Abstract
Introduction
The ren is considered as the most commonly damaged genitourinary organ. In general, blunt kidney traumas (BKT) are mild and can be managed conservatively. We aimed to analyze our own experiences of selective renal artery embolization (RAE) in BKT patients and compare obtained results with other reports.
Material and methods
We analyzed the medical and technical outcomes of RAE in 20 patients with grades II-IV blunt kidney traumas. Indications for RAE were blunt kidney trauma combined with a gross hematuria that could not be stopped conservatively. For evaluating the functioning of kidneys we used radioisotope renography.
Results
According to the American Association for the Surgery of Trauma classification, grade II blunt kidney injury was registered at 2 (10.0%) pts, grade III – at 11 (55.0%) pts and grade IV – at 7 (35.0%) pts. In all patients, the bleeding was stopped with the embolization procedure. 18 (90.0%) patients were treated in a single interventional session and 2 (10.0%) needed further intervention. Different complications were registered as 5 (25%) pts: two or more complications were often combined in each individual case. The function in damaged kidneys was preserved at the 3rd month after RAE sessions.
Conclusions
RAE is an effective, minimally invasive treatment for blunt kidney injury that ensures the cessation of gross hematuria and kidney function preserving.
Keywords: blunt kidney trauma, renal artery embolization, hematuria
INTRODUCTION
Renal trauma occurs in approximately 1-5% of all injuries [1]. The kidney is considered to be the most commonly damaged organ of the urinary system with the male to female ratio being 3:1 [2, 3, 4]. Blunt kidney trauma (BKT) occurs more often than penetrating trauma, being 9 times more common. Both kidneys are at equal disposition for injury [5].
Generally, renal injuries are mild and can be managed conservatively; although blunt kidney trauma can be life threatening and requires urgent open surgery [6]. Kidney damage usually manifests as an urgent accident for both the urologist and the patient, which requires an immediate decision about the treatment option.
Recent advances in the imaging and more precise staging of trauma, as well as in the available treatment options, have decreased the need for surgical intervention and increased the percentage of kidney preservation.
During the few last decades, numerous papers have been published that analyze the indications and advantages of minimally invasive treatment in patients with BKT. However, there is a lack of data results about such techniques. Therefore, we aimed to analyze our own experiences of selective renal artery embolization in BKT patients and compare the obtained results with other investigations on the topic.
MATERIAL AND METHODS
We retrospectively analyzed the medical and technical outcomes of selective renal artery embolization (RAE) in 20 patients with grades II-IV blunt kidney traumas. RAE sessions were performed from December 2005 until May 2014. For trauma staging, we used the American Association for the Surgery of Trauma (AAST) classification [7].
Inclusion criteria were the following:
presence of BKT grades II–IV according to AAST classification;
hemodynamic stability;
gross hematuria that was not able to be stopped conservatively.
RAE sessions consisted of the following steps.
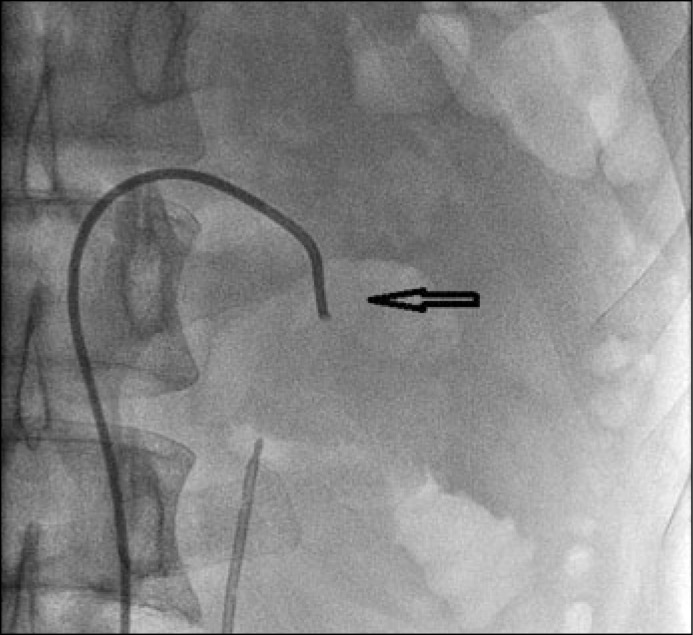
At first, a catheter was inserted transfemorally into the renal artery close to the ruptured branch (Figure 1).
