Abstract
Background
Inhibitors of Apoptosis Proteins (IAPs) are key regulators of apoptosis, and are frequently dysregulated in ovarian cancer. We hypothesized that blocking IAPs with birinapant would increase tumor cell death resulting in objective response for women with platinum-refractory and resistant ovarian cancer.
Methods
In this phase II CTEP-sponsored study, patients received birinapant 47mg/m2 on days 1, 8, 15 of 28-day cycles. Pharmacokinetics were obtained in cycle 1. Plasma, peripheral blood mononuclear cells (PBMC) and percutaneous tumor biopsies were collected prior to cycle 1, and after 6 weeks. The primary endpoint was objective response or progression-free survival lasting greater than 6 months in a mini-max design.
Results
Eleven patients received birinapant, after which accrual was terminated for lack of clinical benefit. Birinapant was well-tolerated, with predominantly grade 2 adverse events (AE) and one grade 3 lymphopenia. Pre-treatment biopsies and PBMCs were collected; paired post-treatment biopsies and PBMC were collected from 7 and 10 patients, respectively. There was consistent downregulation of cIAP1 in tumor (P=0.016) and PBMC (P<0.01). Pro-caspase3 also decreased in tumors (P=0.031) and PBMC (P<0.01); cleaved caspase3 co-localized with gamma-H2AX in tumors after birinapant exposure. Peripheral T- and B-cells decreased significantly post-treatment, but NK-cells did not (P=0.04, P=0.05, P=0.43 respectively).
Conclusion
Birinapant shows consistent target suppression in vivo, without single agent anti-tumor activity in this small population. Single agent pharmacodynamics were necessary to understand drug mechanism of action and set the stage for rational combination therapy. Preclinical studies are ongoing to identify optimal synergistic combinations for future clinical trials.
Keywords: Birinapant, SMAC mimetic, IAP, ovarian cancer, pharmacodynamics
Introduction
Advances in therapy over the past several decades have translated into small improvements in 5-year survival for women with ovarian cancer (1). The need for novel therapy targeting chemoresistant cells is underscored by the high rate of recurrence with conventional chemotherapy. Apoptosis, or programmed cell death, is a highly regulated process that is commonly dysregulated in cancer. Apoptosis signaling follows two pathways, intrinsic (mitochondrial) and extrinsic (death receptor-ligand) pathways. Intracellular damage triggers the intrinsic pathway, resulting in altered mitochondrial membrane permeability and subsequent release of cytochrome c and second mitochondria-derived activator of caspases (SMAC). The release of cytochrome c and SMAC promotes apoptosome formation and degradation of inhibitors of apoptosis proteins (IAP), respectively (2). Ultimately, apoptosis pathways converge on a common platform of cellular destruction mediated by caspases 3 and 7 activation.
Increasing evidence suggests that defective apoptosis is linked with chemotherapy resistance (3, 4). Thus, nodes in apoptotic signaling rapidly became targets of interest for novel cancer therapy development. IAPs bind caspases, blocking their pro-apoptotic function (5). IAPs are frequently dysregulated in ovarian cancer (6), and exploiting the IAP-caspase interaction may be a logical strategy for anti-cancer drug development.
SMAC is an endogenous IAP antagonist released from mitochondria upon cell death signaling (7). SMAC-mimetics are designed to bind IAPs by mimicking the N-terminal Ala-Val-Pro-Ile (AVPI) tetrapeptide sequence of SMAC (8). SMAC-mimetics have shown preclinical activity in both solid tumors and hematologic malignancies (9-11). Birinapant is a divalent SMAC-mimetic specifically designed to target cellular IAPs, cIAP1 and cIAP2. In preclinical models, birinapant depleted cIAP1s, activated caspase 3, and inhibited tumor cell growth (12).
cIAP1s are unique among the IAP proteins in their ability to promote NF-κB activation downstream of cell surface receptors such as TNF receptor (13). Activation of NF-κB causes cancer cell proliferation, inflammation, and anti-apoptosis gene expression. Our laboratory previously demonstrated autonomous activation of NF-kB in ovarian cancers (14). In addition, ovarian cancers are associated with elevated TNF-α (15-16), a factor that can mediate either pro-survival or pro-death signaling (17). In the presence of cIAPs, TNF-α activates pro-survival NF-κB signaling upon binding to its receptor. In the absence of cIAPs, however, TNF-α activates caspase-8 and extrinsic apoptosis (18-19). We therefore hypothesized that by inhibiting cIAPs, birinapant had the potential to be an effective therapy for ovarian cancer. This single-agent phase 2 clinical trial evaluated the drug’s mechanism of action and identified key pharmacodynamic markers for future clinical trials. Understanding such parameters is essential to move birinapant forward clinically, in rationally selected combination with standard and/or novel anti-cancer therapies.
