRNA (Ambion, s6053, sense: GGCUGUACAUUGAGCAGAAtt; antisense: UUCUGCUCAAUGUACAGCCtc); ERα siRNA (Ambion, s4823, sense: ACAUCAUCUCGGUUCCGCAtt; antisense: UGCGGAACCGAGAUGAUGUag); CREB1 siRNA (Ambion, 109994, sense: GGUGGAAAAAUGGACUGGCUtt; antisense: AGCCAGUCCAUUUUCCACCtt); IGFBP-1 siRNA (Santa Cruz, sc-39584) or non-targeting siRNA (Ambion, 4390843, negative control #1). After overnight recovery in maintenance media, the transfection was repeated again followed by growing transfected cells in media containing 10% charcoal-striped FBS and analyzed for protein expression (immunoblot) after 48 hours.
2.3. Total RNA isolation and quantitative PCR analysis
Total RNA was isolated with the PureLink RNA Mini Kit (Life Technologies, Carlsbad CA) with DNase-I following the manufacturer's protocol. cDNA was synthesized from total RNA (1μg) using the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR reactions were performed using SYBR Green Master Mix and the 7300 Real-Time PCR system (Bio-Rad, Hercules, CA). RPL30 gene expression was used in all quantitative real-time PCR reaction as an internal reference gene to normalize relative changes in transcript levels. Primers for amplification of human IGFBP-1: forward 5′-CTA-TGA-TGG-CTC-GAA-GGC-TC-3′, reverse 5′-TTC-TTG-TTG-CAG-TTT-GGC-AG-3′ (XIE, 2014) and RPL30: forward 5′-ACA-GCA-TGC-GGA-AAA-TAC-TAC-3′, reverse: 5′-AAA-GGA-AAA-TTT-TGC-AGG-TTT-3′ (de Jonge et al., 2007).
2.4. Immunoblot Analysis
Whole cell extracts were prepared in RIPA buffer containing protease and phosphatase inhibitor cocktails (87785, 78420, Thermo Scientific, Rockford, IL). Cell extract protein concentrations were determined using BCA assay (Thermo Scientific, Rockford, IL). 30–50 μg of whole cell lysates were resolved using Bolt 4–12% Bis-Tris Plus gels (Life Technologies, Carlsbad, CA) and transferred to PVDF membrane. Blots were blocked in Tris-buffered saline-0.1% Tween 20 (1X TBST) containing 5% fat-free milk at room temperature for 1 hour then incubated with primary antibody at room temperature for 2 hours using the following antibodies: IGFBP-1 (sc-13097, Santa Cruz Biotechnology, Dallas, TX); GPER1 (sc-48825-R, Santa Cruz Biotechnology, Dallas, TX); phospho-IGF-I receptor beta (3024, Cell Signaling Technology, Danvers, MA); IGF-I receptor beta (3027, Cell Signaling Technology Danvers, MA); phospho-AKT (S473) (4060, Cell Signaling Technology Danvers, MA); AKT (4685, Cell Signaling Technology Danvers, MA); phosphop44/42 MAPK (9106, Cell Signaling Technology Danvers, MA); p-44/42 MAPK (9102, Cell Signaling Technology Danvers, MA) and β-actin (sc-47778, Santa Cruz Biotechnology, Dallas, TX). After washing three times with 1X TBST, blots were incubated with anti-IgG horseradish peroxidase-conjugated secondary antibody (sc-81178, Santa Cruz Biotechnology, Dallas, TX). Prior to the addition of chemiluminescence reagent (34076, Thermo Scientific, Rockford, IL), blots were washed three times with 1X TBST. Each blot was stripped using restore plus western blot stripping buffer (46430, Thermo Scientific, Rockford, IL) when necessary to determine equivalent sample loading. Chemiluminescence was detected using Gel Doc™ XR ChemiDoc™ imaging system (BioRad, Hercules, CA) and quantitated using Quantity One® software (BioRad, Hercules, CA).
2.5. Extracellular IGFBP-1 measurement and conditioned medium
Extracellular concentration of IGFBP-1 was determined using human IGFBP-1 ELISA (ab100539, Abcam, Cambridge, MA) following manufacturer's protocol. For immunoblot analysis, protein content of conditioned cell culture medium from treated cells was column concentrated (Pratt and Pollak, 1993) about 10-fold using Centrifugal Filter Devices (Ultracel 3K, Milipore, Germany) (Deng et al., 2010). Also, protease inhibitor cocktail (P1860, Sigma, St. Louis, MA) 10 μl/mL was applied to the cell culture medium during vehicle and drug treatments prior to conditioned medium analysis (Han et al., 2012; Ween et al., 2011). After centrifugation, protein concentration was determined using BCA protein assay (Thermo Scientific, Rockford, IL) and 20 μg of concentrated protein was used for immunoblot analysis as previously described. Experiments using conditioned media involved moving culture medium from 4-OHT-treated cultures to cultures previously treated with vehicle and vice versa. After the media was switched, cells were stimulated for 15 minutes with 50 ng/mL IGF-1.
2.6. IGFBP-1 Neutralization assay
IGFBP-1 neutralization was performed using anti-IGFBP-1 antibody (Sigma, I2032, monoclonal mouse IgG1). After treating MCF-7 cells for 24 hours with 4-OHT to allow for the accumulation of IGFBP-1 in the cell culture medium, the control IgG or IGFBP-1 neutralizing antibody (10 ng/mL) was added for 2h prior to IGF-1 stimulation.
2.7. Cell viability assay
Cells were plated in 96-well plates at a density of 2500 cells per well in DMEM supplemented with 10% fetal bovine serum. 12 hours prior to treatment, media were replaced with DMEM supplemented with 1% charcoal-stripped serum (Fagan et al., 2012), and cell number was determined after five days using Alamar blue reagent (Life Technology, Carlsbad, CA) according to the manufacture's procedure.
2.8. Statistical Analysis
All statistical analysis was performed by one-way ANOVA, Tukey's test using Kaleidagraph (Synergy Software, Reading, PA). Differences were considered significant if p ≤ 0.05 and the error bars are ± SEM.
