Abstract
Many adults with elevated clinic blood pressure (BP) have lower BP when measured outside the clinic. This phenomenon, the “white‐coat effect,” may be larger among older adults, a population more susceptible to the adverse effects of low BP. The authors analyzed data from 257 participants in the Jackson Heart Study with elevated clinic BP (systolic/diastolic BP [SBP/DBP] ≥140/90 mm Hg) who underwent ambulatory BP monitoring (ABPM). The white‐coat effect for SBP was larger for participants 60 years and older vs those younger than 60 years in the overall population (12.2 mm Hg, 95% confidence interval [CI], 9.2–15.1 mm Hg and 8.4 mm Hg, 95% CI, 5.7–11.1, respectively; P=.06) and among those without diabetes or chronic kidney disease (15.2 mm Hg, 95% CI, 10.1–20.2 and 8.6 mm Hg, 95% CI, 5.0–12.3, respectively; P=.04). After multivariable adjustment, clinic SBP ≥150 mm Hg vs <150 mm Hg was associated with a larger white‐coat effect. Studies are needed to investigate the role of ABPM in guiding the initiation and titration of antihypertensive treatment, especially among older adults.
In the United States, antihypertensive medication treatment decisions including those in the 2014 Evidence‐Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) are primarily based on blood pressure (BP) measurements obtained in the clinic setting.1 Previous studies have reported that 20% to 25% of untreated adults and more than 30% of treated adults with elevated clinic BP have nonelevated BP when measured outside of the clinic.2, 3 Therefore, healthcare providers relying on clinic BP measurements may be unnecessarily initiating treatment and treating to a lower BP level than intended.
The optimal systolic BP (SBP) goal for adults 60 years and older is controversial.4 Clinic‐based studies have reported that a larger difference between clinic and out‐of‐clinic daytime BP, a white‐coat effect, may be present in older individuals.5, 6 Identifying a large white‐coat effect among older adults has important implications given the possible increased susceptibility of this population to adverse events associated with low on‐treatment BP.7 The goal of the current study was to determine the white‐coat effect among younger and older adults in a population‐based sample. Additionally, we determined the prevalence of white‐coat hypertension. To achieve these goals, we analyzed data from a population‐based cohort of African Americans participating in the Jackson Heart Study (JHS).
Methods
Study Participants
The JHS is a community‐based observational study of African American adults recruited from urban and rural areas of three counties (Hinds, Madison, and Rankin) that comprise the Jackson, Mississippi, metropolitan area. Baseline data collection occurred between September 2000 and March 2004. Details of the study design and recruitment have been published previously.8, 9, 10 In brief, the study was designed to identify risk factors for cardiovascular disease (CVD) in African Americans. Individuals were selected for enrollment through a combination of drivers’ license registries and commercially available lists. The final cohort includes 5301 African American adults 21 years and older. The study protocol was approved by the institutional review boards governing research in human subjects at the participating centers and all participants provided written consent.
Data Collection
Data used for the current analysis were collected through an in‐home interview, a study examination, and 24‐hour ambulatory BP monitoring (ABPM). Information on age, sex, education, income, cigarette smoking, and history of diabetes were collected during the study interview. Total physical activity was assessed with the modified Baecke questionnaire, which scores self‐reported physical activity in sports, leisure time, and at work on a scale of 1 (low) to 5 (high).11 During the clinic visit, a standardized protocol was followed to obtain two BP measurements, measure waist circumference, and collect blood and urine samples. Information was recorded on all medications, vitamins, mineral supplements, and herbal or home remedies used within the 2 weeks prior to the participant's interview.9
Participants were asked to fast overnight prior to their study visit. Urinary albumin was measured with the Dade Behring BN II nephelometer (Siemens Healthcare Diagnostics, Newark, DE). Serum and urine creatinine were measured using a multi‐point enzymatic spectrophotometric assay on a Vitros 950 analyzer (Ortho‐Clinical Diagnostics, Raritan, NJ). Creatinine values were biochemically calibrated to Cleveland Clinic‐equivalent Minnesota Beckman CX3 assay for analysis purposes.12 Estimated glomerular filtration rate (eGFR) was calculated via the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation,13 and CKD was defined as an albumin/creatinine ratio ≥30 mg/g or eGFR <60 mL/min/1.73 m2. Diabetes was defined as a fasting (≥8 hours) plasma glucose ≥126 mg/dL, glycohemoglobin ≥6.5%, or use of antidiabetes medication.