Patients and Methods
Patients
Eligible women were ≥18 years with histologically confirmed relapsed platinum-refractory or -resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer (collectively EOC), with disease measurable by RECISTv1.1 (20) and amenable to percutaneous core needle biopsy. Platinum refractory was defined as progression of disease while receiving a platinum and platinum-resistant was defined as progression of disease within 6 months of completion of platinum therapy. There was no limitation to prior therapy. Other criteria included adequate end organ function, Eastern Cooperative Oncology Group performance status ≤2, and a minimum of 4 weeks from previous therapy. The study was conducted in accordance with the Declaration of Helsinki, approved by the Institutional Review Board at the National Cancer Institute and registered with Clinicaltrials.gov (NCT01681368). All patients provided written informed consent at study enrollment.
Treatment Plan
This was an open label, non-randomized phase II trial of the SMAC-mimetic birinapant (TL32711; NSC 756502). Birinapant 47mg/m2 was administered intravenously on days 1, 8 and 15 of 28-day cycles until disease progression, unacceptable toxicity, or patient withdrawal (Figure 1). Patients were evaluated every 4 weeks; laboratory studies were completed prior to each birinapant infusion, and as clinically indicated. Response was monitored by CT scan every 2 cycles according to RECISTv1.1 (20). CA-125 was recorded but was not a measure of response. Toxicity was assessed according to the Common Terminology Criteria for Adverse Events (CTCAEv4.0). Dose reductions were indicated for adverse event (AE) grade ≥ 3 (Supplemental Methods).
Figure 1.
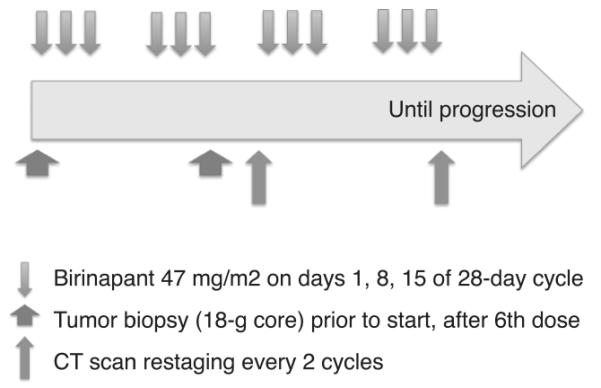
Schematic diagram of trial design. Patients received birinapant 47mg/m2 intravenously weekly for 3 weeks out of 4 (downward arrows). Cycles were 28 days. Tumor biopsies were collected prior to Cycle 1 and within 12-22 hours after cycle 2 day 15 dose (wide upward arrows). Computed tomography (CT) imaging was performed within 28 days of starting cycle 1, and following every 2 cycles (narrow upward arrows). Treatment continued until disease progression in all patients.
Translational Endpoints
Sample acquisition
Whole blood was collected into heparin tubes, separated into plasma and PBMC components by ficoll gradient, aliquoted and stored separately at −80C until use. PBMCs were collected prior to administration of birinapant, and 4h and 24h after the first dose; additional paired specimens were collected prior to cycle 2 day 15 dose (C2D15), and 4h following. Percutaneous tumor biopsies were collected prior to initiation of cycle 1 (C1), and within 12-24 hours after C2D15. Four cores were obtained; cores 1 and 3 were immediately flash frozen and stored in liquid nitrogen, and cores 2 and 4 were fixed in formalin and paraffin-embedded. Time from core acquisition to storage condition (liquid nitrogen or formalin) was uniformly less than 30 seconds.
Pharmacokinetic Assessments
Birinapant concentrations within tumor were assessed from core 3 (frozen) percutaneous tumor biopsies at each timepoint. Plasma samples obtained on C1 day 1 (pre-dose, 30, 60, 120, and 240 minutes after infusion) were used to assess circulating birinapant pharmacokinetics. Both tumor and plasma birinapant concentrations were analyzed using liquid chromatography-tandem mass spectrometry, via proprietary methodology (TetraLogic Pharma, Malvern, PA). A noncompartmental analysis was performed using Phoenix WinNonlin 6.4 (Certara Pharsight, Cary, NC) to obtain individual pharmacokinetic parameters. A predicted mean plasma concentration-time curve was obtained using a two-compartment pharmacokinetic model. Graphical and statistical analyses were performed using GraphPad Prism, v6.
Pharmacodynamic Assessments
Core 1 tumor samples and PBMCs from each timepoint were lysed in T-per buffer (Thermo Scientific) for protein quantification by an automated capillary electrophoresis immunoassay system (Simple Western™). Tumor protein lysate (40-60ng), or PBMC protein lysate (16-77ng) were analyzed according to manufacturer’s instructions (ProteinSimple, Santa Clara, CA). Primary antibodies sources are listed in Supplemental Methods. Reproducibility of this assay has been demonstrated in clinical samples (21-22).