3. Results
3.1. Conditioned medium from 4-OHT-treated MCF-7 cells inhibited IGF-1-stimulated AKT phosphorylation
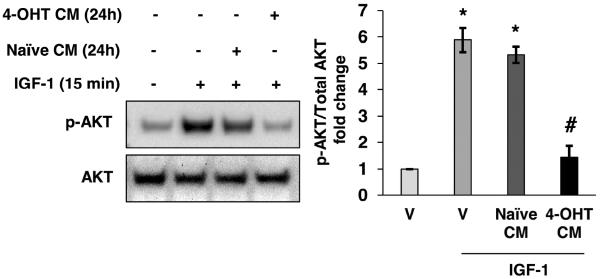
Previous reports show that 4-OHT exposure for 72 hours inhibits IGF-1R phosphorylation in IGF-1-stimulated breast cancer cells (Guvakova and Surmacz, 1997), however, the mechanism of inhibition was not identified. Such inhibition is not observed with a 10 minute or 8 hour treatment with 4-OHT (Lee et al., 1997) suggesting that the observed inhibition may require extracellular accumulation of an inhibitory factor that modulates IGF-1. To determine if inhibition of IGF-1 stimulation in breast cancer cells is dependent on accumulation of an extracellular factor, conditioned medium experiments were performed. AKT phosphorylation was used as an indicator of IGF-1 stimulation in breast cancer cells. After MCF-7 cells were treated for 24 hours with 1μM 4-OHT, conditioned medium was collected and used to replace the medium of naïve, vehicle-treated cells. After the medium switch, cells were stimulated with 50 ng/mL IGF-1 for 15 minutes and the phosphorylation status of AKT (S473) was measured by immunoblot. After stimulation with 50 ng/mL IGF-1 in conditioned medium obtained from MCF-7 cells treated with 4-OHT, phosphorylation of AKT (S473) was decreased compared to stimulation in conditioned medium from vehicle-treated MCF-7 cells (Fig. 1, lane 4 compared to lane 3). To ensure that the observed inhibition of AKT phosphorylation did not result from the remaining 4-OHT in the conditioned medium, a 15 minute 4-OHT treatment was included as a control. Data from this experiment indicated that treatment with 4-OHT for 15 minutes did not affect IGF-1-stimulated AKT phosphorylation (Supplemental Fig. 1). This result is consistent with previously published data showing that a relatively short-term 4-OHT treatment is unable to inhibit IGF-1 signaling (Lee et al., 1997) and indicated that treatment with 4-OHT increased an extracellular factor that modulates IGF-1-stimulation in MCF-7 breast cancer cells.
Fig. 1.
4-OHT-induced extracellular factor inhibits IGF-1-stimulated AKT phosphorylation. Conditioned medium (CM) from vehicle-treated MCF-7 cells (24 hours) was replaced with conditioned medium from 4-OHT-treated cells prior to IGF-1 stimulation (50 ng/mL) for 15 min. Total AKT and phospho-AKT were measured by immunoblot. *, p<0.05 compared to vehicle (V) control, and #, p<0.05 compared to IGF-1 treated sample. The results are representative of three independent experiments, and the error bars are SEM.
3.2. 4-OHT induced IGFBP-1 transcription and extracellular accumulation in MCF-7 cells
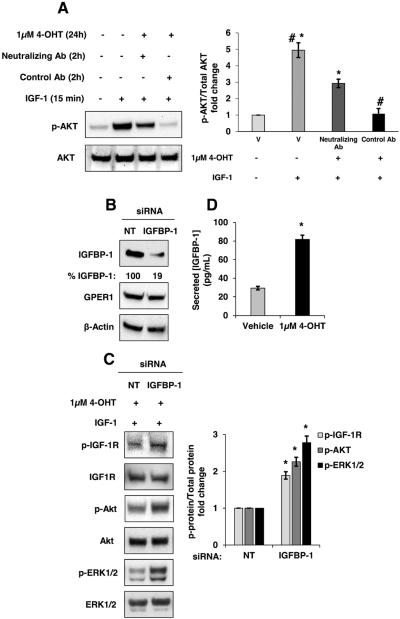
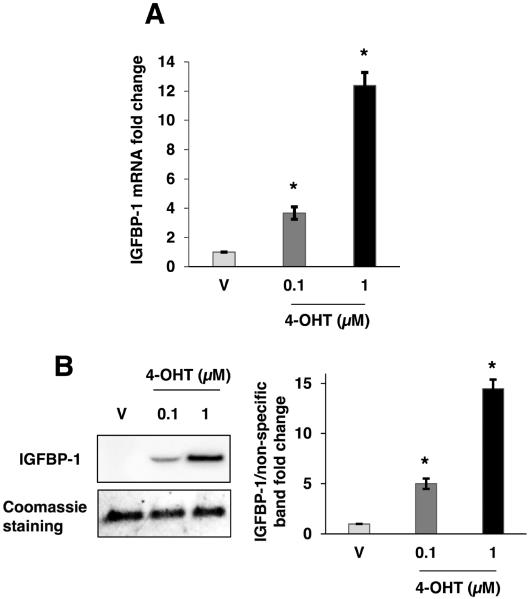
Exogenously expressed IGFBP-1 has been previously demonstrated to regulate MCF-7 breast cancer cells (Yee et al., 1994). To determine if 4-OHT induced endogenous IGFBP-1 transcription, MCF-7 cells were treated with 4-OHT for 24 hours and relative IGFBP-1 transcript levels were determined by quantitative real-time PCR (qPCR) analysis. The results indicated a significant and dose-dependent increase in IGFBP-1 transcription after treatment with 4-OHT compared to vehicle treatment (Fig. 2A). Furthermore, 4-OHT treatment induced the intracellular IGFBP-1 protein levels compared to vehicle treatment (supplemental Fig. 2). Since the observed inhibition of IGF-1 stimulation was conferred using conditioned medium from 4-OHT-treated MCF-7 cells, we determined the extracellular accumulation of IGFBP-1. Since the concentration of IGFBPs are quite low in media from MCF-7 cells (Hermani et al., 2013; Pratt and Pollak, 1993; Yee et al.,1994), the conditioned culture medium from vehicle or 4-OHT-treated MCF-7 cells was column concentrated prior to immunoblot analysis (Deng et al., 2010) to obtain a detectable level of protein in the sample. Using this approach, a dose-dependent increase in extracellular IGFBP-1 was observed after 24 hours of treatment with 4-OHT (Fig. 2B). These data indicated that 4-OHT induced IGFBP-1 transcription and increased extracellular IGFBP-1 protein levels in MCF-7 breast cancer cells.
Fig. 2.
4-OHT induced IGFBP-1 transcription and extracellular accumulation in MCF-7 cells. (A) Relative IGFBP-1 gene transcription determined by quantitative real-time PCR after 24 hours of treatment with vehicle (V), 100 nM 4-OHT, or 1μM 4-OHT. Relative transcription was normalized to the RPL30 transcript. (B) Immunoblot analysis of concentrated MCF-7 cell culture medium after 24 hours of treatment with vehicle (V), 100 nM 4-OHT, or 1μM 4-OHT. Results are representative of at least three independent experiments. *, p<0.05 compared to vehicle control, and the error bars are SEM.