Figure 1.
The catheter was inserted transfemorally into renal artery closely to ruptured branch.
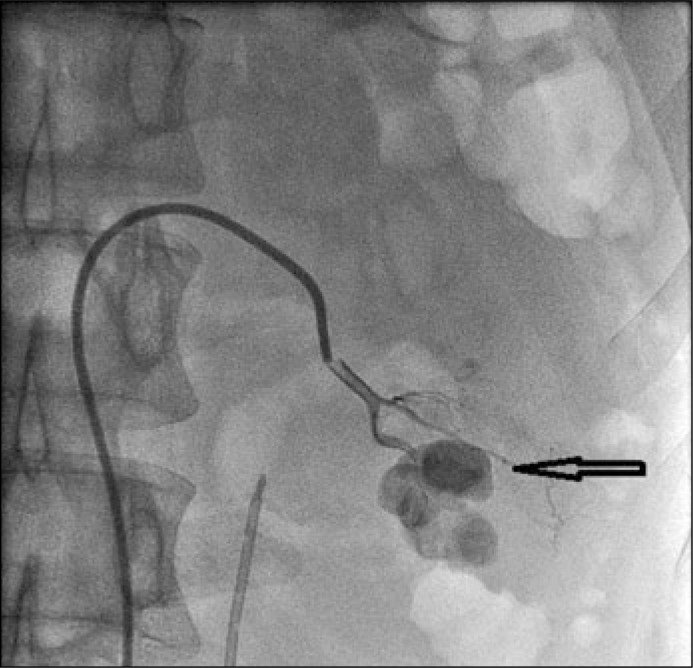
Then an angiography was performed with a 5-F cobra catheter transfemorally (Figure 2).
Figure 2.
The angiogram evaluated source of bleeding (arrow).
After localization of the origin of bleeding, we moved the catheter with a steerable 0.035-inch guide wire (Cook; Cordis) more distally to the bleeding vessel.
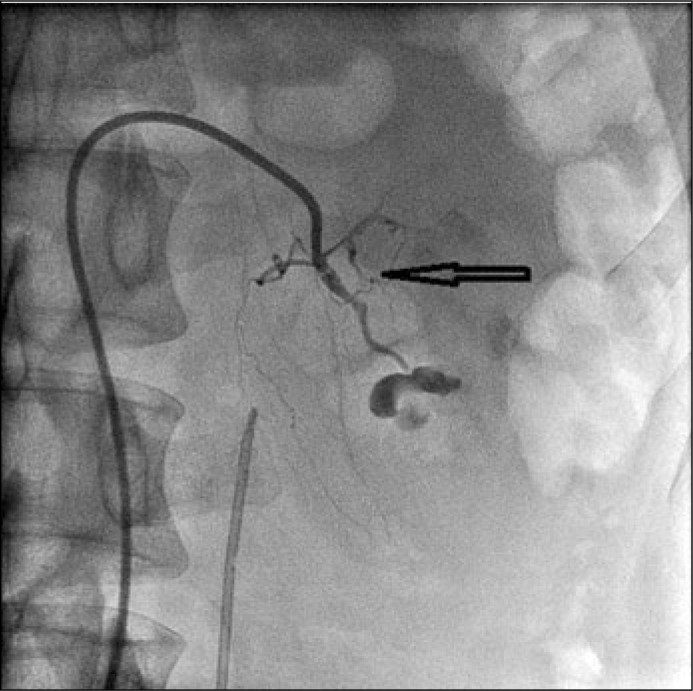
After that, a 3-F superselective coaxial catheter (Terumo) was introduced coaxially into the proximity of the vessel's rupture and an embolization was performed (Figure 3).
Figure 3.
Embolization of ruptured vessel.
Embolization and the preparing of the active solution were performed according to generally approved principles. For embolization, we used polyvinyl alcohol (PVA) particles sized 250–350 µm (Contour; Boston Scientific) in 14 patients and “Gelaspon” granules in 6 patients. The particles were prepared by mixing them with contrast material, usually by adding 5 mL of contrast to the 2- to 5-mL prefilled saline.
