SUMMARY
Escape behaviors deliver organisms away from imminent catastrophe. Here, we characterize behavioral responses of freely swimming larval zebrafish to looming visual stimuli simulating predators. We report that the visual system alone can recruit lateralized, rapid escape motor programs, similar to those elicited by mechanosensory modalities. Two-photon calcium imaging of retino-recipient midbrain regions isolated the optic tectum as an important center processing looming stimuli, with ensemble activity encoding the critical image size determining escape latency. Furthermore, we describe activity in retinal ganglion cell terminals and superficial inhibitory interneurons in the tectum during looming and propose a model for how temporal dynamics in tectal periventricular neurons might arise from computations between these two fundamental constituents. Finally, laser ablations of hindbrain circuitry confirmed that visual and mechanosensory modalities share the same premotor output network. Together, we establish a circuit for the processing of aversive stimuli in the context of an innate visual behavior.
INTRODUCTION
When confronted with threatening stimuli, organisms respond with stereotyped behavioral patterns that promote survival. The most fundamental of these behaviors is the escape response, which delivers the individual away from assault. While these escapes are diverse across phyla (Chalfie et al., 1985; Muijres et al., 2014; Sherrington, 1910), they are nevertheless highly conserved and occupy an ancient and essential corner of the ethogram (Eaton, 1984). Indeed, when examined ontogenetically, escape behaviors typically develop before the organism can feed or make coordinated movements (Armstrong and Higgins, 1971), highlighting the vital importance of these avoidance programs.
The robustness and stereotypy of escape behaviors are of great utility for studies of sensorimotor computations (Dickinson and Moss, 2012; Eaton et al., 2001; Fotowat and Gabbiani, 2011; Korn and Faber, 2005). While studies of escape behaviors have often focused on impulse-like mechanosensory stimulation such as a touch or brief auditory buzz, where stimulus control and behavioral execution is straightforward and where the underlying sensory detection and processing pathways are relatively compact. The visual system, however, is arguably better suited for detecting threatening stimuli, as visual cues can be detected long before the mechanical signatures of an approaching predator reach somatosensory and auditory systems (Billington et al., 2011; Fotowat et al., 2011; Khakhalin et al., 2014; Oliva et al., 2007; Preuss et al., 2006; Yilmaz and Meister, 2013). At the same time, the sensory computations required for the visual detection of threats are potentially more complex, as these must involve the rapid analysis of high-dimensional spatiotemporal sensory streams. Nonetheless, mechanisms of visual escape behavior, typically evoked by signatures of impending collision (looming), have not been well-studied outside of invertebrates (Gabbiani et al., 1999; von Reyn et al., 2014). Here, we use the behavioral, optical, and genetic accessibility of the larval zebrafish, Danio rerio, to address the neural basis of visually evoked escapes in a vertebrate animal.
In response to acoustic or tactile stimulation, larval zebrafish perform a fast, high-angle, stereotyped escape maneuver (the “C-bend”) that is conserved across most anamniotes (Eaton et al., 2001). This escape behavior is recruited by a short ipsilateral arc (minimum 2 synapses) from the ear (in the case of sound) to rhombomere 4 of the hindbrain, where a premotor system dominated by the large, morphologically distinct Mauthner cell (M-cell) effects a high-amplitude turn to the contralateral side. While it is not known in zebrafish whether the M-cell and its associated segmental homologues (collectively, the M-system) mediate any visually guided behaviors, studies in goldfish (Preuss et al., 2006; Zottoli et al., 1987) show that the M-cell may receive visual input from the optic tectum (OT), the homologue of the mammalian superior colliculus.
In turn, a large body of evidence supports a role for the OT in complex visual processing. The OT, by far the largest contiguous larval visual brain structure, is recurrently connected across its laminar architecture and receives direct input from the majority of retinal projections (Burrill and Easter, 1994) in addition to indirect input from accessory visual areas (Vanegas and Ito, 1983). Neurons in the OT show direction, orientation, speed, and size selectivity (Gabriel et al., 2012; Grama and Engert, 2012; Hunter et al., 2013; Niell and Smith, 2005) and respond to aversive (predator-like) and appetitive (prey-like) visual cues in many animals (Dean et al., 1989; Ewert, 1997; Muto et al., 2013). Furthermore, OT neurons in birds (Winkowski and Knudsen, 2008), tadpoles (Khakhalin et al., 2014), frogs (Baranauskas et al., 2012), and fish (Niell and Smith, 2005) respond to looming stimuli. Thus, the OT is well-positioned to mediate visually evoked escape responses by feeding filtered visual input to the hindbrain M-system and associated escape circuitry.
However, so far, a causal link between the hindbrain M-system and visually evoked escapes has not been demonstrated. Furthermore, most analyses of tectal processing have remained descriptive and treat single cells in isolation (Gabriel et al., 2012; Grama and Engert, 2012), independent of behavior and the activity of other visual, motion-sensitive midbrain structures such as the pretectum (Kubo et al., 2014; Portugues et al., 2014). Given its anatomical and functional position, an understanding of population activity in the OT during a well-defined visuomotor behavior would lead to new insights into how the vertebrate central nervous system isolates behaviorally relevant cues from sensory streams and transforms these into behavior.
In this study, we employ a combination of behavioral and calcium imaging techniques to map the sensory and motor systems underlying visually evoked escape behavior and construct a working model of behaviorally relevant stimulus representation in the OT. We establish that freely swimming larval zebrafish respond to visual stimuli representing object approach with directed C-bend escape maneuvers and describe a convergence in the circuits mediating mechanosensory and visual escapes at the premotor level. In addition, we demonstrate that the OT encodes the critical image size associated with escape latency across hundreds of periventricular neurons (PVNs), providing a novel basis for ethologically relevant processing in collicular structures. Furthermore, we measure the activity in the presynaptic terminals of retinal ganglion cells that provide the input to the OT, as well as the response properties of superficial inhibitory interneurons (SINs) that have been shown to serve as an important computational unit in the context of separating large from small moving objects (Del Bene et al., 2010). These data allow us to propose a mechanistic model of how the behaviorally relevant dynamics of PVNs – the putative output neurons of the OT – arise in the context of looming stimuli. Together, these results outline the circuitry and computations controlling a robust, innate visually guided behavior and reveal fundamental principles of neural system organization likely prevalent in subcortical visual structures across phyla.
RESULTS
Looming visual stimuli evoke fast escape maneuvers in larval zebrafish
To test how larval zebrafish respond to looming stimuli, we constructed an arena in which individual freely swimming fish were monitored with a high-speed camera while visual stimuli were presented with closed-loop feedback onto a screen beneath the animal (Figure 1A). This high-speed (506 fps acquisition, 60 Hz stimulus update), closed-loop stabilization generated maximal consistency in visual stimulation across presentations; furthermore, locked egocentric stimuli best matched the conditions used in subsequent imaging experiments.
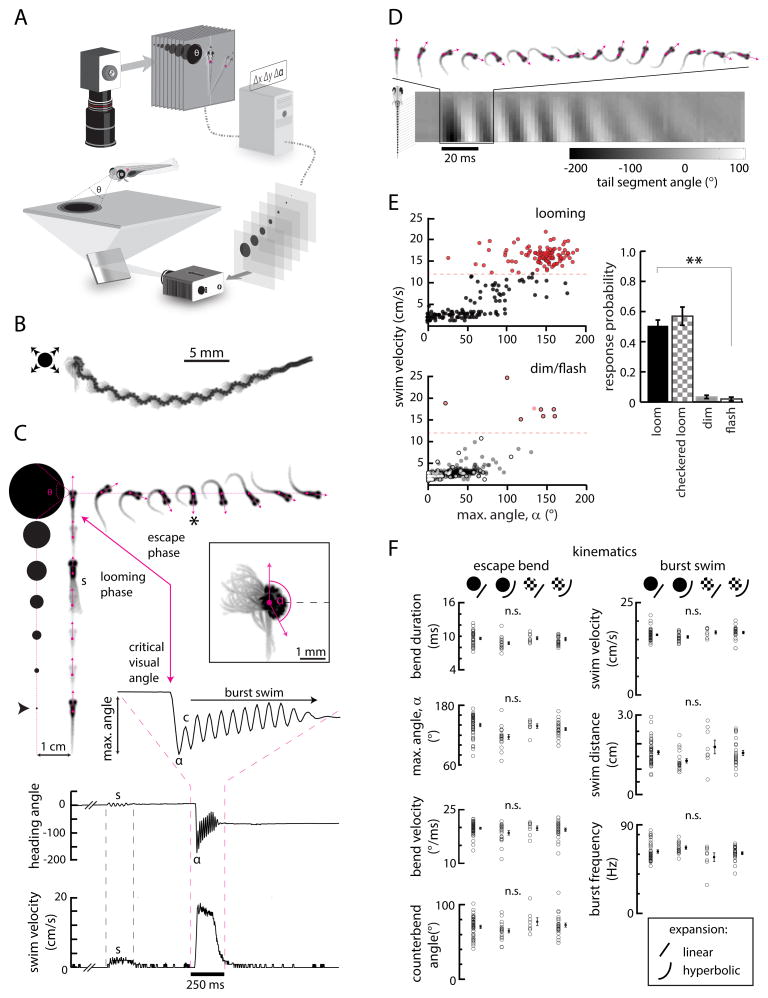
Figure 1. Kinematic analysis of looming-specific escapes.
(A) Schematic of the closed-loop behavior setup used to present visual stimuli and monitor behavior. Video is analyzed online to detect fish position and orientation in real time. This information is used to update stimulus position with closed-loop feedback so that stimuli remain in a fixed position within the fish’s visual field.