During the clinic visit, BP was measured after a 5‐minute rest with a Hawksley random zero sphygmomanometer (Hawksley and Sons Ltd, Langing, United Kingdom) equipped with one of four cuff sizes selected following measurement of each participant's arm circumference. The average of the two measures taken 1 minute apart was used to define clinic BP. On completion of the study visit, all participants were invited to complete ABPM over the next 24 hours. A subset of 1148 participants agreed and subsequently underwent ABPM. ABPM measurements were obtained with a portable, noninvasive oscillometric device (Spacelabs 90207; Medifacts International Ltd, Rockville, MD) with a cuff fitted to the participant's nondominant arm. Trained technicians instructed participants in the proper use of the ABPM device. With the participant seated, three to five simultaneous ABPM and office sphygmomanometer BP readings were taken to confirm that the ABPM device was calibrated. The device was programmed to measure BP every 20 minutes for 24 hours, and participants were instructed to proceed with their normal daily activities but keep their arm still and extended at their side during each BP reading. Participants returned to the clinic after 24 hours for the removal of the device. The monitor was connected to a computer and the BP readings were downloaded with commercially available software (Medicom, version 3.41; Medifacts Ltd, Rockville, MD). Quality control was assured by technician recertification, procedural checklists, and data review.9, 14, 15, 16
Assessment of White‐Coat Effect and White‐Coat Hypertension
Mean daytime out‐of‐clinic SBP and diastolic BP (DBP) were calculated as the average of all ABPM measurements taken between 10 am and 8 pm.17 For SBP and DBP, separately, the white‐coat effect was calculated as clinic BP minus daytime out‐of‐clinic BP. Elevated clinic BP was defined as clinic SBP ≥140 mm Hg or clinic DBP ≥90 mm Hg. Nonelevated daytime out‐of‐clinic BP was defined as ABPM‐derived SBP and DBP <135 mm Hg and <85 mm Hg, respectively.2 White‐coat hypertension was defined as elevated clinic BP with nonelevated daytime out‐of‐clinic BP. In a sensitivity analysis, white‐coat hypertension was defined as elevated clinic BP with nonelevated 24‐hour mean BP (ie, ABPM‐derived SBP and DBP <130 mm Hg and <80 mm Hg, respectively).
Statistical Analysis
The current analysis was limited to participants with elevated clinic BP and valid ABPM data based on the International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO) criteria (n=257). Specifically, we required participants to have 10 or more daytime (defined as 10 am–8 pm) and 5 or more nighttime (defined as 12 pm–6 am) SBP and DBP measurements.17 First, we determined baseline characteristics of study participants younger than 60 years (ie, “younger” adults) and those 60 years and older (ie, “older” adults), overall, and restricted to participants without CKD or diabetes. We performed an analysis of patients without CKD or diabetes since individuals 60 years and older without these conditions have a higher SBP threshold for initiation of treatment and a higher treatment goal according to the recent JNC 8 guideline (SBP ≥150 mm Hg vs SBP ≥140 mm Hg for those without CKD and diabetes).1 For the overall population and, separately, for those without CKD or diabetes, we calculated clinic and daytime out‐of‐clinic SBP and DBP and the white‐coat effect by age group. Differences in the white‐coat effect between age groups were assessed using t tests. Next, we used linear regression to identify factors associated with the white‐coat effect in unadjusted and adjusted models. Initial adjustment included age and sex, with a subsequent model further adjusting for current smoking, total physical activity score, body mass index, diabetes, eGFR, clinic SBP, and antihypertensive medication use. We calculated the prevalence of white‐coat hypertension by age for the overall population and, separately, for those without CKD or diabetes. We also calculated the percentage of study participants with ≥10 mm Hg lower daytime out‐of‐clinic vs clinic SBP and, separately, DBP. We repeated the above analyses stratified by antihypertensive medication use status. In a sensitivity analysis, we calculated the prevalence of white‐coat hypertension by age using the secondary definition described above. We also calculated the percentage of study participants with ≥10 mm Hg lower 24‐hour mean vs clinic SBP and, separately, DBP. Data management and statistical analysis was conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Participant Characteristics
Older participants were less likely to be current smokers and more likely to have an eGFR <60 mL/min/1.73 m2 and take antihypertensive medication compared with their younger counterparts (Table 1). In addition, older participants had lower levels of physical activity. When restricted to participants without CKD or diabetes, older adults were more likely than younger adults to be taking antihypertensive medication.
