Abstract
Introduction
Complex vesico-vaginal fistula (VVF) has a high recurrence rate and so the repair with graft tissues seems to be favorable. Amniotic membrane (AM) plays an increasing role as a scaffold for the repair of defect tissue due to its unique biological properties with regard to promoting wound healing.
Material and methods
An innovative surgical procedure for AM-assisted repair of a complex vesico-vaginal fistula as the Idea Stage following the IDEAL recommendations is presented. The development of amnion preparation and the involved surgical steps are described.
Results
We are able to report a successful repair of VVF by abdominal approach with an amniotic membrane graft. Good functional results, no adverse events and no graft rejection have been detected.
Conclusions
Favorable results confirm the technical simplicity, safety and efficacy of this procedure. Following the IDEAL recommendations, consecutive animal experiments and a cohort study are in progress.
Keywords: amniotic membrane, vesico-vaginal fistula, IDEAL recommendations
INTRODUCTION
Vesico-vaginal fistulae (VVF) are frequently caused by complex onco-gynecologic or pelvic surgery and may be aggravated by oncologically necessary radiotherapy. These procedures are known to lead to symptoms such as permanent incontinence, leakage of urine from the vagina, infection and pain; therefore, significantly deteriorating the quality of living for the affected women [1]. In addition, numerous surgical procedures (in several cases requiring multiple interventions) for fistula repair like urinary diversion, etc. may worsen the situation for these patients [2]. New individual approaches with graft applications are therefore indicated to improve the local anatomical conditions and to reduce the symptoms. Several biological and artificial materials have been used, but can lead to further complications such as rejection, necrosis and infection. First reports of amnion application into the pelvic region have shown fast epithelialization and improved functionality in vaginal and bladder reconstruction, but, so far, there is no standardized procedure [3, 4].
Patient-tailored innovative surgical approaches are difficult to standardize and respective recommendations cannot easily be generalized. A new approach, the IDEAL method has been proposed in 2009 by Mc Culloch and colleagues [5]. The IDEAL procedure clearly provides stages of surgical innovations, which allow for the ability to assign a new method to its specific level of development and evidence. For the first time, we present a VVF repair with amniotic membrane as the “Idea level” following the IDEAL recommendations.
MATERIAL AND METHODS
A 64-year old female was admitted to our hospital with sigma diverticulitis and chronic vesico-vaginal fistula (VVF). She had a previous history of cervical cancer and radio-chemotherapy for anal cancer with complete remission. The resection of the sigma-bowel and fistula were performed in our department of surgery. However, VVF persisted after multiple abdominal operations. The patient suffered due to permanent incontinence and local skin infection. After a recovery period of 3 months, we reevaluated the patient with cystography, cystoscopy, ureteropyelography and vaginal examination. Complex vesico-vaginal fistula of 1.5 cm was detected at the apical anterior vaginal wall leading to the bladder base (Figure 1). Additionally, scarring of the vagina and distal ureters was found and the patient suffered from a persistent MRSA-colonization of the urine. Due to the complex situation, we discussed an individualized treatment strategy with the off-label use of amniotic membrane (AM). Prior to the operation, AM was obtained immediately after elective cesarean sections with normal gestation. The donors were screened for infections including HIV, hepatitis and syphilis after informed consent. The placenta was cleaned with balanced salt solution and the amnion was separated from the chorion by blunt dissection under the laminar flow. The separated membranes were cut and after several rinsing steps the amnion was frozen at -20°C for 24 hours until further use. For further processing, the AM was defrosted in the water, sterilized in peracetic acid andalcohol mixture, then incubated for 2 hours on the shaker. After rinsing the amnion was applied on a sterile silicon scaffold and dried under laminar flow. Prior to surgery, three layers of dried AM were fixed together by a 4-0 Monocryl suture. During the surgery single-shot antibiotic was given and an extraperitoneal abdominal approach was chosen to allow maximum exposure. The peritoneum was dissected off the bladder dome and posterior bladder wall. The incision was carried out through the anterior bladder wall to prepare the fistula. The ureteric orifices were found outside the fistula region. The fistulous tract and all devitalized tissue were excised sparingly. The multilayer AM of 4 cm size was used as a graft to close the defect and was fixed with 4-0 Monocryl sutures at the edges of the normal bladder wall. The anterior bladder wall was then closed and ureteric stents were led out in a suprapubic region to dry the bladder. Topical oestrogen was prescribed for 3 weeks after surgery. Anti-muscarinic therapy was started to restore the bladder capacity.