Then the air was carefully removed and the syringe was gently inverted several times. Then, using a three-way stopcock attached to a smaller 1- to 5-mL syringe, the solution was further mixed. The smaller syringe was used to inject small aliquots of the particles under careful fluoroscopic guidance keeping in mind that constant agitation and remixing of the agent is very important to prevent particle aggregation. A higher-contrast material concentration usually results in precipitation of the particles with a higher tendency to conglomerate and form clusters. That is why the solution tends to become more stable with time, and premixing several minutes in advance was important [8].
The endpoints that have to be achieved were technical and medical successes of the procedure. Success and complications were evaluated according to original assignment proposed by Dinkel et al., 2002 [9].
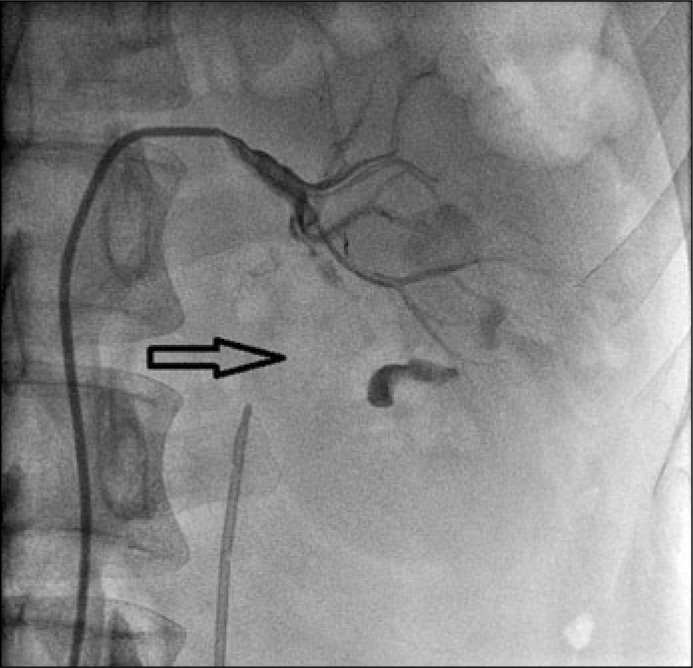
Technical success of the embolization session was defined as the complete and irreversible occlusion of renovascular bleedings with arterial occlusion as registered with arterial angiography at the end of the RAE (Figure 4.)
Figure 4.
Final image of arterial angiography at the end of the RAE with pointing of devascularized area.
Medical success was defined by the cessation of gross hematuria up to the 3rd day after RAE as well as the absence of:
recurrent hematuria and need for erythrocyte administration;
recurrent decrease of hemoglobin by more than 1.5 g/dL (15 g/L);
necessity for angiographic reembolization or subsequent open surgery.
Complications of RAE also were divided into technical and medical, short term (during first 30 days) and long-term (after 30th day from RAE session).
To evaluate renal function we used radioisotope renography with 131-Iodine labelled sodium ortho-iodohippurate (Hippuran) that was performed 3, 6 and 12 months after RAE session in all patients and could be considered as an adequate method of kidney function monitoring [10]. The following parameters were measured from renogram curves:
The time interval from injection to the peak of the tracing (TMAX);
The time interval from injection to the point where the curve decays to 50% of the maximum (T1/2);
The normal range of (TMAX) was considered from 2.5 to 5.5 min, and the normal range of (T1/2) – from 8.2 to 9.9 min [11].The renograms were considered normal if they were within these limits and abnormal if outside.
Differences in the distribution of continuous variables were described in terms of the mean ± standard deviation (M ±SD) and assessed for statistical significance using Student's t test. Results were considered significant if the two-tailed p-value was less than 0.05.
RESULTS
According to the AAST classification, grade II blunt kidney injury was registered at 2 (10.0%) pts, grade III – at 11 (55.0%) pts and grade IV – at 7 (35.0%) pts.
Together with flank pain and local swelling, a gross hematuria following trauma was the leading symptom in all patients. None of the patients underwent open surgery before and after RAE.
The sessions of embolization lasted 54.6 ±3.8 minutes, and the mean term of hospitalization was 9.8 ±1.2 days.