3.3. 4-OHT increased phospho-CREB levels to modulate IGFBP-1 transcription in MCF-7 cells
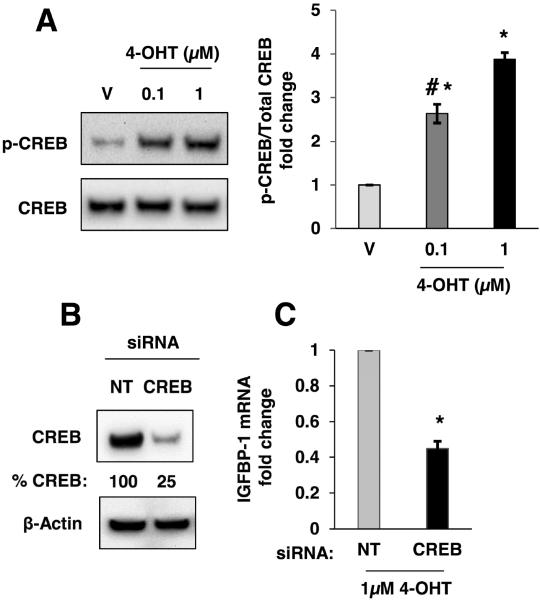
Phosphorylation on CREB transcription factor at serine 133 promotes expression of its target genes via modulation of promoters containing the cAMP-response element (CRE) (Brindle and Montminy, 1992). The CRE has been identified in the human IGFBP-1 promoter and the regulatory role of CREB in IGFBP-1 expression has been determined (Frost et al., 2000; Sugawara et al., 2000). CREB phosphorylation at serine 133 is downstream of the cAMP-PKA axis (Gonzalez and Montminy, 1989). To determine if 4-OHT induced CREB phosphorylation, MCF-7 cells were treated with 4-OHT for 2 hours and phospho-CREB levels were determined by immunoblot. Results demonstrated that 4-OHT (100 nM and 1μM) increased CREB phosphorylation compared with vehicle-treated MCF-7 cells (Fig. 3A). To determine if CREB mediated the observed IGFBP-1 transcription in 4-OHT-treated breast cancer cells, siRNA knockdown of CREB was performed. After CREB knockdown by siRNA, IGFBP-1 transcription was significantly reduced compared to non-targeting control after 4-OHT-treatment (Fig. 3B,C). Knockdown of CREB expression by ~75% compared to non-targeting control CREB expression levels resulted in a ~50% reduction in IGFBP-1 mRNA expression. These data indicated that CREB contributes to IGFBP-1 induction and suggests a role for additional modulators in this system. These data demonstrated the involvement of CREB in the observed IGFBP-1 transcription in 4-OHT-treated breast cancer cells.
Fig. 3.
CREB was phosphorylated after treatment with 4-OHT and CREB knockdown partially inhibited 4-OHT-induced IGFBP-1 transcription in MCF-7 cells. (A) Immunoblot analysis of phospho-CREB (S133) after 2 hours of treatment with 4-OHT using the indicated doses. (B) Immunoblot analysis of CREB expression after transfection of CREB-targeting siRNA. (C) Relative IGFBP-1 gene transcription determined by quantitative real-time PCR in CREB knockdown and control cells (NT) after 24 hours of treatment with 4-OHT. *, p<0.05 compared to control, and #, p<0.05 compared to 4-OHT (1μM) treated sample. The results are representative of three independent experiments, and the error bars are SEM.
3.4. GPER1-mediated CREB phosphorylation in breast cancer cells
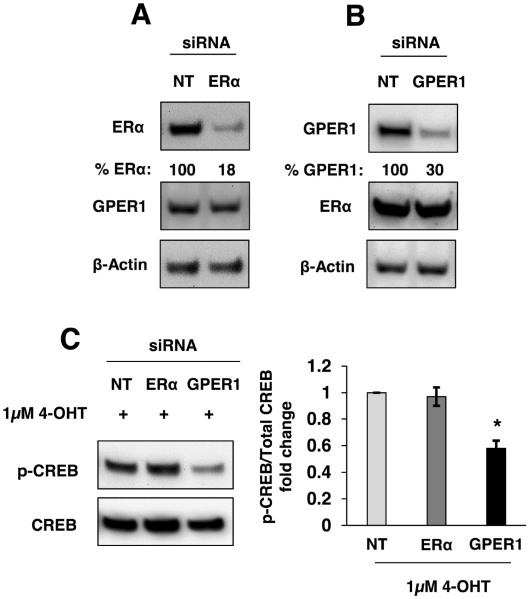
CREB activation by phosphorylation at serine 133 (S133) is downstream of cAMP-activated protein kinase (PKA) (Brindle and Montminy, 1992). While 4-OHT is a well-characterized antagonist for ERα in MCF-7 cells, it has also been shown to function as a GPER1 agonist. Since GPER1 activates adenylyl cyclase (Filardo et al., 2002) and its downstream kinase, PKA, it may play a role in IGFBP-1 expression in 4-OHT-treated breast cancer cells. Furthermore, the involvement of GPER1 in regulation of gene expression through CREB has been previously demonstrated (Karki et al., 2013). To determine the role of ERα and GPER1 in the observed 4-OHT-induced CREB phosphorylation, ERα or GPER1 knockdown cells were treated with 4-OHT followed by Immunoblot analysis of phospho-CREB levels. Phosphorylation of CREB was decreased in GPER1 knockdown cells compared with ERα knockdown and non-targeting control (Fig 4C). Together, these experiments demonstrated that decreased ERα expression does not alter the accumulation of phospho-CREB after treatment with 4-OHT and that GPER1 contributes to 4-OHT-induced CREB phosphorylation. To further demonstrate a role for GPER1 activation in the observed CREB phosphorylation, treatment with the GPER1-agonist G-1 was performed and increased CREB phosphorylation in MCF-7 cells was observed (supplemental Fig. 3A). Furthermore, 4-OHT induced CREB phosphorylation in ERα-negative and GPER1-positive SKBr-3 breast cancer cells (supplemental Fig. 3B). These data support a role for GPER1 in the observed 4-OHT-induced CREB phosphorylation and that ERα is not a key determinant of CREB phosphorylation after 4-OHT treatment in breast cancer cells.
Fig. 4.
GPER1-mediated CREB phosphorylation in MCF-7 cells. (A, B) The total ERα and GPER1 protein levels were reduced using siRNA knockdown. (C) Immunoblot analysis of CREB phosphorylation after 2 hours of treatment with 1μM 4-OHT subsequent to ERα or GPER1 knockdown compared to non-targeting siRNA (NT) cells. *, p<0.05 compared to control. The results are representative of three independent experiments, and the error bars are SEM.
