Abstract
Ghrelin’s receptor, growth hormone secretagogue receptor (GHSR), is highly expressed in the arcuate nucleus (ARC) and in neuropeptide Y (NPY) neurons. Fasting, diet-induced obesity (DIO), and 17β-estradiol (E2) influence ARC Ghsr expression. It is unknown if these effects occur in NPY neurons. Therefore, we examined the expression of Npy, Agrp, and GHSR signaling pathway genes after fasting, DIO, and E2 replacement in ARC and pools of NPY neurons. In males, fasting increased ARC Ghsr and NPY Foxo1 but decreased NPY Ucp2. In males, DIO decreased ARC and NPY Ghsr and Cpt1c. In fed females, E2 increased Agrp, Ghsr, Cpt1c, and Foxo1 in ARC. In NPY pools, E2 decreased Foxo1 in fed females but increased Foxo1 in fasted females. DIO in females suppressed Agrp and augmented Cpt1c in NPY neurons. In summary, genes involved in GHSR signaling are differentially regulated between the ARC and NPY neurons in a sex-dependent manner.
Keywords: arcuate nucleus, ghrelin, neuropeptide y, fasting, diet-induced obesity, 17β-estradiol
1. Introduction
Obesity is a global health concern due, in part, to changes in diet and lifestyle and is a major risk factor for a variety of clinical conditions including cardiovascular disease, type II diabetes, and metabolic syndrome (Mark, 2006). One hallmark of obesity is the dysregulation of the hypothalamic centers that control feeding behavior, energy expenditure, and the peripheral hormones that mediate the communication between the body and the brain (Cai and Liu, 2011). Normally, neuronal populations in the hypothalamus regulate energy homeostasis by responding to circulating nutrients as well as appetite-regulating hormones such as leptin, insulin, and ghrelin (Gao and Horvath, 2007).
The incidence of metabolic disorders associated with obesity exhibit clear sex differences with premenopausal women having fewer metabolic disorders than men. However, metabolic disorders increase dramatically in postmenopausal women (Ford, 2005; Loucks et al., 2007). The loss of the reproductive steroid 17β-estradiol (E2) is, in part, the major cause of these effects especially on body weight gain (Rachoń and Teede, 2010; Stefanska et al., 2015). In ovariectomized rodent models, E2 regulates many aspects of energy homeostasis through both peripheral actions and central mechanisms (reviewed in Mauvais-Jarvis et al., 2013; Roepke, 2009; Shi et al., 2009). E2 suppresses feeding and fat accumulation and augments energy expenditure and activity. To control energy homeostasis, E2 primarily uses the nuclear steroid receptor, ERα, which is highly expressed in the hypothalamus (Roepke, 2009). Total body knockout of ERα or deletion in specific hypothalamic neurons produces an obese phenotype with hyperphagia, higher visceral adiposity, and lower activity and energy expenditure in mice (Geary et al., 2001; Heine et al., 2000; Mamounis et al., 2014; Musatov et al., 2007; Xu et al., 2011).
Among the hypothalamic areas involved in energy homeostasis, the arcuate nucleus (ARC) is of special interest as it is an integration center for homeostatic signals from the periphery and the central nervous system (CNS). The “first order” ARC neurons central to the control of energy homeostasis are the proopiomelanocortin (POMC) and neuropeptide Y (NPY) neurons (Gao and Horvath, 2007). POMC and NPY neurons have opposing actions in the control of energy homeostasis. POMC neurons are anorexigenic primarily through the actions of α-melanocyte-stimulating hormone (α-MSH) via melanocortin receptors (MC3/4) expressed throughout the hypothalamus (Dietrich and Horvath, 2013). NPY neurons are orexigenic primarily through the actions of its neuropeptides, NPY and agouti-related peptide (AgRP), an antagonist for the MC4 receptors. Thus, the actions of POMC and NPY/AgRP neurons are important for the hypothalamic control of feeding and energy expenditure (Gao and Horvath, 2007).
Ghrelin is a brain-gut peptide hormone secreted from the stomach to stimulate food intake by acting on its receptor, growth hormone secretagogue receptor (GHSR). GHSR is expressed throughout the brain and in NPY/AgRP neurons in the ARC (Cowley et al., 2003; Willesen et al., 1999). Ghrelin-expressing neurons are also found in the periventricular region of the hypothalamus dorsal to the ARC (Guan et al., 2003; Mondal et al., 2005). ARC and peripheral ghrelin administration induces NPY/AgRP gene expression (Chen et al., 2004; Goto et al., 2006; Kamegai et al., 2001, 2000) and NPY activation (Wang et al., 2002) and potently depolarizes NPY neurons (Andrews et al., 2008; Cowley et al., 2003). Furthermore, ghrelin activation of GHSR potently excites NPY neurons and controls calcium homeostasis (Andrews et al., 2008; Cowley et al., 2003). These rapid effects of ghrelin potentially involve calcium channels (Kohno et al., 2003) and may involve the inhibition of the M-current as recently reported in striatal neurons (Shi et al., 2013).
Activation of GHSR in NPY neurons initiates a signaling cascade that involves the mitochondrial enzymes uncoupling protein 2 (UCP2) and carnitine palmitoyltransferase 1 (CPT-1) to control Npy/Agrp gene expression through a forkhead box O1 (FoxO1)-mediated mechanism (Andrews, 2011; Andrews et al., 2008; López et al., 2008a). CPT-1 is involved in malonyl-CoA sensing and fatty acid oxidation, while UCP2 is necessary for reactive oxidation species (ROS) buffering and mitochondrial biogenesis (Andrews, 2011; López et al., 2008b). Of the three types of CPT-1, hypothalamic neurons express CPT-1c, which does not have acyltransferase activity, while hypothalamic astroyctes express CPT-1a, which does have acyltransferase activity (Wolfgang et al., 2006). FoxO1 is a transcription factor that negatively regulates adipogenesis, mediates insulin-induced gluconeogenesis, and is an effector of Npy/Agrp gene expression (Cao et al., 2011; Ren et al., 2012).
Fasting and diet-induced obesity (DIO) regulate expression of Npy, Agrp, Ghsr, and Ucp2 in the ARC (Briggs et al., 2014, 2013, 2010; Coppola et al., 2007; Verhulst et al., 2012), which may be a contributing mechanism underlying the ghrelin resistance seen in DIO (Briggs et al., 2014, 2010; Perreault et al., 2004). Furthermore, a high fat diet (HFD) or DIO inhibits ghrelin’s augmentation of hyperphagia (Gardiner et al., 2010; Perez-Tilve et al., 2011). Recently, E2 was shown to increase Ghsr expression in the ARC (Frazao et al., 2014). Interestingly, E2 is an anorexigenic steroid and is known to induce Pomc and suppress Npy gene expression (Roepke, 2009). Because ghrelin is orexigenic, this E2-induced increase in Ghsr expression in the ARC may be independent of its effects on feeding and occur in other ARC neurons.
Because GHSR and the components of its signaling pathway are widely expressed in the heterogeneous cell types of the ARC, determinations of gene expression only in the ARC may lead to incorrect assumptions about their modulation in NPY neurons. Therefore, our hypothesis is that there will be significant differences in gene expression between the ARC and NPY neurons due to fasting and DIO. We also hypothesize that there will be distinct sex differences in the response to these dietary influences due, in part, to E2 in females. To address these hypotheses, we characterized the expression of Ghsr, Ucp2, Cpt1c, Foxo1, and Npy/Agrp in both the ARC and NPY neurons in males after fasting and DIO and in ovariectomized females with or without E2 replacement after fasting and DIO using wild type (WT) C57 mice and GFPNPY transgenic mice.
2. Materials & Methods
2.1. Animal Care and Experimental Design
Animal experiments described in this project are in accordance with institutional guidelines based on National Institutes of Health standards and have been approved by The Rutgers University Institutional Animal Care and Use Committee. All animals were maintained under controlled temperature and photoperiod (12 h:12 h). All mice, both WT C57BL/6J (Jackson Laboratory) and GFP-NPY ((Dr. Bradford Lowell, Harvard University, (van den Pol et al., 2009), were given free access to food and water except where noted. In experiment #1, males (10-15 weeks of age), 13 WT and 12 GFP-NPY, were either fed ad libitum (n=7 WT, n=6 GFP) or fasted (n=6 both) for 24 h prior to tissue collection or cell harvesting. In experiment #2, males (8 weeks of age), 12 WT and 12 GFP-NPY, were fed either a low-fat diet (ND, 10% kCal, n=6) or a high-fat diet (HFD, 45% kCal, n=6) for 12 weeks prior to tissue collection or cell harvesting. In experiment #3, females (10-14 weeks of age), 12 WT and 12 GFP-NPY, were ovariectomized (ovx) under isoflurane anesthesia and allowed to recover for 5 days and fed ad libitum on a low-phytoestrogen standard chow (Lab Diets 5V75). Females were then injected 48 h prior to sacrifice with 0.25 μg estradiol benzoate (EB) or oil followed by a second injection of 1.5 μg EB (n=6) or oil (n=6) 24 h prior to sacrifice. The EB injection paradigm yields physiological levels of E2 (Bosch et al., 2013). In experiment #4, females (10-14 weeks old), 12 WT and 12 GFP-NPY, were ovx under isoflurane anesthesia and allowed to recover for 5 days. Females were then injected 48 h prior to sacrifice with 0.25 μg estradiol benzoate (EB) or oil followed by a second injection of 1.5 μg EB (n=6) or oil (n=6) 24 h prior to sacrifice. These females were fasted 24 h prior to euthanasia. In experiment #5, females (8 weeks of age), 32 WT and 24 GFP-NPY, were ovx under isoflurane anesthesia and fed either ND (n=16 WT; n=12 GFP) or HFD (n=16 WT; n=12 GFP) for 8 weeks prior to tissue collection or cell harvesting. Half of each diet group was administered oil or EB (300 μg/kg) daily perorally via a peanut butter carrier (WT, all groups: n=8; GFP-NPY, all groups: n=6). We chose peroral E2 replacement for the DIO studies to reduce the stress-inducing effects of repeated injection and to maintain a constant systemic level of E2 in the blood (Ingberg et al., 2012). Body weights were measured weekly. All animals were injected with ketamine (100 μl of 100 mg/ml, i.p.) prior to decapitation. All euthanasia occurred at 1000-1100 h. Uteri were weighed in all females.
2.2. Drugs and Diets
Estradiol benzoate (EB) and 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF) were purchased from Sigma-Aldrich. EB was dissolved in 100% ethanol (Sigma) prior to dissolving in sesame oil (Sigma). Ghrelin and tetrodotoxin (TTX) were purchased from Tocris (Bristol, UK) and dissolved in water. DIO diets were purchased from Research Diets (New Brunswick, NJ): low-fat diet (10% kcal fat; D12450B) and high-fat diet (45% kcal fat; D12451).
2.3. ARC Tissue Dissections and RNA Extractions
At the end of the experiments, males and females were decapitated and the brain was extracted from the skull. The basal hypothalamus (BH) was cut using a brain slicer (Ted Pella,, Redding, CA, USA), into one mm thick coronal rostral and caudal blocks corresponding to plates 42-47 and plates 48-53, respectively, from The Mouse Brain in Stereotaxic Coordinates (Paxinos & Franklin, 2008, 3rd edition) (Paxinos and Franklin, 2008). The BH blocks were transferred to RNAlater (Life Technologies, Grand Island, NE, USA) and stored overnight at 4°C. The entire ARC was dissected from the BH slices using a dissecting microscope for storage at −80°C. Total RNA was extracted from the combined ARC using Ambion RNAqueous-Micro Kit (Life Technologies) according to the manufacturer’s protocol. Total RNA was also DNase I-treated, using the extraction kit, at 37°C for 20 min to minimize any genomic DNA contamination. RNA quantity and quality were determined using a NanoDrop ND-2000 spectrophotometer (ThermoFisher, Waltham, MA) and an Agilent 2100 Bioanalyzer with RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA). Samples with an RNA integrity number greater than 7.0 were used for analysis.