Core 4 was subjected to deparaffinization and endogenous peroxidase blocking; antigen retrieval was performed with citrate buffer. Sections (5um) were stained with antibodies against cleaved caspase 3 (R&D Systems, MAB835, 10ug/ml) and biotinylated gamma-H2AX (Millpore, 16-193, 10ug/ml), and detected using anti-rabbit secondary antibody to Alexa 546 and streptavidin Alexa 680 respectively. DAPI counterstained nuclei. Images were acquired using a Leica Aperio Scanscope FL under constant illumination and exposure settings. Field selection used DAPI channel to minimize selection bias. Post image enhancement was used for visualization and applied uniformly across the images. Segmented nuclei were analyzed based on mean intensity threshold in gamma-H2AX channel. Nuclei above a predetermined threshold were considered positive.
Peripheral blood mononuclear cells were characterized by fluorescence-activated cell sorting for T cells (CD3, and CD4 or CD8), B-cells (CD19) and NK-cells (CD56+, CD16+, CD45-bright, CD3-negative). Plasma was analyzed for IL-6, IL-8, TRAIL, and TNF-α cytokines per manufacturer’s instructions (MesoScale Discovery, Rockville, MD).
Statistical design and analysis
The primary endpoint was objective response rate (ORR, confirmed partial or complete response) or progression-free survival lasting ≥6 months (23). Secondary objectives included overall survival, tolerability and safety, and impact on molecular signaling. A modified two-stage design (24) was implemented with a maximum of 40 participants, giving 89% power (alpha 0.05) to detect a true ORR of at least 20% and 85% power to detect a true 6-month PFS rate of at least 45%. The endpoints are those set out by the Gynecologic Oncology Group as deemed the minimum bar required to determine that an agent is worthy of further study in the platinum refractory and resistant ovarian cancer population (25). The first stage of accrual required >1 objective response (5%), and >5 instances of 6-month PFS (25%) among the initial 20 participants to continue to second stage. At least 5 objective responses (12.5%), or at least 15 instances of 6-month PFS (37.5%), were necessary among 40 evaluable study participants for consideration of further testing as single agent therapy in ovarian cancer.
Secondary endpoints were analyzed with exploratory intent. A true population prevalence of 40% was assumed for a particular biomarker, requiring 39 study participants to estimate this prevalence with 10% error and 80% confidence. All exploratory comparisons were performed using Wilcoxon signed rank test, given the non-normal distribution of a small number of samples; p<0.05 was considered significant.
Results
Patients and outcomes
Eleven patients were enrolled. Birinapant achieved no clinical benefit in the first 11 patients. The best response was disease stabilization in 2 patients (4 months and 5 months). CA125 tumor marker showed varying trends across patients (Figure S1). The statistical design allowed 20 patients in the first stage, with at least 2 patients attaining objective response and at least 6 patients maintaining stable disease for greater than 6 months. This design would require 8 of the next 9 patients to achieve clinical benefit in order to proceed to second stage of accrual. After discussions with the study sponsor, we determined this to be an unrealistic goal and closed accrual at 11 patients in order to limit exposure to possible toxicity and risks associated with biopsies with an extremely low likelihood of clinical benefit. The median age of patients was 59 years, 82% were white, 54.5% had serous ovarian cancer histology, and the median number of prior therapies was 5 (Table 1).
Table 1.
Patient characteristics
| Patient characteristics | No. of patients (n = 11) |
|---|---|
| Median age (range), in years | 59 (48-69) |
| Race | |
| White | 9 |
| Black | 1 |
| Unknown | 1 |
| ECOG Performance status | |
| 0 | 0 |
| 1 | 10 |
| 2 | 1 |
| Histologic subtype | |
| Serous | 6 |
| Endometrioid | 1 |
| Clear cell | 1 |
| Unclassified | 3 |
| Median no. of prior therapies | 5 (3-10) |
| ≤3 | 1 |
| 4-6 | 6 |
| ≥7 | 4 |
| Types of prior therapies | |
| Chemotherapy | 11 |
| Biologic | 6 |
| Hormonal | 5 |
| Vaccine | 1 |
ECOG, Eastern Cooperative Oncology Group.
Birinapant was well-tolerated, with predominantly grade 2 adverse events (AE), one grade 3 lymphopenia, and no grade 4-5 AEs (Table 2). No AEs required dose reduction. One patient experienced grade 2 Bell’s palsy; neurologic exam demonstrated palsy of bilateral cranial nerves (CN) VII, right CN VI and left CN V one week after the second infusion during C1. Bell’s palsy was reported in other clinical trials using birinapant, and thus was not unexpected. MRI demonstrated faint enhancement of left optic nerve, without other significant findings. The patient received oral prednisolone 60mg daily for 7 days followed by a taper over 7 days, and symptoms resolved completely. Cerebrospinal fluid (CSF) was collected during diagnostic evaluation, and the patient consented to additional measurement of birinapant in the CSF. Interestingly, birinapant was detected in the CSF (0.0711ng/mL). The C1 day 15 dose was held and cycle 2 delayed one week, as permitted by protocol. This patient was re-treated with three additional weekly doses of birinapant without dose reduction, to complete cycle 2, and experienced no further AEs.
Table 2.