3.5. 4-OHT-induced IGFBP-1 expression was GPER1-mediated and inhibits IGF-1 stimulation in MCF-7 cells
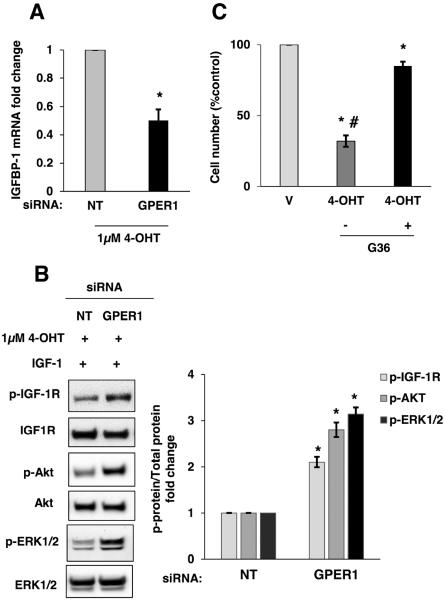
To further support our findings that GPER1 mediated the increased IGFBP-1 expression after treatment with 4-OHT, MCF-7 cells were treated with the GPER1-specific agonist G-1. A significant increase in IGFBP-1 transcription was observed after treatment with 100 nM G-1 when compared to vehicle treatment in MCF-7 cells (Supplemental Fig. S4A). GPER1 knockdown was performed prior to treatment with 4-OHT to demonstrate the involvement of GPER1 in the observed IGFBP-1 induction. When GPER1 protein was reduced in MCF-7 cells, 4-OHT-dependent IGFBP-1 induction was decreased compared with control, non-targeting siRNA (Fig. 5A). Additionally, 4-OHT significantly increased IGFBP-1 transcription in ERα-negative, GPER1-positive SKBr-3 cells, and GPER1 knockdown significantly reduced IGFBP-1 transcription in SKBr-3 cells after treatment with 4-OHT (Supplemental Fig. S4B, C). Since GPER1 knockdown reduced IGFBP-1 transcription in 4-OHT-treated cells, the impact on the ability of 4-OHT to inhibit IGF-1 signaling was determined by measuring the changes in phosphorylation of IGF-I receptor β (Y1135/1136), AKT (S473), and ERK1/2 after IGF-1 stimulation. In GPER1 knockdown cells, the inhibition of IGF-1 stimulation after 4-OHT treatment was significantly less than control cells (Fig. 5B). To determine if GPER1 contributes to decreased MCF-7 cell viability after 4-OHT treatment, cells were treated with 4-OHT in the presence or absence of the GPER1 antagonist G36 (Dennis et al., 2011) and cell viability was determined after five-days of treatment. Consistent with results from others (Fagan et al., 2012; Mo et al., 2013), these data showed that 4-OHT significantly decreased MCF-7 cell viability, and this effect is inhibited by GPER1 antagonism (Fig. 5C). These data demonstrated that in 4-OHT-treated breast cancer cells, GPER1 contributes to the inhibition of IGF-1 signaling and decreases cell viability.
Fig. 5.
4-OHT-induced IGFBP-1 transcription and inhibition of IGF-1 stimulation is decreased after GPER1 knockdown in MCF-7 cells. (A) Relative IGFBP-1 gene transcription determined by quantitative real-time PCR in GPER1 knockdown and non-targeting control cells (NT) after 24 hours of treatment with vehicle or 1μM 4-OHT. (B) Immunoblot analysis p-IGF-1R, p-AKT and p-ERK1/2 after IGF-1 stimulation in control and GPER1 knockdown MCF-7 cells. (C) MCF-7 cell viability after treatment with 1μM 4-OHT with and without 1μM G36 treatment. *, p<0.05 compared to vehicle and #, p<0.05 compared to G36- treated sample. The results are representative of three independent experiments, and the error bars are SEM.
3.6. PKA inhibitor decreased IGFBP-1 induction in 4-OHT-treated MCF-7 cells
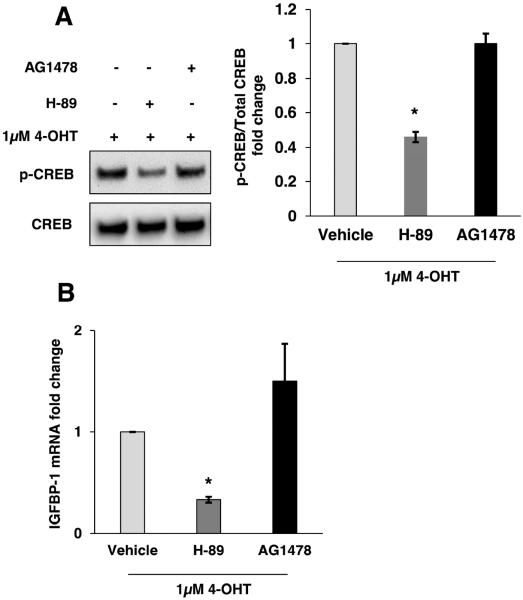
Our data showed that GPER1 contributes to 4-OHT-induced CREB phosphorylation and IGFBP-1 transcription. GPER1 activation can also result in EGFR activation by release of heparin-bounded EGF (Filardo et al., 2000), and it has been shown that EGFR signaling pathway can induce CREB phosphorylation at S133 (Vanhoutte et al., 1999; Xing et al., 1998). To determine if the observed IGFBP-1 induction is cAMP-dependent or EGFR-mediated, MCF-7 cells were pretreated with either the PKA inhibitor H-89 or the EGFR inhibitor AG-1478 prior to 4-OHT treatment. Immunoblot analysis demonstrated that pretreatment with H-89, but not AG1478, inhibited 4-OHT-induced CREB phosphorylation (Fig. 6A). Furthermore, the effect of each inhibitor on 4-OHT-induced IGFBP-1 transcription was determined. Consistent with reduced CREB phosphorylation after pretreatment with PKA inhibitor, 4-OHT-induced IGFBP-1 transcription was also reduced (Fig. 6B). These results indicated that GPER1-mediated IGFBP-1 transcription dependent on the phosphorylation of CREB by PKA.
Fig. 6.
4-OHT-induced IGFBP-1 transcription is mediated by PKA in MCF-7 cells. (A) Immunoblot analysis of CREB phosphorylation in PKA-selective inhibitor (H-89) and EGFR-selective inhibitor (AG-1478) followed by 4-OHT treatment for 2h. (B) Relative IGFBP-1 gene transcription determined by quantitative real-time PCR in 20 μM H-89 and 20 μM AG1478 treated MCF-7 cells after 24 hours of treatment with 1μM 4-OHT. *, p<0.05 compared to control. The results are representative of three independent experiments, and the error bars are SEM.