Complementary DNA (cDNA) was synthesized from 200 ng of total RNA using 50 U Superscript III reverse transcriptase (RT) (Life Technologies, Inc.), 4 μl 5x Superscript Buffer, 25 mM MgCl2, 10 mM dNTP (Clontech Laboratories, Mountain View, CA), 100 ng random hexamer primers (Promega, Madison, WI), 40 U/μl RNasin (Promega), and 100 mM DTT in DEPC-treated water (Gene Mate, Kaysville, UT) in a total volume of 20 μl. Reverse transcription was conducted using the following protocol: 5 min at 25°C, 60 min at 50°C, 15 min at 70°C. The cDNA was diluted 1:20 with nuclease-free water (Gene Mate, Bioexpress) for a final cDNA concentration of 0.5 ng/μl and stored at −20°C. BH test tissue RNA was used for positive and negative controls (no reverse transcriptase) and processed simultaneously with the experimental samples.
2.3. Cell Harvesting of Dispersed NPY Neurons
All GFP-NPY males and females were killed by decapitation after sedation. The brain was quickly removed from the skull and submerged in cold (4°C) oxygenated (95% O2, 5% CO2) high-sucrose artificial CSF (aCSF, in mmol): 208 sucrose, 2 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 2 MgSO4, 1 MgCl2, 10 HEPES (pH 7.3, 300 mOsm). Four coronal slices (250 μm) from the BH were cut on a vibratome and bathed for ~10 min in high-sucrose CSF at 4°C. The slices were then transferred to an auxiliary chamber where they were kept at room temperature (25°C) in aCSF consisting of (in mmol): 124 NaCl, 5 KCl, 2.6 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, 10 HEPES, 10 glucose (pH 7.3, 310 mOsm) for recovery of ~1 h until cell dispersal. The ARC nucleus was microdissected and incubated in 5-10 ml aCSF (pH 7.35, 300 mOsm) containing 1 mg/ml protease for 15 minutes at 37°C. The tissue was then washed four times in low-calcium CSF (0.1 mM CaCl2) and twice in aCSF. The cells were isolated by trituration with flame-polished Pasteur pipettes, dispersed onto a 60 mm glass-bottomed Petri dish, and perfused continuously with aCSF at a rate of ~2 ml/min. Fluorescent and adjacent, non-fluorescent cells were visualized using a Leica DM-IL inverted fluorescent microscope, patched, and then harvested by applying low negative pressure to the pipette using the Xenoworks manipulator system (Sutter Instruments, Novato, CA). The content of the pipette was expelled into a siliconized microcentrifuge tube containing 1 μl 5X Superscript III Buffer (Life Technologies), 15 U RNasin (Promega), 0.5 μl 100 mM DTT, and DEPC-treated water in a 8 μl volume. GFP-NPY neurons were either harvested as single cells (Fig. 1) or collected into 10-12 pools of 5 NPY neurons each from each animal (all other Fig.s).
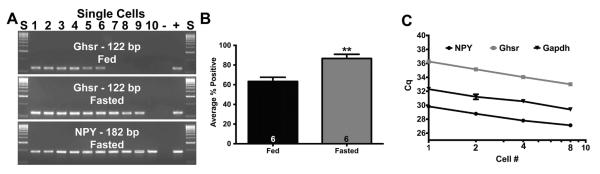
Fig. 1.
Single cell expression and linearity of Ghsr expression in NPY neurons. Fasting increased the percentage of Ghsr-expressing NPY neurons in males shown here in a representative gel (A) and summarized in a bar graph (B). Numbers in bars represent sample size. (C) Linear regression analysis plotting Cq versus the log scale of the number of cells collected in each pool to determine the linearity of mRNA expression in pools of NPY neurons compared to single neurons for Npy, Ghsr, and Gapdh expression collected from fed males (n=6). Data are represented as mean ± SEM. Bar graph was analyzed with a Student’s t-test (P < 0.01).
Each harvested cell or pool of cells was reverse transcribed as described previously (Bosch et al., 2013; Roepke et al., 2011). Briefly, the tubes containing the harvested cell solution and two tubes containing 25 ng of hypothalamic total RNA in 1 μl were denatured for 5 min at 65°C, then cooled on ice for 5 min. Single stranded cDNA was synthesized from cellular RNA by adding 50 U Superscript III RT, 3 μl 5x Superscript Buffer, 5 mM MgCl2, 0.625 mM dNTPs (Clontech), 15 U RNasin, 400 ng anchored oligo(d)T (Life Technologies), 100 ng random hexamers (Promega), 10 mM DTT in DEPC-water in a total volume of 25 μl. One single cell and one tissue RNA tube were used as negative controls and processed as described above but without RT. CSF was also collected every 2-3 pools and analyzed for any contamination. Reverse transcription was conducted using the following protocol: 5 min at 25°C, 60 min at 50°C, 15 min at 70°C. In preliminary investigation of Ghsr expression in NPY neurons, harvested single cells (10-15) were collected from male GFP-NPY mice that were either fed (n=6) or fasted (n=6) for 24 hr. Npy and Ghsr expression were analyzed using standard PCR protocols and gel electrophoresis as previously described (Roepke et al., 2011; Xu et al., 2008). Each reaction was amplified for 50 cycles using a C1000 Thermal Cycler (Bio-Rad, Hercules, CA) at an annealing temperature of 60°C. Negative (cell and tissue samples without RT), collected CSF, and positive tissue controls were analyzed with each PCR run.
2.4. Quantitative Real-time PCR
All primers were designed to span exon-exon junctions and synthesized by Life Technologies, using Clone Manager 5 software (Sci Ed Software, Cary, NC). See Table 1 for a listing of all the primer sequences used for single-cell and quantitative real-time PCR (qPCR). For ARC tissue qPCR, 4 μl cDNA template (an equivalent of 2 ng total RNA) was amplified using either PowerSYBR Green master mix (Life Technologies) or SsoAdvanced SYBR Green (BioRad, Hercules, CA) on CFX-Connect Real-time PCR instrument (BioRad). Standard curves for each primer pair were prepared using serial dilutions of BH cDNA in triplicate to determine the efficiency [E = 10(−1/m)−1, m = slope] of each primer pair. All efficiencies, expressed as % efficiency, were approximately equal (one doubling per cycle, 90-110%, Table 1).
Table 1.
Primer sequences for single cell PCR and qPCR.
| Gene Name |
Product Length |
Primer Eff. (%) |
Primer sequence | Base pair # | Accession # |
|---|---|---|---|---|---|
| Agrp | 146 | 105 | F: CTCCACTGAAGGGCATCAGAA | 287-307 | NM_007427.2 |
| R: ATCTAGCACCTCCGCCAAA | 414-432 | ||||
| Actb | 63 | 100 | F: GCCCTGAGGCTCTTTTCCA | 849-867 | NM_007393.3 |
| R: TAGTTTCATGGATGCCACAGGA |
911-990 | ||||
| Cpt1c | 191 | 96 | F:GGCTGGCATTGGTCAGAATC | 719-738 | NM_153679.2 |
| R:CGTGCAACCTCAGGAAGTC | 892-910 | ||||
| Foxo1 | 243 | 96 | F:CAATGGCTATGGTAGGATGG | 2208-2227 | NM_019739 |
| R:TTTAAATGTAGCCTGCTCAC | 2431-2450 | ||||
|
Gapd
h |
98 | 93 | F: TGACGTGCCGCCTGGAGAAA | 778-797 | NM_008084.2 |
| R: AGTGTAGCCCAAGATGCCCTTCA G |
852-875 | ||||
| Ghsr * | 122 | 111 | F: CAGGGACCAGAACCACAAAC | 1003-1022 | NM_177330 |
| R: AGCCAGGCTCGAAAGACT | 1107-1124 | ||||
| Hprt | 117 | 107 | F:GCTTGCTGGTGAAAAGGACCTC TCGAAG |
631-658 | NM_013556 |
| R:CCCTGAAGTACTCATTATAGTC AAGGGCAT |
718-747 | ||||
| Npy * | 182 | 100 | F: ACTGACCCTCGCTCTATCTC | 106-125 | NM_023456 |
| R: TCTCAGGGCTGGATCTCTTG | 268-287 | ||||
| Ucp2 | 194 | 105 | F: CATTGGCCTCTACGACTC | 668-685 | NM_011671 |
| R: CGACAGTGCTCTGGTATC | 844-861 |
Forward primer (F) is listed first with the reverse primer (R) second. AgRP, agouti-related peptide; Cpt-1c, carnitine palmitoyltransferase 1c; Foxo1, forkhead box O1; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Ghsr, Growth Hormone Secretagogue Receptor; Hprt, hypoxanthine-guanine phosphoribosyltransferase; NPY, neuropeptide Y; Ucp2, uncoupling protein 2.
also used for single-cell PCR.
The relative mRNA expression data were analyzed using the ΔΔCq method (Livak and Schmittgen, 2001; Pfaffl, 2001; Schmittgen and Livak, 2008). The amplification protocol for all the genes was as follows: initial denaturing at 95°C for 10 min (PowerSYBR) or 3 min (SsoAdvanced) followed by 40-45 cycles of amplification at 94°C for 10 sec (denaturing) and 60°C for 45 sec (annealing), and completed with a dissociation step for melting point analysis with 60 cycles of 95°C for 10 sec, 65°C to 95°C (in increments of 0.5°C) for 5 sec, and 95°C for 5 sec. The reference genes used were Actb (β-actin), Gapdh (glyceraldehyde-3-phosphate dehydrogenase), and Hprt (Hypoxanthine-guanine phosphoribosyltransferase). Positive (BH) and negative controls (BH no RT) were added to the amplification run as well as one water blank. Quantification values were generated only from samples showing a single product at the expected melting point.
Final relative quantitation was calculated using a calibrator of diluted (1:20) cDNA from the BH of an untreated, intact male. The data were reported as relative mRNA expression. To determine the Cq for each transcript, the threshold was consistently set at the lowest point of the exponential curve where the slope of the curve was the steepest for all plates. All gene expression data were calculated using the geometric mean of the Cq values for Actb, Gapdh, and Hprt to calculate the ΔCq. The relative linear quantity of target molecules was calculated using the formula 2-ΔΔCT. The n-fold difference was averaged for each treatment.
For quantification of gene expression in NPY pools, two target genes and one reference gene were analyzed in duplicate from each pool of neurons using 4 μl of sample per well with 45 cycles of amplification (Bosch et al., 2013; Roepke et al., 2008, 2011). Relative gene expression was analyzed by first averaging the relative gene expression of 3 pools from each animal. The pool average was then analyzed within each treatment (n = 6 animals, n = 3 pools/animal). Due to the small sample volume (25 μl), only Gapdh was used as a reference gene.
2.5. Preparation of Basal Hypothalamic Slices
Slices for electrophysiology were prepared as described previously (Roepke et al., 2012, 2011). Transgenic GFP-NPY mice were killed quickly by decapitation at 1000 h. The brain was rapidly removed from the skull and a block containing the BH was immediately dissected. The BH block was submerged in cold (4°C) oxygenated (95% O2, 5% CO2) high-sucrose aCSF (aCSF; in mmol): 208 sucrose; 2 KCl; 26 NaHCO3; 10 glucose; 1.25 NaH2PO4; 2 MgSO4; 1 MgCl2; 10 HEPES (pH 7.3; 300 mOsm). Coronal slices (250 μm) from the BH were cut on a vibratome and bathed for ~10 min in high-sucrose CSF at 4°C. The slices were then transferred to an auxiliary chamber in which they were kept at room temperature (25°C) in aCSF consisting of (in mM): 124 NaCl; 5 KCl; 2.6 NaH2PO4; 2 MgCl2; 2 CaCl2; 26 NaHCO3; 10 glucose (pH 7.35; 310 mOsm) until recording (recovery for 1-2 h). A single slice was transferred to the recording chamber which was mounted on an Olympus BX51W1 upright microscope equipped with video-enhanced, infrared-differential interference contrast (DIC) and Exfo X-Cite 120 Series fluorescence light source (Mississauga, Ontario, Canada). The slice was continually perfused with warm (35°C), oxygenated aCSF at 1.5 ml/min. Targeted neurons were viewed using both IR-DIC and blue excitation light with an Olympus 40X water-immersion lens.