Adverse events
| Grade (no. of patients) |
||||
|---|---|---|---|---|
| Toxicity | 2 | 3 | 4 | 5 |
| Fatigue | 1 | |||
| Anemia | 8 | |||
| Pleural effusion | 1 | |||
| Hypercalcemia | 1 | |||
| Hypoalbuminemia | 1 | |||
| Decreased lymphocyte count | 9 | 1 | ||
| Depression | 1 | |||
| Back pain | 1 | |||
| Diarrhea | 1 | |||
| Constipation | 2 | |||
| Urinary retention | 1 | 1 | ||
| Urinary tract infection | 1 | |||
| Thromboembolic event | 1 | |||
| Upper respiratory infection | 1 | |||
| Skin pain | 1 | |||
| Facial nerve disorder | 2 | |||
| Eye disorder | 1 | |||
| Headache | 1 | |||
| Extremity pain | 1 | |||
| Vomiting | 1 | 1 | ||
| Dehydration | 1 | |||
| Bowel obstruction | 1 | 1 | ||
Pharmacokinetics
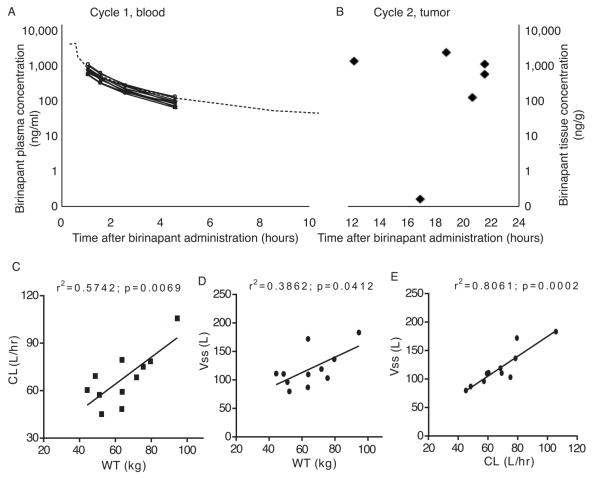
Drug concentration was evaluated in both plasma and tumor. Plasma birinapant concentrations were measured following C1 infusion (Figure 2A). Sparse sampling in our study limits true pharmacokinetic profiling. Observed exposures, however, are consistent with those reported in a previous study (Amaravadi et al submitted). Percutaneous tumor biopsy was performed prior to initiation of C1 in 11 patients, and a second tumor biopsy was performed within 12-22 hours after C2D15 infusion in 7 of those patients, and birinapant concentrations were measured. Although there is no direct comparison between plasma and tumor concentrations on C2D15, tumor concentrations ranged from 185-1830ng birinapant per gram of tissue (Figure 2B) as late as 22 hours post dose. The limited sample size prevents accurate description of birinapant’s true pharmacokinetic profile, which has demonstrated tri-phasic elimination (Amaravadi et al., submitted). Our observations, using limited sampling and sample size, however, are consistent with birinapant following at least a biphasic disposition, where the early phase involves birinapant tissue distribution followed by a slower terminal phase elimination. The calculated Vss of 119 L further supports the claim of extensive birinapant distribution into tissues, as this is much larger than normal plasma volumes (5-7 L). Our data suggest that drug remains in tumor much longer than in plasma, where it is rapidly cleared (68 L/hr). This longer half-life in tumor is consistent with a more comprehensive dataset where plasma half-life was estimated to be 30 hours, and tumor tissue half-life estimated to be 60 hours (Amaravadi et al submitted). Body weight appeared to be related to both volume of distribution (r2=0.386; p=0.04) and clearance (r2=0.574; p=0.007) (Figure 2C). Further, dose-normalization to body surface area produced comparable exposures in the eleven patients (Figure S2).
Figure 2.
Concentrations of birinapant in patients. (A) Birinapant was detected in plasma obtained at 30, 60, 120 and 180 minutes after administration of the first dose, and followed expected pharmacokinetics in all patients. The heavy black dots represent the observed plasma concentrations and the dashed line represents the predicted birinapant plasma concentration when dosed at 47mg/m2 in a phase 1 study (n=11). (B) Levels of birinapant were measured in core needle biopsies of tumors that had been frozen at the time of acquisition. Paired samples prior to treatment and following cycle 2 were obtained in 7 patients. No birinapant was detected in any of the pre-exposure biopsies, and birinapant was measurable in all but one biopsy obtained after cycle 2 day 15 dose. (C) Birinapant clearance and volume of distribution correlated with each other and were influenced by body weight.