3.7. Inhibition of IGF-1-stimulated cell signaling after 4-OHT treatment required IGFBP-1
Data thus far demonstrate that conditioned medium collected from MCF-7 cells treated with 4-OHT for 24 hours inhibited IGF-1 stimulation. In addition, IGFBP-1 transcription and extracellular accumulation were observed after treatment with 4-OHT for 24 hours. To determine if extracellular IGFBP-1 inhibited IGF-1 stimulation in MCF-7 cells, both antibody-mediated neutralization and knockdown of IGFBP-1 were performed. Antibody-mediated neutralization of IGFBP-1 (10 ng/mL, ~66.6 pM) rescued IGF-1 stimulation in 4-OHT-treated MCF-7 cells (Fig. 7A, lane 3 compared to lane 4). A magnitude higher dose of neutralizing antibody (100 ng/mL) had a similar effect on IGF-1 stimulation while a magnitude lower concentration (1 ng/mL) was slightly less effective compare to 10 ng/mL (data not shown). Addition of control antibody (10 ng/mL for 2h) did not alter the previously observed phosphorylation of IGF-1R-mediated signal transduction components after IGF-1 stimulation (Fig. 7A, lane 4). However, the neutralizing antibody did not result in a complete reversal of inhibition suggesting that other extracellular factors in the conditioned medium from 4-OHT-treated cells may modulate IGF-1 signaling. IGFBP-1 knockdown significantly reduced IGFBP-1 expression in MCF-7 cells (Fig. 7B) and reduced the ability of 4-OHT treatment to inhibit IGF-1-dependent cell signaling (Fig. 7C). These results support a role for IGFBP-1 in the inhibition of IGF-1 stimulation in 4-OHT-treated breast cancer cells. One potential mechanism of the observed inhibition is sequestration of IGF-1 via binding to IGFBP-1. It has been reported that an equimolar or near equimolar concentration for the IGF and IGFBP is required for inhibition by sequestration (Butt et al., 1999; Cullen et al., 1990; Figueroa et al., 1993; Firth and Baxter, 2002; Firth et al., 2002; Jones and Clemmons, 1995; Kijimura et al., 2005, Yee et al., 1994). To determine if the observed induction of IGFBP-1 results in a sufficient increase in IGFBP-1 molarity to achieve inhibition IGF-1 signaling by sequestration, the concentration of IGFBP-1 in conditioned medium was determined by ELISA. The concentration of IGFBP-1 in conditioned medium from MCF-7 cells was significantly increased from 29.4 (+/− 1.9) pg/mL to 81.8 (+/− 4.6) pg/mL for vehicle and 1 μM 4-OHT-treated cells, respectively. 81.8 pg/mL of IGFBP-1 is approximately 2.7 pM and the IGF-1 concentration in our studies is approximately 7 nM. These results do not support sequestration of IGF-1 by IGFBP-1 as the mechanism of action in this system as such a mechanism would require a higher molarity for IGFBP-1. However, siRNA knockdown of IGFBP-1 and antibody neutralization experiments demonstrate that IGFBP-1 is a critical component of the observed inhibition of IGF-1-dependent cell signaling after 4-OHT treatment in breast cancer cells.
Fig. 7.
Antibody-mediated IGFBP-1 neutralization or IGFBP-1 knockdown reverses the inhibition of IGF-1 stimulation in 4-OHT-treated MCF-7 cells. (A) Two hours prior to IGF-1 stimulation (15 minutes), control IgG or IGFBP-1 neutralizing antibody (10 ng/mL each) were added to MCF-7 cells treated with vehicle (V) or 4-OHT-treated for 24 hours. Total AKT and phospho-AKT levels were determined by immunoblot. (B) siRNA mediated reduction of IGFBP-1 protein expression. (C) After transfection with IGFBP-1 siRNAs or non-targeting control (NT), MCF-7 cells were treated with 1μM 4-OHT for 24 hours followed by 50 ng/mL IGF-1 stimulation for 15 minutes followed by the immunoblot analysis of p-IGF-1R (Y1135/1136), p-AKT (S473) and p-ERK1/2. (D) Extracellular IGFBP-1 protein concentration determined by ELISA. *, p<0.05 compared to vehicle, and #, p<0.05 compared to IGFBP-1 blocking Ab-treated. The results are representative of three independent experiments, and the error bars are SEM.
Discussion
In an effort to identify potential extracellular components of tamoxifen action in breast cancer cells, the expression and accumulation of IGFBP-1 in 4-OHT-treated breast cancer cell cultures was identified. Data herein show that GPER1 and CREB mediate the observed IGFBP-1 induction after 4-OHT treatment and this effect is independent of ERα. Although the concentration of extracellular IGFBP-1 is not likely sufficient for the inhibition of IGF-1-dependent cell signaling by IGF-1 sequestration, IGFBP-1 knockdown demonstrated that IGFBP-1 is, at least in part, required for the inhibition of IGF-1-dependent cell signaling after 4-OHT treatment. Furthermore, antibody-mediated neutralization experiments support a role for extracellular IGFBP-1 in the observed inhibition. Taken together, these data suggest that GPER1-mediates the accumulation of extracellular IGFBP-1 in 4-OHT-treated breast cancer cells and that this accumulation of IGFBP-1 results in the inhibition of IGF-1-dependent cell signaling.
E2-dependent proliferation of ERα-positive breast cancer cells by activation of ERα has been well studied and ERα antagonism is a critical component of tamoxifen action in this cell type (Frasor et al., 2004; Johnston et al., 1992; Ring and Dowsett, 2004; Sharma et al., 2006). In addition to E2-dependent proliferation mediated by ERα, breast cancer cell proliferation is also induced by IGF-1R activation (Lee et al., 1997). Several mechanisms of crosstalk between ERα and IGF-1R have been identified. For example, E2 induces IGF-1R expression and activation in ERα-positive breast cancer cells (Huynh et al., 1996; Lee et al., 1997; Song et al., 2007). Also, IGF-1 stimulates ligand-independent ERα-mediated transcription that may lead to cell proliferation (Becker et al., 2011; Castano et al., 1997). Less understood is the modulation of ERα/IGF-1R crosstalk after tamoxifen treatment in ERα-positive breast cancer cells. However, there is evidence that 4-OHT inhibits E2-stimulated IGF-1R expression (Lee et al., 1999). Data herein provide evidence for a novel mechanism of action by which 4-OHT treatment increases extracellular IGFBP-1 to inhibit IGF-1 stimulation in breast cancer cells. These findings contribute to the understanding of the complex crosstalk mechanisms between E2 and IGF-1 signaling in breast cancer cells.
Activated CREB induces IGFBP-1 transcription via cAMP response element (CRE) in the IGFBP-1 promoter (Frost et al., 2000; Sugawara et al., 2000). More recently, it has been shown that 4-OHT treatment activates CREB downstream of GPER1 in rat astrocytes (Karki et al., 2013). Based on these published results, it was hypothesized that CREB activation downstream of GPER1 induced IGFBP-1 expression in 4-OHT-treated MCF-7 cells. In this submission, CREB knockdown experiments demonstrated the involvement of CREB in the observed IGFBP-1 expression after 4-OHT treatment. This result suggested that CREB activation in this context inhibits breast cancer cell proliferation by inhibiting growth factor signaling. Although CREB binds directly to the IGFBP-1 promoter in other cell types (Suwanichkul et al., 1993), CREB binding to the IGFBP-1 promoter was not determined in breast cancer cells. Future experimentation will need to be performed to determine the molecular mechanisms IGFBP-1 transcription in the presence of 4-OHT with emphasis on CREB and other transcription factors such as FoxO1 that has been shown to modulate IGFBP-1 in other cell types (Durham et al., 1999; Kim et al., 2003).
GPER1 activation in MCF-7 cells induces p21 expression in a p53-dependent manner (Ariazi et al., 2010; Wei et al., 2014) and it has been shown that p-CREB potentiates p53 transcriptional activity (Giebler et al., 2000). Taken together, these results suggest that CREB activation downstream of GPER1 may play an inhibitory role in ERα-positive breast cancer cells. In contrast, CREB activation induces proliferation in tamoxifen-resistant breast cancer cells and other cancer cell types (Catalano et al., 2014; Daniel et al., 2014; Nguyen et al., 2014; Sofi et al., 2003). Furthermore, increased CREB expression in breast tumors is associated with poor prognosis, shorter survival and higher risk of metastasis (Chhabra et al., 2007; Fan et al., 2012). Additional experimentation will be required to gain a thorough understanding with regard to the role of CREB during tamoxifen treatment.