2.6. Visualized Whole-cell Patch Recording
Normal aCSF and pipette solutions were used in electrophysiological recording (Roepke et al., 2012, 2011). Standard whole-cell patch recording procedures and pharmacological testing were as previously described (Roepke et al., 2012, 2011). Whole-cell voltage and current clamp recordings were performed using pipettes made of borosilicate glass and pulled using a P-97 Flaming/Brown Micropipette Puller (Sutter Instrument). Pipettes were filled with normal internal solution consisting of (in mM): 10 NaCl; 128 K-gluconate; 1 MgCl2; 10 HEPES; 1 ATP; 1.1 EGTA; 0.25 GTP (pH 7.3; 300 mOsm) with a 3-5 MΩ resistance. An Axopatch 200A amplifier, Digidata 1322A Data Acquisition System, and pCLAMP software (version 9.2, Molecular Devices) were used for data acquisition and analysis. Input resistance, series resistance, and membrane capacitance were monitored throughout the experiments. Only cells with stable series resistance (< 30 MΩ, < 20% change) and an input resistance > 500 MΩ were used for analysis. The access resistance was 80% compensated, and the calculated liquid junction potential (10 mV) was corrected. To display reversal potential and rectification characteristics of the ligand-activated currents, I-V plots were constructed by voltage steps from −50 to −140 mV at 10 mV increments applied at 1 s intervals from a holding potential of −60 mV. The input resistance was determined from the I-V plot as the ratio of the voltage (−60 to −80 mV) divided by the change in current (pA). In voltage clamp, a deactivation protocol was used to measure the M-current. The deactivation protocol measured the currents elicited during 500 ms voltage steps from −25 to −75 mV after a 300 ms prepulse to −20 mV, which included the membrane potential at which the maximal M-current could be obtained (Roepke et al., 2012, 2011). The amplitude of M-current relaxation or deactivation was measured as the difference between the initial (< 10 ms) and sustained current (> 475 ms) of the current trace in the control conditions (TTX only, 0.5 μM, 5 min) minus the difference in ghrelin (100 nM, 0.5 μM TTX, 10 min) conditions. The deactivation protocol was repeated twice for each bath solution and averaged for analysis.
2.7. Hormone Serum Analysis
Terminal trunk blood was collected for each WT animal immediately after decapitation. Blood samples were collected using K+ EDTA tubes. The protease inhibitor AEBSF (1 mg/ml) was quickly added to the sample and mixed. Plasma was isolated after centrifugation and the supernatant was stored at −20°C until analysis. Total ghrelin (EZRGRT-91K; Millipore, Billerica, MA) was measured as per manufacturer’s instructions. Total plasma estradiol levels were measured using Mouse/Rat Estradiol ELISA kit (ES180S-100) (Calbiotech, Spring Valley, CA).
2.8. Data Analysis
All data were analyzed using GraphPad Prism software (GraphPad Software, La Jolla, CA) and were expressed as mean ± SEM. In all cases, effects were considered significant at α ≤ 0.05. All data from quantitative real-time PCR experiments were analyzed using a two-way ANOVA followed by a post-hoc Bonferroni multiple comparison test with gene and treatment (fasting, diet, or steroid) as the two factors. Hormone data were analyzed by a two-way ANOVA followed by a post hoc Bonferroni multiple comparison test or a Student’s t-test (unpaired). Comparisons of the I-V plots between control and ghrelin perfusion were performed using a repeated measures, two-way ANOVA analysis with the post hoc Bonferroni multiple comparison test.
3. Results
3.1. Linearity of Ghsr expression in NPY neurons
To initially determine the detectability of Ghsr in NPY neurons and its regulation by fasting in males, we harvested individual GFP-NPY neurons from 6 fed males and 6 fasted males and conducted single-cell RT-PCR for Ghsr (Fig. 1A and B). Fasting increased the detection of Ghsr from 63.3 ± 4.2% in fed males to 86.7 ± 4.2% in fasted males (p < 0.01, Fig. 1B). These data suggest that fasting increases the expression of Ghsr specifically in NPY neurons. Furthermore, to determine if the linearity of mRNA expression in pools of NPY neurons compared to that in single neurons, we quantified the expression of Npy, Ghsr, and Gapdh in collection tubes containing 1, 2, 4, or 8, cells/tube from fasted males (n = 6 tubes/cell number from 2 males). Using 4 μl of pool cDNA, NPY primers produced Cq values of 29.8 ± 0.3, 28.8 ± 0.3, 27.8 ± 0.2, and 27.1 ± 0.2, for 1, 2, 4, and 8, cells/tube, respectively. GHSR primers, using 4 μl of pool cDNA, produced Cq values of 36.3 ± 0.2, 35.1 ± 0.1, 34.0 ± 0.2, and 33.0 ± 0.1 for 1, 2, 4, and 8, cells/tube, respectively. GAPDH primers, using 2 μl of pool cDNA, produced Cq values of 32.3 ± 0.2, 31.2 ± 0.4, 30.6 ± 0.3, and 29.4 ± 0.1 for 1, 2, 4, and 8, cells/tube, respectively (Fig. 2C). These data suggest that as NPY cell number increases, the quantification of Npy, Ghsr, and Gapdh gene expression increases linearly.
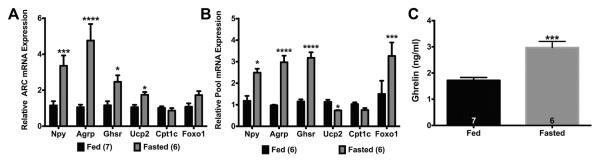
Fig. 2.
Fasting for 24 hr increased expression of GHSR pathway genes in ARC and NPY neurons in males. (A) Fasting increased Npy, Agrp, Ghsr, and Ucp2 gene expression in the ARC compared to the fed males. (B) Fasting increased Npy, Agrp, Ghsr, and Foxo1 gene expression in NPY pools while decreasing Ucp2 in NPY pools. (C) Fasting also increased plasma ghrelin (ng/ml) levels. Data are represented as mean ± SEM normalized to fed males. Data were analyzed by a two-way ANOVA with post hoc Bonferroni multiple comparison test (A & B) or Student’s t-test (C) (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). Numbers in parentheses or bars represent sample size.
3.2. Effects of Fasting on Gene Expression and Ghrelin
Previously, fasting has been shown to affect NPY neuronal activity (Roepke et al., 2011; Takahashi and Cone, 2005), gene expression (Palou et al., 2009; Roepke et al., 2011), and synaptic inputs (Dietrich and Horvath, 2013). Fasting has also been shown to increase the expression of Ghsr expression in the hypothalamus (Kim et al., 2003). To determine if fasting had a specific effect on the expression of the genes involved in the GHSR signaling pathway in the ARC and specifically in NPY neurons, both WT males and GFP-NPY males were either fed or fasted for 24 h prior to tissue collection or cell harvesting. In the ARC, there was a significant effect of fasting (F(1, 66) = 50.26, p < 0.0001; (Fig. 2A)), gene (F(5, 66) = 8.51, p < 0.0001), and fasting*gene (F(5, 66) = 8.064, p < 0.0001). Fasting increased the expression of Npy (p < 0.05), Agrp (p < 0.0001), Ghsr (p < 0.0001), and Ucp2 (p < 0.05). In NPY neurons, there was a significant effect of fasting (F(1, 59) = 38.36, p < 0.0001; (Fig. 2B)), gene (F(5, 59) = 8.866, p < 0.0001), and fasting*gene (F(5, 59) = 6.934, p < 0.0001). Fasting increased Npy (p < 0.05), Agrp (p < 0.0001), Ghsr (p < 0.0001), and Foxo1 (p < 0.001) expression, but decreased Ucp2 expression. As expected, fasting increased plasma ghrelin concentrations in males by ~ 73% (p < 0.001, Fig. 2C).
3.3. Effects of DIO on gene expression and ghrelin
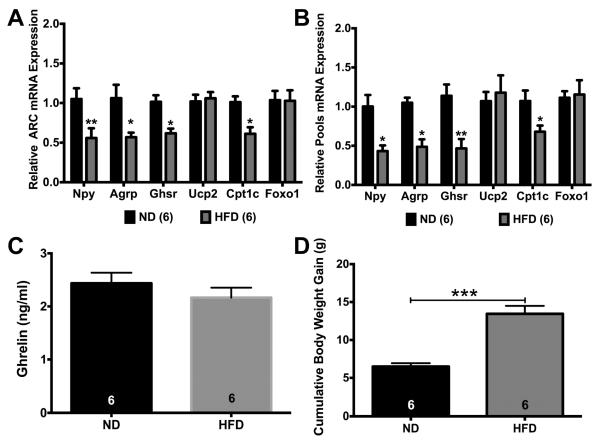
DIO has been shown to affect NPY neuronal activity, gene expression, and ghrelin sensitivity in the ARC (Briggs et al., 2014, 2013, 2010). To determine if DIO had an effect on the GHSR signaling pathway in the ARC and in NPY neurons, WT males and GFP-NPY males were fed a low-fat diet (ND) or a high-fat diet (HFD) for 12 weeks prior to tissue collection or cell harvesting. In the ARC, there was a significant effect of diet (F(1, 60) = 22.97, p < 0.0001; (Fig. 3A)), gene (F(5, 60) = 2.421, p < 0.05), and diet*gene (F(5, 60) = 2.644, p < 0.05). DIO suppressed Npy (p < 0.01), Agrp (p < 0.05), Ghsr (p < 0.05), and Cpt1c (p < 0.05) expression in ARC tissue, In pools of NPY neurons, there was a significant effect of diet (F(1, 60) = 20.72, p < 0.0001; (Fig. 3B)), gene (F(5, 60) = 3.94, p < 0.01), and fasting*gene (F(5, 60) = 3.337, p = 0.01). DIO suppressed Npy (p < 0.05), Agrp (p < 0.05), Ghsr (p < 0.01), and Cpt1c (p < 0.05) expression. DIO had no effect on plasma ghrelin concentrations in males (Fig. 3C) while doubling cumulative body weight gain over 12 weeks of HFD (ND: 6.5 ± 0.4 g vs. HFD: 13.5 ± 1.0 g; p < 0.001; Fig. 3D).
Fig. 3.
DIO suppressed expression of GHSR pathway genes in ARC and NPY neurons in males. (A) DIO decreased expression of Npy, Agrp, Ghsr, and Cpt1c in ARC tissue. (B) DIO decreased expression of Npy, Agrp, Ghsr, and Cpt1c in NPY pools. (C) DIO had no effect on plasma ghrelin levels. (D) HFD increased cumulative body weight gain over 12 weeks in males. Data are represented as mean ± SEM normalized to ND. Data were analyzed by a two-way ANOVA with post hoc Bonferroni multiple comparison test (A & B) or Student’s t-test (C & D) (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). Numbers in parentheses or bars represent sample size.
3.4. Effects of EB and Fasting on Gene Expression and Ghrelin
Because E2 is known to modulate ARC gene expression (Roepke et al., 2007; Roepke, 2009) including Ghsr expression (Frazao et al., 2014), we examined the effects of EB replacement in fed and fasted, ovariectomized (ovx) female mice. To determine if EB had an effect on GHSR signaling pathway in the ARC and in NPY neurons from fed females, WT and GFP-NPY females were ovx and injected with either two daily doses of oil or EB prior to tissue collection or harvesting of cells. In the ARC, there was a significant effect of steroid (F(1, 57) = 39.59, p < 0.0001; (Fig. 4A)), gene (F(5, 57) = 6.416, p < 0.0001), and steroid*gene (F(5, 57) = 5.613, p < 0.001). In fed females, EB replacement increased Agrp (p < 0.05), Ghsr (p < 0.01), Cpt1c (p < 0.001), and Foxo1 (p < 0.0001) expression in the ARC. In NPY pools, there was a significant effect of steroid (F(1, 58) = 7.766, p < 0.01; (Fig. 4A), gene (F(5, 58) = 3.354, p < 0.01), and steroid*gene (F(5, 58) = 3.533, p < 0.01). Unlike in the ARC, only Agrp expression was higher in EB-treated mice (p < 0.01), while EB replacement suppressed Foxo1 expression (p < 0.05). These data suggest that the positive effects of EB replacement on Foxo1 expression in the ARC of fed mice are specific to non-NPY expressing neurons.