Table 1.
Characteristics of Jackson Heart Study Participants With Clinic Systolic Blood Pressure ≥140 mm Hg or Diastolic Blood Pressure ≥90 mm Hg By Age
| All Participants (N=257) | Participants Without CKD or Diabetes (n=130) | |||||
|---|---|---|---|---|---|---|
| Age <60 y (n=114) | Age ≥60 y (n=143) | P Value | Age <60 y (n=65) | Age ≥60 y (n=65) | P Value | |
| Age, y | 51.3 (6.1) | 69.1 (5.5) | <.001 | 50.1 (6.5) | 68.7 (5.6) | <.001 |
| Women, % | 62.3 | 66.4 | .49 | 67.7 | 66.2 | .85 |
| Current smoker, % | 15.8 | 7.8 | .05 | 12.3 | 6.3 | .24 |
| Physical activity, exercise unitsa | 8.6 (2.3) | 7.9 (2.7) | .03 | 8.7 (2.4) | 8.2 (2.8) | .29 |
| Body mass index, kg/m2 | 31.5 (7.0) | 30.9 (6.2) | .45 | 30.3 (6.0) | 31.3 (7.2) | .42 |
| Diabetes, % | 21.2 | 31.2 | .07 | 0 | 0 | — |
| eGFR <60 mL/min/1.73 m2, % | 3.5 | 11.4 | .02 | 0 | 0 | — |
| Albuminuria, % | 14.0 | 12.6 | .73 | 0 | 0 | — |
| Taking antihypertensive medications, % | 53.5 | 74.8 | <.001 | 43.1 | 64.6 | .01 |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate. aPhysical activity score ranges from 1 to 20, with a higher score indicating higher physical activity.
White‐Coat Effect
The white‐coat effect for SBP was larger for older, compared with younger, participants, overall (12.2 mm Hg; 95% confidence interval [CI], 9.2, 15.1 mm Hg vs 8.4 mm Hg; 95% CI, 5.7, 11.1 mm Hg, respectively; P=.06) and among those without diabetes or CKD (15.2 mm Hg; 95% CI, 10.1, 20.2 mm Hg vs 8.6 mm Hg; 95% CI, 5.0, 12.3 mm Hg, respectively; P=.04 [Table 2]). The white‐coat effect for DBP was smaller for older, compared with younger, participants (3.0 mm Hg; 95% CI, 1.1, 4.9 mm Hg vs 6.2 mm Hg; 95% CI, 4.2, 8.1 mm Hg, respectively; P=.02) but was similar among older and younger adults without diabetes or CKD (5.9 mm Hg; 95% CI, 2.7, 9.0 mm Hg and 7.3 mm Hg; 95% CI, 5.1, 9.5 mm Hg, respectively; P=.46). Table S1 shows the white‐coat effect stratified by age for participants not taking and taking antihypertensive medication, separately.
Table 2.
Clinic and Out‐of‐Clinic Daytime Blood Pressure Among Jackson Heart Study Participants With Clinic Systolic Blood Pressure ≥140 mm Hg or Diastolic Blood Pressure ≥90 mm Hg
| All Participants (N=257) | Participants Without CKD or Diabetes (n=130) | |||||
|---|---|---|---|---|---|---|
| Age <60 y (n=114) | Age ≥60 y (n=143) | P Value | Age <60 y (n=65) | Age ≥60 y (n=65) | P Value | |
| SBP, mm Hg | ||||||
| Clinic | 143.4 (140.6–146.2) | 151.9 (149.5–154.2) | <.001 | 140.9 (137.6–144.2) | 149.9 (145.9–153.9) | <.001 |
| Out‐of‐clinic daytime | 135.0 (132.4–137.6) | 139.7 (137.5–142.0) | .01 | 132.2 (129.4–135.1) | 134.8 (131.8–137.7) | .22 |
| Difference | 8.4 (5.7–11.1) | 12.2 (9.2–15.1) | .06 | 8.6 (5.0–12.3) | 15.2 (10.1–20.2) | .04 |
| DBP, mm Hg | ||||||
| Clinic | 91.3 (89.8–92.8) | 81.1 (79.3–82.9) | <.001 | 92.2 (90.3–94.2) | 82.6 (79.9–85.2) | <.001 |
| Out‐of‐clinic daytime | 85.1 (83.4–86.8) | 78.1 (76.4–79.7) | <.001 | 84.9 (82.9–87.0) | 76.7 (74.3–79.1) | <.001 |
| Difference | 6.2 (4.2–8.1) | 3.0 (1.1–4.9) | .02 | 7.3 (5.1–9.5) | 5.9 (2.7–9.0) | .46 |
Abbreviations: CKD, chronic kidney disease; DBP, diastolic blood pressure; SBP, systolic blood pressure. Values are expressed as mean systolic or diastolic blood pressure (95% confidence interval).
