Abstract
Objective
To assess intrinsic and extrinsic risk factors in the development of posterior glottic stenosis (PGS) in intubated patients.
Methods
PGS patients diagnosed between September 2012 – May 2014 at three tertiary care university hospitals were included. Patient demographics, comorbidities, duration of intubation, ETT size, and indication for intubation were recorded. PGS patients were compared to control patients represented by patients intubated in intensive care units (ICU).
Results
Thirty-six PGS patients were identified. After exclusion, 28 PGS patients (14 male, 14 female) and 112 (65 male, 47 female) controls were studied. Multivariate analysis demonstrated ischemia (p<0.05), diabetes (p<0.01), length of intubation (p<0.01) were significant risk factors for the development of PGS. 14/14 (100%) males were intubated with a size 8 or larger ETT compared to 47/65 (72.3%) male controls (p<0.05). PGS (p<0.01), length of intubation (p<0.001), and obstructive sleep apnea (p<0.05) were significant risk factors for tracheostomy.
Conclusion
Duration of intubation, ischemia, diabetes mellitus, and large ETT size (8 or greater) in males were significant risk factors for the development of PGS. Reducing the use of size 8 ETTs and earlier planned tracheostomy in high-risk patients may reduce the incidence of PGS and improve ICU safety.
Keywords: laryngotracheal stenosis, fibrosis, intubation, hospital safety, tracheostomy
Introduction
Posterior Glottic Stenosis (PGS) is a life-threatening condition in which the vocal folds are fixed in a midline position. This results in a severely narrowed glottic airway with limited vocal fold abduction and subsequent ventilatory collapse. PGS is most frequently caused by intubation injury to the interarytenoid mucosa and underlying cartilage ultimately resulting in interarytenoid fibrosis, contracture and/or cricoarytenoid joint fixation (1–4). In Whited‘s 1984 prospective study of 200 intubated patients the incidence of PGS was 5% in patients intubated between 5–10 days and 12% in those intubated between 11–24 days (5). Other described etiologies responsible for PGS include radiation changes, systemic autoimmune disease (specifically sarcoidosis and rheumatoid arthritis), external trauma, and caustic ingestion (3, 6–7). At times PGS can present in combination with subglottic stenosis, making it a more challenging disease process to treat.
PGS may be considered an abnormal wound healing response of the posterior commissure. With an ultrastructural relationship between epithelium and cartilaginous suprastructure mirroring subglottic and tracheal anatomy, the posterior commissure is comprised of thin mucosa overlying cartilage. The endotracheal tube (ETT) places pressure at the posterior commissure as it bends in a caudal direction in the around the tongue base to allow the distal end to sit coaxial within the proximal trachea, positioning helped by the balloon cuff (4). In PGS, this pressure results in mucosal ulceration, prolonged inflammation, granulation tissue, and fibrosis that ultimately contracts the arytenoids and often extends into the cricoarytenoid joint (Figure 1) (4, 6, 8–9). Bogdasarian proposed a classification system with an interarytenoid scar band with posterior sinus tract (Class I), limited motion of arytenoids (Class II), fixation of one cricoarytenoid joint (Class III), and ultimately fixation of both cricoarytenoid joints (Class IV) however, this classification system has not been assessed to be predictive of therapy or outcomes (2). More recently, Sandhu proposed a grading system based on cricoarytenoid joint mobility and interarytenoid granulation tissue versus mature scar showing fewer interventions in patients with granulation tissue (Grade III or lower) than those with mature scar (Grade IV) (10). At a cellular level, PGS is similar to the proliferative fibrotic process of laryngotracheal stenosis (11) yet distinct from the effects of vocal fold scar, with tissue loss and scar vocal fold fibroblasts that are less proliferative than normal (12).
Figure 1.
Laryngoscopic images capture the evolution of posterior glottic stenosis. Initial mucosal ulceration and inflammation (A) progresses to posterior laryngeal granulation tissue (B) that ultimately contracts the arytenoids forcing the vocal folds into a bilateral midline position (C).