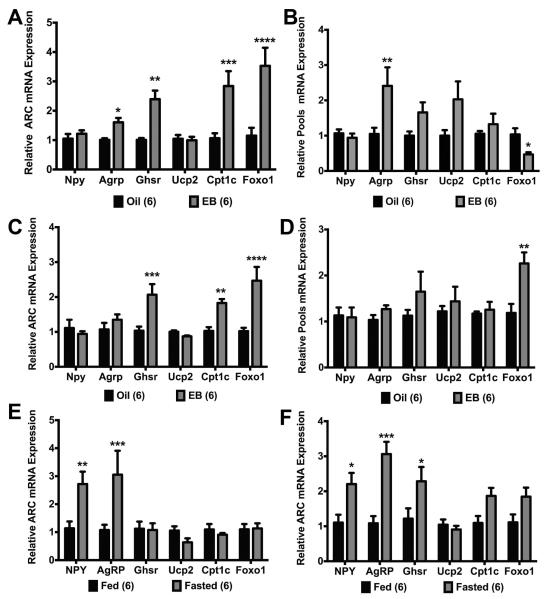
Fig. 4.
EB differentially regulated expression of GHSR pathway genes between the ARC and NPY neurons in ovx females. In fed females, (A) EB increased Agrp, Ghsr, Cpt1c, and Foxo1 expression in the ARC and (B) increased Agrp expression and decreased Foxo1 expression in NPY pools. In fasted females, (C) EB increased Ghsr, Cpt1c, and Foxo1 expression in the ARC and (D) increased Foxo1 expression in NPY pools. (E) In oil-treated ARC, fasting increased Npy, Agrp, and Ghsr expression. (F) In EB-treated ARC, fasting increased Npy and Agrp expression. Data are represented as mean ± SEM normalized to oil (A-D) or fed (E-F). Gene expression data were analyzed by a two-way ANOVA with post hoc Bonferroni multiple comparison test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). Numbers in parentheses represent sample size.
To determine if fasting altered the effects of E2 replacement on the GHSR signaling pathway in both the ARC and NPY neurons, WT and GFP-NPY females were ovx and injected with either two daily doses of oil or EB and fasted 24 h prior to tissue collection or harvesting of cells. In the ARC, there was a significant effect of steroid (F(1, 60) = 25.47, p < 0.0001; (Fig. 4C)), gene (F(5, 60) = 5.652, p < 0.001), and steroid*gene (F(5, 60) = 6.208, p = 0.0001). EB replacement did not increase Agrp expression as it had in the fed females nor did EB replacement affect Npy and Ucp2 expression. However, Ghsr expression (p < 0.1) was higher in EB-treated females as was Cpt1c (p < 0.05) and Foxo1 (p < 0.0001). In pools of NPY neurons, there was a significant effect of steroid (F(1, 59) = 7.839, p < 0.01; (Fig. 4D)). EB replacement doubled Foxo1 expression (p < 0.01) but had no effect on any other gene in the pools of NPY neurons.
To determine if fasting had an effect separate from steroid treatment, we analyzed gene expression in ARC tissue within each steroid treatment (oil or EB) using samples from fed and fasted females. As expected, fasting increased Npy (p < 0.05) and Agrp (p < 0.001) expression in the ARC samples from oil-treated females as well as Ghsr (p < 0.05) expression in the ARC (fasting: F(1,58) = 37.36, p < 0.0001; Fig. 4E). In EB-treated females, fasting increased Npy (p < 0.01) and Agrp (p < 0.001) expression but had no effect on any other gene in the GHSR pathway (fasting: F(1,60) = 6.649, p < 0.05; Fig. 4F). When diet and steroid were analyzed together for each gene, there were significant effects of diet on Npy expression (F(1, 19) = 17.35, p < 0.001) and Agrp expression (F(1, 20) = 16.79, p < 0.001). There were also steroid effects on Ghsr (F(1, 20) = 4.507, p < 0.05) and a steroid effect (F(1, 20) = 6.894, p < 0.05) and steroid*diet effect (F(1, 20) = 6.894, p < 0.05) on Cpt1c expression.
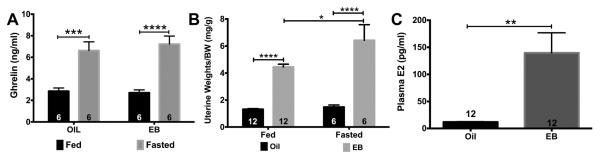
Fasting also increased plasma ghrelin levels in both oil-treated (p < 0.01) and EB-treated (p < 0.001) females while acute EB replacement had no effect on plasma ghrelin levels (fasting: F(1,20) = 50.69, p < 0.0001; Fig. 5A). Elevated uterine weights are a standard biomarker for physiological levels of E2 (and EB). For uterine weights, there was a significant effect of steroid (F(1, 32) = 89.6, p < 0.0001), fasting (F(1, 32) = 6.281, p < 0.05), and steroid*fasting (F(1, 32) = 4.491, p < 0.05 (Fig. 5B). EB replacement 5 days post-ovx increased uterine weights (shown as normalized to body weights) in both fed (p < 0.0001) and fasted (p < 0.0001) females while fasting increased normalized uterine weights of EB-treated females. Interestingly, normalized uterine weights were higher in the fasted females treated with EB than the fed females treated with EB (p < 0.05). Plasma E2 levels were higher in EB-treated females (p < 0.01; Fig. 5C). Fed and fasted samples were reported collectively because no significant differences between fed or fasted females were found (data not shown).
Fig. 5.
Plasma levels of ghrelin and E2 in oil- and EB-treated females. (A) Fasting increased plasma ghrelin in both oil- and EB-treated, ovx females. (B) EB increased uterine weights in both fed and fasted, ovx females. (C) Plasma E2 levels were higher in EB-treated than oil- treated females. Data are represented as mean ± SEM. Data were analyzed by a two-way ANOVA with post hoc Bonferroni multiple comparison test (A & B) or Student’s t-test (C) (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). Numbers in bars represent sample size.
3.5. Effects of EB and DIO on gene expression and ghrelin
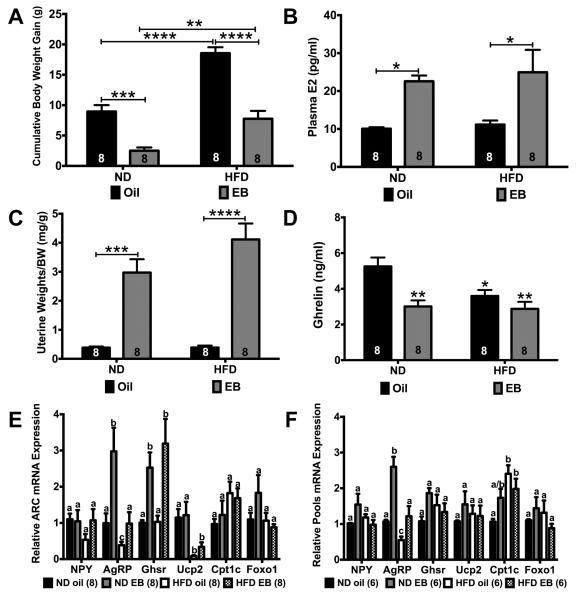
It is well known that E2 replacement suppresses the post-ovariectomy body weight gain in wild type female mice fed a normal chow diet through actions in the CNS including the ARC (Geary et al., 2001; Mamounis et al., 2014; Roepke, 2009). Because DIO and E2 both regulate ARC gene expression (see Figs. 3 and 4), we examined the combined effects of DIO and EB replacement in ovx females by feeding them either a low-fat (10%) or high-fat (45%) diet with oral dosing with oil or EB for 8 weeks. The cumulative body weight gain in both diets was suppressed by oral EB dosing (p < 0.001 in ND and p < 0.0001 in HFD) and greater in females fed a HFD compared to the ND-fed females (p < 0.0001 in oil and p < 0.01 in EB, Fig. 6A). There was a significant effect of diet (F(1, 28) = 55.36, p < 0.0001), steroid (F(1, 28) = 74.44, p < 0.0001), and steroid x diet (F(1, 28) = 4.734, p < 0.05). There was no difference in weekly energy intake between oil and EB-treated animals within each diet although, as expected, females fed a HFD had a higher weekly energy intake than the ND-fed females (ANOVA: F(3,24) = 3.681, p < 0.05; data not shown). Plasma E2 levels, collected 24 h after the last dosing of EB, were higher in both EB-treated females than oil-treated (steroid: F(1, 28) = 16.70, p < 0.001; Fig. 6B). Uterine weights (normalized to body weight) were also higher in EB-treated females (p < 0.0001 for both diets) compared to oil-treated (steroid: F(3,28) = 27.14, p < 0.0001; Fig. 6C). Plasma ghrelin levels were suppressed by EB replacement in the ND-fed females (p < 0.05) but not in the HFD-fed females, although ghrelin levels in HFD-EB treated females were significantly lower than the ND-oil treated females (p < 0.01). HFD alone decreased plasma ghrelin levels in oil-treated females (p < 0.05) but not in EB-treated females (diet: F(1,28) = 5.237, p < 0.5; steroid: F(1,28) = 14.34, p < 0.001; Fig. 6D).
Fig. 6.
Interaction of EB replacement and DIO (HFD) in ovx females on expression of GHSR pathway genes in ARC and NPY neurons. (A) EB replacement suppressed post-ovariectomy weight gain in both ND- and HFD-fed females. (B) Plasma E2 was higher in both ND- and HFD- fed females on oral dosing of EB (300 μg/kg/day). (C) Uterine weights were higher in EB-treated females in both ND- and HFD-fed females. (D) HFD and EB suppressed plasma ghrelin. Asterisks denote comparison to ND-oil. (E & F) Interactions of EB treatment and HFD on gene expression in ARC tissue and NPY pools. Weight and hormone data were analyzed by a two way ANOVA with post hoc Bonferroni multiple comparison test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). Gene expression data were analyzed by a two-way ANOVA with post hoc Bonferroni multiple comparison test within genes (common letters denote similar expression). Data are represented as mean ± SEM normalized to ND-oil. Numbers in bars or parentheses represent sample size.
In the ARC, neither diet nor steroid had an effect on Npy or Foxo1 expression (Fig. 6E). Both diet and EB had an effect on Agrp expression (steroid: F(1, 28) = 11.20, p < 0.01; diet: F(1, 28) = 11.40, p < 0.01). EB replacement did increase Agrp expression by threefold in ND-fed females (p < 0.01), which was abrogated in the HFD-fed females. HFD in oil-treated females suppressed Agrp expression (p < 0.05). EB replacement alone altered Ghsr expression in the ARC by two- to threefold in both ND-fed (p < 0.01) and HFD-fed (p < 0.01) females (F(1,28) = 20.15, p < 0.001). Regardless of steroid, HFD (oil: p < 0.05, EB: p < 0.05) suppressed Ucp2 expression in the ARC to ~ 25% of ND-fed females (F(1, 28) = 18.30, p < 0.001). There was also an effect of diet on Cpt1c expression (diet: F(1, 28) = 4.726, p < 0.05).
In pools of NPY neurons, steroid and diet had no effect on Npy, Ghsr, Ucp2, or Foxo1 expression (Fig. 6F), but there was interaction of steroid and diet on Npy and Ghsr expression (Npy: F(1, 20) = 4.472, p < 0.05; Ghsr: F(1, 20) = 4.865, p < 0.05). Both steroid and diet altered Agrp expression (steroid: F(1, 20) = 28.64, p < 0.0001; diet: F(1, 20) = 21.37, p < 0.001; steroid x diet: F(1, 20) = 5.163, p < 0.05). Agrp expression was augmented in NPY neurons by EB treatment in ND-fed females (p < 0.001) and suppressed by HFD in both oil-treated (p < 0.05) and EB-treated (p < 0.001) females. As in the acute EB-treated females from the Experiment #3 and unlike in the ARC, Cpt1c expression in NPY pools was augmented twofold in HFD-fed, oil-treated females (p < 0.01) (diet: F(1, 20) = 12.30, p < 0.01; steroid x diet: F(1, 20) = 5.778, p < 0.05), indicating that this gene is regulated by HFD (DIO) differentially in NPY neurons compared to the heterogeneous ARC neuronal population.