This study is the first to our knowledge to characterize the mechanism of endogenous IGFBP1 production in breast cancer cells and the first to identify extracellular factors that mediate tamoxifen action. Interestingly, elevated IGFBP-1 levels are observed in sera obtained from breast cancer patients treated with tamoxifen (Bonanni et al., 2001; Helle et al., 1996; Johansson et al., 2008; Lahti et al., 1994; Lonning et al., 1992), but the mechanism for the observed increase in IGFBP-1 has not been studied. Besides IGFBP-1, other IGFBPs may be induced by tamoxifen treatment in our experiments and function to modulate IGF-1-dependent cell signaling. The induction of IGFBP-3 protein in 4-OHT-treated MCF-7 cells has been shown previously (Pratt and Pollak, 1993), however, IGFBP-4 expression reduced by 4-OHT in MCF-7 cells (Pratt and Pollak, 1993; Qin et al., 1999). Further studies aimed at understanding the role of IGFBPs (and other secreted proteins) during tamoxifen treatment will need to be completed to gain a thorough understanding of extracellular mechanisms of tamoxifen action.
IGFBPs can modulate cells via both intracellular and extracellular mechanisms and previous studies on IGFBP function have demonstrated various mechanisms of action other than classical, IGF-1 sequestration (Figueroa et al., 1993; Firth and Baxter, 2002; Lee et al., 1997; Murphy, 1998). While the data presented here do not support sequestration of IGF-1 by IGFBP-1 as a mechanism for the observed modulation of IGF-1-depdendent signaling, IGFBP-1 knockdown and antibody neutralization experiments demonstrate that IGFBP-1 is part of the mechanism of inhibition. The observed inhibition of IGF-1-dependent cell signaling in these studies could also be modulated by other IGFBPs or secreted factors that have not yet been identified. The discovery of an alternate mechanism if IGFBP-1-mediated inhibition and the identification of additional secreted factors involved in the observed inhibition will require further investigation.
Sustained GPER1 activation results in the development of tamoxifen resistance using in vitro models (Ignatov et al., 2010; Mo et al., 2013) suggesting a role for GPER1 in tamoxifen action and the development of tamoxifen resistance. Interestingly, tamoxifen-resistant breast cancer cells have decreased IGF-1R expression and an increased dependence on other growth factors such as EGF (Fagan et al., 2012; Fan et al., 2007). In the context of the present data, it is hypothesized that increased extracellular IGFBP-1 downstream of GPER1 activation during tamoxifen treatment inhibits IGF-1-dependent cell signaling and provides selective pressure in favor of breast cancer cells that are not dependent on IGF-1R signaling for survival and proliferation. Additional studies are required to determine if increased extracellular IGFBP-1 accumulation may be involved in the development of tamoxifen resistance.
Supplementary Material
IGF-1-dependent cell signaling is inhibited in tamoxifen-treated breast cancer cells
Tamoxifen induces the accumulation of extracellular IGFBP-1
GPER1 is required for induction of IGFBP-1 in tamoxifen-treated breast cancer cells
Acknowledgements
This work was funded by the NM-INBRE grant from the National Institutes of Health (8 P20 GM103451).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have nothing to disclose.
References
- Ariazi EA, Brailoiu E, Yerrum S, Shupp HA, Slifker MJ, Cunliffe HE, Black MA, Donato AL, Arterburn JB, Oprea TI, Prossnitz ER, Dun NJ, Jordan VC. The G Protein-Coupled Receptor GPR30 Inhibits Proliferation of Estrogen Receptor-Positive Breast Cancer Cells. Cancer Res. 2010;70:1184–1194. doi: 10.1158/0008-5472.CAN-09-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga CL, Kitten LJ, Coronado EB, Jacobs S, Kull FC, Allred DC, Osborne CK. Blockade of the type-I somatomedin receptor inhibits growth of human-breast cancer-cells in athymic mice. J. Clin. Invest. 1989;84:1418–1423. doi: 10.1172/JCI114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga CL. Interference of the IGF system as a strategy to inhibit breast-cancer growth. Breast Cancer Res. Treat. 1992;22:101–106. doi: 10.1007/BF01833338. [DOI] [PubMed] [Google Scholar]
- Baxter RC. Insulin-like growth factor binding protein-3 (IGFBP-3): Novel ligands mediate unexpected functions. J. Cell Commun. Signal. 2013;7:179–189. doi: 10.1007/s12079-013-0203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MA, Ibrahim YH, Cui X, Lee AV, Yee D. The IGF Pathway Regulates ER alpha through a S6K1-Dependent Mechanism in Breast Cancer Cells. Mol. Endocrinol. 2011;25:516–528. doi: 10.1210/me.2010-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanni B, Johansson H, Gandini S, Guerrieri-Gonzaga A, Torrisi R, Sandri MT, Cazzaniga M, Mora S, Robertson C, Lien EA, Decensi A. Effect of low dose tamoxifen on the insulin-like growth factor system in healthy women. Breast Cancer Res. Treat. 2001;69:21–27. doi: 10.1023/a:1012241505717. [DOI] [PubMed] [Google Scholar]
- Burgaud JL, Resnicoff M, Baserga R. Mutant IGF-I receptors as dominant negatives for growth and transformation. Biochem. Biophys. Res. Commun. 1995;214:475–481. doi: 10.1006/bbrc.1995.2311. [DOI] [PubMed] [Google Scholar]
- Butt AJ, Firth SM, Baxter RC. The IGF axis and programmed cell death. Immunol. Cell Biol. 1999;77:256–262. doi: 10.1046/j.1440-1711.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- Castano E, Vorojeikina DP, Notides AC. Phosphorylation of serine-167 on the human oestrogen receptor is important for oestrogen response element binding and transcriptional activation. Biochem. J. 1997;326:149–157. doi: 10.1042/bj3260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano S, Giordano C, Panza S, Chemi F, Bonofiglio D, Lanzino M, Rizza P, Romeo F, Fuqua SAW, Maggiolini M, Ando S, Barone I. Tamoxifen through GPER upregulates aromatase expression: a novel mechanism sustaining tamoxifen-resistant breast cancer cell growth. Breast Cancer Res. Treat. 2014;146:273–285. doi: 10.1007/s10549-014-3017-4. [DOI] [PubMed] [Google Scholar]
- Chhabra A, Fernando H, Watkins G, Mansel RE, Jiang WG. Expression of transcription factor CREB1 in human breast cancer and its correlation with prognosis. Oncol. Rep. 2007;18:953–958. [PubMed] [Google Scholar]
- Chrivia JC, Kwok RPS, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated creb binds specifically to the nuclear-protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Cullen KJ, Yee D, Sly WS, Perdue J, Hampton B, Lippman ME, Rosen N. Insulin-like growth-factor receptor expression and function in human-breast cancer. Cancer Res. 1990;50:48–53. [PubMed] [Google Scholar]