Factors Associated With White‐coat Effect
In the overall population, clinic SBP ≥150 mm Hg vs <150 mm Hg was associated with a larger white‐coat effect in unadjusted models and after multivariable adjustment (Table 3). Among those without CKD or diabetes, older age and clinic SBP ≥150 mm Hg vs <150 mm Hg were associated with a larger white‐coat effect in unadjusted models. These associations remained present after multivariable adjustment.
Table 3.
Factors Associated With White‐Coat Effect for Systolic Blood Pressure Among Jackson Heart Study Participants With Clinic Systolic Blood Pressure ≥140 mm Hg or Clinic Diastolic Blood Pressure ≥90 mm Hg
| All Participants (N=257) | Participants Without CKD or Diabetes (n=130) | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Unadjusted | Model 1 | Model 2 | |
| Age, y | ||||||
| <60 | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| ≥60 | 3.8 (2.1) | 3.8 (2.1) | 1.3 (2.2) | 6.5 (3.1)c | 6.5 (3.1)c | 5.9 (2.8)c |
| Women | 0.4 (2.2) | 0.2 (2.2) | 0.7 (2.1) | −3.5 (3.3) | −3.3 (3.3) | −0.04 (2.9) |
| Current smoker | −4.9 (3.2) | −4.3 (3.2) | −6.0 (3.2) | 3.1 (5.3) | 3.8 (5.2) | −3.6 (4.6) |
| Total physical activity score, exercise unitsb | ||||||
| Tertile 1 (3.29–7.13) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| Tertile 2 (7.14–9.57) | 0.6 (2.5) | 1.5 (2.6) | 1.0 (2.4) | 4.5 (3.9) | 5.8 (3.9) | 4.3 (3.3) |
| Tertile 3 (9.59–15.64) | −3.0 (2.7) | −2.4 (2.7) | −0.8 (2.5) | −5.0 (4.0) | −4.1 (4.0) | −3.3 (3.4) |
| Body mass index, kg/m2 | ||||||
| <25 | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| 25–29 | −1.7 (3.1) | −2.8 (3.2) | −0.2 (3.1) | −1.7 (4.5) | −3.8 (4.5) | −4.4 (4.0) |
| ≥30 | −0.03 (3.0) | −0.4 (3.0) | 2.7 (3.0) | −0.6 (4.4) | −0.8 (4.3) | −0.5 (3.9) |
| Diabetes | −1.4 (2.4) | −1.9 (2.4) | −3.9 (2.3) | — | — | — |
| eGFR <60 mL/min/1.73 m2 | 6.1 (3.9) | 5.2 (3.9) | 3.1 (4.0) | — | — | — |
| Clinic SBP, mm Hg | ||||||
| <150 | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) |
| ≥150 | 14.4 (1.9)d | 14.5 (2.0)d | 13.6 (2.2)d | 22.3 (2.9)d | 21.9 (2.9)d | 20.0 (3.0)d |
| Taking antihypertensive medication | 1.3 (2.2) | 0.4 (2.3) | −2.1 (2.3) | −3.1 (3.2) | −4.3 (3.2) | −5.0 (2.8) |
Abbreviations: eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure. Model 1 includes adjustment for age and sex. Model 2 includes all variables listed in the left column. Values are expressed as mean (standard error) from a linear regression model. aOverall (left panel) and after further restriction to participants without chronic kidney disease (CKD) or diabetes (right panel). bPhysical activity score ranges from 1 to 20, with a higher score indicating higher physical activity. c P<.05. d P<.001.