3.6. Ghrelin Inhibits the M-current in NPY neurons
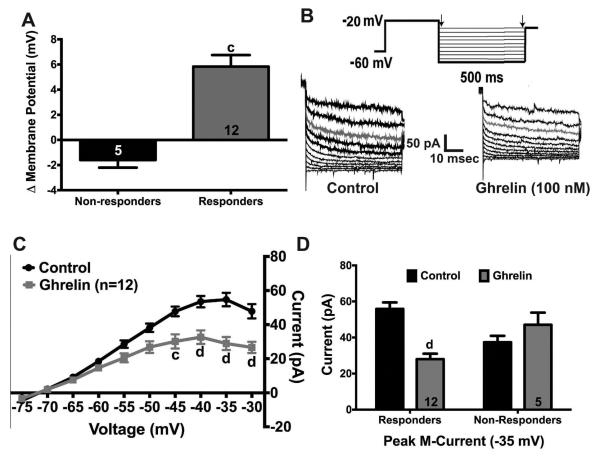
Previously, we characterized the regulation of the M-current in NPY neurons by fasting in male mice and E2 replacement in female mice (Roepke et al., 2011). Since ghrelin has recently been shown to inhibit the M-current in striatal neurons (Shi et al., 2013), we hypothesized that ghrelin would also affect the M-current in NPY neurons. Using whole-cell patch clamp electrophysiology in GFP-NPY from fed male mice, ghrelin perfusion depolarized 70.6% (12/17) of NPY neurons by 5.8 ± 0.9 mV (Fig. 7A). To determine the effects of ghrelin on M-current activity, we used standard voltage clamp deactivation protocol (Fig. 7B) (Roepke et al., 2012, 2011). Perfusion of ghrelin (100 nM) suppressed the potassium current elicited by the deactivation protocol after 10 min (ghrelin: F(1,22) = 10.90, p < 0.01; Fig. 7C). The peak current at −35 mV was suppressed by ghrelin perfusion by ~50% (12/17, p < 0.0001). Furthermore, there was a significant interaction between treatment and responders (ANOVA: F(1, 30)=18.18, p < 0.001). Interestingly, the peak current in non-responders under control conditions was lower than the responders (p<0.05), but after ghrelin perfusion, the peak current was higher in the non-responders (p<0.05). Ghrelin perfusion did not affect the M-current in the NPY neurons (n = 5) that did not respond to ghrelin perfusion (5/17, Fig. 7D).
Fig. 7.
Ghrelin suppresses the activity of the M-current in NPY neurons from fed males. (A) 70% (12/17) of NPY neurons were depolarized (+5.8 ± 0.9 mV) by ghrelin (100 nM). (B) Representative traces of the deactivation protocol from NPY neurons in control conditions (TTX, 500 nM) and after ghrelin perfusion (10 min). (C) I-V plot of the deactivation traces illustrates the decrease in current after ghrelin perfusion. (D) Control peak current was 55.8 ± 3.6 pA, and ghrelin peak current was 28.0 ± 3.0 pA in the 12 responders. Letter denotes comparison between control and ghrelin. Data are represented as mean ± SEM. Numbers in bars (A & D) represent sample size. Data (C & D) were analyzed by a two-way ANOVA (C: repeated measures) followed by post hoc Bonferroni multiple comparison test (c = P < 0.001; d = P < 0.0001).
4. Discussion
Understanding the impact of caloric restriction and high fat diets on the neuroendocrine control of energy homeostasis is key to addressing the obesity epidemic and other metabolic disease. Most studies in this field only examine male rodent models due, in part, to the influence of circulating E2 on energy homeostasis in females during the estrous cycle. Another weakness of this field is the prevalence of studies examining genomic effects of these conditions on the heterogeneous nuclei of the hypothalamus without regard to potential differences in cell-type responses. Therefore, we set out to illustrate the importance of cell-type specific effects and steroid-driven sex differences in the neuroendocrine responses to caloric restriction and DIO using specific neuronal pools coupled with quantitative real-time PCR in intact males and ovx females with or without E2 replacement. We clearly demonstrate that the regulation of the GHSR signaling pathway by fasting and DIO in both ARC tissue and in pools of NPY neurons occurs in a sex-dependent manner due to the regulation of these genes by E2.
We showed that fasting and DIO have opposing effects on Npy/Agrp and Ghsr expression in the ARC that is recapitulated in pools of NPY neurons from male mice. However, two genes involved in GHSR signaling, Ucp2 and Foxo1, were differentially regulated in between the ARC and NPY neurons in fasted males. EB replacement in fed, ovx females increased Agrp, Ghsr, Cpt1c, and Foxo1 in the ARC but only Agrp in pools of NPY neurons. In fasted females, EB replacement also increased Ghsr, Cpt1c, and Foxo1 expression in the ARC but only Foxo1 in NPY pools. In ovx, ND-fed females, EB replacement augmented Agrp, which was abrogated by HFD in both the ARC and NPY pools; however, regulation of Ghsr expression by EB was not affected by HFD in the ARC nor did EB increase Ghsr expression in NPY pools. Therefore, our data suggest that fasting, HFD (DIO), and E2 have differential effects on GHSR signaling in between the heterogeneous ARC and homogenous pools of NPY neurons in a sex-specific manner. A summary of the differential regulation of GHSR signaling molecules is presented in Table 2.
Table 2.
A summary of results
| Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Fed vs. Fasted | ND vs. HFD | ||||||||
|
| |||||||||
| Gene Name |
Fast | HFD | Oil Fast |
EB Fast |
Fed EB |
Fast EB |
Diet | EB | Diet × EB |
| Npy |
 Arc Arc NPY NPY |
 Arc Arc NPY NPY |
 Arc Arc |
 Arc Arc |
* NPY | ||||
| Agrp |
 Arc Arc NPY NPY |
 Arc Arc NPY NPY |
 Arc Arc |
 Arc Arc |
 NPY NPY |
✱ Arc ✱ NPY |
✱ Arc ✱ NPY |
✱ NPY | |
| Ghsr |
 Arc Arc NPY NPY |
 Arc Arc NPY NPY |
 Arc Arc |
✱ Arc | ✱ NPY | ||||
| Ucp2 |
 Arc Arc NPY NPY |
✱ Arc | |||||||
| Cpt1c |
 Arc Arc NPY NPY |
✱ Arc ✱ NPY |
✱ NPY | ||||||
| Foxo1 |
 NPY NPY |
 NPY NPY |
 NPY NPY |
||||||
|
| |||||||||
| Fig. 2 | Fig. 3 | Fig. 4E | Fig. 4F | Fig. 4B | Fig. 4D | Fig. 6E & 6F | |||
For male fasted and HFD and female fasted experiments:  denotes an increase in expression;
denotes an increase in expression;  denotes a decrease in expression. For female ND vs. HFD: * denotes significant effect of diet, EB, or interaction. ARC = arcuate nucleus. NPY = pools of NPY neurons. Treatment headings are in comparison to Fed and ND for males. For female fed vs. fasted, Fast is in comparison to fed within steroid treatment in the ARC and EB is in comparison to oil within fed or fasted state in NPY pools.
denotes a decrease in expression. For female ND vs. HFD: * denotes significant effect of diet, EB, or interaction. ARC = arcuate nucleus. NPY = pools of NPY neurons. Treatment headings are in comparison to Fed and ND for males. For female fed vs. fasted, Fast is in comparison to fed within steroid treatment in the ARC and EB is in comparison to oil within fed or fasted state in NPY pools.
4.1. The Effects of Fasting in Males
Fasting alters ARC gene expression and, in particular, increases expression of orexigenic neuropeptides (Npy and Agrp) and genes involved in ghrelin signaling (Ghsr, Ucp2) (Briggs et al., 2013, 2010; Coppola et al., 2007; Palou et al., 2009; Verhulst et al., 2012). In our study, fasting increased Npy, Agrp, Ghsr, and Ucp2 expression in the ARC. In pools of NPY neurons, fasting increased the neuropeptides and Ghsr but decreased Ucp2 expression. In our study and others (Verhulst et al., 2012), fasting increased plasma ghrelin, a potent orexigenic peptide hormone. Presumably, the elevation of plasma ghrelin and Ghsr expression in NPY neurons intensifies neuronal sensitivity to ghrelin to drive the post-fasting hyperphagia (Bagnasco et al., 2003; Becskei et al., 2009; Kinzig et al., 2009; Teubner et al., 2013) and augment Npy/Agrp expression, which is found in fasted male mice (Verhulst et al., 2012).
Ghrelin binding to GHSR leads to the activation of an AMPK-mediated cascade that stimulates CPT-1, which acylates carnitine with palmitic acid and other long-chain fatty acids. CPT-1 (along with CPT-2) allows the translocation of acyl-carnitine (palmitoylcarnitine) from the cytosol into the mitochondrial matrix for beta-oxidation (Andrews et al., 2008; López et al., 2008a; Wolfgang and Lane, 2011; Wolfgang et al., 2008, 2006). Fatty acid beta-oxidation leads to an increase in reactive oxygen species (ROS), which trigger UCP2 to buffer ROS and promote mitochondrial biogenesis (Andrews et al., 2008; Du et al., 2006; Lam et al., 2005). An increase in UCP2 activity also enhances the efficiency of the transcription of Npy/Agrp genes, leading to an increase in orexigenic behavior (Andrews et al., 2008). Interestingly, the elevated plasma levels of ghrelin are not correlated with an increase in Cpt1c gene expression, which has been previously reported for Cpt1 (Andrews et al., 2008). Although hypothalamic malonyl-CoA levels decrease during fasting (Hu et al., 2003; Wolfgang et al., 2007), the expression of Cpt1c in the ARC and in NPY neurons during fasting is not sensitive to this change.
Greater activation of the GHSR signaling cascade due to elevated ghrelin during fasting will augment uncoupling respiration (UCP2 activity) in the mitochondria. Fasting increases Ucp2 expression in the ARC and in cortical mitochondria (Coppola et al., 2007; Davis et al., 2008), as does ghrelin in the hypothalamus (Andrews et al., 2008). However, Ucp2 expression is suppressed by fasting in pools of NPY neurons by ~50%. This decrease in Ucp2 expression contradicts the hypothesis that UCP2 is required for the ghrelin-induced increase in Npy/Agrp gene expression, neuronal activity, and the drive to feed during negative states of energy balance (fasting) (Andrews et al., 2008), although a reduction in gene expression does not necessarily correlate with a reduction in UCP2 activity, as previously reported with ghrelin administration (Lage et al., 2010).
Alternatively, the decrease in Ucp2 expression, which could interfere with the activation of Npy/Agrp gene expression, is compensated by the increase in Foxo1, the transcription factor that mediates, in part, the regulation of Npy/Agrp gene expression by ghrelin (Lage et al., 2010). FoxO1 activity in ARC AgRP-expressing neurons regulates locomotor activity, oxygen consumption, food intake, glucose homeostasis, and ghrelin sensitivity (Cao et al., 2011; Kim et al., 2012; Ren et al., 2012). Interestingly, Foxo1 expression in the ARC was not affected by fasting despite an increase in plasma ghrelin. Previous studies have demonstrated that ghrelin administration augments Foxo1 expression in the ARC (Lage et al., 2010). However, fasting did increase Foxo1 expression by two- to threefold in NPY neurons. The difference in expression between the ARC and NPY neurons suggests that the increase in Foxo1 expression in NPY neurons was offset by a decrease in Foxo1 expression in other ARC neurons.