(B) The trajectory of a single looming-evoked behavioral response, shown here as the multiple movie frames superimposed (106 frames, 210 ms). An expanding, looming disk presented in the left visual field evokes a high-angle, long-distance maneuver to the right. In this representation, the eyes and swim bladder appear as dark spots, and the gray shades are the tail.
(C) Each individual trial can be separated into a looming phase (fish images, bottom to top), where the stimulus expands while locked to a fixed position from the fish center of mass (frames separated by 250 ms to illustrate dynamics, black arrowhead denotes stimulus start time), and an escape phase (fish images, top, left to right), during which the fish executes a high-angle, high-velocity escape maneuver (frames separated by the true frame period, 1.98 ms at 506 fps). Heading direction (pink vectors) and center of mass (pink dots) are extracted from each frame and used to characterize behavioral responses. (s), a spontaneous swim bout that occurs before this fish initiates an escape response (shown here as multiple superimposed movie frames), illustrating the need for closed-loop stimulus presentation. The same event is marked in the heading angle and swim velocity plots below. (*), The frame corresponding to the maximum change in heading direction (inset and time series, angle α). Bottom, plots of heading angle and instantaneous swim velocity isolated from video. A zoom-in of heading direction reveals a high-angle bend followed by a small counterbend (c) and a high-frequency burst swim lasting hundreds of milliseconds.
(D) An example of a looming-evoked escape with a large counterbend. The angle of 20 equally spaced points along the tail relative to the fish body axis can be extracted from high-resolution video, revealing detailed tail kinematics.
(E) Top left, a scatterplot of maximum heading angle α and swim velocity for all swim events initiated during looming stimulus presentation (N = 37 fish, n = 315 swim events). Bottom left, scatterplot for all swim events initiated while presenting dimming (filled circles, N = 26, n = 116) or flashed (open circles, N = 10, n = 1097) stimuli. Red, high-angle, high-velocity swim events above a threshold maximum swim velocity of 12.0 cm/s (dashed red line). Right, a quantification of escape response probability across multiple stimulus conditions. For this responsivity analysis, fish were only included if they responded at least once to at least one stimulus in the stimulus set. Looming black (N = 33 fish and checkered (N = 8) spots elicit escape-like maneuvers much more often than dimming (N = 27) or flashed (N = 6) stimuli. Looming stimuli expanded with constant radial velocity (linear expansion). Error bars represent mean ± SEM across fish. **, p < 10−5, permutation test.
(F) Analysis of 7 kinematic variables across 4 different classes of looming stimuli that vary in either radial expansion dynamics (linear or hyperbolic) or relative contrast (black or checkered isoluminant). Left, kinematics associated with the initial high-angle bends (α) of classified escapes. Right, kinematics associated with the burst swim phase of behavioral responses. While expansion dynamics may affect kinematics slightly, all variables are statistically similar across all 4 conditions after applying the Bonferroni correction ([significance level]0.05/7 ≈ 0.007; p = 0.008 maximum angle, p = 0.199 bend duration, p = 0.241 bend velocity, p = 0.086 swim distance, p = 0.077 burst frequency, p = 0.125 swim velocity, p = 0.175 counterbend angle, F-statistic (ANOVA) permutation test). Offset points and error bars are mean ± SEM across fish. Each open circle is the mean for a single fish. N = 49 fish, n = 301 responses, black linear expansion; N = 19, n = 192, black hyperbolic expansion; N = 8, n = 65, checkered linear expansion; N = 25, n = 322, checkered hyperbolic expansion.
Looming dark spots, which mimic approaching objects (Gabbiani et al., 1999), were presented on a neutral gray background to 5–6 days post-fertilization (dpf) larvae. These spots started at singular points offset orthogonally from the fish midline and expanded as disks with either constant radial velocity (linear expansion) or constant approach velocity (hyperbolic expansion), the former corresponding to a decelerating approach. Stimuli were presented either to the left or right side of the animal, remaining exclusively within each respective monocular visual field (Bianco et al., 2011) for at least the first half of the expansion period. These looming stimuli typically evoked high-velocity, high-angle, long-distance swim maneuvers (Figure 1B–C, Movie S1) that we quantified using detailed kinematic analysis (Figure 1D).
To better distinguish looming-evoked escape responses from other maneuvers in the larval zebrafish behavioral repertoire (e.g. routine turns or spontaneous swimming), we plotted the maximum instantaneous linear velocities and bend angles of all locomotion events initiated after stimulus onset but before the stimulus had stopped expanding. This analysis revealed a cluster of high-velocity, high-angle events separated from routine turns and swimming, demonstrating that looming stimuli consistently evoke escape-like responses that are distinct from other behaviors (Figure 1E). To probe whether these responses were indeed specific to looming stimuli, we also presented spots that appeared instantaneously (flashed stimuli) or spots that dimmed with the same temporal dynamics as the looming stimuli. A response probability metric – the probability of maximum swim velocity exceeding 12.0 cm/s (Supplemental Figure 1A–C) – indicated looming stimuli induced escapes about half of the time (51.0 ± 3.8% for linear expansion, 46.0 ± 9.3% for hyperbolic expansion), whereas high-velocity escape maneuvers almost never occurred during presentation of dimming and flashed stimuli (3.4 ± 1.1% and 2.0 ± 1.3%, respectively).
To test in more detail which stimulus feature generated the escape behavior during looming stimulus presentation, we evaluated whether object expansion was the key trigger. Expanding disks decrease overall luminance; although larvae did not escape to dimming alone, it is possible that a conjunction of looming and dimming is required for triggering escapes. Therefore, we presented checkered looming stimuli (Fotowat and Gabbiani, 2007), which were subjectively isoluminant over the time course of expansion. These stimuli were equally efficacious in evoking escape responses (57.0 ± 6.1% of the time for linear expansion, Figure 1E; 60.2% ± 4.7% for hyperbolic expansion), providing further evidence that this behavior employs complex, luminance-independent visual computations to detect expanding borders, consistent with looming-evoked escape responses in other species (Gabbiani et al., 2001; Landwehr et al., 2013; de Vries and Clandinin, 2012).
Zebrafish escape behavior has so far been described primarily in the context of mechanosensory C-starts (Burgess and Granato, 2007a; Gahtan et al., 2012; Kohashi and Oda, 2008; Liu and Fetcho, 1999; O’Malley et al., 1996), which are characterized by stereotyped kinematics. To better define looming-evoked behavior and compare it to these C-start escapes, we analyzed 7 different kinematic variables across 4 looming stimulus conditions: black or checkered constant radial expansion and black or checkered hyperbolic expansion. While the former pair simulated a decelerating approach trajectory, the latter pair simulated object approach at constant velocity, the stimulus most commonly used in other organisms (Hatsopoulos et al., 1995). We found that most kinematic variables tested were indistinguishable across the four stimuli with the exception of maximum bend angle, which varied slightly depending on the temporal dynamics of expansion (Figure 1F). Applying the Bonferroni correction (Dunn, 1961) for multiple comparisons, however, eliminates this significance. Thus, all four types of looming stimuli trigger indistinguishable motor programs.
Across all stimulus conditions, looming-evoked behaviors are characterized by at least 3 unique phases. First, larvae initiated a rapid (bend duration 9.4 ± 0.1 ms; maximum bend velocity 19.5 ± 0.2 °/ms), high-angle bend (133.4 ± 2.1°), that quickly reverses heading direction. Second, fish performed a counterbend that re-oriented the body (70.4 ± 1.3°). Third, fish executed a high-velocity burst swim (velocity 16.4 ± 0.2 cm/s; burst (undulation) frequency 62.7 ± 0.9 Hz) away from the starting position (distance 1.5 ± 0.1 cm, mean ± SEM across fish). These kinematics closely resemble the C-start escape behaviors elicited by mechanosensory modalities (Budick and O’Malley, 2000; Eaton et al., 1988, Supplemental Figure 1D–F) and are starkly different from the high-angle dark flash o-bend (Burgess and Granato, 2007b; Huang et al., 2013) or large spot avoidance (Bianco et al., 2011) behaviors previously described. Thus, this response to looming stimuli is the first description of a rapid escape behavior elicited by a visual stimulus in freely swimming larval zebrafish.