In all patients, the bleeding was stopped with the embolization procedure that was registered by arterial angiography at the end of the RAE session.
Eighteen (90.0%) patients were treated in a single interventional session with clear urine within 1–3 days after the procedure. The other 2 (10.0%) needed a further intervention – reembolization, stipulated by repeated gross hematuria in a short-term post-session period on the second and third day after RAE.
Among our patients, the following technical complications of RAE were registered:
accidental embolization of unruptured arterial branches of vascularised territories as a consequence;
puncture-site bleeding.
Medical complications were classified as short-term (i.e., registered up to the 30th day after the RAE) or delayed (i.e., registered after 30th day from RAE performing).
Short-term complications included:
postembolization syndrome (i.e., back pain and fever not registered before);
decrease in renal function (defined as serum creatinine levels ≺130 mol/L);
arterial hypertension;
repeated persistent gross hematuria.
Long-term complications included:
arterial hypertension;
decrease in renal function;
renal abscess.
Observed medical and technical complications of RAE in our patients complications are systematized in the Table 1.
Table 1.
Medical and technical complications of RAE
| Complications | Technical | Medical | TOTAL (events) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accidental embolization of unruptured arteria | Puncture-site bleeding | Short-term | Long-term | ||||||||
| Postembolization syndrome | Decrease in renal function | Arterial hypertension | Repeated persistent gross hematuria | Decrease in renal function | Renal abscess | Arterial hypertension | |||||
| n | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 15 | |
| % | 10.0 | 5.0 | 10.0 | 5.0 | 10.0 | 10.0 | 10.0 | 5.0 | 10.0 | ||
As presented in Table 1, 15 events of RAE complications were registered. Pointed events were registered in 5 (25%) patients: two or more complications were often combined in each individual case. For example, in a 35-year-old male with Grade IV blunt kidney trauma, an accidental, undesirable embolization of unruptured arteria was performed during the RAE session, followed by postembolization syndrome and decrease in renal function and arterial hypertension. In another 37-year-old female with Grade III blunt kidney trauma, one day after complete embolization, postembolization syndrome manifested; 3 days after the RAE session, persistent gross hematuria occurred, that caused reembolization accompanied with repeated puncture-site bleeding and was followed by long-term decrease in renal function.
In general, our results demonstrate perspectives of minimally invasive treatment in patients with kidney injury, complicated by intensive gross hematuria. Complications that have been registered did not seriously influence postoperative management and were corrected conservatively.
Table 2 presents means of TMAX and T1/2 that were calculated after radioisotope renography performed in damaged kidneys at 3rd, 6th and 12th months after RAE sessions.
Table 2.
Means of TMAX and T1/2 in damaged kidneys calculated after radioisotope renography
| Variables | M ±SD | p | ||
|---|---|---|---|---|
| 3rd month | 6th month | 12th month | ||
| TMAX, min | 11.32 ±1.67 | 7.51 ±0,53 | 6.12 ±0.47 | <0.05 |
| T1/2, min | 24.33 ±3.04 | 16.31 ±2.38 | 12.25 ±1.43 | <0.05 |
As it was shown in Table 2, function in damaged kidneys was preserved at the 3rd month after RAE sessions. However, means of TMAX and T1/2 in damaged kidneys were considered as abnormal because they differed from normal limits. After 6th and 12th months, reducing means of TMAX and T1/2 were registered with a tendency towards normalizing.
DISCUSSION
The presented study aimed to analyze the efficacy and safety of selective renal artery embolization in patients with blunt kidney injuries based on own and other researchers experiences.
The data of numerous studies with similar aims were published during the last period.
Breyer et al. in 2008 reported about their experience of RAE performed in 26 patients with acute renal hemorrhage as a consequence of kidney injury (10 of them – after blunt injury). The authors noted that embolization proved effective for grade 4 blunt renal trauma for which conservative therapy failed [12]. Accordingly to researchers, RAE indications include cases of kidney injury with: persistent gross hematuria, inefficacy of conservative treatment aimed at blood pressure stabilization; increasing perirenal hematoma, renal parenchyma ruptures, renal arterio-venous fistulas or pseudoaneurism formation. In the authors’ experiences, minimally invasive treatment failed in all grade 5 acute renal injuries (100%) caused by external trauma.