Pharmacodynamic Studies
Cell survival and apoptosis
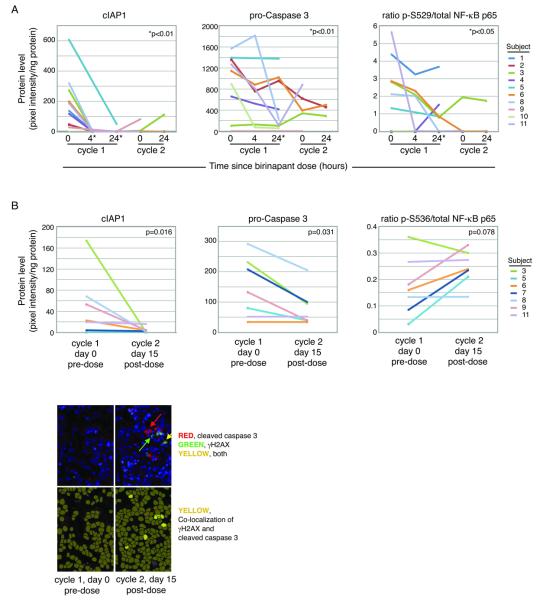
We sought to determine on-target activity of birinapant in PBMCs and tumor specimens, by investigating both downregulation of anti-apoptotic pathways and initiation of pro-apoptotic cascades. We measured the direct target, cIAP, as well as pro-caspase3 as a marker of apoptosis induction, and phosphorylated NF-kB-p65 representing the known role of IAPs in NF-kB signaling. The SimpleWestern™ assay allows quantification of multiple proteins from minute volumes of sample. Adequate assay sensitivity and reproducibility has been demonstrated with highly quantitative data in both laboratory and clinical samples (26). Birinapant induced rapid degradation of cIAP1, the primary target, in serial PBMC protein lysates (Figure 3A). Pro-caspase3 and phospho-NF-κB-p65 also showed downregulation in PBMC at 24 hours following birinapant administration (Figure 3A).
Figure 3.
Birinapant induced pharmacodynamic changes in target proteins, in both peripheral blood mononuclear cells (PBMC) and tumors. (A) Protein lysates from PBMC collected at the indicated time points following birinapant administration showed rapid and sustained loss of birinapant’s primary target, cIAP1, as measured by SimpleWestern™. Similar measurement of pro-caspase 3 and pS529-NF-kB p65 showed similar patterns of downregulation at 24 hours. (B) SimpleWestern™ analysis of protein lysates from tumor biopsies consistently showed loss of cIAP1 after birinapant. Pro-caspase 3 decreased after birinapant, suggesting cleavage. The ratio of phosphorylated to total NF-kB p65 protein showed a non-significant trend towards increase in the tumor biopsies. (C) Immunofluorescent detection of gamma-H2AX in tumor biopsies suggested early apoptosis, and co-localized with cleaved caspase 3. Few cells were positive after birinapant exposure.
In core needle biopsies of tumor, cIAP1 showed a downward trend, consistent with on-target activity of birinapant (n=7, p=0.016, Figure 3B). Unexpectedly, cIAP1 was not uniformly high, but was measured across a wide concentration range among the specimens. For the majority of patients, cIAP2 signals were below the limits of detection for accurate quantification (not shown). Pro-caspase 3 was significantly decreased after birinapant, suggesting that its cleavage may have occurred; the assay did not detect the cleaved form (p=0.031, Figure 3B). Phospho-NF-κB-p65 trended towards significance (p=0.078, Figure 3B). Other NF-κB markers tested included IκB-alpha, NF-κB-p50, NF-κB-p52, NF-κB-p100, NF-κB-p105, PARP, c-FLIP, and RIP; none demonstrated significant changes (not shown).
We developed an immunohistochemistry assay to measure co-expression of cleaved caspase 3 and gamma-H2AX in fixed specimens. Core 4 (FFPE) tumor biopsy was used to measure co-expression of these proteins as a surrogate for early apoptosis. Only two pairs of adequate pre- and post-treatment biopsies were available for analysis. The remaining paired samples were inadequate for IHC due to necrotic debris in the post-treatment biopsy (1 specimen) or contamination with blood (4 specimens). This finding suggests that 4 cores may exceed the limit for obtaining high quality specimens for molecular analyses. In the usable tissues, the percentage of dually positive cells increased approximately 2-fold and 4-fold following birinapant administration, consistent with early apoptotic activity (Figure 3C).
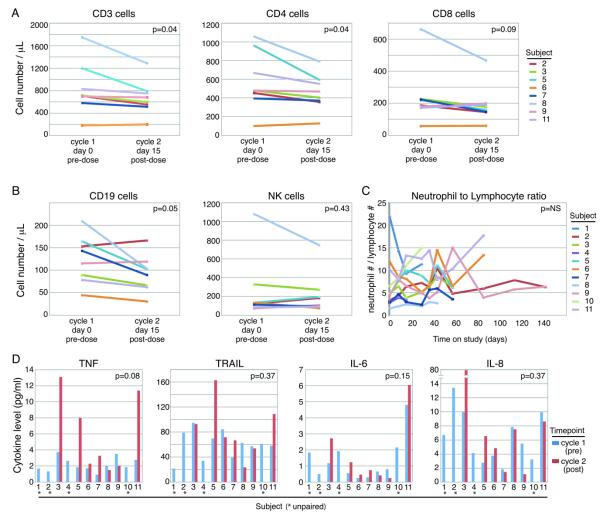
Immune parameters
We assessed whether birinapant modulated the composition of circulating immune cells, given the proposed effects of birinapant on NF-κB signaling and the overwhelming importance of NF-κB in immune cells. Indeed, dose-dependent lymphocytopenia was observed in previous studies with birinapant (Amaravadi et al submitted). PBMCs were analyzed for T-cell, B-cell, and NK-cell counts. All T-cell subsets decreased following exposure to birinapant (P=0.04 to 0.09; Figure 4A). B-cells also decreased (P=0.05), and no significant change in NK-cells was observed (P=0.43; Figure 4B). The neutrophil to lymphocyte ratio (NLR) has been proposed as a general marker of inflammation in cancer (18). NLR showed variable trends across patients but followed no consistent pattern (Figure 4C).