Escape trajectories are dictated by stimulus position within the visual field
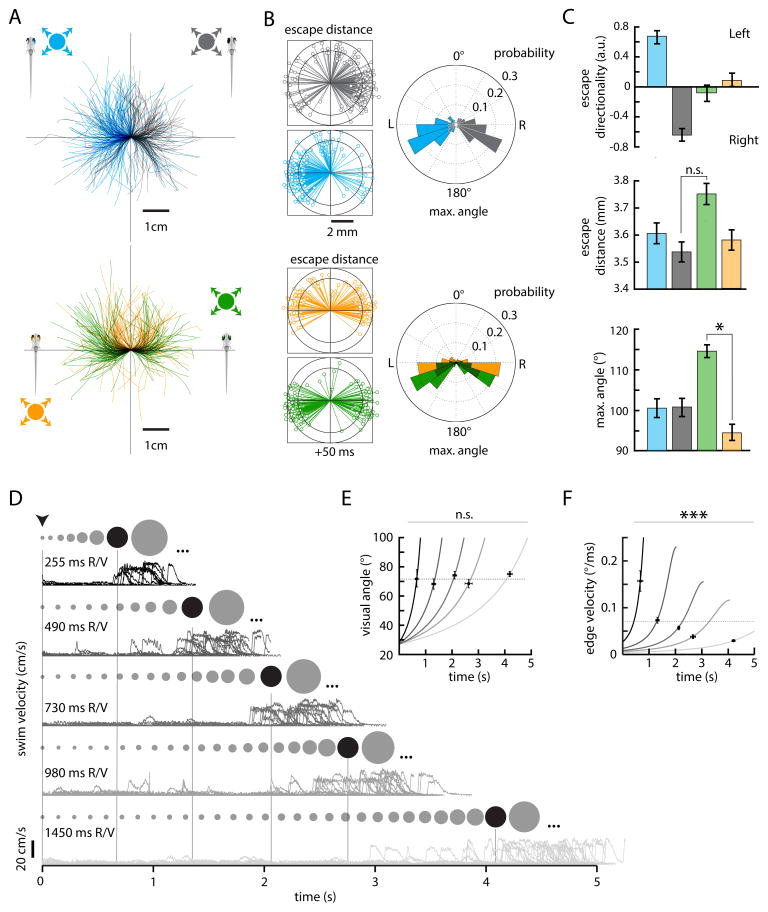
The directionality of escape can often be influenced by the location of the eliciting stimulus, reflecting an obvious but important strategy to effectively distance oneself from threats. Touch-evoked escapes in larval zebrafish are coarsely directional (Kohashi et al., 2012), and looming-evoked behaviors in locusts (Santer et al., 2005), flies (Card and Dickinson, 2008), and adult goldfish (Eaton and Emberley, 1991) show a dependence on incident angle. To probe whether looming-evoked behavior in larval zebrafish is influenced by stimulus position, we compared the escape trajectories elicited by looming stimuli presented in fixed positions in either the front, back, right, or left visual field (0, 180, 270, and 90° relative to the fish center of mass, respectively). In 33 fish, stimuli in the right visual field consistently evoked escapes to the left, and vice versa. This relationship is readily identifiable when escape trajectories are rotated and aligned onto the body axis for each condition (Figure 2A). Despite differences in escape direction (Figure 2B, angular histograms), the velocity of escape maneuvers is similar across all conditions, as evidenced by plots of fish position 50 ms after escape initiation (Figure 2B). Quantification of response preference across fish formalizes a strong positional dependence for left and right stimuli (0.68 ± 0.09 and −0.69 ± 0.09, respectively) and a lack of directional bias for binocular front and back stimuli (−0.14 ± 0.11 and 0.10 ± 0.10, respectively) (Figure 2C). Further analysis reveals that the observed trajectory bias reflects differences in absolute maximum turn angle (100.5 ± 2.3° for left; 100.7 ± 2.2° for right; 114.6 ± 1.5° for front, 94.5 ± 2.0° for back stimuli), with back stimuli eliciting significantly shallower (smaller turn angle) responses (p = 0.002, permutation test). As indicated, the distance traveled by larvae after 50 ms does not depend on stimulus position (3.61 ± 0.04 mm for left, 3.54 ± 0.04 mm for right, 3.75 ± 0.04 mm for front, 3.58 ± 0.04 for back stimuli; back, front p = 0.127, permutation test). It is worth noting that even in response to the same stimulus type, individual trajectories are highly variable; this suggests that larvae might employ a protean evasion strategy (Domenici et al., 2008; Humphries and Driver, 1970) that makes it harder for predators to predict and foil escapes once they are triggered. Nevertheless, these data demonstrate that larvae utilize a sensorimotor transformation that conserves positional stimulus information and alters escape motor programs accordingly.
Figure 2. Stimulus position and dynamics dictate escape direction and latency.
(A) Top, escape trajectories elicited by looming dark spots in the right (blue, N = 34, n = 214) or left (black, N = 33, n = 198) visual field. Bottom, escape trajectories elicited by looming dark spots centered in the nasal[back] (orange, N = 21, n = 164) or temporal[front] (green, N = 23, n = 177) visual field.
(B) Left, radial plots of fish position 50 ms after escape initiation. Right, angular histograms of the maximum turn angle (see Experimental Procedures) for all events in (A).
(C) Quantification of behavior across all 4 stimulus positions. Top, bar plots of left-right preference [(# left turns − # right turns)/(# left turns + # right turns)] for right (blue, N = 26), left (gray, N = 26), back (orange, N = 18), and front (green, N = 17) stimuli for fish with at least 5 escape responses. Left and right stimuli consistently evoke responses directed away from the starting stimulus position, whereas back and front stimuli evoke responses to the left and right with near-equal probability. Middle, bar plots of mean distance traveled after 50 ms. Bottom, bar plots of mean maximum turn angle across all 4 conditions. (*) p < 0.001, permutation test. Error bars are mean ± SEM across trials.
(D) Records of swim velocity over time for escapes elicited by stimuli simulating approach at 5 different velocities (255 ms, 490 ms, 730 ms, 980 ms, 1450 ms R/V, top to bottom). The ratio R/V is a unique identifier of expansion trajectory [visual angle = θ(t) = 2*arctan(R/(−Vt)), t <= 0, where t is time to simulated collision]. The spots above each trace represent the size of the looming stimulus before a critical size is reached (dark spot), after which an escape is initiated with a fixed delay. Stimuli continue to expand (ellipses) until the end of the allotted trial time.
(E) Plots of visual angle (solid lines, velocity decreasing from left to right) evaluated at the average response latency for each velocity condition (crosses). The visual angle subtending the axis of the stimulus parallel to the fish body axis 81 ms before escape initiation is not significantly different across all 5 conditions. We determined the fixed delay (Δt = 81 ms) by minimizing the threshold visual angle standard deviation across all trials (see Experimental Procedures).
(F) Plots of edge velocity, θ̇(t) evaluated at the average response latency for each velocity condition (crosses). Stimulus edge velocity 81 ms before escape initiation is significantly different across stimulus conditions (*** p < 10−5, F-statistic (ANOVA) permutation test). Dotted lines represent the mean visual angle or edge velocity 81 ms prior to escape initiation across all conditions. For 255 ms R/V, N = 9, n = 11; 490 ms, N = 12, n = 20; 730 ms, N = 18, n = 25; 980 ms, N = 19, n = 31; 1450 ms, N = 21, n = 41. Error bars are mean ± SEM across trials.
Escapes are triggered when stimuli reach a critical visual angle
To probe the effect of stimulus expansion velocity on visually evoked escape behaviors, we next presented a set of 5 stimuli that mimicked disks of constant radius approaching larvae from below at different velocities. When projected onto a flat surface, these stimuli can be described by functions of spot radius over time arising from fixed ratios of simulated disk radius and approach velocity (R/V, Supplemental Figure 2) (Hatsopoulos et al., 1995). Escape latency was strongly modulated by stimulus velocity, with faster stimuli reliably eliciting escapes with shorter latencies (Figure 2D). When functions of stimulus image size and edge velocity are evaluated at times of escape onset (minus a fixed processing lag, Δt = 81 ms, Experimental Procedures), an average threshold in angular image size (72.0 ± 1.3°, p = 0. 198, F-statistic (ANOVA) permutation test, Figure 2E) but not edge velocity (Figure 2F) emerges (p < 10−5, F-statistic (ANOVA) permutation test). This result is similar to descriptions of looming-evoked escape behaviors in other organisms (Fotowat and Gabbiani, 2007) and suggests that the circuits processing looming stimuli may primarily use stimulus size information when determining when and if an escape should be initiated.
Looming stimuli are primarily represented in the optic tectum
Our classification of looming-evoked escape behavior allowed us to explore the representation of this novel and ethologically relevant stimulus across various visual brain regions using calcium imaging in pan-neuronal Tg(elval3:GCaMP5G) 5–6 dpf larvae (Ahrens et al., 2013) (Figure 3A). To this end, larvae were fully embedded in agarose and imaged with a two-photon laser scanning microscope during stimulus presentation (Ahrens et al., 2012) to screen neurons for response selectivity. Looming stimuli and flashed stimuli evoked responses throughout the midbrain, which we segregated into 3 main regions based on anatomical boundaries and functional similarities: the optic tectum (OT), the pretectum/thalamus (PT/TH), and the midbrain tegmentum (MB) (Figure 3B). Responses to stimuli were diverse, but, within the scope of this study, were categorized based only on significant differences in activity during looming and flashed stimulus epochs compared to baseline (Experimental Procedures). This reduction allowed us to analyze stimulus selectivity, an indicator of processing specificity, throughout visual processing regions.
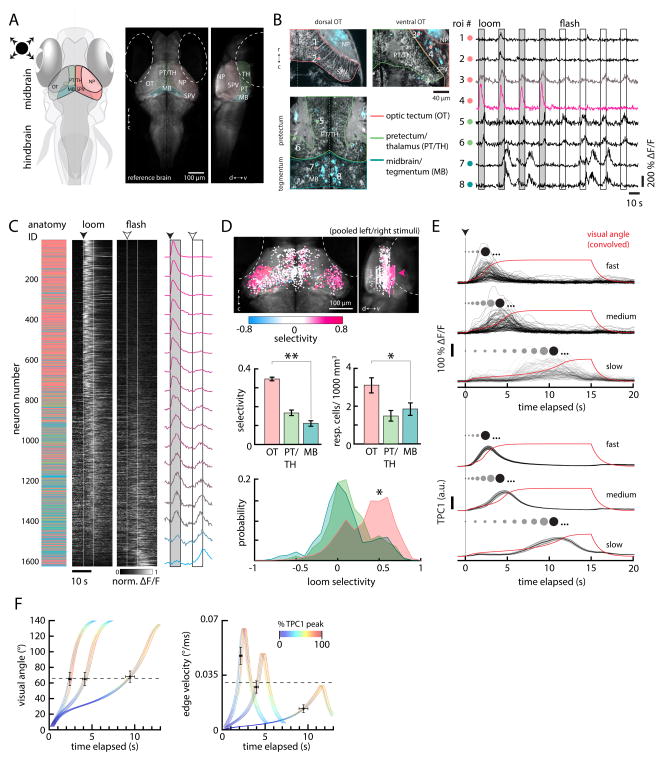
Figure 3. Looming-specific neurons in the optic tectum encode critical size.