PGS is challenging to successfully treat, with fibrosis within the small and complex laryngeal anatomy. While early steroid injection into the cricoarytenoid joint may be successful at reducing inflammation, there are currently no medical therapies that are successful at reversing fibrotic processes. Surgical therapy to excise or release the scar, including incision of interarytenoid scar and dilation of glottic stenosis has limited success at restoring laryngeal function and maintaining the delicate balance between voice, airway, and swallow. More frequently, surgical therapies disrupt that balance to create a functional airway, preserve swallow, and maintain tolerable voice. These procedures include posterior cricoid split, suture lateralization, posterior cordotomy with partial arytenoidectomy, and tracheostomy (2–4). The limitation of current therapies is evidenced by the myriad approaches and techniques described over the last 70 years (13). Iatrogenic PGS has a massive effect on respiratory function. Preventative efforts to preserve laryngeal function could have a significant impact on reducing associated morbidity.
Prevention is predicated on identification of predictive factors and has the potential to identify intubated patients at high risk for developing PGS. Other than Whited’s study that demonstrated an increasing incidence of PGS with increasing duration of intubation, there have been small case series published that have proposed risk factors to include endotracheal tube size, patient movement during intubation, multiple intubations over a short period of time, gastroesophageal reflux, diabetes mellitus, congestive heart failure, stroke, and local bacterial infections (3–5, 14–18). The purpose of this investigation is to evaluate risk factors for PGS with the goal of identifying predictors that could influence ICU and hospital management strategies to reduce its incidence in intubated patients. We hypothesize that conditions promoting ischemia such as hemorrhage, other conditions associated with poor perfusion, diabetes, large endotracheal tubes, in addition to length of intubation are associated with the development of PGS.
Methods
Study Design and Patients
This study was approved by the Institutional Review Boards at The Johns Hopkins University, Emory University, and Vanderbilt University. It was designed as a multi-institutional case-controlled study and matched PGS patients with intubated patients at a ratio of 1:4.
Subjects diagnosed with laryngeal stenosis (ICD-9: 478.74) between January 2012 and December 2014 were identified. For the purposes of this study, patients were defined as having PGS if they had at least unilateral cricoarytenoid joint fixation (Bogdasarian grade 3 or 4) and mature interarytenoid scar (Sandhu grade 4) following intubation as well as the clinical criteria of dyspnea (2, 10). Cricoarytenoid joint fixation was assessed during suspension microlaryngoscopy. Exclusion criteria for this study included patients with systemic disease potentially affecting the larynx (i.e. rheumatoid arthritis, sarcoidosis), patients with subglottic stenosis, patients who received radiation therapy, patients who had mobility in both vocal cords, patients with a potential neurogenic etiology and those with incomplete data sets.
The control cohort was defined as patients intubated in intensive care units (ICUs) for at least 24 hours from 11/15/2013 to 1/1/2014. Control patients were matched by ICU as a proxy for underlying disease and to include a diverse cohort of patients requiring intubation. Patients were obtained from different intensive care units (ICU) including the cardiovascular surgery ICU (CVSICU), neuro critical care unit (NCCU), surgical ICU (SICU), and medical ICU (MICU), and were recruited on 4 separate days at least 2 weeks apart with overlapping patients in the consecutive recruitment days excluded. The recruitment was performed on different days of the week each time to avoid the repeat inclusion of same specialties’ patients. Control patients were followed until they were extubated, underwent tracheostomy, or deceased to determine the total intubation period. None of the control patients were diagnosed with posterior glottic stenosis.
Data Collected and Outcomes
Patient characteristics extracted from medical records included age, gender, body mass index (BMI), comorbidities including hypertension, obstructive sleep apnea (OSA), gastro-esophageal reflux (GERD), diabetes mellitus (DM), ischemia, duration of intubation, ETT size, and smoking status. Comorbidities including GERD, DM, hypertension, and OSA were determined by identifying ICD-9 code on chart review. Patients were categorized as having ischemia if they had cardiopulmonary bypass surgery, myocardial infarction (MI), or hemorrhagic shock or anaphylactic shock (19–21). Patients were staged using the Charlson Comorbidity Index (CCI). Presence of a tracheostomy was the primary outcome of this study.