Prevalence of White‐Coat Hypertension
Over 30% of younger and older adults with elevated clinic BP had white‐coat hypertension based on daytime BP (Figure 1, top panel). Furthermore, 44.7% of younger adults and 49.7% of older adults had a ≥10 mm Hg lower out‐of‐clinic daytime vs clinic SBP and 33.3% of younger adults and 24.5% of older adults had a ≥10 mm Hg lower out‐of‐clinic daytime vs clinic DBP. These prevalence estimates were higher among participants without CKD or diabetes (Figure 1, bottom panel). Results were similar in a sensitivity analysis defining white‐coat hypertension based on 24‐hour mean BP rather than out‐of‐clinic daytime BP (Figure 2). Figure S1 shows the percentage of older and younger adults with elevated clinic BP who had nonelevated out‐of‐clinic daytime BP and ≥10 mm Hg lower out‐of‐clinic daytime vs clinic SBP and DBP stratified by antihypertensive medication use.
Figure 1.
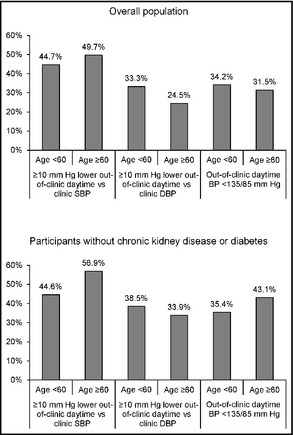
White‐coat hypertension by age for the overall population with clinic systolic blood pressure (SBP) ≥140 mm Hg or clinic diastolic blood pressure (DBP) ≥90 mm Hg (top panel) and after further restriction to participants without chronic kidney disease or diabetes (bottom panel). BP indicates blood pressure.
Figure 2.
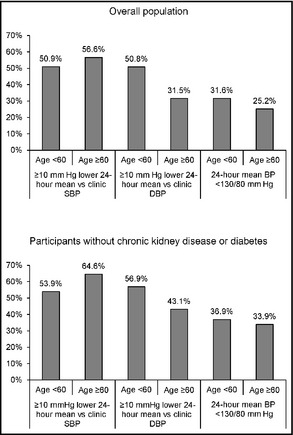
White‐coat hypertension by age for the overall population with clinic systolic blood pressure (SBP) ≥140 mm Hg or clinic diastolic blood pressure (DBP) ≥90 mm Hg (top panel) and after further restriction to participants without chronic kidney disease or diabetes (bottom panel). BP indicates blood pressure.
Discussion
Among participants 60 years and older with elevated clinic BP in the current study, clinic SBP was on average 12.2 mm Hg higher than daytime SBP. Further, almost half of this population had a clinic SBP ≥10 mm Hg higher than their out‐of‐clinic daytime BP. The white‐coat effect and the percentage of participants with a clinic SBP ≥10 mm Hg higher than their out‐of‐clinic daytime BP were even larger in those without CKD or diabetes. Higher clinic SBP was associated with a larger white‐coat effect in the overall population. In addition, among those without CKD or diabetes, older age and clinic SBP ≥150 mm Hg vs <150 mm Hg were associated with a larger white‐coat effect.
The recently published JNC 8 recommends treating older adults without diabetes or CKD to an SBP <150 mm Hg.1 This recommendation represents a fundamental shift from previous guidelines, including the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7), which recommended treating SBP in this population to <140 mm Hg.18 Although the new recommendation is based on lack of evidence from randomized controlled trials showing a benefit of a goal SBP <140 mm Hg,1 it has been controversial because of the high rate of CVD among older adults and a large body of observational data on the increased risk for CVD at higher levels of SBP.4 However, overtreatment among older adults has also raised concern as this population may be more susceptible to adverse events (eg, falls) associated with low on‐treatment BP.19 Using data from the National Health and Nutrition Examination Survey 2005–2010, we previously reported that 13.1% and 15.8% of untreated and treated adults 60 years and older without CKD or diabetes, respectively, had a clinic SBP of 140 mm Hg to 149 mm Hg.20 Hence, a large number of older adults stand to be affected by the new BP treatment guidelines.