(A) Left, schematic of the larval zebrafish brain indicating the positions of the optic tectum (OT) with its neuropil (NP) and cell body layers (stratum periventriculare, SPV), the pretectum/thalamus (PT/TH), and the midbrain tegmental region (MB). Right, a transverse average intensity projection of a 5 days post-fertilization (dpf) Tg(elval3:GCaMP2) larval brain and accompanying sagittal view. (TH), thalamus. (PT), pretectum. Dotted lines denote the position of each eye. (r) rostral; (c) caudal; (d) dorsal; (v) ventral.
(B) Left, single planes showing anatomy (gray) and activity (blue) in the dorsal and ventral OT, the PT/TH, and MB. Individual ROI numbers (ROIs shown as colored circles) correspond to the traces on the right, which illustrate the general pattern of activity observed across midbrain visual areas in response to looming stimuli. Neurons in the dorsal OT (1 and 2) respond weakly to looming stimuli. Neurons in the ventral OT (3 and 4) show more varied responses but typically respond strongly to and favor looming stimuli. Neurons in PT/TH (5 and 6) respond to both looming and flashed stimuli. Neurons in MB (7 and 8) were typically active spontaneously and non-stimulus-locked. Boxes represent stimulus presentation periods. Traces are re-ordered and concatenated from longer recordings.
(C) Middle, trial-averaged normalized ΔF/F evoked by looming and flashed stimuli from 1613 active neurons across 12 fish. Each neuron is sorted according to its selectivity index (see Experimental Procedures) in descending order (1 = looming exclusive, −1 = flash exclusive). Left, the corresponding anatomical location of each neuron, color-coded as in (B). Right, the average normalized ΔF/F binned across 100 neurons from the sorted list. Traces are normalized to [0 1] after trial-averaging. Normalization values were skewed to the right (maximum 586.24% ΔF/F, minimum 21.02% ΔF/F, skewness 1.28). Dotted lines and boxes represent stimulus presentation periods, with start times indicated by arrowheads.
(D) Top, all neurons from (C) mapped to a reference brain and colored according to selectivity index. Arrowhead, preponderance of looming-selective neurons in the ventral OT. Differences in the number of OT neurons between the left and right hemispheres reflects a sampling bias (data from stimuli presented in the left and right visual fields were pooled); most OT imaging was unilateral. Middle, bar plots quantifying mean selectivity (left) and responsive cell density (right) across the OT (n = 60 imaging planes), PT (n = 34), and MB (n = 44). Bottom, histograms showing the distribution of looming selectivity across neurons in the OT (n = 973 cells), PT (n = 279), and MB (n = 361). (**) p < 10−5, (*) p < 0.01, permutation test. N = 12 larvae. (r) rostral; (c) caudal; (d) dorsal; (v) ventral.
(E) Top, the responses of 110 OT neurons in 1 fish to looming stimuli simulating approach at 3 different velocities (R/V 545 ms, 1090 ms, 2730 ms, top to bottom). The stimulus size (angle) over time for each condition, convolved with a calcium impulse response function (CIRF) (τ = 962 ms), is shown in red. Bottom, the first temporal principal component (TPC1, ± SEM across fish) averaged over 8 fish, 1533 neurons. The spots above each trace schematize the size of the looming stimulus before the TPC1 threshold (dark spot). Stimuli continue to expand (ellipses) until the end of the stimulus epoch.
(F) Quantifications of average convolved stimulus visual angle (left) and edge velocity (right) at TPC1 threshold times (81% TPC1 response after normalization to maximum across all stimulus conditions) for all 3 velocity conditions. The colored curves show the convolved stimulus size and edge velocity for each condition, fanned to show TPC1 dynamics for individual fish. Each color value corresponds to the normalized amplitude of TPC1 activity during expansion. Crosses show the average value of each stimulus variable at the TPC1 threshold for each velocity condition. Error bars are SEM for TPC1 threshold timing (horizontal) and respective stimulus variables (vertical) across fish. Dotted lines represent the mean visual angle and edge velocity at TPC1 threshold across all conditions.
To quantify the anatomical distribution of looming-selective neurons across brain regions and fish, we mapped active neurons to a standard fish brain (Ahrens et al., 2012) after assigning a loom/flash selectivity index (SI, (zloom − zflash)/(zloom + zflash), Experimental Procedures) that effectively classified neural responses, with more positive values reflecting greater looming selectivity (Figure 3C). This analysis revealed a preponderance of looming-selective neurons in the ventral OT compared to the other midbrain regions we analyzed (Figure 3D). Furthermore, looming-selective activity in the OT typically peaked prior to the end of expansion, consistent with the timing of escape initiations (see examples in Figure 3B). On average, the OT was more than twice as selective as PT/TH or MB (0.35 ± 0.01, 0.17 ± 0.01, 0.11 ± 0.01 SI, respectively; OT, PT/TH p < 10−5; OT, MB p < 10−5, permutation test) and contained almost twice as many responsive neurons per unit volume (3.32 ± 0.40, 1.68 ± 0.27, 2.16 ± 0.32 active neurons/1000 μm3, respectively; OT, PT/TH p = 0.001; OT, MB p = 0.010, permutation test). Furthermore, responses in the OT were also lateralized (Movie S2), with neurons in the left OT responding to looming stimuli in the right visual field and vice versa, consistent with the contralateral segregation of retinal streams and providing a putative mechanism for the observed lateralization of escape trajectory. These results suggest that the OT serves as a primary nucleus involved in looming detection within the larval zebrafish brain.
Population activity encodes critical image size during looming
If the OT is fundamentally involved in looming processing, activity in the OT should reflect the input-output relationships observed in freely swimming fish. To test this, we presented a set of monocular looming stimuli expanding at 3 different R/V ratios (545 ms, 1090 ms, 2730 ms) while imaging neural responses in the periventricular neurons (PVNs) of the ventral OT.
Like ventral OT responses to constant radial expansion, activity in ventral OT neurons typically peaked prior to the end of expansion under different velocity conditions (Figure 3E, top, Supplemental Figure 3C). The timing of these peaks relative to stimulus onset, however, was strongly influenced by expansion velocity, with responses to the slowest stimulus peaking nearly 8 seconds after responses to the fastest; this trend was reminiscent of the velocity-latency relationship we observed in freely swimming fish (Figure 2D). In order to better evaluate this correspondence, we performed principal component analysis in order to provide an unbiased estimate of looming representation across the OT neuronal population.
After aligning convolved stimulus size over time with the temporal evolution of the first principal component (temporal principal component, TPC1), which explained between 44% and 82% of the neural response variance (Supplemental Figure 3A, B), a clear link between TPC1 and stimulus size is revealed (Figure 3E, bottom). Across all 3 velocity conditions, the OT population consistently signals a common angular image size (66.0 ± 4.5°, mean ± SEM across all conditions from 10 fish, 1816 neurons, dotted black line in Figure 3F, left; see Experimental Procedures; p = 0.955, 1-way ANOVA; compare to Figure 2D,E) during expansion as its activity crosses a fixed threshold (81% of the normalized peak TPC1 across stimuli, see Experimental Procedures). In order to estimate the visual angle signaled by TPC1, which reflects activity convolved with indicator dynamics that temporally shift underlying representations, this analysis was performed using convolved stimulus variables (GCaMP5G kernel, τ = 962 ms (Chen et al., 2013)); a similar threshold angular image size is found when relating deconvolved TPC1 dynamics to raw visual angle (64.9 ± 5.0°, Supplemental Figure 3D). This threshold angular image size represented by the OT population is in close agreement with the critical image size found to trigger the behavior in freely swimming experiments (72.0 ± 1.3°). Also similar to the behavior, TPC1 does not reach a threshold at a coherent edge velocity (Figure 3F, right). These data argue that the OT is capable of encoding a critical looming visual angle as an ensemble, providing an example of a putative mechanism for salient expansion encoding across a collicular population.
RGC terminals and SINs in the OT encode diverse features of looming stimuli
In order to dissect the role of individual neural components of tectal circuitry in generating the population activity encoding critical angle, we analyzed activity patterns in two distinct neural cell types: retinal ganglion cells (RGCs) that send axons into the four input layers of the OT (SO, SFGS, SGC, SAC, (Nikolaou et al., 2012)), and superficial inhibitory interneurons (SINs), which have been shown to play a significant role in filtering out large (Del Bene et al., 2010) as well as small (Preuss et al., 2014) moving visual stimuli. In order to measure activity in RGC terminals, we generated a transgenic fish line (UAS:SyGCaMP6s) in which GCaMP6s (Chen et al., 2013) is linked to synaptophysin and expression in RGCs is driven by an Isl2b:Gal4 line (Figure 4A (Nikolaou et al., 2012)). Presentation of looming stimuli at three different approach speeds revealed diverse dynamics in RGC terminals. Using a regression-based clustering approach (Experimental Procedures, (Bianco and Engert, 2015)) we identified four major clusters capturing distinct properties of the rapidly evolving looming stimuli (Figure 4B). Notably, none of these clusters sufficiently match the population activity signatures extracted from the PVN population (see Figure 5), arguing that significant processing occurs between retinal OT inputs and downstream PVNs.