4.2. The Effects of Diet-induced Obesity in Males
HFD or DIO induces ghrelin insensitivity in mice (Andrews, 2011; Briggs et al., 2013), possibly through dysregulating the GHSR signaling pathway including CPT-1 and UCP2. For example, HFD or DIO will suppress Npy, Agrp, and Ghsr expression in the ARC in several model species (Briggs et al., 2010; Kurose et al., 2005). Briggs and colleagues (2010) found that DIO suppressed both hypothalamic Ghsr expression and ghrelin (total and active) in male mice (Briggs et al., 2010), unlike in our study where DIO had no effect on total ghrelin. However, another study in male rats fed a HFD from weaning to 6 months of age found that hypothalamic Ghsr expression was not altered compared to ND-fed males (Priego et al., 2009). We have demonstrated in our study that by 12 weeks of DIO suppressed Npy, Agrp, and Ghsr in both the ARC and specifically in NPY pools. The decrease in NPY neuronal expression of Ghsr is a potential mechanism underlying diet-induced ghrelin resistance and NPY insensitivity (Briggs et al., 2013, 2010). The decrease in expression of ghrelin’s receptor would certainly lead to attenuation of the fasting-induced hyperphagia (Briggs et al., 2010).
Unlike in fasted males, Ucp2 expression was unchanged in the ARC of HFD-fed males compared to ND-fed males. Previous studies have demonstrated that whole hypothalamic Ucp2 expression is elevated by prolonged (33 weeks) HFD in male mice (Kocalis et al., 2012). It is possible that Ucp2 levels remained unchanged in our study due to differences in expression amongst the various hypothalamic nuclei. Alternatively, unaltered UCP2 levels will serve to maintain mitochondrial ROS buffering capacity in response to elevated fatty acid beta-oxidation due to excess lipids from the HFD. On the other hand, Cpt1c expression was suppressed in both ARC and in NPY pools. The reduction in Cpt1c expression in HFD-fed males is not involved in fatty acid beta-oxidation during DIO but may be relevant to the malonyl-CoA sensing in the mitochondria and its role in hypothalamic glucose-sensing (Wolfgang et al., 2006). Therefore, the reduction in Cpt1c may play a role in the dysregulation of glucose homeostasis by the ARC during DIO.
4.3. Integration of the Effects of 17β-estradiol, Fasting, and DIO in Females
E2 replacement in multiple models and replacement paradigms suppresses Npy expression ((Dhillon and Belsham, 2011, reviewed in Brown and Clegg, 2010; Roepke, 2009), NPY orexigenic activity (Santollo and Eckel, 2008), and Agrp expression (Cheng et al., 2009; Olofsson et al., 2009). However, in our study, acute EB treatment did not alter Npy expression in fed, fasted, or DIO females. Certainly, the differences between our studies and others involve variations in methodology in E2 replacement (injection, capsules, pellets, oral, etc.), rodent models, housing, and strain effects (Nagy et al., 2002; Simon et al., 2013). With regard to Agrp expression, it was augmented by EB in fed females but not in fasted females, indicating that fasting is a primary driver of Agrp expression and overwhelms the effects of EB. Furthermore, Npy and Agrp regulation by fasting in females is independent of EB since fasting augmented the expression of both genes in oil-treated and EB-treated females.
Little is known about the interactions of HFD/DIO and E2/EB on Npy/Agrp expression in the ARC. In our study, Npy is not suppressed by HFD in females in both ARC tissue and NPY pools as it is in male mice, which highlights the importance of examining sex differences in neuroendocrine responses to HFD and obesity. Conversely, Agrp expression is augmented by EB in ND-fed females and suppressed by HFD in both oil-treated and EB-treated females, as it is in male mice. In NPY neurons, the suppression of Agrp expression by HFD in females is not correlated with a suppression of Ghsr as it is in male mice, suggesting that DIO-induced ghrelin resistance occurs through different mechanisms in males and females. Interestingly, acute EB treatment affected more genes in the ARC than long-term EB treatment (See Table 2). This divergent response highlights the difference of the physiological relevance of acute vs. long-term E2 replacement paradigms. In most rodent models, circulating E2 rise and fall during the estrous cycle every 4-5 days. Our acute paradigm is similar to this cyclical pattern and closely mimics the proestrous levels of endogenous E2, while the long-term effects may be compensated for by receptor-mediated mechanism such as ERα downregulation due to long-term steroid exposure (Roepke et al., 2008).
The consistent effect of EB treatment, whether acute or long-term, to increase Agrp expression in our study is not commonly reported. In many studies, E2 effects on Agrp expression or secretion are either suppressive or not significant (reviewed in (Brown and Clegg, 2010; Roepke, 2009). However, long-term E2 replacement in female mice did produce an increase in Agrp expression in the hypothalamus (Cheng et al., 2009). Furthermore, in clonal hypothalamic neurons that express both NPY and AgRP, E2 treatment augments Agrp expression depending on the temporal ratio of ERα/ERβ expression wherein greater ERβ activation produces positive Agrp expression by E2 (Titolo et al., 2006) to potentially function as a mediator of peripheral hormone signaling on the HPG axis (Sheffer-Babila et al., 2013). Both acute and long-term E2 treatment suppresses ERα expression in the ARC (Roepke et al., 2008; Yang et al., unpublished data), which may be sufficient to alter the ERα/ERβ ratio leading to an increase in Agrp expression.
Our data suggests that a unique interaction between circulating E2, ghrelin, and Ghsr expression in the rodent ARC during fasting and DIO. In previous studies, plasma ghrelin levels were lower in intact female rats than in ovx females (Clegg et al., 2007). E2 replacement also suppresses ghrelin expression in the stomach (Matsubara et al., 2004). In our study, acute E2 did not alter plasma ghrelin in fed or fasted females, but long-term EB replacement did suppress ghrelin in ND-fed females. Plasma ghrelin level was also reduced in HFD-fed, oil-treated females, yet EB replacement did not further suppress ghrelin in HFD-fed females. Others have found that both acute and long-term (8 weeks) peripheral ghrelin administration augmented Npy and Agrp expression in male and female mice (Egecioglu et al., 2008; Goto et al., 2006). The reduction in plasma ghrelin by HFD or EB in females, without the suppression of Ghsr expression in NPY neurons, may counteract the regulation of Npy gene expression typically found by HFD-fed animals.
In fed, fasted, or DIO ovx females, EB augmented the expression of Ghsr in the ARC but not in NPY neurons, indicating that E2’s action on Ghsr expression does not directly affect the ARC melanocortin-neuropeptide Y circuitry. While Ghsr is expressed in ARC growth hormone releasing hormone (GHRH) neurons, dopaminergic neurons, and KNDy neurons (Frazao et al., 2014; Osterstock et al., 2010; Willesen et al., 1999; Zigman et al., 2006), the effects of E2 on Ghsr expression in these neurons are not fully characterized. Frazao and colleagues (2014) reported that E2 administration in ovx females (through capsules inserted during ovx) increased Ghsr expression in the ARC and in Kiss1-expressing neurons (Frazao et al., 2014). In our study, fasting only increased ARC Ghsr expression in oil-treated females. This suggests that the interaction of fasting and E2 differentially regulates Ghsr in the ARC. While Ghsr expression does not change during the estrous cycle in female rats, ghrelin’s effects on food intake are only found during the diestrus cycle, when E2 levels are lower (Sakurazawa et al., 2013). The differential effect on ghrelin sensitivity and Ghsr expression may be due, in part, to changes in expression of downstream effectors of GHSR signaling (e.g., Cpt1c, Foxo1) as we have found in the ARC and in NPY pools.
EB did not regulate Ucp2 expression in the ARC or NPY neurons. We did expect to find EB-induced Ucp2 regulation because previous studies have reported that E2 alters the expression of Ucp2 in adipocyte and breast cancer cell lines (Nadal-Serrano et al., 2012). Unlike in males, fasting did not have an effect on Ucp2 expression in either tissue or cell type in females while HFD suppressed Ucp2 expression in the ARC of females independent of steroid treatment. The differences in Ucp2 expression between males and females in response to fasting and HFD highlight again the need to examine sex differences in genes controlling energy homeostasis in the hypothalamus.
To our knowledge, this is the first study reporting an effect of E2 (EB) on expression of brain-specific Cpt1c gene in the ARC, although a previous study found a small, but significant, reduction in hypothalamic Cpt1c expression by E2, when administered by pellet over 2 months (Cheng et al., 2009). In our study, EB replacement 5 days post-ovx increased Cpt1c expression in the ARC regardless of fed state but not in NPY neurons. Presumably, Cpt1c is ubiquitously expressed in ARC neurons, and elevated expression of Cpt1c is correlated with the increase in ARC Ghsr expression by EB in other non-NPY neurons (see discussion above). Elevated Cpt1c by EB would augment the ability of ARC neurons to respond to changes in malonyl-CoA concentrations, which correlates with feeding behavior (Hu et al., 2003; Wolfgang et al., 2007). Interestingly, EB treatment and HFD in females increased Cpt1c in NPY neurons, unlike in male ARC and NPY pools. The elevation of Cpt1c may be involved in the resistance of females to DIO-induced impairments of glucose homeostasis since it acts as a sensor for malonyl-CoA and hypothalamic glucose sensing (Wolfgang et al., 2006).
E2, through its nuclear receptors, impacts FoxO1 signaling in the brain, the reproductive tract, breast cancer cells, and other cell lines via phosphorylation through Akt or Pak1 (Kemper et al., 2014; Koh, 2006; Lengyel et al., 2007; Mazumdar et al., 2003; Won et al., 2006). However, there is no data suggesting that E2 directly regulates the Foxo1 gene in the brain. In our study, Foxo1 expression was augmented by acute EB treatment in the ARC in fed and fasted females and in NPY neurons in fasted females. Lage and colleagues (2010) found that central ghrelin administration increased FoxO1 and pFoxO1 protein expression in the female hypothalamus, which was correlated with an increase in Npy/Agrp expression (Lage et al., 2010). However, in our study, E2 suppressed Foxo1 expression in NPY neurons in fed females suggesting that EB may blunt the effects of ghrelin in NPY neurons by suppressing Foxo1 expression in the fed state but augment ghrelin’s action through an increase in Foxo1 in fasted females.
4.4. Ghrelin Inhibits the M-current in NPY Neurons
In a previous study, we have shown that fasting suppressed the M-current in NPY neurons in males (Roepke et al., 2011). Recently, ghrelin has been shown to inhibit the M-current in striatal neurons through a PLC-PKC-mediated signaling pathway (Shi et al., 2013). Because ghrelin activates Ca2+ channels (N-type) in NPY neurons (Kohno et al., 2007, 2003) and in GHRH neurons (Osterstock et al., 2010) and unidentified voltage-gated K+ channels in GH3 cells (Han et al., 2005), we hypothesized that ghrelin would also inhibit the M-current in NPY neurons. In approximately 70% of NPY neurons from fed males, ghrelin perfusion depolarized NPY neurons while suppressing the activity of the M-current. Presumably, in NPY neurons, ghrelin also inhibits the M-current via a similar PLC-PKC-mediated pathway recently characterized in striatal neurons (Shi et al., 2013). Because the M-current is suppressed transcriptionally (reduction in KCNQ2 and KCNQ3 expression) in NPY neurons by fasting (Roepke et al., 2011) while Ghsr expression is augmented (the current study), the role of M-current inhibition in the stimulation of NPY neuronal activity during fasting may be reduced, supplanted by another cation channel (Kohno et al., 2007, 2003), or compensated by the increased Ghsr expression and activity. This reduction in the M-current may play a role in the attenuation of the ghrelin-induced hyperphagia after food restriction that is found in DIO male mice and hamsters (Briggs et al., 2010; Teubner et al., 2013).
5. Conclusion
Our experiments have confirmed the effects of fasting, DIO, and E2 on the expression of Ghsr, Npy, and Agrp in the ARC (Briggs et al., 2013, 2010; Brown and Clegg, 2010; Coppola et al., 2007; Palou et al., 2009; Roepke et al., 2008; Verhulst et al., 2012). However, gene expression in pools of NPY neurons does not fully reflect findings in the heterogeneous ARC for other genes involved in GHSR signaling. Clearly, neuronal cell type should be considered when studying the expression of ubiquitously expressed genes and proteins in hypothalamic nuclei. The quantitative analysis of pools of GFP-tagged neurons by real-time quantitative PCR from treated males and females will greatly enhance our understanding of the sex-dependent, cell-type-specific effects of fasting, DIO, and E2 on hypothalamic homeostatic functions.