Figure 4.
Birinapant had modest effects on circulating immune cell subsets or cytokines. (A) Most patients showed decreases in circulating T cells, as measured by CD3+ cells on flow cytometry. CD4+ T cells appeared to decrease more than CD8+ T cells. (B) B cells, as measured by CD19, also showed mild decrease after birinapant exposure. There was no significant change in circulating NK cells, as measured by CD3neg, CD45+ and CD16+ cells. (D) Cytokine TNF was measured by multiplex ELISA (Mesoscale), and showed a trend towards increasing in patients’ plasma. Plasma levels of TRAIL did not significantly change with birinapant administration, nor did IL-6 and IL-8 levels.
Plasma collected prior to C1 and following C2D15 was analyzed for IL-6, IL-8, TRAIL, and TNF-α cytokines. Interestingly, a trend towards increased TNF-α was measured following treatment (P= 0.08; Figure 4D); no trend, however, was observed in the other cytokines. There was no relationship between change in TNF-α level and time on study.
Discussion
Cell death signaling is a complex process commonly dysregulated in malignancy. The present study tested the ability of the SMAC-mimetic, birinapant, to release a block to apoptosis in ovarian tumors by downregulating cIAPs and NF-κB signaling. Detailed pharmacodynamic endpoints were included to better understand in vivo effects of this drug, in order to leverage this compound optimally in combination therapies moving forward. The major limitation of this study was the small number of enrolled patients due to lack of clinical benefit in the first stage of accrual. Accrual was halted early on ethical grounds, in order to avoid subjecting subsequent patients to the risks of the study (adverse events, tumor biopsies, and radiation from associated imaging) when there was no signal of efficacy. Even the small cohort, however, provided valuable information. In line with prior studies, birinapant was well tolerated. Prospectively collected correlative studies demonstrated good penetration of drug into tumors, and consistent target downregulation of cIAP1 protein in tumors and PBMC. Study of the single agent was necessary to confirm drug penetration into tumors, define on-target activity of the compound, and allow better understanding of the relevance of IAPs in chemorefractory ovarian cancer.
Normal cells have redundancy in pathways to maintain plasticity of signaling, but this redundancy is often breached in the transformation to malignancy, when cancer cells develop “addiction to” a single signaling node, thus introducing a point for therapeutic intervention. This did not appear to be the case for cIAP1 in multiply recurrent ovarian cancers. The lack of clinical responses or disease stabilization suggests that downregulation of cIAP1 alone is insufficient to decrease tumor burden in women with recurrent ovarian cancer, and that IAP1 does not, in itself, sustain the viability of ovarian cancer cells. Thus, SMAC mimetics may be better suited to augment apoptosis in combination with anti-cancer agents. While a combination of reproducible on-target suppression and clinical efficacy are the key ingredients for proceeding with further development of novel “targeted” agents when they are intended as single agent, this particular targeted agent shows a compelling mechanism of action suggesting that it should be logically combined with other agents to augment their ability to induce programmed cell death. For example, we measured a significant decrease of procaspase-3 in tumor biopsies suggesting that caspase-3 cleavage had occurred. Trends were also seen in suppression of NF-κB activity, the pro-survival pathway. Thus, birinapant combined with agents that act on similar signaling nodes could maximize clinical benefit.
NF-κB activity also affects programmed cell death. Pro-inflammatory cytokines such as TNF-α induce pro-survival and anti-apoptotic NF-κB signaling that opposes TNF-α-induced apoptosis (27). In our current study, patients showed a trend towards increased circulating TNF-α. A recent trial of DEBIO1143 (AT-406) also reported increased TNF-α levels following study agent administration in 4 of 5 patients (28). Cytokine-induced inflammation may trigger some adverse events associated with birinapant, a bivalent SMAC-mimetic. Both monovalent and bivalent SMAC mimetics have been developed. Bivalent SMAC mimetics have higher binding affinities that correspond with higher potency, and potentially better efficacy in preclinical models (29-30). Bell’s palsy has only been observed with bivalent SMAC mimetics (31-32). This adverse event was reported in 11 of 238 (4.6%) patients in previous trials with birinapant, and 4 of 54 (7.4%) patients on a phase 1 trial with HGS1029. In the current study, the constellation of clinical findings was consistent with increased cranial nerve inflammation rather than immune suppression typically associated with viral reactivation-associated palsy. In this case of Bell’s palsy, nerve function returned after brief anti-inflammatory treatment, and the patient received additional birinapant without further complications. We observed no significant alterations in cytokines in our limited study population, although individual patients did show elevations in TNF, TRAIL, IL-6 and IL-8. In this patient, cytokines had decreased at C2D15, after treatment and resolution of the event. Despite the infrequency of Bell’s palsy, early diagnosis and prompt treatment are imperative to minimize long-term deleterious effects.