- Daniel P, Filiz G, Brown DV, Hollande F, Gonzales M, D'Abaco G, Papalexis N, Phillips WA, Malaterre J, Ramsay RG, Mantamadiotis T. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis. 2014;3:1–10. doi: 10.1038/oncsis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge HJM, Fehrmann RSN, de Bont E, Hofstra RMW, Gerbens F, Kamps WA, de Vries EGE, van der Zee AGJ, Meerman GJT, ter Elst A. Evidence Based Selection of Housekeeping Genes. Plos One. 2007;2:e898. doi: 10.1371/journal.pone.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Feng J, Broaddus RR. The novel estrogen-induced gene EIG121 regulates autophagy and promotes cell survival under stress. Cell Death Disease. 2010;e32:1–12. doi: 10.1038/cddis.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi SI, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J. Steroid Biochem. Mol. Biol. 2011;127:358–366. doi: 10.1016/j.jsbmb.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham SK, Suwanichkul A, Scheimann AO, Yee D, Jackson JG, Barr FG, Powell DR. FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology. 1999;140:3140–3146. doi: 10.1210/endo.140.7.6856. [DOI] [PubMed] [Google Scholar]
- Fagan DH, Uselman RR, Sachdev D, Yee D. Acquired resistance to tamoxifen is associated with loss of the type I Insulin-like growth factor receptor: implications for breast cancer treatment. Cancer Res. 2012;72:3372–3380. doi: 10.1158/0008-5472.CAN-12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CF, Mao XY, Wang EH. Elevated p-CREB-2 (ser 245) expression is potentially associated with carcinogenesis and development of breast carcinoma. Mol. Med. Rep. 2012;5:357–362. doi: 10.3892/mmr.2011.657. [DOI] [PubMed] [Google Scholar]
- Fan P, Wang JP, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–1360. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- Figueroa JA, Sharma J, Jackson JG, McDermott MJ, Hilsenbeck SG, Yee D. Recombinant insulin-like growth-factor binding protein-1 inhibits IGF-I, serum, and estrogen-dependent growth of MCF-7 human breast-cancer cells. J. Cell Physiol. 1993;157:229–236. doi: 10.1002/jcp.1041570204. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-induced activation of ERK-1 and ERK-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR, Bland KI. Action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002;6:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Firth SM, Mcdougall F, Mclachlan AJ, Baxter RC. Impaired blockade of Insulin-like gowth factor I (IGF-I)-induced hypoglycemia by IGF binding protein-3 analog with reduced ternary complex-forming ability. Endocrinology. 2002;143:1669–1676. doi: 10.1210/endo.143.5.8764. [DOI] [PubMed] [Google Scholar]
- Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS. Selective estrogen receptor modulators: Discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res. 2004;64:1522–1533. doi: 10.1158/0008-5472.can-03-3326. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Stimulation of insulin-like growth factor finding protein-1 synthesis by interleukin-1 beta: Requirement of the mitogen-activated protein kinase pathway. Endocrinology. 2000;141:3156–3164. doi: 10.1210/endo.141.9.7641. [DOI] [PubMed] [Google Scholar]
- Galiano RD, Zhao LL, Clemmons DR, Roth SI, Lin X, Mustoe TA. Interaction between the insulin-like growth factor family and the integrin receptor family in tissue repair processes. Evidence in a rabbit ear dermal ulcer model. J. Clin. Invest. 1996;98:2462–2468. doi: 10.1172/JCI119064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebler HA, Lemasson I, Nyborg JK. p53 recruitment of CREB binding protein mediated through phosphorylated CREB: a novel pathway of tumor suppressor regulation. Mol. Cell Biol. 2000;20:4849–4858. doi: 10.1128/mcb.20.13.4849-4858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronbaek H, Tanos V, Meirow D, Peretz T, Raz I, Flyvbjerg A. Effects of tamoxifen on insulin-like growth factors, IGF binding proteins and IGFBP-3 proteolysis in breast cancer patients. Anticancer Res. 2003;23:2815–2820. [PubMed] [Google Scholar]
- Guvakova MA, Surmacz E. Tamoxifen interferes with the insulin-like growth factor I receptor (IGF-IR) signaling pathway in breast cancer cells. Cancer Res. 1997;57:2606–2610. [PubMed] [Google Scholar]
- Han JZ, Townes-Anderson E. Cell Specific Post-Translational Processing of Pikachurin, A Protein Involved in Retinal Synaptogenesis. Plos One. 2012;7:e50552. doi: 10.1371/journal.pone.0050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle SI, Holly JMP, Tally M, Hall K, Vander Stappen J, Lonning PE. Influence of treatment with tamoxifen and change in tumor burden on the IGF-system in breast cancer patients. Int. J. Cancer. 1996;69:335–339. doi: 10.1002/(SICI)1097-0215(19960822)69:4<335::AID-IJC17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Huynh H, Nickerson T, Pollak M, Yang XF. Regulation of insulin-like growth factor I receptor expression by the pure antiestrogen ICI 182780. Clin. Cancer Res. 1996;2:2037–2042. [PubMed] [Google Scholar]
- Ignatov A, Ignatov T, Roessner A, Costa SD, Kalinski T. Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Res. Treat. 2010;123:87–96. doi: 10.1007/s10549-009-0624-6. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao YP, Xu JQ, Thun MJ. Cancer statistics, 2009. CA. Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Johansson H, Gandini S, Guerrieri-Gonzaga A, Iodice S, Ruscica M, Bonanni B, Gulisano M, Magni P, Formelli F, Decensi A. Effect of Fenretinide and Low-Dose Tamoxifen on Insulin Sensitivity in Premenopausal Women at High Risk for Breast Cancer. Cancer Res. 2008;68:9512–9518. doi: 10.1158/0008-5472.CAN-08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SRD, Dowsett M, Smith IE. Towards a molecular-basis for tamoxifen resistance in breast-cancer. Ann. Oncol. 1992;3:503–511. doi: 10.1093/oxfordjournals.annonc.a058251. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Jones JI, Doerr ME, Clemmons DR. Cell migration: interactions among integrins, IGFs and IGFBPs. Prog. Growth Factor Res. 1995;6:319–327. doi: 10.1016/0955-2235(95)00015-1. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Aida K, Duan C. Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. Proc. Natl. Acad. Sci. USA. 2005;102:1240–1245. doi: 10.1073/pnas.0407443102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Webb A, Smith K, Lee K, Son DS, Aschner M, Lee E. cAMP Response Element-binding Protein (CREB) and Nuclear Factor kappa B Mediate the Tamoxifen-induced Up-regulation of Glutamate Transporter 1 (GLT-1) in Rat Astrocytes. J. Biol. Chem. 2013;288:28975–28986. doi: 10.1074/jbc.M113.483826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG. Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol. Reprod. 2003;68:24–30. doi: 10.1095/biolreprod.102.009316. [DOI] [PubMed] [Google Scholar]
- Kwok RPS, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SGE, Green MR, Goodman RH. Nuclear-protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- Lahti EI, Knip M, Laatikainen TJ. Plasma insulin-like growth-factor-I and its binding protein-1 and protein-3 in postmenopausal patients with breast-cancer receiving long-term tamoxifen. Cancer. 1994;74:618–624. doi: 10.1002/1097-0142(19940715)74:2<618::aid-cncr2820740213>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lee AV, Jackson JG, Gooch JL, Hilsenbeck SG, Coronado-Heinsohn E, Osborne CK, Yee D. Enhancement of insulin-like growth factor signaling in human breast cancer: Estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol. Endocrin. 1999;13:787–796. doi: 10.1210/mend.13.5.0274. [DOI] [PubMed] [Google Scholar]