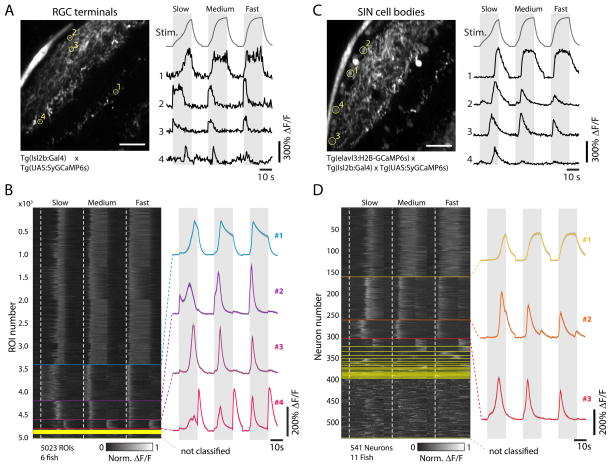
Figure 4. Retinotectal processing of looming stimuli.
(A) Left, representative imaging plane from a 5 dpf Tg(Isl2b:Gal4;UAS:SyGCaMP6s) fish, which specifically expresses GCaMP6s in the axon terminals of RGCs. Scale bar 20 μm. Right, traces from the 4 ROIs indicated on the left (yellow circles), with convolved looming stimulus time courses for slow (R/V = 2730 ms), medium (R/V = 1090 ms), and fast (R/V = 545 ms) stimuli shown on top for reference. Grey intervals denote stimulus duration.
(B) Left, raster plot after regression cluster analysis of normalized ΔF/F responses for n = 5023 RGC terminal ROIs across N = 6 fish, sorted according to the number of individual traces assigned to each respective cluster. Start frames for each stimulus are denoted by the dotted white, vertical lines. Different clusters are separated by horizontal lines. Right, mean traces of the 4 main clusters (containing at least 2% of the total ROIs from at least 5 fish, top to bottom: clusters 1–4, N = 3420, 788, 413, 192ROIs, respectively).
(C) Left, representative imaging plane from a 5 dpf Tg(elavl3:H2B-GCaMP6s;Isl2b:Gal4;UAS-SyGCaMP6s) fish, which labels SINs. Scale bar 20 μm. Right, traces from the 4 ROIs indicated on the left (yellow circles).
(D) Left, raster plot after regression cluster analysis of normalized ΔF/F responses for n = 541 SIN neurons across N = 11 fish, sorted according to the number of individual traces assigned to each respective cluster. Right, mean traces of the 3 main clusters (containing at least 2% of the total neurons from at least 8 fish, top to bottom: clusters 1–3, N = 163, 101, 44 neurons, respectively).
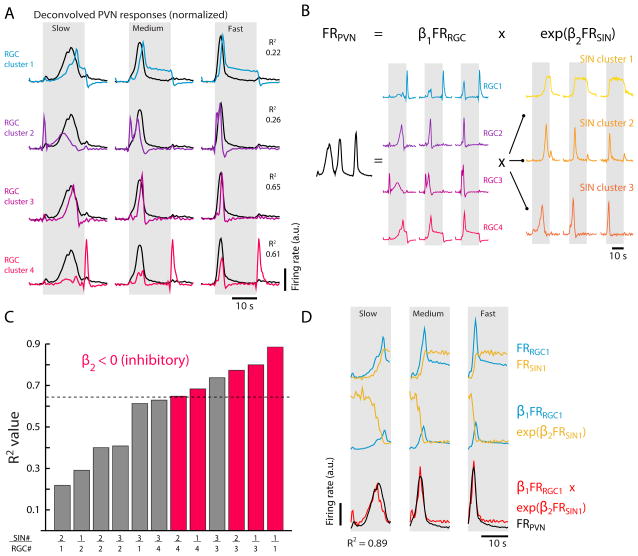
Figure 5. Regression models predict PVN responses to looming stimuli.
(A) Deconvolved mean RGC cluster traces (colored) overlaid on the deconvolved primary PVN trace (black, see Supplemental Figure S4A, derived from the dataset in Figure 3E) collected in response to slow (R/V = 2730 ms), medium (R/V = 1090 ms), and fast (R/V = 545 ms) looming stimuli. R2 values are calculating during stimulus period, gray. Each RGC trace is normalized to the maximum of the PVN trace across stimuli.
(B) Schematic of the eta-like non-linear regression model used to explore whether inhibition from SIN clusters can be combined with excitation from RGC clusters to tune PVN responses. The model attempts to explain the deconvolved firing rate of PVNs (FRPVN) using a combination of scaled deconvolved RGC firing rate (FRRGC) and exponentially weighted deconvolved SIN firing rate (FRSIN), pairwise for each identified response cluster.
(C) Bar plot of the best-fit R2 values for each SIN-RGC response pair, as determined by the non-linear regression in (B). Four combinations predict PVN responses better than the best-matched RGC cluster (black dotted horizontal line, R2 = 0.65). Best-fit individual regression models that assign negative β2 coefficients, and thus accurately treat GABAergic SINs as inhibitory, are shown in pink.
(D) Illustration of the best SIN-RGC regression model (right-most bar in (C), R2 = 0.89). Top, Normalized FRRGC (blue) and FRSIN (gold) for clusters 1 and 1, respectively. Middle, individual model terms incorporating FRRGC (blue) and FRSIN (gold) and their respective best-fit coefficients. These traces are multiplied to arrive at a prediction of PVN activity (bottom, red). Note that FRRGC is suppressed significantly by FRSIN as stimuli increase in size, effectively shifting the FRRGC peak to better match FRPVN (bottom, black).
In order to isolate putative processing units within the OT that might transform RGC inputs into the observed PVN population responses, we used a previously described line expressing nuclearly localized GCaMP6s (Freeman et al., 2014) under the elavl3 promoter, which, among other tectal neurons, also labels the SIN population. In this line, SINs are easily separated from other neurons in the OT because they are spatially segregated within neuropil layers (Figure 4C). Regression-based cluster analysis identified three main SIN response types, characterized by SIN responses to looming stimuli of different speeds (Figure 4D).
Non-linear regression identifies two SIN response types as potential computational modules honing RGC input in the OT
We next tested whether one or several of the SIN response types are sufficient to generate the signals recorded in the PVNs when they are allowed to operate on the input signals arriving in the OT via RGC projections. Figure 5A shows the four deconvolved RGC response types overlaid on the deconvolved average trace of the primary PVN response type extracted via cluster analysis (Supplemental Figure 4); we focused on this PVN cluster because its activity appeared to shape TPC1 almost exclusively (Supplemental Figure 4). It is clear that there is no perfect overlap between the PVN response and any of the RGC responses. The presence of well-characterized, wide-field GABAergic inhibitory interneurons (SINs) operating on the excitatory RGC inputs, however, suggested that the PVN population might inherit its response profile in a manner consistent with invertebrates, where excitatory and inhibitory inputs are nonlinearly combined to create receptive fields that encode critical angle (Gabbiani et al., 1999). To ask whether a similar model, using excitation from RGCs and inhibition from SINs, might explain PVN responses, we tested pairwise combinations of RGC and SIN response types using a nonlinear regression analysis (Figure 5B). Across all pairs, four show significantly enhanced R2 values, with three pairs, including the best model, properly incorporating SIN activity as inhibitory (Figure 5C). Of these three pairs, two share a common RGC type, and two share a common SIN type. Notably, the best model uses an RGC type (no. 1) that, on its own, explains PVN activity poorly (alone R2 = 0.22, with SINs R2 = 0.89), illustrating that SIN inhibition may contribute to PVN dynamics significantly. This fit is also better than that achieved with a linear regression (R2 = 0.79) combining all 4 RGC types. Furthermore, the best RGC-SIN model uses the most frequent RGC type (no. 1) and SIN type (no. 1), suggesting that OT looming computations are salient and comprise fundamental functional units established by direct retinal excitation and indirect, processed retinal inhibition.
The Mauthner system dictates looming-evoked escape direction
After looming stimuli are processed by the OT, the OT must recruit a specific motor program that completes the sensorimotor transformation (see proposed model, Figure 6A). In adult teleost fish, activity in the Mauthner cell (M-cell), a large hindbrain spinal projection neuron involved in mechanosensory escapes, is correlated with looming-evoked escapes, (Preuss et al., 2006) and the M-cell receives projections from the OT on its ventral dendrite (Zottoli et al., 1987). Given this history and the kinematic similarities between the escape responses evoked by mechanosensory and looming stimuli, we hypothesized that the M-cell and its segmental homologues, morphologically and functionally similar neurons in rhombomeres 4–6 (Liu and Fetcho, 1999), would govern visually evoked escape behavior.
