Abstract
Ureteroscopy is fast becoming the first line treatment option for the majority of urinary tract stones. Ureteroscopy training can be performed in a variety of ways including simulation, hands on ureteroscopy courses and supervised operative experience. We report an “expert consensus view” from experienced endourological surgeons, on all aspects of basic ureteroscopic techniques, with a particular focus on avoiding and getting out of trouble while performing ureteroscopy. In this paper we provide a summary of treatment planning, positioning, cannulation of ureteric orifice, guidewire placement, rigid ureteroscopy and stone fragmentation.
Keywords: urolithiasis, ureteroscopy, stent, ureterorenoscopy
Ureteroscopy is fast becoming the first-line treatment option for the majority of urinary tract stones. This is reflected in current guidelines, with ureteroscopy showing greater stone clearance rates for all stones in the ureter, except stones <1 cm in the upper ureter and for a significant proportion of intra-renal stones [1]. In the UK, the number of rigid and flexible ureteroscopy procedures performed has increased rapidly over the last 10 years. Recent data further support this rise, representing a dramatic increase in ureteric procedures and retrograde intrarenal surgery (RIRS) [2]. Interestingly, the proportion of shock wave lithotrispy (SWL) and percutaneous nephrolithotomy (PCNL) has remained relatively static over this same period [2].
As with all forms of endourology, operative technique needs to be modified for patient-specific factors. Despite surgeon preference for ureteroscopy, intra-operative obstacles can arise. Previously standard techniques of ureteroscopy and equipment requirements have been reported in the literature [3]. Training via simulation, hands-on ureteroscopy courses and supervised operative experience is required throughout the training programme in order to perform ureteroscopy independently.
As with any “tips and tricks” guide, the techniques and caveats described below are not based on randomised trials, or even on case studies, but on the collective experience and practice of 105 years’ cumulative consultant practice in endourology. In the majority of these centres between 150–300 ureteroscopic procedures are performed annually. The corresponding author (BS) performs >200 ureteroscopic procedures annually. Whilst compiling this review, we found we shared a similar approach and technique the majority of the time, but all of us surgeons are open to new ideas and suggestions, as we hope you are too.
General points, planning and positioning
According to a quote often attributed to Einstein, the definition of insanity “is doing the same thing over and over again and expecting different results”. No truer statement could be applicable to upper tract endoluminal surgery, in which operative techniques have become refined with experience, discussion with colleagues/experts and by consulting published data.
The most important aspect to remember about ureteroscopy and upper tract surgery is that each case should be judged individually on its merits. Planning is imperative and this includes patient factors, consent, preoperative imaging, correct-side marking, ensuring the relevant imaging is displayed in theatre, having appropriately trained theatre staff, using the correct fragmentation device, availability of intra-operative fluoroscopy and having easy access to additional specialised equipment or disposables in times of difficulty. Imaging on the morning of surgery for opaque stones can be useful, especially in patients with a solitary stone. Antibiotic prophylaxis is recommended by international guidelines [1], and the treatment choice and duration should be dictated by the local microbiology department policy.
Establishing a routine is helpful and we recommend that each case is set up the same way: ensuring drapes (often transurethral resection (TUR) drapes are very good for stone work), cameras, laser machine, scrub nurse, imaging stack and fluoroscopy are kept constant. The best advice for any endourological procedure is if one gets into trouble or feels unsafe, seek help or insert a ureteric stent and come back another day. This is by far the safest option and as endourological stone surgeons we should never forget it!
Fluoroscopy has an associated radiation dose and hence is best used by taking a minimalist approach. A simple ”flash” will often suffice, rather than continuous screening. A retrograde study, prior to any ureteric manipulation can be of use as a road map, highlighting anatomy and stone position. Of course, contrast can hide the position of a radio-opaque stone, particularly in the kidney. Contrast is extremely useful if there is uncertainty about orientation, concern regarding potential ureteric/pelvic injury or in the diagnosis of suspected upper tract tumor or unexplained haematuria.
Ureteric orifice cannulation
There is a lot of debate about whether a safety wire is always needed for ureteroscopy, with some surgeons not routinely using them [4, 5]. For the purpose of this paper we do advocate their use, per international guidelines, whilst noting that the level of evidence is limited [1]. The benefit of the safety wire is for easy and safe stent insertion if one gets into trouble. Without such a wire, the patient may require a nephrostomy and then subsequent antegrade stent insertion to escape the problem of compete loss of retrograde access to the kidney.