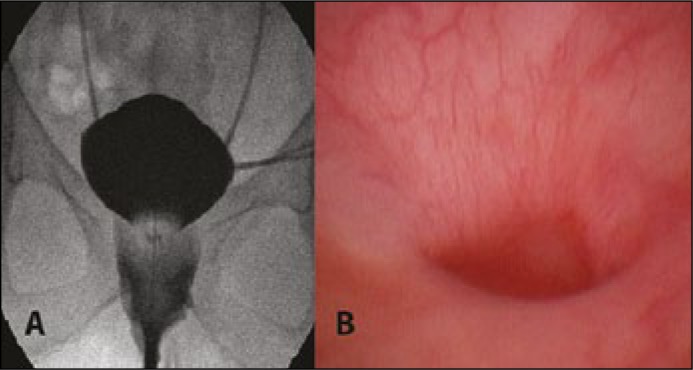
Figure 1.
Preoperative examination. A. Cystography showing the vaginal contrast leakage, indwelling ureteral stents. B. Cystoscopy showing the fistula canal at the bottom of the bladder.
RESULTS
Seven days after the surgery a cystography was performed showing no leakage. In the three following months a reevaluation with cystography, cystoscopy and vaginal examination was performed with no signs of recurrent fistula and full recovery of the vaginal epithelium was presented (Figure 2). The ureter stents were internalized and the patient was able to micturate without incontinence. The bladder capacity was restored to the volume of 250 ml. No severe complications and no signs of graft rejection were observed during the follow-up of 6 months. The MRSA-colonization in the urine was successfully eradicated.
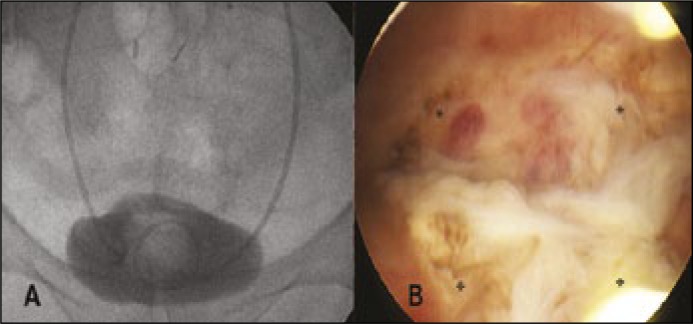
Figure 2.
Postoperative examination 3 months after the surgery. A. Cystography showing no contrast leakage, indwelling ureteral stents and catheter. B. Cystoscopy showing the amnion graft. AM merged with the surrounding bladder tissue at the margins but was still clearly demarcated with no signs of overgrowing urothelium or stroma. *, fixation points with suture.
DISCUSSION
Complex fistulae are characterized by a size >3–4 cm, involvement of the urethra, presence of ureteric orifices, distinct vaginal scarring and multiple localization. They usually present a poor outcome with high recurrence rates and a devastating course [1, 2, 6, 7]. Literature on the post-radiation fistulae is still scarce. Radiation-induced recurrent vesico-vaginal fistulae have the lowest success rate in terms of successful treatment and require the most demanding interventions [1]. The surgical approach to the repair of VVF depends on the surgeon's experience, the type and location of the fistula, and the patient's specific preferences [1, 2, 6]. The trans-vaginal approach is preferred in the case of distal fistulae and has been associated with lower morbidity rates. However, the abdominal or even laparoscopic approaches are favorable for deep and large fistulae. For the repair of this specific condition an interposition flap is needed to substitute the defect. The type of vascularized interposition flap is determined by how proximal (peritoneal, O′Conor approach, muscle flap) or distal (Martius labial fat) the genitourinary fistula is located in the vagina (85–100% success) [1]. The used flaps have the risk of infection, necrosis, loss of function after time and long-term morbidity. To avoid these problems different xeno-, homo- and autolog grafts have been proposed for the repair of VVF recently [1, 8]. Vascularization of the graft or flap is the main requirement for successful healing, otherwise the recurrence of fistulae occurs frequently.