Statistical Analysis
Statistical analyses were performed with Stata 11.0 (Stata-Corp, College Station, TX). Mean and standard deviations were calculated for interval data, and frequencies and percentages were calculated for categorical data. We compared interval data between two groups using the Student t test for variables with a parametric distribution and the Mann-Whitney test for variables with a non-parametric distribution. Categorical data was compared using the Pearson Chi Square for variables with greater than 10 counts and Fisher exact tests for variables with less than 10 counts. Univariate and multivariate logistic regression analyses were performed to identify if any of the patient characteristics predicted the development of PGS. Multivariate analysis with clustered logistic regression was performed in order to match cases and controls based on ICU of origin. Univariate analyses that revealed a P value of < 0.20 were retained for the multivariate logistic regression analysis. Statistical significance for the remaining analyses was set at P < 0.05.
Results
A total of 36 patients with PGS were identified. Eight patients were excluded due to etiology other than intubation (n=4) or incomplete records (n=4). Of the 28 that met inclusion criteria, 8 were from the MICU, 8 were from the CVSICU, 8 were from the SICU, and 4 were from the NCCU. One-hundred-and-twelve control patients were identified matched by ICU, i.e. 8 MICU patients with PGS were matched with 32 controls taken from intubated patients in the MICU.
Univariate Analysis
Variables that were retained for multivariate analysis included length of intubation (Figure 2), diabetes, ischemia, hypertension, obstructive sleep apnea, and CCI. While endotracheal tube size was not significant for all patients, analysis was stratified by gender due to distinct shifts in distribution of ETT sizes used to intubate males and females (Figure 3A). There was a significant difference in ETT size in males with and without PGS (p=0.018), with 14 of 14 males (100%) with PGS versus 47 of 65 males (72%) without PGS intubated with size 8 ETT or larger (Figure 3B). Variables that were retained for multivariate analysis for the primary outcome of tracheostomy included PGS, length of intubation, hypertension, and obstructive sleep apnea. Figure 4A demonstrates the difference (p<0.001) for patients with PGS (22 of 28, 79%) and without PGS (29 of 112, 26%) who required a tracheostomy. Figure 4B demonstrates the percentage of patients with tracheostomy at last follow-up visit in the two groups (p<0.001).
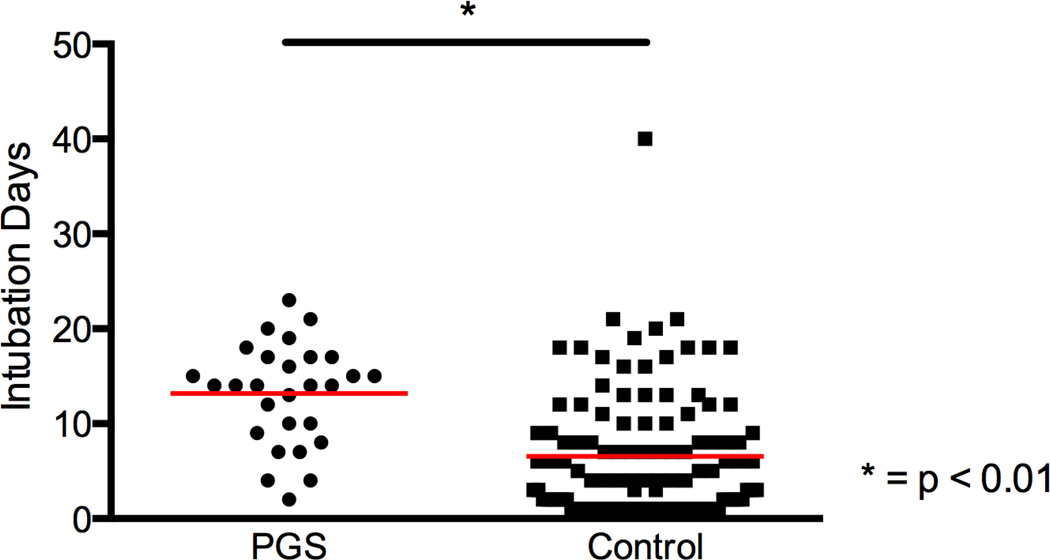
Figure 2.
Average length of intubation was significantly greater for posterior glottic stenosis (PGS) patients than controls. Dot plot graph with mean (red line) of days intubated in posterior glottic stenosis (PGS) and control cohorts.
Figure 3.