Cannulation of the ureteric orifices (UO) is usually straightforward, but can occasionally be extremely difficult for a variety of reasons. One must be aware of the bladder neck and avoid unnecessary trauma to it when performing cystoscopy, especially in men with an enlarged, vascular prostate gland. Once a cystoscopy has been performed and the UO located, inserting the wire into the scope, whilst in the middle of the bladder, will remove the influence of the bladder neck. Having the cystoscope beak as close as sensible to the UO will also take out the influence of the bladder neck. After noting the position of the UO, if it looks like a difficult angle to cannulate, consider turning the cystoscopy 90–180° (utilising a 30° scope) to aid wire passage. A hydrophilic tip wire often aids passage through the UO and in tortuous ureters.
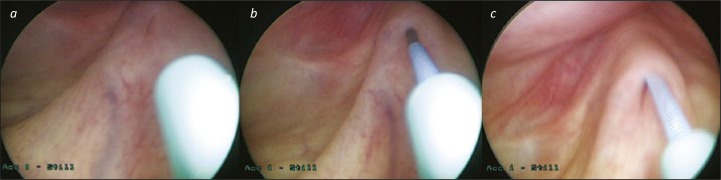
If one is unable to pass the wire through the UO, pre-loading the wire in an open ended, 5–6Ch ureteric catheter often helps (see Figure 1). The ureteric catheter can be advanced up to the UO and the wire can then often be inserted (the ureteric catheter can act as a moveable guide and pointer). If difficulty persists, consider a retrograde study to define the lower ureter; often ”fish hook” ureters can be extremely difficult to negotiate initially. Try to avoid air bubbles by flushing the ureteric catheter before intubating the UO.
Figure 1.
Ureteric catheter as a pointer. 1a. A 6Fr open-ended ureteric catheter is aimed at the Left ureteric orifice. 1b. A pre-loaded hydrophilic tipped “stone wire” is advanced towards the UO – the direction of the wire is determined by the direction of the ureteric catheter; there is no risk that the wire will twist in the scope and exit in the wrong direction. 1c. The wire can be advanced under vision and fluoroscopic control as normal. If necessary, the ureteric catheter can be advanced over the wire, either for additional support of the wire, or for a retrograde contrast study.
If one is still unable to pass a wire through the UO, use a rigid ureteroscope, placing the tip of the scope at the UO, and insert the wire under direct vision via the ureteroscope (+/- retrograde study which can be performed simultaneously).
In order to reduce the fluoroscopy screening dose, there is often no need to screen a safety wire during insertion; a single flash at the level of the respective kidney will usually confirm the safe positioning of the upper end of the wire. If a radio-opaque stone is present, one can perform screening centred on the stone as the wire passes. If a stone is radio-lucent, then fluoroscopy should be centred on the knowledge of the stone's position from preoperative imaging. If no ureteric stone is expected and the wire passes smoothly then a simple flash at the level of the kidney should suffice. This is usually ‘sensed’ when the wire meets resistance and no more of it can be inserted without additional force.
If pus is noted to drain from the ureter following insertion of the safety wire, it is best to deploy a stent and return for the definitive procedure at a later date. This should be in conjunction with urine culture obtained at the time of the procedure and must be covered with appropriate antibiotics. Once the safety wire is in place it is best secured by either placing the excess caudal end of the wire back into the plastic coil and taping it to the drape or forming a loop with the wire and cliping it to the drape. This should reduce the risk of any accidental dislodgement of the wire.
Passing the ureteric orifice with the ureteroscope
Once a safety wire is in place, this often helps keep the UO open and aids passage of the rigid ureteroscope. Often approaching the UO infero-laterally to the safety wire will aid its passage. After placement of the safety wire and before introducing the ureteroscope, the bladder should be emptied to avoid compression of the intramural part of the ureter, which may impede ureteroscope passage.
If initially it is difficult to enter the UO, turn the semi-rigid ureteroscope 90–180°, enabling the beak of the scope to pass easily through the UO. Consider adding 20 ml syringe of saline to the 2nd ureteroscope irrigation channel, then use pulsed irrigation via the syringe to open up the intra-mural ureter.