Cooperative interactions between chemotherapeutics and SMAC-mimetics have been achieved in the preclinical setting at relatively lower concentrations that should minimize chemotherapy-related morbidity (33-35). A Phase 1b/2a clinical trial established safety and tolerability of birinapant combined with five different chemotherapy regimens (NCT01188499). Birinapant with docetaxel was well tolerated, and may offer a logical experimental option for women with recurrent ovarian cancer, to whom docetaxel is frequently administered in third-line. The combination of birinapant with a TRAIL death receptor agonist is also promising. In the preclinical setting, TRAIL and TNF-α sensitized head-and-neck squamous cell carcinoma cell lines to birinapant treatment while docetaxel was synergistic with birinapant. The combination of birinapant with TRAIL and docetaxel decreased cell density in vitro while the combination of birinapant and docetaxel delayed tumor growth to a greater extent than monotherapy birinapant in xenografts (33). A phase 1-2 study is currently underway to test the safety and activity of birinapant combined with TRAIL agonist antibody conatumumab in women with relapsed epithelial ovarian cancer (NCT01940172).
Birinapant demonstrated reproducible on-target suppression, but minimal single-agent activity. Correlative studies included in this clinical trial confirmed on target activity of birinapant, and established that IAP is not a viable target in isolation for ovarian cancer. Rational combination therapies with SMAC-mimetics are likely required to improve anti-tumor activity. Given the good tolerability of birinapant, the results of our phase 2 clinical trial support moving forward with this promising agent in combination therapy for cancer treatment. Future clinical trials with birinapant should incorporate similar markers of activity, and search for predictive markers of response to combination therapy. Innovative research incorporating high-throughput drug screening will help identify clinically relevant synergistic drug combinations with birinapant to exploit apoptotic signaling for treating ovarian cancer.
Supplementary Material
Figure S1: CA125 measurements were recorded for patients prior to the start of each cycle.
Figure S2: Dose normalization by BSA produced comparable exposures, with no apparent trend with absolute dose.
Acknowledgments
The authors thank all of the participating patients and their families, the participating investigators, research nurses, and study coordinators. We are indebted to Seth Steinberg, NCI, for providing expert statistical advice.
Financial support: Intramural Research Program, NCI (CMA, WDF, LC); Division of Cancer Treatment and Diagnosis, NCI (RK); Cancer Therapy Evaluation Program, NCI (NT)
Footnotes
Conflicts of interest: none
REFERENCES
- 1.SEER Cancer Statistic Review, 1975-2012. National Cancer Institute; 2015. [Google Scholar]
- 2.Cain K, Bratton SB, Langlais C, et al. Apaf-1 oligomerizes into biologically active approximately 700-kDa and inactive approximately 1.4-MDa apoptosome complexes. J Biol Chem. 2000;275:6067–70. doi: 10.1074/jbc.275.9.6067. [DOI] [PubMed] [Google Scholar]
- 3.Liu JR, Opipari AW, Tan L, et al. Dysfunctional apoptosome activation in ovarian cancer: implications for chemoresistance. Cancer Res. 2002;62:924–31. [PubMed] [Google Scholar]
- 4.Antoon JW, Lai R, Struckhoff AP, et al. Altered death receptor signaling promotes epithelial-to-mesenchymal transition and acquired chemoresistance. Sci Rep. 2012;2:539. doi: 10.1038/srep00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–52. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 6.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int J Oncol. 2002;21:315–20. [PubMed] [Google Scholar]
- 7.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. United States. 2000:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Chai J, Suber TL, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–12. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 9.Gatti L, De Cesare M, Ciusani E, et al. Antitumor activity of a novel homodimeric SMAC mimetic in ovarian carcinoma. Mol Pharm. 2014;11:283–93. doi: 10.1021/mp4004578. [DOI] [PubMed] [Google Scholar]
- 10.Krepler C, Chunduru SK, Halloran MB, et al. The novel SMAC mimetic birinapant exhibits potent activity against human melanoma cells. Clin Cancer Res. United States: 2013 Aacr. 2013:1784–94. doi: 10.1158/1078-0432.CCR-12-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scavullo C, Servida F, Lecis D, et al. Single-agent Smac-mimetic compounds induce apoptosis in B chronic lymphocytic leukaemia (B-CLL) Leuk Res. 2013;37:809–15. doi: 10.1016/j.leukres.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Benetatos CA, Mitsuuchi Y, Burns JM, et al. Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-kappaB activation, and is active in patient-derived xenograft models. Mol Cancer Ther. United States. 2014:867–79. doi: 10.1158/1535-7163.MCT-13-0798. [DOI] [PubMed] [Google Scholar]