Comparison of endotracheal tube (ETT) size by gender and disease state. ETT distribution stratified by gender (A) demonstrating distinct shifts in distribution of ETT sizes used to intubate males and females. There was a significant difference between posterior glottic stenosis (PGS) and control patients in males intubated with size 8 ETT or larger (B).
Figure 4.
PGS patients have a significantly higher risk of a tracheostomy. The outcome of tracheostomy was significantly higher for posterior glottic stenosis (PGS) patients than in controls (A). Tracheostomy at last follow-up visit was also significantly higher in the PGS cohort (B).
Multivariate Analysis
Multivariate regression analysis was performed to determine independent predictors of posterior glottic stenosis (Table 1). Each additional day of intubation increased the odds of development of PGS by 21% (odds ratio [OR] 1.21; 95% CI 1.07 – 1.37; p = 0.003). Furthermore the comorbidity of diabetes was associated with an 888% increased odds (OR 8.88; 95% CI 2.27 – 34.72; p = 0.002) and an ischemic condition was associated with a 374% (OR 3.74; 95% CI 1.09 – 12.90; p = 0.037) increased odds of PGS.
Table 1.
Demographics and Risk Factors for Posterior Glottic Stenosis
| PGS (n = 28) |
Control (n = 112) |
Odds Ratio |
95% CI | P-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 55.68 (25 – 76) | 60.04(21 – 94) | 0.98 | 0.91 – 1.03 | NS |
| Gender | NS | ||||
| Male | 50.00 (14) | 58.04 (65) | |||
| Female | 50.00 (14) | 41.96 (47) | |||
| Race | NS | ||||
| Caucasian | 35.71 (10) | 27.68 (31) | |||
| African-American | 60.71 (17) | 64 (72) | |||
| Other | 3.57 (1) | 8.04 (9) | |||
| BMI | 31.92 (18.9 – 57.2) | 29.6 (16.0 – 70.1) | NS | ||
| Risk Factors | |||||
| Length of Intubation (days) | 13.18 (2 – 23) | 6.54 (1 – 40) | 1.21 | 1.07 – 1.37 | 0.003 |
| ETT Size ≥ 8 | 64.29 (18) | 52.68 (59) | 1.48 | 0.42 – 5.21 | NS |
| Diabetes | 53.57 (15) | 12.50 (14) | 8.88 | 2.27 – 34.72 | 0.002 |
| Ischemia | 53.57 (15) | 29.46 (33) | 3.74 | 1.09 – 12.90 | 0.037 |
| HTN | 57.14 (16) | 24.11 (27) | 3.38 | 0.93 – 12.30 | NS |
| OSA | 28.57 (8) | 10.71 (12) | 1.38 | 0.39 – 4.83 | NS |
| CCI | 3.85 (0 – 11) | 4.71 (0 – 12) | 0.79 | 0.54 – 1.18 | NS |
Values are shown as % (n) or mean (minimum – maximum range).
PGS = Posterior Glottic Stenosis; BMI = Body Mass Index. ETT = Endotracheal tube; HTN = Hypertension; OSA = Obstructive Sleep Apnea; CCI = Charlson Comorbidity Index.
Independent predictors of tracheostomy (Table 2) included PGS (OR 38.88; 95% CI 2.71 – 557.82; p = 0.007), length of intubation (OR 1.29; 95% CI 1.17 – 1.42; p < 0.001), and OSA (OR 3.60; 95% CI 1.16 – 11.23; p = 0.027). While the presence of hypertension was a statistical predictor of avoiding the outcome of tracheostomy, the clinical significance of this finding is unclear.
Table 2.
Predictors of Tracheostomy Outcome
| Tracheostomy (n = 51) |
No Tracheostomy (n = 89) |
Odds Ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| Comorbidities* | |||||
| PGS | 43.14 (22) | 6.74 (6) | 38.88 | 2.71 – 557.82 | 0.007 |
| Length of Intubation (days) | 13.16 (1 – 40) | 4.84 (1 – 21) | 1.29 | 1.17 – 1.42 | 0.000 |
| HTN | 21.57 (11) | 35.96 (32) | 0.02 | 0.00 – 0.32 | 0.006 |
| OSA | 21.57 (11) | 10.11 (9) | 3.60 | 1.16 – 11.23 | 0.027 |
Values are shown as % (n) or mean (minimum – maximum range).