The treatment decisions in the JNC 8 guidelines are based on BP measurements obtained in the clinic setting. However, previous studies suggest that many adults with elevated clinic BP have normal BP when measured outside the clinic, and that there may be a larger white‐coat effect among older adults.21 ABPM can quantify the white‐coat effect in older adults, and prior research demonstrates that the use of ABPM to diagnose white‐coat hypertension is cost‐effective.22 However, data from a 5% random sample of Medicare beneficiaries showed a very low percentage (approximately 0.1%) of beneficiaries had claims for ABPM between 2007 and 2010, and 95% of those with an ABPM claim were taking antihypertensive medication, suggesting that ABPM is not currently widely used among older adults, especially for diagnosing white‐coat hypertension among untreated individuals.23
The recently released draft BP screening recommendations by the United States Preventive Services Task Force (USPSTF) endorsed ABPM to confirm high BP before the diagnosis of hypertension in adults 18 years and older.24 This is based, in part, on the findings of a systematic review of published studies showing up to 65% of patients with elevated clinic BP did not have elevated BP on ABPM.21 Furthermore, elevated 24‐hour SBP was consistently associated with increased CVD risk, independent of clinic BP measurements.21
White‐coat hypertension is associated with a lower risk for CVD when compared with sustained hypertension.25 Therefore, healthcare providers may be unnecessarily treating older individuals and/or treating to lower BP levels than anticipated. A recent study of Medicare beneficiaries reported antihypertensive medication to be associated with serious fall injuries (adjusted hazard ratios [95% CIs], 1.40 [1.03–1.90] and 1.28 [0.91–1.80] for moderate‐intensity and high‐intensity antihypertensive medication groups compared with nonusers).7 However, randomization to an SBP goal <120 mm Hg vs <140 mm Hg among people with type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was not associated with an increased risk for falls.26 Future research is needed on the association between clinic and out‐of‐clinic BP and the risk for falls and other adverse events among older adults.
Study Limitations and Strengths
Since the JHS is restricted to African American participants, we could not assess racial differences in the white‐coat effect. In a recent analysis of young adults (mean age, approximately 30 years) participating in the Coronary Artery Risk Development in Young Adults (CARDIA) study,27 the prevalence of white‐coat hypertension was 3.3% and 3.9% among African Americans and whites, respectively.27 In addition, a 2005 meta‐analysis by Agyemang and colleagues28 showed no differences in the white‐coat effect between blacks and whites.28 Specifically, the weighted mean differences in the white‐coat effect between African Americans and whites (ie, the white‐coat effect for African Americans minus the white‐coat effect for whites) were 0.31 (95% CI, −1.96 to 2.57; P=.79) and 0.18 (95% CI, −1.70 to 1.35; P=.82) for SBP and DBP, respectively.28 Additional research is needed to determine whether the larger white‐coat effect at older age observed among African Americans in the current study is also present among whites.
Strengths of the JHS include the large population‐based sample of African American adults, collection of ABPM following a standardized protocol, and extensive data collection that allowed us to adjust for several potential confounders. Most prior studies of ABPM in the United States have included clinic populations or have had scarce minority representation. Despite these strengths, the findings of the current study should be considered in the context of certain limitations. Clinic BP was measured on a single occasion. Some participants may not have had elevated clinic BP if measured on a separate day. ABPM was also measured on a single occasion, which may have resulted in some misclassification of the white‐coat effect. Some participants may not have demonstrated a white‐coat effect on re‐testing. Finally, ABPM was conducted in only a sample of JHS participants and, of those, only 257 had elevated clinic BP. This resulted in a small sample size for some analyses.
Conclusions
Clinic SBP was substantially higher than out‐of‐clinic SBP in the current study. This difference was larger among participants ≥60 vs <60 years of age. Also, 32% of older individuals with elevated clinic BP had non‐elevated out‐of‐clinic daytime BP. The white‐coat effect and percentage of individuals with elevated clinic but non‐elevated daytime BP were even larger for individuals without CKD or diabetes. Among older adults, the cardiovascular risk reduction benefits of antihypertensive medication initiation and intensification for individuals with elevated clinic BP should be balanced with potential adverse side effects associated with lower out‐of‐clinic BP. Given the large white‐coat effect present, future studies are needed to investigate the role of ABPM in guiding antihypertensive treatment among older adults.
Supporting information
Table S1. Clinic and out‐of‐clinic daytime blood pressure among Jackson Heart Study participants with clinic systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.
Figure S1. White‐coat effect by age for the overall population with clinic systolic blood pressure ≥140 mm Hg or clinic diastolic blood pressure ≥90 mm Hg (top panel) and after further restriction to those without CKD or diabetes (bottom panel), stratified by antihypertensive medication use.