- 13.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. United States. 1995:1243–52. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez L, Hsu SC, Davidson B, Birrer MJ, Kohn EC, Annunziata CM. Activation of NF-kappaB signaling by inhibitor of NF-kappaB kinase beta increases aggressiveness of ovarian cancer. Cancer Res. United States: 2010 Aacr. 2010:4005–14. doi: 10.1158/0008-5472.CAN-09-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Jaco P, Asselain B, Orlandi C, Fridman WH, Teillaud JL. Evaluation of circulating tumor necrosis factor-alpha in patients with gynecological malignancies. Int J Cancer. 1991;48:375–8. doi: 10.1002/ijc.2910480311. [DOI] [PubMed] [Google Scholar]
- 16.Piura B, Medina L, Rabinovich A, Dyomin V, Levy RS, Huleihel M. Distinct expression and localization of TNF system in ovarian carcinoma tissues: possible involvement of TNF-alpha in morphological changes of ovarian cancerous cells. Anticancer Res. Greece. 2014:745–52. [PubMed] [Google Scholar]
- 17.Baker SJ, Reddy EP. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- 18.Varfolomeev E, Goncharov T, Fedorova AV, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. United States. 2008:24295–9. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertrand MJ, Milutinovic S, Dickson KM, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. United States. 2008:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. England. 2009:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen JQ, Heldman MR, Herrmann MA, et al. Absolute quantitation of endogenous proteins with precision and accuracy using a capillary Western system. Anal Biochem. 2013;442:97–103. doi: 10.1016/j.ab.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JQ, Lee JH, Herrmann MA, et al. Capillary isoelectric-focusing immunoassays to study dynamic oncoprotein phosphorylation and drug response to targeted therapies in non-small cell lung cancer. Mol Cancer Ther. United States: 2013 Aacr. 2013:2601–13. doi: 10.1158/1535-7163.MCT-13-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose PG, Tian C, Bookman MA. Gynecol Oncol. United States: 2010. Published by Elsevier Inc.; 2010. Assessment of tumor response as a surrogate endpoint of survival in recurrent/platinum-resistant ovarian carcinoma: a Gynecologic Oncology Group study; pp. 324–9. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee A, Tsiatis AA. Adaptive two-stage designs in phase II clinical trials. Stat Med. 2006;25:3382–95. doi: 10.1002/sim.2501. [DOI] [PubMed] [Google Scholar]
- 25.Rose PG, Tian C, Bookman MA. Assessment of tumor response as a surrogate endpoint of survival in recurrent/platinum-resistant ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;117:324–9. doi: 10.1016/j.ygyno.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Fan AC, Deb-Basu D, Orban MW, Gotlib JR, Natkunam Y, O'Neill R, et al. Nanofluidic proteomic assay for serial analysis of oncoprotein activation in clinical specimens. Nat Med. United States. 2009:566–71. doi: 10.1038/nm.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 28.Hurwitz HI, Smith DC, Pitot HC, et al. Safety, pharmacokinetics, and pharmacodynamic properties of oral DEBIO1143 (AT-406) in patients with advanced cancer: results of a first-in-man study. Cancer Chemother Pharmacol. 2015;75:851–9. doi: 10.1007/s00280-015-2709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun H, Nikolovska-Coleska Z, Lu J, et al. Design, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAP. J Am Chem Soc. 2007;129:15279–94. doi: 10.1021/ja074725f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H, Nikolovska-Coleska Z, et al. Design of small-molecule peptidic and nonpeptidic Smac mimetics. Acc Chem Res. 2008;41:1264–77. doi: 10.1021/ar8000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NV F, S L, J M, et al. A Phase I Study Using Single Agent Birinapant in Patients with Relapsed Myelodysplastic Syndrome and Acute Myelogenous Leukemia. Blood. 2014 [Google Scholar]
- 32.BI S, SG E, G G, et al. Safety, pharmacokinetcs (PK), and pharmacodynamics (PD) of HGS1029, an inhibitor of apoptosis protein (IAP) inhibitor, in patients (Pts) with advanced solid tumors: Results of a phase I study. J Clin Oncol. 2012;29(suppl) (abstr 3008) [Google Scholar]
- 33.Eytan DF, Snow GE, Carlson SG, Schiltz S, Chen Z, Van Waes C. Combination effects of SMAC mimetic birinapant with TNFalpha, TRAIL, and docetaxel in preclinical models of HNSCC. Laryngoscope. 2014 doi: 10.1002/lary.25056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulda S. Smac mimetics as IAP antagonists. Semin Cell Dev Biol. 2015;39:132–8. doi: 10.1016/j.semcdb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Tian A, Wilson GS, Lie S, et al. Synergistic effects of IAP inhibitor LCL161 and paclitaxel on hepatocellular carcinoma cells. Cancer Lett. 2014;351:232–41. doi: 10.1016/j.canlet.2014.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: CA125 measurements were recorded for patients prior to the start of each cycle.
Figure S2: Dose normalization by BSA produced comparable exposures, with no apparent trend with absolute dose.