The AM is the innermost layer of the placenta and is being increasingly applied in reconstructive surgery and tissue engineering (wound healing, ophthalmology) due to its mechanical durability and “scaffolding” quality [9, 10]. The postoperative outcome after fistula repair significantly depends on the degree of angiogenesis and inflammation, which are regulated by the AM through cytokines (TGF-ß, IL-10) and growth factors (VEGF) [10]. The AM also has an immunomodulatory effect, with no relevant tissue rejection being described in previous allogen or xenogen experiments [10, 11]. AM might also be used as an economically reasonable and alternative biomaterial for the treatment of VVF in developing countries with a high VVF incidence.
Introducing new surgical methods, innovation, as well as medical devices does not yet follow clear paradigms as is the case for the approval process of new drugs. The IDEAL procedure suggests and clearly provides stages, which allow to assign every method to its particular level of development and evidence. A new surgical method at the first “Idea level” should aim at proving a concept on a highly selected single-to-few patient basis. The suggested method of reporting should be a structured case report.
For the first time, we present a structured implementation of a new method for VVF repair following the IDEAL recommendations [5]. After positive results of this first case we plan consecutive prospective developmental studies in an animal model and cohort studies.
CONCLUSIONS
We reported a successful repair of VVF by an abdominal approach with an AM graft. Favorable results confirmed the technical simplicity, safety and efficacy of this procedure in the first case. Further research with controlled and randomized trials will show if a general application of the amnion graft to repair defects like VVF can be recommended.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Ghoniem GM, Warda HA. The management of genitourinary fistula in the third millennium. Arab J Urol. 2014;12:97–105. doi: 10.1016/j.aju.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampel C, Neisius A, Thomas C, Thüroff JW, Roos F. Vesicovaginal fistula. Incidence, etiology and phenomenology in Germany. Urologe A. 2015;54:349–358. doi: 10.1007/s00120-014-3679-x. [DOI] [PubMed] [Google Scholar]
- 3.Fishman IJ, Flores FN, Scott FB, Spjut HJ, Morrow B. Use of fresh placental membranes for bladder reconstruction. J Urol. 1987;138:1291–1294. doi: 10.1016/s0022-5347(17)43586-5. [DOI] [PubMed] [Google Scholar]
- 4.Mhaskar R. Amniotic membrane for cervical reconstruction. Int J Gynaecol Obstet. 2005;90:123–127. doi: 10.1016/j.ijgo.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann R. Vesico-vaginal fistula. In: Hofmann R, Wagner U, editors. Incontinence- and Descensus Surgery. 2nd edition. Springer; 2015. pp. 241–249. [DOI] [Google Scholar]
- 7.Starownik R, Michalak J, Bar K, Plaza P, Muc K, Rechberger T. An uncommon case of inflammatory infiltration of the urinary bladder in the long-term process of the purulent inflammation of the cervix and vaginal fornix, complicated with vesicovaginal fistula of unknown etiology. Cent European J Urol. 2013;66:101–103. doi: 10.5173/ceju.2013.01.art30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robles JE, Saiz A, Rioja J, et al. Collagen graft interposition in vesicovaginal fistula treatment. Urol Int. 2009;82:116–118. doi: 10.1159/000176038. [DOI] [PubMed] [Google Scholar]
- 9.Meller D, Pauklin M, Thomasen H, et al. Amniotic membrane transplantation in the human eye. Dtsch Arztebl Int. 2011;108:243–248. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeffelbein DJ, Rohleder NH, Eddicks M. Evaluation of human amniotic membrane as a wound dressing for split-thickness skin-graft donor sites. Biomed Res Int. 2014:572183. doi: 10.1155/2014/572183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akle CA, Adinolfi M, Welsh KI, et al. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]