Acknowledgments
The JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD). Support was also partially provided through P01‐HL047540 (Dr Shimbo) from NHLBI. Dr Ogedegbe was supported in part by grant K24HL111315 from NHLBI. Dr Muntner received an institutional grant from Amgen, Inc, and has served on an advisory board for Amgen, Inc. Dr Seals and Dr Tanner have no disclosures. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
J Clin Hypertens (Greenwich). 2016;18:139–145. DOI: 10.1111/jch.12644. © 2015 Wiley Periodicals, Inc.
References
- 1. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 2. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 3. MacDonald MB, Laing GP, Wilson MP, Wilson TW. Prevalence and predictors of white‐coat response in patients with treated hypertension. CMAJ. 1999;161:265–269. [PMC free article] [PubMed] [Google Scholar]
- 4. Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G. Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160:499–503. [DOI] [PubMed] [Google Scholar]
- 5. Helvaci MR, Seyhanli M. What a high prevalence of white coat hypertension in society!. Intern Med. 2006;45:671–674. [DOI] [PubMed] [Google Scholar]
- 6. Reddy AK, Jogendra MR, Rosendorff C. Blood pressure measurement in the geriatric population. Blood Press Monit. 2014;19:59–63. [DOI] [PubMed] [Google Scholar]
- 7. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sempos CT, Bild DE, Manolio TA. Overview of the Jackson Heart Study: a study of cardiovascular diseases in African American men and women. Am J Med Sci. 1999;317:142–146. [DOI] [PubMed] [Google Scholar]
- 9. Taylor HA Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;4(suppl 6):4–17. [PubMed] [Google Scholar]
- 10. Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African‐American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;4(suppl 6):S18–S29. [PubMed] [Google Scholar]
- 11. Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical activity and cardiovascular disease in African Americans in Atherosclerosis Risk in Communities. Med Sci Sports Exerc. 2013;45:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fox ER, Benjamin EJ, Sarpong DF, et al. The relation of C–reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study. BMC Nephrol. 2010;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wyatt SB, Akylbekova EL, Wofford MR, et al. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51:650–656. [DOI] [PubMed] [Google Scholar]
- 15. Ogedegbe G, Spruill TM, Sarpong DF, et al. Correlates of isolated nocturnal hypertension and target organ damage in a population‐based cohort of African Americans: the Jackson Heart Study. Am J Hypertens. 2013;26:1011–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hickson DA, Diez Roux AV, Wyatt SB, et al. Socioeconomic position is positively associated with blood pressure dipping among African‐American adults: the Jackson Heart Study. Am J Hypertens. 2011;24:1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stolarz‐Skrzypek K, Thijs L, Li Y, et al. Short‐term blood pressure variability in relation to outcome in the International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO). Acta Cardiol. 2011;66:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 19. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123:2434–2506. [DOI] [PubMed] [Google Scholar]
- 20. Shimbo D, Tanner RM, Muntner P. Prevalence and characteristics of systolic blood pressure thresholds in individuals 60 years or older. JAMA Intern Med. 2014;174:1397–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: an updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162;192–204. [DOI] [PubMed] [Google Scholar]
- 22. Krakoff LR. Cost‐effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47:29–34. [DOI] [PubMed] [Google Scholar]
- 23. Shimbo D, Kent ST, Diaz KM, et al. The use of ambulatory blood pressure monitoring among Medicare beneficiaries in 2007‐2010. J Am Soc Hypertens. 2014;8:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. USPSTF . Draft recommendation statement – high blood pressure in adults: screening. 2014; http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementDraft/hypertension-in-adults-screening-and-home-monitoring#Pod12. Accessed January 2, 2015.
- 25. Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19:801–807. [DOI] [PubMed] [Google Scholar]
- 26. Margolis KL, Palermo L, Vittinghoff E, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med. 2014;29:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muntner P, Lewis CE, Diaz KM, et al. Racial differences in abnormal ambulatory blood pressure monitoring measures: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens. 2015;28:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agyemang C, Bhopal R, Bruijnzeels M, Redekop WK. Does the white‐coat effect in people of African and South Asian descent differ from that in White people of European origin? A systematic review and meta‐analysis. Blood Press Monit. 2005;10:243–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinic and out‐of‐clinic daytime blood pressure among Jackson Heart Study participants with clinic systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg.
Figure S1. White‐coat effect by age for the overall population with clinic systolic blood pressure ≥140 mm Hg or clinic diastolic blood pressure ≥90 mm Hg (top panel) and after further restriction to those without CKD or diabetes (bottom panel), stratified by antihypertensive medication use.