PGS = Posterior Glottic Stenosis; HTN = Hypertension; OSA = Obstructive Sleep Apnea.
Discussion
This represents one of the largest studies on iatrogenic PGS and the first designed to determine independent predictors of intubation-related PGS. In it, we present data supporting diabetes, ischemic conditions, and length of intubation to be significant risk factors to develop PGS. Furthermore, males intubated with endotracheal tubes larger than 7.5 also demonstrated greater risk of developing PGS. Patients with iatrogenic PGS were at significantly greater risk (82%) of requiring a tracheostomy versus 26% in the control cohort of intubated patients (Figure 4A). PGS patients were also at significantly higher risk of tracheostomy at last follow-up (46% vs. 12%, Figure 4B). The other significant predictors for tracheostomy in this study were the length of intubation and the presence of obstructive sleep apnea.
When clustering PGS with other intubation-related diseases of the larynx and trachea like tracheal stenosis, common risk factors including diabetes, ETT size, and length of intubation consistently prove significant. Both Gelbard et al. and Wu et al. demonstrated diabetic patients to have a higher likelihood of developing tracheal stenosis following intubation and subsequent long-term tracheostomy dependence (22–23). Ischemia similarly contributes to poor wound healing. Patients with systemic hypo perfusion are at higher risk for endotracheal tube-related pressure injury to the laryngotracheal mucosa combining to result in cartilage exposure, perichondritis, granulation tissue, and pathologic wound healing leading to scar formation and contracture (1, 14, 16, 24). The risk of local pressure injury on the posterior glottis increases with the length of intubation and size of endotracheal tube (5, 16, 25). Whited’s prospective study of intubated patients demonstrated length of intubation to be a causal factor of PGS while Halum et al. showed ETT size greater than 7.5 to be a risk factor for tracheal stenosis in obese patients (5, 25). Bishop et al. demonstrated increasing ETT size increased ischemia in the larynx during 8 hours of intubation in an animal study (16, 25). The finding that males are at risk of PGS with large ETT intubation in this study adds to the literature on risks of large ETT associated with laryngotracheal injury. ETT was not a predictor of PGS in female patients in this study; in a larger cohort of females with PGS, ETT size might be revealed as a factor.
The preference for larger ETT sizes placed in the ICU, OR, and emergency department (ED) is justified by arguments including reduced airway resistance in patients with a large body habitus, improved ability to suction secretions through the tube, and allowance for therapeutic bronchoscopy while maintaining ventilation. Combined with a recent trend to use ETT equipped with subglottic suction, which have a larger outer diameter than traditional ETT, patients are at risk of long-term intubation with larger outer diameter ETT. While the range of intubation for patients in this study was 2–23 days, 19 of 28 were intubated for 11 days or greater, the category demonstrated by Whited to be at highest risk for developing PGS (5). Increasing awareness about morbidity associated with long-term intubation and large ETT size may contribute to changing patterns of practice.
Reducing the incidence of PGS is in keeping with health care goals of improving patient safety. While intrinsic risk factors to poor wound healing such as diabetes or ischemia may be harder to control, hospital ICUs can more easily target extrinsic factors and develop smart care plans for high-risk patients. Literature addressing extrinsic factors demonstrates that ETT size selection in male patients should consider height as a predictive measure of tracheal size rather than BMI (26 – 27). To this point, D’Anza showed a trend of smaller airways with increasing BMI and concluded that avoiding larger tubes in obese patients would reduce airway injury (27). Halum et al. did not show increased incidence of stenosis in obese patients intubated with a 7.5 or smaller sized ETT, suggesting size 7.5 ETT may be used safely in appropriate patients (25). Therefore, eliminating or significantly reducing the use of ETT larger than 7.5 and reducing the length of intubation in patients with diabetes or ischemic conditions has the potential to reduce the incidence of PGS. As otolaryngologists are primarily responsible for the treatment of PGS patients, involvement with hospital safety committees along with education of ED and ICU physicians can increase awareness about intubation-related laryngotracheal injury. This would improve communication with the physicians who manage the intubation (9). Other potential interventions include the development of nationally mandated ICU quality metrics to track the incidence of PGS development and the development of hospital-wide protocols to more rapidly convert from intubation to tracheostomy in high-risk patients. While earlier tracheostomy should be balanced with the risk of complications from the procedure, Young et al. demonstrated no difference in mortality, ICU length of stay, or acute complications in early versus late tracheostomy patients (28).