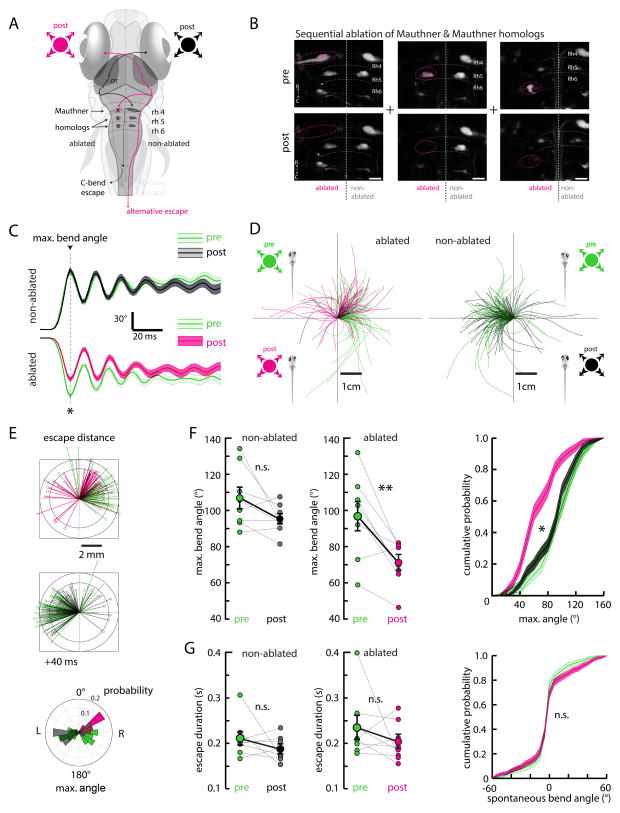
Figure 6. Laser ablation of the Mauthner system alters escape trajectory and reduces initial bend angle.
(A) Schematic of the zebrafish brain and hypothesized information flow from the eye, through the contralateral OT, to the contralateral hindbrain Mauthner system (M-system) comprising the Mauthner cell (M-cell) and homologues in rhombomeres 4–6.
(B) Two-photon micrographs showing an example of the M-cell (left), MiD2 (center), and MiD3 (right) pre- and post- unilateral ablation. For each fish, the M-cell and MiD2/MiD3 clusters were ablated together. Ablations were specific to targeted neurons and did not affect nearby cells. Cells were backfilled with dextran-conjugated dye. Scale bar is 20 μm.
(C) The average maximum turn angle during escapes is significantly altered for maneuvers contralateral to the ablated side, with the largest change evident at the first (maximum) bend. The average escape ipsilateral to the ablated side remained unchanged. All traces are aligned to the time point of the first bend. Error is SEM across all trials, N = 8 fish. Pre, non-ablated, n = 60; pre, ablated, n = 73; post, non-ablated, n = 88; post, ablated, n = 66. (*) p = 0.002.
(D) Left, escape trajectories pre- and post-ablation (green and pink, respectively) elicited by stimuli ipsilateral to the ablated side. Right, escape trajectories pre- and post-ablation (green and black, respectively) elicited by stimuli contralateral to the ablated side.
(E) Top, stick diagrams representing fish position 40 ms after escape initiation for the ablated and non-ablated sides, pre- and post-ablation. The shift in escape trajectory post-ablation is more apparent on this time scale. Bottom, angular histogram of the maximum turn angle pre- and post-ablation for the ablated and non-ablated sides.
(F) Left, quantification of maximum turn angle across fish pre- and post-ablation for the ablated and non-ablated sides. (**) p = 0.002, (n.s.) p = 0.093, permutation test. Right, this change is also apparent in histograms of bend angle across all trials, p <10−5 ablated side, p = 0.175 non-ablated side, permutation test. Error is bootstrapped SEM.
(G) Left, quantification of escape duration. No significant change is apparent on either side after ablation. Non-ablated, p = 0.073. Ablated, p = 0.101, permutation test. Error bars are SEM across fish. Right, the distribution of spontaneous turns does not change after ablation, providing further evidence of ablation specificity; pre-ablation n = 744, post-ablation n = 911 spontaneous swim events, (n.s.) p = 0.725, permutation test. Error is bootstrapped SEM.
To test this hypothesis, we backfilled the hindbrain reticulospinal system (Huang et al., 2013; O’Malley et al., 1996) to label the M-cell and its homologues (MiD2 and MiD3) and target them for laser ablation with a two-photon microscope. A short (100 ms to 2 s), high power (~100 mW at sample) laser pulse (Orger et al., 2008) was sufficient to cause a loss of cell morphology and fluorescence specific to the targeted neuron and not its labeled neighbors (Figure 6B). Because escape direction is lateralized and easily separated by left-right stimulus position, we performed unilateral ablations of the M-cell and its homologues, using the intact contralateral side as an internal control. Analysis of monocular looming-evoked escapes before and after unilateral ablation revealed a pronounced decrease in maximum turn angle (Figure 6C). Only escape responses contralateral to the ablated M-system (M-cell, MiD2, and MiD3) were perturbed (non-ablated side, pre 102.5 ± 3.6°, post 97.7 ± 3.5°, p = 0.093; ablated side, pre 99.1 ± 3.3°, post 71.8 ± 3.4°, p = 0.002). This change is consistent with the laterality conferred by the descending axons of the M-system (Gahtan and O’Malley, 2003) and is not explained by non-specific perturbations of other spinal projection neurons like the ventromedially located spinal projection neurons (Huang et al., 2013), which determine the direction of ipsilateral turns. As a result of this turn deficit, escape trajectories also changed (Figure 6D). However, the reduction in turn angle and trajectory was not concomitant with an obvious decrease in escape velocity or distance (Figure 6E). The turn deficit was confirmed on a fish-by-fish basis (Figure 6F), and cumulative distribution plots of maximum turn angle revealed a significant shift in turn angle for the ablated side across all trials (p<10−5).
Other kinematic parameters like escape duration (Figure 6G) remained unchanged for responses to both the non-ablated and ablated side (non-ablated side, pre 187.0 ± 9.7 ms, post 170.9 ± 5.1 ms, p = 0.073, permutation test; ablated side, pre 194.7 ± 9.8 ms, post 176.4 ± 5.9 ms, p = 0.101, permutation test), suggesting that an independent population of neurons may control the late phase of the visually evoked escape response. We also analyzed histograms of spontaneous turn angles, which were not significantly different post-ablation (Figure 6G, right). As spontaneous turns are thought to be governed by separate premotor circuitry (Huang et al., 2013), we conclude that ablations were specific to the intended M-system targets.
These data provide strong evidence for multi-modal convergence of sensory signals within the M-system of larval zebrafish and is the first study to establish a necessary role of the M-system in visually evoked behavior. The M-system assumes an essential role in the sensorimotor transformation from looming stimuli to escape behavior, providing a functional scaffold for the zebrafish to quickly evade threats identified with their eyes alone.
DISCUSSION
We have shown, to the best our knowledge, the first quantification of visually evoked escape behavior in freely swimming larval zebrafish. This escape behavior is elicited specifically by looming stimuli and not by flashed or dimming spots, illustrating a highly tuned system for processing image expansion within the zebrafish visual system. High-angle turns elicited by whole-field dark flashes have been described previously (Burgess and Granato, 2007b; Chen and Engert, 2014; Huang et al., 2013), but these maneuvers (“o-bends”) are relatively slow and are not followed by high-velocity burst swims. Furthermore, o-bend directionality appears linked to either turn history (Chen and Engert, 2014) or asymmetries in field luminance (Burgess and Granato, 2007b), not stimulus position. Thus, the dark flash response appears more to re-orient larvae than to propel them away from harm. Given these differences, we believe that looming-evoked escapes represent a separate, novel class of visually evoked behavior in larval zebrafish.
Analysis of the relationship between escape latency and approach velocity under our experimental conditions revealed a threshold angular image size for behavior initiation that is reminiscent of looming size thresholds in other organisms (Fotowat and Gabbiani, 2011; Oliva et al., 2007). In locusts and flies, the link between stimulus dynamics and behavior has been traced to a pair of wide-field neurons whose spike rates during looming can be described by a function (typically labeled eta) that peaks with a fixed delay before a critical visual angle (Hatsopoulos et al., 1995) and the onset of escape (Fotowat and Gabbiani, 2007). We provide evidence that a similar non-linear computation involves primary RGC and SIN types, but the specific way in which these components are combined to affect population activity in the OT are, as of yet, unknown.
In order to gain additional insight into the specific neural computation underlying looming detection, we functionally imaged main retino-recipient midbrain structures as they responded to looming and flashed stimuli and uncovered a preponderance of looming-selective neurons within the ventrocaudal OT. This selectivity may be conferred by the integration of motion-selective inputs from the retina (Nikolaou et al., 2012) or from within the OT itself (Gabriel et al., 2012; Grama and Engert, 2012). Although we have not assessed the necessity of the OT for looming-evoked escapes, the high density of looming-selective responses in the ventrocaudal OT provides strong correlative evidence that the OT is involved in processing looming stimuli; extra-tectal neurons and arborization fields may process other visual cues like whole-field motion and luminance changes. Given the broad responsiveness of neurons within the OT and the nature of our stimulus delivery (onto the dorsal retina), it is likely that the concentration of looming selectivity in the ventrocaudal OT arises primarily due to established retinotopy (Nevin et al., 2010) and does not reflect a specialized processing region. However, the broad spatial distribution of activity within the OT, along with our principal component analysis, does suggest that the computation and isolation of looming-related features from the visual scene may operate at a network level before activity is projected out of the OT to recruit downstream motor programs.