The RAE could be used successfully even in the cases of anatomically abnormal kidney injury. Molina Escudero R. et al. in 2012 reported about effective use of selective embolization by means of arteriography of a branch of the right renal artery and placement of a double J stent due to urinary extravasation in the lower left horseshoe kidney pole [13].
In 2009 Reay al. also performed embolization in blunt kidney trauma of adult polycystic kidney [14].
It is important to select patients for RAE. Lin et al. in 2013 suggested that contrast extravasation, changing of perirenal hematoma rim distance and the extent of hematoma could be considered as simple and sensitive indicators of patients with kidney trauma for minimally invasive treatment [15]. Radiographic findings correlate with necessity of intervention for renal hemorrhage [16].
However, presently there is no consensus to predict or exclude an indication of kidney angioembolization with a computed tomography (CT) scan. Charbit et al. in 2011 concluded that none of the CT criteria had a strong negative predictive value for the outcomes of renal embolization. They presented their experience of the successful use of nephron sparing selective arterial cessation associated with a perirenal hematoma rim distance <25 mm as an indication to exclude embolization [17].
Except for hemorrhage cessation, one more positive feature of RAE is able to preserve damaged kidney function. As Chatziioannou et al. in 2004 considered, super selective embolization resulted in permanent cessation of bleeding as well as preventing serious parenchymal infarction and allowed serum creatinine levels in patients to return to the pre-bleeding values [18].
Morita et al. in 2013 suggested that the function of the injured kidney could be preserved in the majority of patients with grade IV blunt renal trauma that indicated the efficacy of embolization in such cases [19].
Heyns & Stellmacher in 2005 concluded that the reduction of kidney function occurs even after successful selective renal artery embolization in 50% of cases [20].
Huber et al. in 2011 reported about the high percentage of re-embolization in patients with posttraumatic renal hemorrhage as well as the necessity of nephrectomy when embolisation failed. Embolization was performed in 19 out of 21 patients with posttraumatic kidney bleeding and hemoglobin decrease of more than 2 gm/dl. The primary clinical success was observed in 12 (63%) patients, including 2 cases with grade V parenchymal injury. In the cases when primary treatment failed, transarterial embolization was repeated in 86% (6 of 7 patients). It resulted in clinical success in 4 out of 6 patients (67%) with equal efficiency (p = 1). Three patients (16%) who could not be sufficiently treated with transarterial embolization underwent nephrectomy [21].
According to Yanagi et al., 2013, in patients with type III renal injury classified by the Japanese Association for the Surgery of Trauma staging system, transcatheter arterial embolization could be an effective method of choice. Sixteen patients were treated successfully with transcatheter arterial embolization in the 2 hospitals, and 15 of them were classified as type III renal injury. Thus, the authors believe that nephrectomy should be avoided in such patients because of the benefits offered by embolization [22, 23].
Hemodynamically stable patients with a grade IV or V BRI could be safely managed without operation as van der Wilden GM et al. concluded in 2013. The authors compared treatment outcomes in 206 patients; 52 (25.2%) of them were operated on immediately, and 154 (74.8%) were managed conservatively (with the assistance of angiographic embolization for 25 patients). By multivariate analysis, the authors identified two independent predictors of non-operative treatment failure: age over 55 years and a road traffic crash as the mechanism of injury. When both risk factors were present, non-operative treatment failure occurred for 27.3% of the patients; when both were absent there were no conservative management failures.
The renal salvage rate was 76.2% for the entire population and 90.3% among patients selected for non-operative management [24].
Rao et al. in 2014 concluded that superselective transcatheter renal artery embolization is a highly effective, minimally invasive therapy for the treatment of non-iatrogenic BKT III-V Grade hemorrhages. In the presented series, there was only one patient who died of cerebral trauma 3 days after the minimally invasive treatment, although his macroscopic hematuria had disappeared at that time [25].
In Table 3 our results are where obtained results are compared with others’ data.
Table 3.