The factors that did not prove to be predictive of PGS are also interesting to note. GERD has been a speculated to be a risk factor in laryngotracheal stenosis, and our finding of no difference between groups was consistent with Gelbard et al’s results in laryngotracheal stenosis (22). Furthermore, comorbidities and obesity rates were not significantly different between groups. This may be due to the retrospective study design or the control group used in our study, which may not be representative of intubation patterns at non-tertiary care medical centers. While the control group may not be a perfect comparison, we did match controls with PGS by ICU as proxy for underlying disease and reason for intubation. In addition, data collection was limited by a number of patients developing PGS at outside medical institutions where the length of intubation or ETT size was unavailable for review, which excluded them from this study. The challenge of retrieving data also did not allow for assessment of factors such as the number of intubations or intubation attempts during the patient’s ICU stay. Similarly, the timing of tracheostomy in PGS patients was not assessed. We suspect the number of patients with intubation-related injury to the glottis leaving the hospital without a diagnosis is relatively high, resulting in progressive shortness of breath mandating tracheostomy after discharge. Post-intubation and tracheostomy complications like PGS often present insidiously, but once manifest they are difficult to ameliorate. Thus, frontline critical care physicians must be aware that future voice, swallowing, and airway rehabilitation depends on optimal early airway management (29).
Conclusion
PGS is a proliferative fibrotic process that results in arytenoid contracture with significant impairment of glottic airflow. In this study diabetes, ischemia, and length of intubation were found to be significant predictors of developing intubation-related PGS. Furthermore, males intubated with endotracheal tubes larger than 7.5 also demonstrated greater risk of developing PGS. Patients with PGS were at significantly increased risk of requiring a tracheostomy. Identification of high-risk intubated patients and initiation of tailored treatment plans including use of smaller ETT and shorter intubation duration has the potential to reduce the incidence of this devastating iatrogenic condition.
Footnotes
This work was accepted for presentation at the Annual Meeting of the American Broncho-Esophagological Association held April 22–23, 2015 in Boston, MA.
References
- 1.Whited RE. Posterior commissure stenosis post long-term intubation. Laryngoscope. 1983;93(10):1314–1318. doi: 10.1002/lary.1983.93.10.1314. [DOI] [PubMed] [Google Scholar]
- 2.Bogdasarian RS, Olson NR. Posterior glottic laryngeal stenosis. Otolaryngol Head Neck Surg. 1980;88(6):765–772. doi: 10.1177/019459988008800625. [DOI] [PubMed] [Google Scholar]
- 3.Gardner GM. Posterior glottic stenosis and bilateral vocal fold immobility: diagnosis and treatment. Otolaryngol Clin North Am. 2000;33(4):855–878. doi: 10.1016/s0030-6665(05)70248-6. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin B, Holinger LD. Laryngeal complications of endotracheal intubation. Ann Otol Rhinol Laryngol. 2008;117(9Supp):1–20. [Google Scholar]
- 5.Whited RE. A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope. 1984;94(3):367–377. doi: 10.1288/00005537-198403000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Roh JL. Prevention of posterior glottic stenosis by mitomycin C. Ann Otol Rhinol Laryngol. 2005;114(7):558–562. doi: 10.1177/000348940511400712. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery WW. Posterior and complete laryngeal (glottic) stenosis. Arch Otolaryngol. 1973;98:170–175. doi: 10.1001/archotol.1973.00780020178007. [DOI] [PubMed] [Google Scholar]
- 8.Courey MS, Bryant GL, Jr, Ossoff RH. Posterior glottic stenosis: a canine model. Ann Otol Rhinol Laryngol. 1998;107(10 Pt 1):839–846. doi: 10.1177/000348949810701005. [DOI] [PubMed] [Google Scholar]