- Lee AV, Weng CN, Jackson JG, Yee D. Activation of estrogen receptor-mediated gene transcription by IGF-I in human breast cancer cells. J. Endocrinol. 1997;152:39–47. doi: 10.1677/joe.0.1520039. [DOI] [PubMed] [Google Scholar]
- Li RS, Pourpak A, Morris SW. Inhibition of the Insulin-like Growth Factor-1 Receptor (IGF1R) Tyrosine Kinase as a Novel Cancer Therapy Approach. J. Med. Chem. 2009;52:4981–5004. doi: 10.1021/jm9002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonning PE, Hall K, Aakvaag A, Lien EA. Influence of tamoxifen on plasma-levels of insulin-like growth factor-I and insulin-like growth-factor binding protein-I in breast-cancer patients. Cancer Res. 1992;52:4719–4723. [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17 beta-estradiol and phytoestrogens in breast cancer cells. J. Biol. Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell. Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Mo ZQ, Liu MR, Yang FF, Luo HJ, Li ZH, Tu G, Yang GL. GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer. Breast Cancer Res. 2013;15:R114. doi: 10.1186/bcr3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TTP, Lim SC, Kim YM, Kang KW. Aromatase induction in tamoxifen-resistant breast cancer: Role of phosphoinositide 3-kinase-dependent CREB activation. Cancer Lett. 2014;351:91–99. doi: 10.1016/j.canlet.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Oh Y, Muller HL, Lamsona G, Rosenfeld RG. Insulin-like growth factor (1GF)-independent action of IGF-binding protein-3 in Hs578T human breast cancer cells. J. Biol. Chem. 1993;268:14964–14971. [PubMed] [Google Scholar]
- Osborne CK, Boldt DH, Clark GM, Trent JM. Effects of tamoxifen on human-breast cancer cell-cycle kinetics-accumulation of cells in early G1-phase. Cancer Res. 1983;43:3583–3585. [PubMed] [Google Scholar]
- Pandey DP, Lappano R, Albanito L, Madeo A, Maggiolini M, Picard D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. Embo J. 2009;28:523–532. doi: 10.1038/emboj.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt SE, Pollak MN. Estrogen and antiestrogen modulation of MCF7 human breast cancer cell proliferation is associated with specific alterations in accumulation of insulin-like growth factor-binding proteins in conditioned media. Cancer Res. 1993;53:5193–5198. [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GrPR30. Annual Rev. Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol. Cell. Endocrinol. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupo M, Pisano A, Lappano R, Santolla MF, De Francesco EM, Abonante S, Rosano C, Maggiolini M. Bisphenol A Induces Gene Expression Changes and Proliferative Effects through GPER in Breast Cancer Cells and Cancer-Associated Fibroblasts. Environ. Health Perspect. 2012;120:1177–1182. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Singh P, Safe S. Transcriptional activation of insulin-like growth factor-binding protein-4 by 17beta-estradiol in MCF-7 cells: role of estrogen receptor-Sp1 complexes. Endocrinology. 1999;140:2501–2508. doi: 10.1210/endo.140.6.6751. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr. Relat. Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Yee D. The IGF system and breast cancer. Endocr. Relat. Cancer. 2001;8:197–209. doi: 10.1677/erc.0.0080197. [DOI] [PubMed] [Google Scholar]
- Sharma D, Saxena NK, Davidson NE, Vertino PM. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: Tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer Res. 2006;66:6370–6378. doi: 10.1158/0008-5472.CAN-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofi M, Young MJ, Papamakarios T, Simpson ER, Clyne CD. Role of CRE-binding protein (CREB) in aromatase expression in breast adipose. Breast Cancer Res. Treat. 2003;79:399–407. doi: 10.1023/a:1024038632570. [DOI] [PubMed] [Google Scholar]
- Song RXD, Zhang ZG, Chen YC, Bao YD, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–4101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara J, Tazuke SI, F-Suen L, Powell DR, Kaper F, Giaccia AJ, Giudice LC. Regulation of insulin-like growth factor-binding protein 1 by hypoxia and 3',5'-cyclic adenosine monophosphate is additive in HepG2 cells. J. Clin. Endocrinol. Metab. 2000;85:3821–3827. doi: 10.1210/jcem.85.10.6866. [DOI] [PubMed] [Google Scholar]
- Suwanichkul A, DePaolis LA, Lee PD, Powell DR. Identification of a promoter element which participates in cAMP-stimulated expression of human insulin-like growth factor-binding protein-1. J. Biol. Chem. 1993;268:9730–9736. [PubMed] [Google Scholar]
- Tang H, Zhang Q, Yang L, Dong Y, Khan M, Yang F, Brann DW, Wang R. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol. Cell. Endocrinol. 2014;389:92–98. doi: 10.1016/j.mce.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol. Cell Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Ando S, Maggiolini M. 17 beta-Estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the G protein-coupled receptor GPR30. Mol. Pharmacol. 2006;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- Ween MP, Lokman NA, Hoffmann P, Rodgers RJ, Ricciardelli C, Oehler MK. Transforming growth factor-beta-induced protein secreted by peritoneal cells increases the metastatic potential of ovarian cancer cells. Int. J. Cancer. 2011;128:1570–1584. doi: 10.1002/ijc.25494. [DOI] [PubMed] [Google Scholar]
- Wei W, Chen ZJ, Zhang KS, Yang XL, Wu YM, Chen XH, Huang HB, Liu HL, Cai SH, Du J, Wang HS. The activation of G protein-coupled receptor 30 (GPR30) inhibits proliferation of estrogen receptor-negative breast cancer cells in vitro and in vivo. Cell Death Disease. 2014;5:e1428–e1428. doi: 10.1038/cddis.2014.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE Y. Regulation of insulin-like growth factor signaling by metformin in endometrial cancer cells. Oncol. Lett. 2014;8:1993–1999. doi: 10.3892/ol.2014.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Kornhauser JM, Xia ZG, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol. Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee D, Jackson JG, Kozelsky TW, Figueroa JA. Insulin-like growth-factor binding protein-1 expression inhibits insulin-like growth factor-i action in MCF-7 breast-cancer cells. Cell Growth Differ. 1994;5:73–77. [PubMed] [Google Scholar]
- Yee D, Paik SY, Lebovic GS, Marcus RR, Favoni RE, Cullen KJ, Lippman ME, Rosen N. Analysis of insulin-like growth factor-I gene-expression in malignancy-evidence for a paracrine role in human-breast cancer. Mol. Endocrinol. 1989;3:509–517. doi: 10.1210/mend-3-3-509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.