A final concern is the relevance of peripheral vs. central ghrelin (from BH ghrelin neurons) (Guan et al., 2003) in the activation of NPY neurons and other ARC neurons (Frazao et al., 2014). Peripheral administration of ghrelin does activate ARC NPY neurons (Wang et al., 2002), but does not eliminate the role of catecholamine hindbrain neurons in mediating the actions of peripheral ghrelin on feeding behavior and NPY activation (Date et al., 2006, 2002; Emanuel and Ritter, 2010). Therefore, the alterations in plasma ghrelin may not be a significant contributor to the ARC and NPY changes in gene expression.
Results from the current study focusing on the genes involved in the GHSR signaling pathway in NPY neurons provide some insight into the interactions of orexigenic ghrelin signaling and anorexigenic E2 signaling in the control of energy homeostasis in females. Indeed, sex differences in ghrelin and GHSR activity are driven, in part, by the interaction of E2 with fasting and DIO (Priego et al., 2009). In summary, these studies emphasize the importance of considering cell type and sex while delineating the effects of ghrelin and potentially other peripheral hormones on hypothalamic gene expression and homeostatic functions.
Fasting in males had differential effects on expression of Foxo1 and Ucp2 expression.
In males, DIO suppressed ARC and NPY Ghsr and Cpt1c expression.
In females, E2 augmented ARC Ghsr, Cpt1c, and Foxo1 but suppressed NPY Foxo1.
In females, DIO suppressed ARC Agrp and augmented NPY Cpt1c.
Ghrelin suppressed M-current activity by ~50% in NPY neurons from fed male mice.
Acknowledgments
The author must thank Drs. Wendie Cohick and Sara Campbell for their assistance with the 17β-estradiol and ghrelin assays, respectively. This research is supported by funds from NIH R00DK083457, R00DK083457-S1, P30ES005022, and NJ06107 (USDA-NIFA).
Glossary
- ARC
Arcuate Nucleus
- AgRP
Agouti-related Peptide
- CPT-1c
Carnitine Palmitoyltransferase 1c
- CNS
Central Nervous System
- DIO
Diet-Induced Obesity
- E2
17β-estradiol
- EB
Estradiol Benzoate
- FoxO1
Forkhead Box O1
- GFP
Green Fluorescent Protein
- GHSR
Growth Hormone Secretagogue Receptor
- NPY
Neuropeptide Y
- POMC
Proopiomelanocortin
- UCP2
Uncoupling Protein 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews ZB. Central mechanisms involved in the orexigenic actions of ghrelin. Peptides. 2011;32:2248–2255. doi: 10.1016/j.peptides.2011.05.014. doi:10.1016/j.peptides.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Liu Z-W, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao X-B, Horvath TL, Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. doi:10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnasco M, Tulipano G, Melis MR, Argiolas A, Cocchi D, Muller EE. Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regul. Pept. 2003;111:161–167. doi: 10.1016/s0167-0115(02)00283-5. [DOI] [PubMed] [Google Scholar]
- Becskei C, Lutz TA, Riediger T. Diet-derived nutrients mediate the inhibition of hypothalamic NPY neurons in the arcuate nucleus of mice during refeeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R100–R110. doi: 10.1152/ajpregu.91014.2008. doi:10.1152/ajpregu.91014.2008. [DOI] [PubMed] [Google Scholar]
- Bosch MA, Tonsfeldt KJ, Rønnekleiv OK. mRNA expression of ion channels in GnRH neurons: Subtype-specific regulation by 17β-estradiol. Mol. Cell. Endocrinol. 2013;367:85–97. doi: 10.1016/j.mce.2012.12.021. doi:10.1016/j.mce.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:4745–4755. doi: 10.1210/en.2010-0556. doi:10.1210/en.2010-0556. [DOI] [PubMed] [Google Scholar]
- Briggs DI, Lockie SH, Benzler J, Wu Q, Stark R, Reichenbach A, Hoy AJ, Lemus MB, Coleman HA, Parkington HC, Tups A, Andrews ZB. Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology. 2014;155:2411–2422. doi: 10.1210/en.2013-1861. doi:10.1210/en.2013-1861. [DOI] [PubMed] [Google Scholar]
- Briggs DI, Lockie SH, Wu Q, Lemus MB, Stark R, Andrews ZB. Calorie-restricted weight loss reverses high-fat diet-induced ghrelin resistance, which contributes to rebound weight gain in a ghrelin-dependent manner. Endocrinology. 2013;154:709–717. doi: 10.1210/en.2012-1421. doi:10.1210/en.2012-1421. [DOI] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. doi:10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Liu T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann. N. Y. Acad. Sci. 2011;1243:1–39. doi: 10.1111/j.1749-6632.2011.06388.x. doi:10.1111/j.1749-6632.2011.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Nakata M, Okamoto S, Takano E, Yada T, Minokoshi Y, Hirata Y, Nakajima K, Iskandar K, Hayashi Y, Ogawa W, Barsh GS, Hosoda H, Kangawa K, Itoh H, Noda T, Kasuga M, Nakae J. PDK1-Foxo1 in agouti-related peptide neurons regulates energy homeostasis by modulating food intake and energy expenditure. PLoS One. 2011:6. doi: 10.1371/journal.pone.0018324. doi:10.1371/journal.pone.0018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan X-M, Ye Z, Nargund RP, Smith RG, Van der Ploeg LHT, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. doi:10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Cheng H, Isoda F, Mobbs CV. Estradiol impairs hypothalamic molecular responses to hypoglycemia. Brain Res. 2009;1280:77–83. doi: 10.1016/j.brainres.2009.05.017. doi:10.1016/j.brainres.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. doi:10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, Diano S. A Central Thermogenic-like Mechanism in Feeding Regulation: An Interplay between Arcuate Nucleus T3 and UCP2. Cell Metab. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. doi:10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. doi:10.1016/S0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in Ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. doi:10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006;4:323–331. doi: 10.1016/j.cmet.2006.09.004. doi:10.1016/j.cmet.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Davis LM, Rho JM, Sullivan PG. UCP-mediated free fatty acid uncoupling of isolated cortical mitochondria from fasted animals: Correlations to dietary modulations. Epilepsia. 2008;49:117–119. doi: 10.1111/j.1528-1167.2008.01854.x. doi:10.1111/j.1528-1167.2008.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-α in clonal, immortalized hypothalamic neurons. Int. J. Obes. (Lond) 2011;35:198–207. doi: 10.1038/ijo.2010.124. doi:10.1038/ijo.2010.124. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Horvath TL. Hypothalamic control of energy balance: Insights into the role of synaptic plasticity. Trends Neurosci. 2013;36:65–73. doi: 10.1016/j.tins.2012.12.005. doi:10.1016/j.tins.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Du X, Edelstein D, Obici S, Higham N, Zou M-H, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J. Clin. Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. doi:10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Stenstrom B, Pinnock SB, Tung LYC, Dornonville de la Cour C, Lindqvist A, Hakanson R, Syversen U, Chen D, Dickson SL. Hypothalamic gene expression following ghrelin therapy to gastrectomized rodents. Regul. Pept. 2008;146:176–182. doi: 10.1016/j.regpep.2007.09.006. doi:10.1016/j.regpep.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Emanuel AJ, Ritter S. Hindbrain catecholamine neurons modulate the growth hormone but not the feeding response to ghrelin. Endocrinology. 2010;151:3237–3246. doi: 10.1210/en.2010-0219. doi:10.1210/en.2010-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- Frazao R, Dungan Lemko HM, da Silva RP, Ratra DV, Lee CE, Williams KW, Zigman JM, Elias CF. Estradiol modulates Kiss1 neuronal response to ghrelin. Am. J. Physiol. Endocrinol. Metab. 2014;306:E606–14. doi: 10.1152/ajpendo.00211.2013. doi:10.1152/ajpendo.00211.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu. Rev. Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. doi:10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Gardiner JV, Campbell D, Patterson M, Kent A, Ghatei MA, Bloom SR, Bewick GA. The hyperphagic effect of ghrelin is inhibited in mice by a diet high in fat. Gastroenterology. 2010;138:2468–76. 2476.e1. doi: 10.1053/j.gastro.2010.02.012. doi:10.1053/j.gastro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. doi:10.1210/en.142.11.4751. [DOI] [PubMed] [Google Scholar]
- Goto M, Arima H, Watanabe M, Hayashi M, Banno R, Sato I, Nagasaki H, Oiso Y. Ghrelin increases neuropeptide Y and agouti-related peptide gene expression in the arcuate nucleus in rat hypothalamic organotypic cultures. Endocrinology. 2006;147:5102–5109. doi: 10.1210/en.2006-0104. doi:10.1210/en.2006-0104. [DOI] [PubMed] [Google Scholar]
- Guan JL, Wang QP, Kageyama H, Takenoya F, Kita T, Matsuoka T, Funahashi H, Shioda S. Synaptic interactions between ghrelin- and neuropeptide Y-containing neurons in the rat arcuate nucleus. Peptides. 2003;24:1921–1928. doi: 10.1016/j.peptides.2003.10.017. doi:10.1016/j.peptides.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Han XF, Zhu YL, Hernandez M, Keating DJ, Chen C. Ghrelin reduces voltage-gated potassium currents in GH3 cells via cyclic GMP pathways. Endocrine. 2005;28:217–224. doi: 10.1385/ENDO:28:2:217. doi:10.1007/s12020-011-9520-z. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. doi:10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Cha SH, Chohnan S, Lane MD. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12624–12629. doi: 10.1073/pnas.1834402100. doi:10.1073/pnas.1834402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17β-estradiol administration to mice. Gen. Comp. Endocrinol. 2012;175:188–193. doi: 10.1016/j.ygcen.2011.11.014. doi:10.1016/j.ygcen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–4800. doi: 10.1210/endo.141.12.7920. doi:10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- Kemper MF, Stirone C, Krause DN, Duckles SP, Procaccio V. Genomic and non genomic regulation of PGC1 isoforms by estrogen to increase cerebral vascular mitochondrial biogenesis and reactive oxygen species protection. Eur. J. Pharmacol. 2014;723:322–329. doi: 10.1016/j.ejphar.2013.11.009. doi:10.1016/j.ejphar.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Donato J, Berglund ED, Choi YH, Kohno D, Elias CF, DePinho RA, Elmquist JK. FOXO1 in the ventromedial hypothalamus regulates energy balance. J. Clin. Invest. 2012;122:2578–2589. doi: 10.1172/JCI62848. doi:10.1172/JCI62848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-S, Yoon C-Y, Park K-H, Shin C-S, Park K-S, Kim S-Y, Cho B-Y, Lee H-K. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport. 2003;14:1317–1320. doi: 10.1097/01.wnr.0000078703.79393.d2. doi:10.1097/01.wnr.0000078703.79393.d2. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Tao EE. Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am. J. Physiol. Endocrinol. Metab. 2009;296:E282–E290. doi: 10.1152/ajpendo.90523.2008. doi:10.1152/ajpendo.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocalis HE, Turney MK, Printz RL, Laryea GN, Muglia LJ, Davies SS, Stanwood GD, McGuinness OP, Niswender KD. Neuron-specific deletion of peroxisome proliferator-activated receptor delta (PPARΔ) in mice leads to increased susceptibility to diet-induced obesity. PLoS One. 2012:7. doi: 10.1371/journal.pone.0042981. doi:10.1371/journal.pone.0042981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh P. 17β -estradiol prevents the glutamate-induced decrease of Akt and Its downstream targets in HT22 cells. J Vet Med Sci. 2006;69:285–288. doi: 10.1292/jvms.69.285. [DOI] [PubMed] [Google Scholar]
- Kohno D, Gao H, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with Neuropeptide Y-containing neurons in the rat arcuate nucleus. Diabetes. 2003;52:948–956. doi: 10.2337/diabetes.52.4.948. [DOI] [PubMed] [Google Scholar]
- Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, Shimazaki T, Okano H, Onaka T, Yada T. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148:2251–2263. doi: 10.1210/en.2006-1240. doi:10.1210/en.2006-1240. [DOI] [PubMed] [Google Scholar]