Studies of RAE efficacy and success rates
| Study | N | AAST grades | Reembolization rate (%) | Renal salvage rate (%) |
|---|---|---|---|---|
| Breyer et al. [12] | 10 | IV | – | 100 |
| Dinkel et al. [9] | 9 | III-V | – | 100 |
| van der Wilden et al. [24] | 25 | IV-V | – | 90.3 |
| Rao et al. [25] | 16 | III-V | – | 100 |
| Our | 20 | II-IV | 10.0 | 100 |
N – number of patients
As presented in Table 3, RAE performed in patients with BKT Grades II-V ensures high renal salvage rates ranging from 90.3 to 100%. According our data, the reembolization rate was 10%, but it does not influence the technical success.
Thus, our presented results and the researches of numerous authors demonstrate the proved efficacy of selective renal artery embolization in hemodynamically stable BKT patients. We considered that indications to perform RAE are the presence of blunt kidney trauma grades II-IV, combined with a gross hematuria that cannot be stopped conservatively.
The absolute advantage of minimally invasive treatment could be considered the preserving of damaged kidney function.
The unanswered question at the present time is the efficacy of RAE in Grade V kidney injuries. We agree with Breyer et al., 2008 that the majority of these patients should be treated operatively because of the trauma's high severity and emergency [12].
Randomized prospective multicenter trials are necessary to define the real value of RAE in patients with BKT; however, keeping in mind the strict inclusion criteria, this aim is very difficult to realize.
CONCLUSIONS
RAE is an effective, minimally invasive treatment of blunt kidney injury accompanied with gross hematuria that stopped kidney bleeding.
Complications of RAE were registered in 25% of our patients, two or more complications may present simultaneously in one patient.
RAE contributes to the preservation of the damaged kidney function.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Meng MV, Brandes SB, McAninch JW. Renal trauma: indications and techniques for surgical exploration. World J Urol. 1999;17:71–77. doi: 10.1007/s003450050109. [DOI] [PubMed] [Google Scholar]
- 2.Herschorn S, Radomski SB, Shoskes DA, Mahoney J, Hirshberg E, Klotz L. Evaluation and treatment of blunt renal trauma. J Urol. 1991;146:274–276. doi: 10.1016/s0022-5347(17)37768-6. [DOI] [PubMed] [Google Scholar]
- 3.Kristjansson A, Pedersen J. Management of blunt renal trauma. Br J Urol. 1993;72:692–696. doi: 10.1111/j.1464-410x.1993.tb16249.x. [DOI] [PubMed] [Google Scholar]
- 4.Thanapaisal C, Sirithanaphol W. Management of blunt renal trauma in Srinagarind Hospital: 10-year experience. J Med Assoc Thai. 2013;(suppl 4):S124–128. [PubMed] [Google Scholar]
- 5.Miller KS, McAninch JW. Radiographic assessment of renal trauma: our 15-year experience. J Urol. 1995;154:352–355. doi: 10.1097/00005392-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Danuser H, Wille S, Zöscher G, Studer U. How to treat blunt kidney ruptures: primary open surgery or conservative treatment with deferred surgery when necessary? Eur Urol. 2001;39:9–14. doi: 10.1159/000052405. [DOI] [PubMed] [Google Scholar]
- 7.Moore EE, Shackford SR, Pachter HL, et al. Organ injury scaling: Spleen, liver, and kidney. J Trauma. 1989;29:1664–1666. [PubMed] [Google Scholar]
- 8.Lopera JH. Embolization in trauma: principles and techniques. Semin Intervent Radiol. 2010;27:14–28. doi: 10.1055/s-0030-1247885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinkel HP, Danuser H, Triller J. Blunt renal trauma: minimally invasive management with microcatheter embolization experience in nine patients. Radiology. 2002;223:723–730. doi: 10.1148/radiol.2233011216. [DOI] [PubMed] [Google Scholar]
- 10.Smith PH, Smith AH. I-131 hippuran renography. An evaluation with observations on technique and interpretation. BJU. 1968;40:501–514. doi: 10.1111/j.1464-410x.1968.tb11841.x. [DOI] [PubMed] [Google Scholar]