While it remains possible that a subset of looming-selective neurons in the OT form a specialized class of looming detectors that drive behavior, a distributed representation of critical visual angle presents several distinct advantages. First, the majority of tectal neuron spatial receptive fields (as assayed by moving spots) are smaller than the critical image size that appears to trigger the escape behavior (Niell and Smith, 2005). Large stimuli may thus be encoded best by a combination of multiple tectal neurons staggered over retinotopic space. Second, a distributed representation of stimuli may increase the overall flexibility of stimulus representation in the OT. Because animals must extract relevant information from complex visual scenes occupying a large space of possible stimulus combinations, a functional platform for encoding many different stimuli across the OT population can guide behavior more amenably. The ensemble encoding of critical visual angle during stimulus expansion thus constitutes a small subset of the total response space likely spanned by the OT. Last, high-dimensional representations of stimuli have been shown to improve animal performance on tasks by increasing the degeneracy of available input-output relationships in readout neurons (Rigotti et al., 2013). It is possible that the escape circuitry presented here utilizes a similar strategy to ensure that escapes are triggered reliably in individual animals and across stimulus presentations.
We have shown that the OT population representation of looming stimuli may require distinct computational modules that contribute uniquely to the encoding of critical visual angle during stimulus expansion. GCaMP6s expression in RGC presynaptic boutons allowed us to measure RGC activity patterns in response to looming stimuli. We used these patterns to construct a model predicting that OT ensemble activity is generated through a modulation of one class of RGC inputs by a specific subset of SINs that may act individually, but also in concert, to shape the population code across PVNs. These PVNs could then innervate the M-cell via synapses that might further be gated or modulated by independent or parallel inputs. The experiments reported here thus provide a framework for a quantitative model of looming selectivity, whose precise details remain to be identified by future experiments.
A recent study analyzed looming-evoked behavior in head-restrained zebrafish larvae (Temizer et al., 2015) and found that escapes are elicited by expanding dark or bright disks in the visual field. Consistent with our study, the authors discovered a size threshold for the behavior, albeit of a value different from our own observations (21.7° visual angle). This discrepancy probably results from differences in experimental conditions (i.e. head-restrained side-projection vs. freely swimming bottom-projection); future analyses, such as consideration of visual solid angle and Snell’s law (Wolf and Krotzsch, 1994), should help reconcile these results. In addition, Temizer et al. show that RGC arbors in the SFGS of the OT are selective for looming over dimming stimuli. We show, however, that simply integrating over RGCs is insufficient to explain the timecourse of the PVN dynamics correlated with critical angle. Rather, we suggest that further intratectal processing via interneurons in the upper layers of the OT is necessary.
Last, we sought to shed light on the involvement of the M-cells in the context of looming evoked escapes. While canonical C-start escape responses are preceded by a spike in the M-cell, ablation of the M-system only affects escape latency and bend velocity, not bend angle, when assayed with acoustic or tactile stimuli (Burgess and Granato, 2007a; Liu and Fetcho, 1999). These results have cemented an idea of parallel hindbrain escape circuitries that form a redundant pathway for escape behavior. However, ablation of the M-system results in a specific bend deficit in response to looming stimuli, suggesting that visual stimuli recruits only a subset of the available escape circuitry, perhaps reflecting a functional bias towards more reliable modalities (e.g. mechanosensation). How the escape circuitry receives signals from visual areas to trigger an escape is still unclear, however. Anatomical and functional evidence from adult goldfish has suggested a direct pathway from the OT to the ventral dendrite of the M-cell (Zottoli et al., 1987), but this pathway has yet to be confirmed in larval zebrafish, leaving open the possibility of either a direct or indirect path from the OT. Taken together, our study provides an overview of a potential circuit mediating a visually evoked escape behavior in a vertebrate model organism and provides an important foundation for future studies of ethologically relevant tectal processing.
EXPERIMENTAL PROCEDURES
Behavior
Larvae (5–6 dpf) were monitored at 506 fps using a high-speed camera (Mikrotron GmbH) in a 9.2 cm petri dish (VWR), 3–5 mm water height. Custom-written C# software extracted fish position and orientation and updated stimuli in a closed-loop configuration. For constant radial expansion trials, the stimulus was centered 0.5 cm to either the left or right of the fish center of mass. Stimuli expanded with a constant radial velocity of 0.5 or 0.6 cm/s until reaching r = 1 cm and disappeared 5 seconds after expansion commenced.
Calcium imaging
To assay neural responses, 5–6 dpf Tg(elval3:GCaMP5G) larvae (Ahrens et al., 2013) were paralyzed with alpha-bungarotoxin (1 mg/mL, Invitrogen) and embedded in 2% low melting point agarose before being imaged with a custom-built two-photon laser scanning microscope. Stimuli were presented onto a screen underneath the fish using a DLP projector (Dell M109S), similar to freely swimming experiments.
For more details regarding behavior analyses, imaging analyses, ablations, and statistics, see Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We are grateful to James Fitzgerald for aiding in the initial analysis of the imaging data and to Jeremy Freeman for providing advice on statistical analysis. We thank Drew Robson, Jennifer Li, and Michael Orger for generating the Tg(elval3-GCaMP5G) fish line. We thank Jessica Miller, Steve Zimmerman, and Alex Schier for fish husbandry. We thank Evan Feinberg, Arjun Krishnaswamy, Joseph Bell, Isaac Bianco and Christopher Harvey for providing helpful feedback on the manuscript. T.W.D. was supported by the National Science Foundation Graduate Research Fellowship Program, and his collaboration with C.G. and F.D.B. was made possible by the NSF Graduate Research Opportunities Worldwide Fellowship. E.A.N. was supported by a Marie Curie fellowship. F.D.B. was supported by an ATIP/AVENIR-program starting grant and by ERC-StG no. 311159, CNRS, INSERM and Institut Curie core funding. We thank the Developmental Biology Curie imaging facility (PICT-IBiSA@BDD, Paris, France, UMR3215/U934) member of the France-BioImaging national research infrastructure for their help and advice with confocal microscopy. The Del Bene laboratory “Neural Circuits Development” is part of the Laboratoire d’Excellence (LABEX) entitled DEEP (ANR -11-LABX-0044), and of the École des Neurosciences de Paris Ile-de-France network. C.G. was supported by a postdoctoral fellowship from the Fondation pour la Recherche Médicale (FRM).
Footnotes
AUTHOR CONTRIBUTIONS
T.W.D, M.B.A., F.E., and F.D.B conceived the project; T.W.D carried out the experiments and analyzed the data; C.G. analyzed data and conceived the SIN experiments. C.R. generated transgenic fish lines. T.W.D., C.G., E.A.N, M.B.A, F.E., and F.D.B wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrens MB, Li JM, Orger MB, Robson DN, Schier AF, Engert F, Portugues R. Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature. 2012;485:471–477. doi: 10.1038/nature11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods. 2013;10 doi: 10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- Armstrong PB, Higgins DC. Behavioral encephalization in the bullhead embryo and its neuroanatomical correlates. J Comp Neurol. 1971;143:371–384. doi: 10.1002/cne.901430307. [DOI] [PubMed] [Google Scholar]
- Baranauskas G, Svirskiene N, Svirskis G. 20Hz membrane potential oscillations are driven by synaptic inputs in collision-detecting neurons in the frog optic tectum. Neurosci Lett. 2012;528:196–200. doi: 10.1016/j.neulet.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Del Bene F, Wyart C, Robles E, Tran a, Looger L, Scott EK, Isacoff EY, Baier H. Filtering of Visual Information in the Tectum by an Identified Neural Circuit. Science. 2010;330:669–673. doi: 10.1126/science.1192949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Engert F. Visuomotor Transformations Underlying Hunting Behavior in Zebrafish. Curr Biol. 2015;25:831–846. doi: 10.1016/j.cub.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Kampff AR, Engert F. Prey capture behavior evoked by simple visual stimuli in larval zebrafish. Front Syst Neurosci. 2011;5:101. doi: 10.3389/fnsys.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington J, Wilkie RM, Field DT, Wann JP. Neural processing of imminent collision in humans. Proc Biol Sci. 2011;278:1476–1481. doi: 10.1098/rspb.2010.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budick SA, O’Malley DM. Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol. 2000;203:2565–2579. doi: 10.1242/jeb.203.17.2565. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Sensorimotor gating in larval zebrafish. J Neurosci. 2007a;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. J Exp Biol. 2007b;210:2526–2539. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Easter SS. Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio) J Comp Neurol. 1994;346:583–600. doi: 10.1002/cne.903460410. [DOI] [PubMed] [Google Scholar]