- 9.Weymuller EA. Laryngeal injury from prolonged endotracheal intubation. Laryngoscope. 1988;98(8 Pt 2) Suppl 45:1–15. doi: 10.1288/00005537-198808001-00001. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu GS. Doctoral thesis. University College London; 2011. Management of adult benign laryngotracheal stenosis. [Google Scholar]
- 11.Namba D, Ma G, Ding D, Pandian V, Elisseeff JH, Horton MR, Hillel AT. Rapamycin inhibits laryngotracheal stenosis-derived fibroblast proliferation, metabolism, and function in vitro. Otolaryngol Head Neck Surg. doi: 10.1177/0194599815573708. [Published online Mar 9, 2015]. pii. 0194599815573708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jette ME, Hayer SD, Thibeault SL. Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope. 2013;123:738–745. doi: 10.1002/lary.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King BT. New and function-restoring operation for bilateral abductor cord paralysis: Preliminary report. JAMA. 1939;112:814–823. [Google Scholar]
- 14.Gould SJ, Howard S. The histopathology of the larynx in the neonate following endotracheal intubation. J Pathol. 1985;146:301–311. doi: 10.1002/path.1711460403. [DOI] [PubMed] [Google Scholar]
- 15.Weymuller EA, Bishop MJ, Fink BR, Hibbard AW, Spelman FA. Quantification of intralaryngeal pressure exerted by endotracheal tubes. Ann Otol Rhinol Laryngol. 1983;92:444–447. doi: 10.1177/000348948309200506. [DOI] [PubMed] [Google Scholar]
- 16.Bishop MJ. Mechanisms of laryngotracheal injury following prolonged tracheal intubation. Chest. 1989;96(1):185–186. doi: 10.1378/chest.96.1.185. [DOI] [PubMed] [Google Scholar]
- 17.Volpi D(1), Lin PT, Kuriloff DB, Kimmelman CP. Risk factors for intubation injury of the larynx. Ann Otol Rhinol Laryngol. 1987;96(6):684–686. doi: 10.1177/000348948709600614. [DOI] [PubMed] [Google Scholar]
- 18.Bishop MJ, Weymuller EA, Fink BR. Laryngeal effects of prolonged intubation. Anesth Analg. 1984;63:335–342. [PubMed] [Google Scholar]
- 19.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133–1138. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Dutton RP. Current concepts in hemorrhagic shock. Anesthesiology Clin. 2007;25:23–34. doi: 10.1016/j.atc.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Kounis NG, Soufras GD, Hahalis G. Anaphylactic shock: Kounis hypersensitivity-associated syndrome seems to be the primary cause. North Am J Med Sci. 2013;5:631–636. doi: 10.4103/1947-2714.122304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope. 2015;125:1137–1143. doi: 10.1002/lary.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Su ZZ, Hu LJ, et al. Analysis of the risk factors causing tracheal stenosis after tracheotomy for mechanical ventilation in 560 patients. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2007;42:839–842. [PubMed] [Google Scholar]
- 24.Gordin A, Chadha NK, Campisi P, Luginbuehl I, Taylor G, Forte V. An animal model for endotracheal tube-related laryngeal injury using hypoxic ventilation. Otolaryngol Head Neck Surg. 2011;144(2):247–251. doi: 10.1177/0194599810392894. [DOI] [PubMed] [Google Scholar]
- 25.Halum SL, Ting JY, Plowman EK, et al. A multi-institutional analysis of tracheostomy complications. Laryngoscope. 2012;122(1):38–45. doi: 10.1002/lary.22364. [DOI] [PubMed] [Google Scholar]
- 26.Karmakar A, Pate MB, Solowski NL, Postma GL, Weinberger PM. Tracheal Size Variability Is Associated With Sex: Implications for Endotracheal Tube Selection. Ann Otol Rhinol Laryngol. 2015 Feb;124(2):132–136. doi: 10.1177/0003489414549154. [DOI] [PubMed] [Google Scholar]
- 27.D’Anza B, Knight J, Greene JS. Does Body Mass Index Predict Tracheal Airway Size? Laryngoscope. 2015 May;125(5):1093–1097. doi: 10.1002/lary.24943. [DOI] [PubMed] [Google Scholar]
- 28.Young D, Harrison DA, Cuthbertson BH, et al. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–2129. doi: 10.1001/jama.2013.5154. [DOI] [PubMed] [Google Scholar]
- 29.Altman KW, Merati AL. Timing of tracheostomy. JAMA. 2013;310(12):1286. doi: 10.1001/jama.2013.277954. [DOI] [PubMed] [Google Scholar]