- 11.Kathel BL. Radioisotope renography as a renal function test in the newborn. Arch Dis Child. 1971;46:314–320. doi: 10.1136/adc.46.247.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breyer BN, McAninch JW, Elliott SP, Master VA. Minimally invasive endovascular techniques to treat acute renal hemorrhage. J Urol. 2008;179:2248–2252. doi: 10.1016/j.juro.2008.01.104. [DOI] [PubMed] [Google Scholar]
- 13.Molina ER, Cancho G MJ, Husillos A A, Lledó GE, Herranz AF, Ogaya PG, et al. Traumatic rupture of horseshoe kidney. Urol Int. 2012;88:112–114. doi: 10.1159/000330800. [DOI] [PubMed] [Google Scholar]
- 14.Reay EK, McEleny K, McDonald S, Thorpe AC. Blunt renal trauma in adult polycystic kidney disease and the use of nephron sparing selective arterial embolization. J Trauma. 2009;66:564–566. doi: 10.1097/01.ta.0000221549.85785.20. [DOI] [PubMed] [Google Scholar]
- 15.Lin WC, Lin CH, Chen JH, et al. Computed tomographic imaging in determining the need of embolization for high-grade blunt renal injury. J Trauma Acute Care Surg. 2013;74:230–235. doi: 10.1097/TA.0b013e318270e156. [DOI] [PubMed] [Google Scholar]
- 16.Myers JB, Brant WO, Broghammer JA. High-grade renal injuries: radiographic findings correlated with intervention for renal hemorrhage. Urol Clin North Am. 2013;40:335–341. doi: 10.1016/j.ucl.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Charbit J, Manzanera J, Millet I, et al. What are the specific computed tomography scan criteria that can predict or exclude the need for renalangioembolization after high-grade renal trauma in a conservative management strategy? J Trauma. 2011;70:1219–1227. doi: 10.1097/TA.0b013e31821180b1. [DOI] [PubMed] [Google Scholar]
- 18.Chatziioannou A, Brountzos E, Primetis E, Kelekis D, et al. Effects of superselective embolization for renal vascular injuries on renal parenchyma and function. Eur J Vasc Endovasc Surg. 2004;28:201–206. doi: 10.1016/j.ejvs.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Morita S, Inokuchi S, Tsuji T, et al. Arterial embolization in patients with grade-4 blunt renal trauma: evaluation of the glomerular filtration rates by dynamic scintigraphy with 99mTechnetium-diethylene triamine pentacetic acid. Scand J Trauma Resusc Emerg Med. 2010;18:11. doi: 10.1186/1757-7241-18-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyns CF, Stellmacher GA. Selective renal artery embolization in the management of non-iatrogenic renal trauma – experience in 28 patients. Afr J Urol. 2005;11:89–94. [Google Scholar]
- 21.Huber J, Pahernik S, Hallscheidt P, Sommer CM, Wagener N, Hatiboglu G, Haferkamp A, Hohenfellner M. Selective transarterial embolization for posttraumatic renal hemorrhage: a second try is worthwhile. J Urol. 2011;185:1751–1755. doi: 10.1016/j.juro.2010.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Nishizawa S, Mori T, Shintani Y, Kohjimoto Y, Inagaki T, Hara I. Applicability of blunt renal trauma classification of Japanese Association for the Surgery of Trauma (JAST) Int J Urol. 2009;16:862–867. doi: 10.1111/j.1442-2042.2009.02392.x. [DOI] [PubMed] [Google Scholar]
- 23.Yanagi M, Kondo Y, Endo Y, et al. Role of transcatheter arterial embolization (TAE) for deep renal injury. Nihon Hinyokika Gakkai Zasshi. 2013;104:688–696. doi: 10.5980/jpnjurol.104.688. [DOI] [PubMed] [Google Scholar]
- 24.van der Wilden GM, Velmahos GC, Joseph DK, et al. Successful nonoperative management of the most severe blunt renal injuries: a multicenter study of the research consortium of New England Centers for Trauma. JAMA Surg. 2013;148:924–931. doi: 10.1001/jamasurg.2013.2747. [DOI] [PubMed] [Google Scholar]
- 25.Rao D, Yu H, Zhu H, Yu K, Hu X, Xie L. Superselective transcatheter renal artery embolization for the treatment of hemorrhage from non-iatrogenic blunt renal trauma: report of 16 clinical cases. Ther Clin Risk Manag. 2014;10:455–458. doi: 10.2147/TCRM.S59671. [DOI] [PMC free article] [PubMed] [Google Scholar]