- Card G, Dickinson MH. Visually mediated motor planning in the escape response of Drosophila. Curr Biol. 2008;18:1300–1307. doi: 10.1016/j.cub.2008.07.094. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Engert F. Navigational strategies underlying phototaxis in larval zebrafish. Front Syst Neurosci. 2014;8:39. doi: 10.3389/fnsys.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr Ra, Orger MB, Jayaraman V, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GW. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Dickinson M, Moss CF. Neuroethology. Curr Opin Neurobiol. 2012;22:177–179. doi: 10.1016/j.conb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Domenici P, Booth D, Blagburn JM, Bacon JP. Cockroaches Keep Predators Guessing by Using Preferred Escape Trajectories. Curr Biol. 2008;18:1792–1796. doi: 10.1016/j.cub.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn OJ. Multiple Comparisons Among Means. J Am Stat Assoc. 1961;56:52–64. [Google Scholar]
- Eaton RC, Emberley DS. How stimulus direction determines the trajectory of the Mauthner-initiated escape response in a teleost fish. J Exp Biol. 1991;161:469–487. doi: 10.1242/jeb.161.1.469. [DOI] [PubMed] [Google Scholar]
- Eaton RC, DiDomenico R, Nissanov J. Flexible Body Dynamics of the Goldfish C-Start: Implications for Reticulospinal Command Neurons. J Neurosci. 1988;8:2758–2768. doi: 10.1523/JNEUROSCI.08-08-02758.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton RC, Lee RK, Foreman MB. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol. 2001;63:467–485. doi: 10.1016/s0301-0082(00)00047-2. [DOI] [PubMed] [Google Scholar]
- Ewert JP. Neural correlates of key stimulus and releasing mechanism: a case study and two concepts. Trends Neurosci. 1997;20:332–339. doi: 10.1016/s0166-2236(96)01042-9. [DOI] [PubMed] [Google Scholar]
- Fotowat H, Gabbiani F. Relationship between the phases of sensory and motor activity during a looming-evoked multistage escape behavior. J Neurosci. 2007;27:10047–10059. doi: 10.1523/JNEUROSCI.1515-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotowat H, Gabbiani F. Collision Detection as a Model for Sensory-Motor Integration. Annu Rev Neurosci. 2011;34:1–19. doi: 10.1146/annurev-neuro-061010-113632. [DOI] [PubMed] [Google Scholar]
- Fotowat H, Harrison RR, Gabbiani F. Multiplexing of Motor Information in the Discharge of a Collision Detecting Neuron during Escape Behaviors. Neuron. 2011;69:147–158. doi: 10.1016/j.neuron.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Vladimirov N, Kawashima T, Mu Y, Sofroniew NJ, Bennett DV, Rosen J, Yang C-T, Looger LL, Ahrens MB. Mapping brain activity at scale with cluster computing. Nat Methods. 2014 doi: 10.1038/nmeth.3041. [DOI] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Laurent G. Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci. 1999;19:1122–1141. doi: 10.1523/JNEUROSCI.19-03-01122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Mo C, Laurent G. Invariance of angular threshold computation in a wide-field looming-sensitive neuron. J Neurosci. 2001;21:314–329. doi: 10.1523/JNEUROSCI.21-01-00314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel JP, Trivedi CA, Maurer CM, Ryu S, Bollmann JH. Layer-Specific Targeting of Direction-Selective Neurons in the Zebrafish Optic Tectum. Neuron. 2012;76:1147–1160. doi: 10.1016/j.neuron.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Gahtan E, O’Malley DM. Visually Guided Injection of Identified Reticulospinal Neurons in Zebrafish: A Survey of Spinal Arborization Patterns. J Comp Neurol. 2003 doi: 10.1002/cne.10621. [DOI] [PubMed] [Google Scholar]
- Gahtan E, Sankrithi N, Campos JB, Malley DMO, Low SE, Amburgey K, Horstick E, Linsley J, Sprague SM, Cui W, et al. Evidence for a Widespread Brain Stem Escape Network in Larval Zebrafish. J Neurophysiol. 2012:608–614. doi: 10.1152/jn.00596.2001. [DOI] [PubMed] [Google Scholar]
- Grama A, Engert F. Direction selectivity in the larval zebrafish tectum is mediated by asymmetric inhibition. Front Neural Circuits. 2012;6:59. doi: 10.3389/fncir.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsopoulos N, Gabbiani F, Laurent G. Elementary Computation of Object Approach by a Wide-Field Visual Neuron. Science. 1995;270:1000–1003. doi: 10.1126/science.270.5238.1000. [DOI] [PubMed] [Google Scholar]
- Huang K-H, Ahrens MB, Dunn TW, Engert F. Spinal Projection Neurons Control Turning Behaviors in Zebrafish. Curr Biol. 2013:1–8. doi: 10.1016/j.cub.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries DA, Driver PM. Protean Defence by Prey Animals. Oecologia. 1970;5:285–302. doi: 10.1007/BF00815496. [DOI] [PubMed] [Google Scholar]
- Hunter PR, Lowe AS, Thompson ID, Meyer MP. Emergent properties of the optic tectum revealed by population analysis of direction and orientation selectivity. J Neurosci. 2013;33:13940–13945. doi: 10.1523/JNEUROSCI.1493-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakhalin AS, Koren D, Gu J, Xu H, Aizenman CD. Excitation and inhibition in recurrent networks mediate collision avoidance in Xenopus tadpoles. Eur J Neurosci. 2014:1–15. doi: 10.1111/ejn.12664. [DOI] [PubMed] [Google Scholar]
- Kohashi T, Oda Y. Initiation of Mauthner- or non-Mauthner-mediated fast escape evoked by different modes of sensory input. J Neurosci. 2008;28:10641–10653. doi: 10.1523/JNEUROSCI.1435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohashi T, Nakata N, Oda Y. Effective sensory modality activating an escape triggering neuron switches during early development in zebrafish. J Neurosci. 2012;32:5810–5820. doi: 10.1523/JNEUROSCI.6169-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kubo F, Hablitzel B, Dal Maschio M, Driever W, Baier H, Arrenberg AB. Functional Architecture of an Optic Flow-Responsive Area that Drives Horizontal Eye Movements in Zebrafish. Neuron. 2014;81:1344–1359. doi: 10.1016/j.neuron.2014.02.043. [DOI] [PubMed] [Google Scholar]
- Landwehr K, Brendel E, Hecht H. Luminance and contrast in visual perception of time to collision. Vision Res. 2013;89:18–23. doi: 10.1016/j.visres.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Muijres FT, Elzinga MJ, Melis JM, Dickinson MH. Flies evade looming targets by executing rapid visually directed banked turns. Science. 2014;344:172–177. doi: 10.1126/science.1248955. [DOI] [PubMed] [Google Scholar]
- Muto A, Ohkura M, Abe G, Nakai J, Kawakami K. Real-time visualization of neuronal activity during perception. Curr Biol. 2013;23:307–311. doi: 10.1016/j.cub.2012.12.040. [DOI] [PubMed] [Google Scholar]
- Nevin LM, Robles E, Baier H, Scott EK. Focusing on optic tectum circuitry through the lens of genetics. BMC Biol. 2010:1–10. doi: 10.1186/1741-7007-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Smith SJ. Functional imaging reveals rapid development of visual response properties in the zebrafish tectum. Neuron. 2005;45:941–951. doi: 10.1016/j.neuron.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Nikolaou N, Lowe AS, Walker AS, Abbas F, Hunter PR, Thompson ID, Meyer MP. Parametric functional maps of visual inputs to the tectum. Neuron. 2012;76:317–324. doi: 10.1016/j.neuron.2012.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley DM, Kao YH, Fetcho JR. Imaging the Functional Organization of Zebrafish Hindbrain Segments during Escape Behaviors. Neuron. 1996;17:1145–1155. doi: 10.1016/s0896-6273(00)80246-9. [DOI] [PubMed] [Google Scholar]
- Oliva D, Medan V, Tomsic D. Escape behavior and neuronal responses to looming stimuli in the crab Chasmagnathus granulatus (Decapoda: Grapsidae) J Exp Biol. 2007;210:865–880. doi: 10.1242/jeb.02707. [DOI] [PubMed] [Google Scholar]
- Orger MB, Kampff AR, Severi KE, Bollmann JH, Engert F. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat Neurosci. 2008;11:327–333. doi: 10.1038/nn2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugues R, Feierstein CE, Engert F, Orger MB. Whole-brain activity maps reveal stereotyped, distributed networks for visuomotor behavior. Neuron. 2014;81:1328–1343. doi: 10.1016/j.neuron.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss SJ, Trivedi CA, Berg-maurer CM, Ryu S. Classification of Object Size in Retinotectal Microcircuits. Curr Biol. 2014:2376–2385. doi: 10.1016/j.cub.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Preuss T, Osei-bonsu PE, Weiss SA, Wang C, Faber DS. Neural Representation of Object Approach in a Decision-Making Motor Circuit. J Neurosci. 2006;26:3454–3464. doi: 10.1523/JNEUROSCI.5259-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Reyn CR, Breads P, Peek MY, Zheng GZ, Williamson WR, Yee AL, Leonardo A, Card GM. A spike-timing mechanism for action selection. Nat Neurosci. 2014;17:962–970. doi: 10.1038/nn.3741. [DOI] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang XJ, Daw ND, Miller EK, Fusi S. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–590. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer RD, Yamawaki Y, Rind FC, Simmons PJ. Motor activity and trajectory control during escape jumping in the locust Locusta migratoria. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:965–975. doi: 10.1007/s00359-005-0023-3. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temizer I, Donovan JC, Baier H, Semmelhack JL. A Visual Pathway for Looming-Evoked Escape in Larval Zebrafish. Curr Biol. 2015:1–12. doi: 10.1016/j.cub.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Ito H. Morphological Aspects of the Teleostean Visual System: A Review. Brain Res Rev. 1983;6:117–137. doi: 10.1016/0165-0173(83)90036-x. [DOI] [PubMed] [Google Scholar]
- De Vries SEJ, Clandinin TR. Loom-sensitive neurons link computation to action in the Drosophila visual system. Curr Biol. 2012;22:353–362. doi: 10.1016/j.cub.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Distinct mechanisms for top-down control of neural gain and sensitivity in the owl optic tectum. Neuron. 2008;60:698–708. doi: 10.1016/j.neuron.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf KB, Krotzsch G. Geometry and dynamics in refracting systems. Eur J Phys. 1994;14 [Google Scholar]
- Yilmaz M, Meister M. Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol. 2013;23:2011–2015. doi: 10.1016/j.cub.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottoli SJ, Hordes aR, Faber DS. Localization of optic tectal input to the ventral dendrite of the goldfish Mauthner cell. Brain Res. 1987;401:113–121. doi: 10.1016/0006-8993(87)91170-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.