- Kurose Y, Iqbal J, Rao A, Murata Y, Hasegawa Y, Terashima Y, Kojima M, Kangawa K, Clarke IJ. Changes in expression of the genes for the leptin receptor and the growth hormone-releasing peptide/ghrelin receptor in the hypothalamic arcuate nucleus with long-term manipulation of adiposity by dietary means. J. Neuroendocrinol. 2005;17:331–340. doi: 10.1111/j.1365-2826.2005.01318.x. doi:10.1111/j.1365-2826.2005.01318.x. [DOI] [PubMed] [Google Scholar]
- Lage R, Vázquez MJ, Varela L, Saha AK, Vidal-Puig A, Nogueiras R, Diéguez C, López M. Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 2010;24:2670–2679. doi: 10.1096/fj.09-150672. doi:10.1096/fj.09-150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TKT, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat. Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. doi:10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- Lengyel F, Vertes Z, Kovacs KA, Kornyei JL, Sumegi B, Vertes M. Effect of estrogen and inhibition of phosphatidylinositol-3 kinase on Akt and FOXO1 in rat uterus. Steroids. 2007;72:422–428. doi: 10.1016/j.steroids.2007.03.001. doi:10.1016/j.steroids.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodríguez-Cuenca S, Deoliveira RM, Castañeda T, Datta R, Dong JZ, Culler M, Sleeman MW, Álvarez CV, Gallego R, Lelliott CJ, Carling D, Tschöp MH, Diéguez C, Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008a;7:389–399. doi: 10.1016/j.cmet.2008.03.006. doi:10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- López M, Saha AK, Diéguez C, Vidal-Puig A. The AMPK-Malonyl-CoA-CPT1 axis in the control of hypothalamic neuronal function-reply. Cell Metab. 2008b;8:176. doi: 10.1016/j.cmet.2008.07.009. doi:10.1016/j.cmet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Magnusson KT, Cook S, Rehkopf DH, Ford ES, Berkman LF. Socioeconomic position and the metabolic syndrome in early, middle, and late life: evidence from NHANES 1999-2002. Ann. Epidemiol. 2007;17:782–790. doi: 10.1016/j.annepidem.2007.05.003. doi:10.1016/j.annepidem.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mamounis KJ, Yang J. a., Yasrebi A, Roepke TA. Estrogen response element-independent signaling partially restores post-ovariectomy body weight gain but is not sufficient for 17β-estradiol’s control of energy homeostasis. Steroids. 2014;81:88–98. doi: 10.1016/j.steroids.2013.10.018. doi:10.1016/j.steroids.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark AL. Dietary therapy for obesity is a failure and pharmacotherapy is the future: A point of view. Clin. Exp. Pharmacol. Physiol. 2006;33:857–862. doi: 10.1111/j.1440-1681.2006.04454.x. doi:10.1111/j.1440-1681.2006.04454.x. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Sakata I, Wada R, Yamazaki M, Inoue K, Sakai T. Estrogen modulates ghrelin expression in the female rat stomach. Peptides. 2004;25:289–297. doi: 10.1016/j.peptides.2003.12.020. doi:10.1016/j.peptides.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. doi:10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar A, Kumar R, Hanoune J. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535:6–10. doi: 10.1016/s0014-5793(02)03846-2. doi:10.1016/S0014-5793(02)03846-2. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, Nakazato M. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul. Pept. 2005;126:55–59. doi: 10.1016/j.regpep.2004.08.038. doi:10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. doi:10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal-Serrano M, Sastre-Serra J, Pons DG, Miro AM, Oliver J, Roca P. The ERalpha/ERbeta ratio determines oxidative stress in breast cancer cell lines in response to 17beta-estradiol. J. Cell. Biochem. 2012;113:3178–3185. doi: 10.1002/jcb.24192. doi:10.1002/jcb.24192. [DOI] [PubMed] [Google Scholar]
- Nagy TR, Krzywanski D, Li J, Meleth S, Desmond R. Effect of group vs. single housing on phenotypic variance in C57BL/6J mice. Obes. Res. 2002;10:412–415. doi: 10.1038/oby.2002.57. doi:10.1038/oby.2002.57. [DOI] [PubMed] [Google Scholar]
- Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15932–15937. doi: 10.1073/pnas.0904747106. doi:10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterstock G, Escobar P, Mitutsova V, Gouty-Colomer LA, Fontanaud P, Molino F, Fehrentz JA, Carmignac D, Martinez J, Guerineau NC, Robinson ICAF, Mollard P, Méry PF. Ghrelin stimulation of growth hormone-releasing hormone neurons is direct in the arcuate nucleus. PLoS One. 2010:5. doi: 10.1371/journal.pone.0009159. doi:10.1371/journal.pone.0009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palou M, Sanchez J, Rodriguez AM, Priego T, Pico C, Palou A. Induction of NPY/AgRP orexigenic peptide expression in rat hypothalamus is an early event in fasting: relationship with circulating leptin, insulin and glucose. Cell. Physiol. Biochem. 2009;23:115–124. doi: 10.1159/000204100. doi:10.1159/000204100. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates, Compact, Third Edition: The coronal plates and diagrams. 3rd ed Academic Press; 2008. [Google Scholar]
- Perez-Tilve D, Heppner K, Kirchner H, Lockie SH, Woods SC, Smiley DL, Tschop M, Pfluger P. Ghrelin-induced adiposity is independent of orexigenic effects. FASEB J. 2011;25:2814–2822. doi: 10.1096/fj.11-183632. doi:10.1096/fj.11-183632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M, Istrate N, Wang L, Nichols AJ, Tozzo E, Stricker-Krongrad A. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int. J. Obes. Relat. Metab. Disord. 2004;28:879–885. doi: 10.1038/sj.ijo.0802640. doi:10.1038/sj.ijo.0802640. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. doi:10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priego T, Sanchez J, Pico C, Palou A. Sex-associated differences in the leptin and ghrelin systems related with the induction of hyperphagia under high-fat diet exposure in rats. Horm. Behav. 2009;55:33–40. doi: 10.1016/j.yhbeh.2008.07.010. doi:10.1016/j.yhbeh.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Rachoń D, Teede H. Ovarian function and obesity-Interrelationship, impact on women’s reproductive lifespan and treatment options. Mol. Cell. Endocrinol. 2010;316:172–179. doi: 10.1016/j.mce.2009.09.026. doi:10.1016/j.mce.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Ren H, Orozco IJ, Su Y, Suyama S, Gutiérrez-Juárez R, Horvath TL, Wardlaw SL, Plum L, Arancio O, Accili D. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012;149:1314–1326. doi: 10.1016/j.cell.2012.04.032. doi:10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA. Oestrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms. J. Neuroendocrinol. 2009;21:141–150. doi: 10.1111/j.1365-2826.2008.01814.x. doi:10.1111/j.1365-2826.2008.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. doi:10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Smith AW, Ronnekleiv OK, Kelly MJ. Serotonin 5-HT2C receptor-mediated inhibition of the M-current in hypothalamic POMC neurons. Am. J. Physiol. Endocrinol. Metab. 2012;302:E1399–E1406. doi: 10.1152/ajpendo.00565.2011. doi:10.1152/ajpendo.00565.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. doi:10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J. Neurosci. 2011;31:11825–35. doi: 10.1523/JNEUROSCI.1395-11.2011. doi:10.1523/JNEUROSCI.1395-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurazawa N, Mano-Otagiri A, Nemoto T, Shibasaki T. Effects of intracerebroventricular ghrelin on food intake and Fos expression in the arcuate nucleus of the hypothalamus in female rats vary with estrous cycle phase. Neurosci. Lett. 2013;541:204–208. doi: 10.1016/j.neulet.2013.02.006. doi:10.1016/j.neulet.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav. Brain Res. 2008;191:173–177. doi: 10.1016/j.bbr.2008.03.019. doi:10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. doi:10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sheffer-Babila S, Sun Y, Israel DD, Liu S-M, Neal-Perry G, Chua SC. Agouti-related peptide plays a critical role in leptin’s effects on female puberty and reproduction. Am. J. Physiol. Endocrinol. Metab. 2013;305:E1512–20. doi: 10.1152/ajpendo.00241.2013. doi:10.1152/ajpendo.00241.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front. Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. doi:10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Bian X, Qu Z, Ma Z, Zhou Y, Wang K, Jiang H, Xie J. Peptide hormone ghrelin enhances neuronal excitability by inhibition of Kv7/KCNQ channels. Nat. Commun. 2013;4:1435. doi: 10.1038/ncomms2439. doi:10.1038/ncomms2439. [DOI] [PubMed] [Google Scholar]
- Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, Sorg T, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14:R82. doi: 10.1186/gb-2013-14-7-r82. doi:10.1186/gb-2013-14-7-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanska A, Bergmann K, Sypniewska G. Metabolic syndrome and menopause: pathophysiology, clinical and diagnostic significance. Adv. Clin. Chem. 2015;72:1–75. doi: 10.1016/bs.acc.2015.07.001. doi:10.1016/bs.acc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. doi:10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- Teubner BJW, Garretson JT, Hwang Y, Cole PA, Bartness TJ. Inhibition of ghrelin O-acyltransferase attenuates food deprivation-induced increases in ingestive behavior. Horm. Behav. 2013;63:667–673. doi: 10.1016/j.yhbeh.2013.02.001. doi:10.1016/j.yhbeh.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titolo D, Cai F, Belsham DD. Coordinate regulation of neuropeptide Y and agouti-related peptide gene expression by estrogen depends on the ratio of estrogen receptor (ER) alpha to ERbeta in clonal hypothalamic neurons. Mol. Endocrinol. 2006;20:2080–2092. doi: 10.1210/me.2006-0027. doi:10.1210/me.2006-0027. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Yao Y, Fu L-Y, Foo K, Huang H, Coppari R, Lowell BB, Broberger C. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J. Neurosci. 2009;29:4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. doi:10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst PJ, Janssen S, Tack J, Depoortere I. Role of the AMP-activated protein kinase (AMPK) signaling pathway in the orexigenic effects of endogenous ghrelin. Regul. Pept. 2012;173:27–35. doi: 10.1016/j.regpep.2011.09.001. doi:10.1016/j.regpep.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci. Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Rømer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. doi:10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Wolfgang MJ, Cha SH, Millington DS, Cline G, Shulman GI, Suwa A, Asaumi M, Kurama T, Shimokawa T, Lane MD. Brain-specific carnitine palmitoyl transferase-1c: Role in CNS fatty acid metabolism, food intake, and body weight. J. Neurochem. 2008;105:1550–1559. doi: 10.1111/j.1471-4159.2008.05255.x. doi:10.1111/j.1471-4159.2008.05255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang MJ, Cha SH, Sidhaye A, Chohnan S, Cline G, Shulman GI, Lane MD. Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19285–19290. doi: 10.1073/pnas.0709778104. doi:10.1073/pnas.0709778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, Matsumoto S-I, Cha SH, Shimokawa T, Lane MD. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2006;103:7282–7287. doi: 10.1073/pnas.0602205103. doi:10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang MJ, Lane MD. Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity. FEBS J. 2011;278:552–558. doi: 10.1111/j.1742-4658.2010.07978.x. doi:10.1111/j.1742-4658.2010.07978.x. [DOI] [PubMed] [Google Scholar]
- Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci. Lett. 2006;398:39–43. doi: 10.1016/j.neulet.2005.12.060. doi:10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Xu C, Roepke TA, Zhang C, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone (GnRH) activates the M-current in GnRH neurons: An autoregulatory negative feedback mechanism? Endocrinology. 2008;149:2459–2466. doi: 10.1210/en.2007-1178. doi:10.1210/en.2007-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. doi:10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. doi:10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]