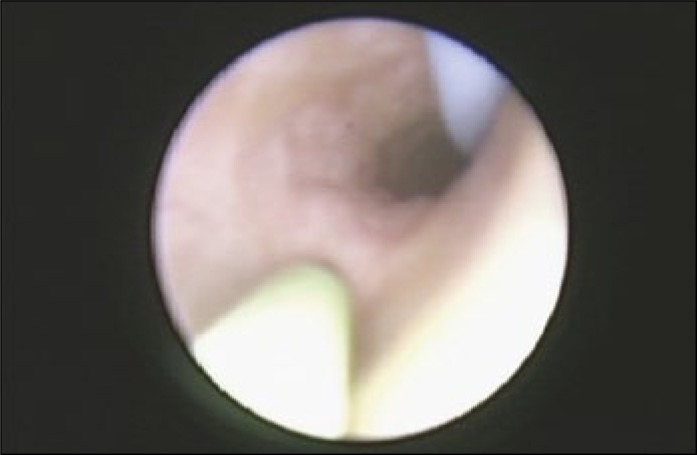
A second, standard PTFE (polytetrafluoroethylene) wire can be placed (the “navigating” wire – see Figure 2), which will often open up the UO inferiorly enabling passage of the ureteroscope between the wires. For stones impacted in the distal ureter, it is often easier to use a hydrophilic-tipped wire to ensure safe passage past the stone, rather than causing mucosal trauma, which can happen with a standard guidewire. Alternatively, the second wire can be placed through the semi-rigid ureteroscope, passing through the UO; the ureteroscope can then be gently guided over this wire, with or without pulsed irrigation.
Figure 2.
A “Navigating” wire. A pre-placed safety wire can be seen heading up the ureter towards the kidney. The green wire, just a few mm beyond the tip of the ureteroscope (i.e. in its flexible segment) can be used like a proboscis for “feeling” the path ahead, and allow the scope to pass smoothly over the contour of the ureter, such as narrow segments, the middle third of the ureter, or to bypass a ureteric stone.
If the ureter feels tight, despite using two wires, it is imperative not to push hard and perforate the ureter. As long as the mucosa is moving past the scope, constant and gentle manipulation of the advancing ureteroscope is reasonable. If the mucosa is not moving, it is best to stop, relax and withdraw the ureteroscope slowly back down the ureter. If no mucosal flap or tear is apparent then repeated controlled gentle advancing could be tried again. If a mucosal flap or tear is evident, then it is safer to deploy a stent over the safety wire and defer the definitive procedure. If the ureter is impassable due to small intra-luminal size, then placing a stent and performing ureteroscopy in 2–4 weeks’ time would vastly improve the chance of success as most ureters will have dilated sufficiently well by this time.
Guiding the wire beyond an obstruction
We suggest using a hydrophilic tip wire as a standard “stone and stent wire”. This wire will aid passage beyond an impacted stone, when compared with a standard PTFE wire. However, some obstructions are still impassable; and alternative options may be necessary.
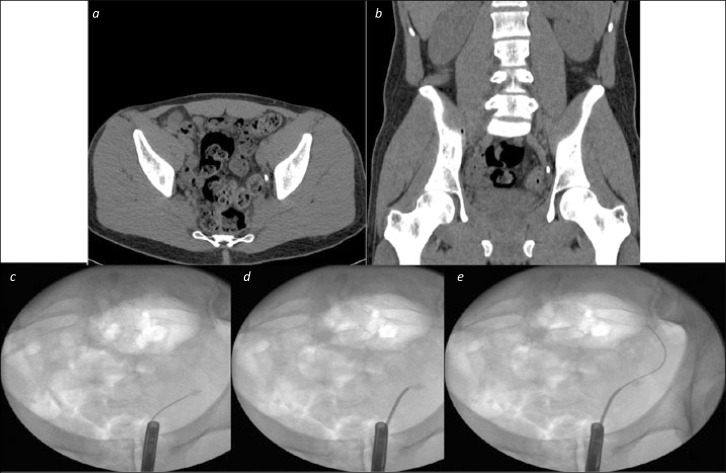
An open ended 5-6Ch ureteric catheter, passed over the working wire, can be placed distal to the obstruction to try and re-pass the wire. This will often help due to increased wire stability distal to the obstruction (see Figure 3). If a hydrophilic tip wire is still unsuccessful at this stage, consider repeating the above step using a full-length hydrophilic guide wire or an angled tip (J-tip or Shepherd’ hook) guidewire. Once the wire has successfully passed the stone, place the wire in the kidney and advance the ureteric catheter into the kidney, enabling wire exchange for a standard ureteroscopy safety wire.
Figure 3.
Ureteric catheter to negotiate difficult stone. 3a and 3b. Axial and Coronal CT images showing a distal ureteric stone. 3c. An initial attempt to pass a wire beyond the stone failed with buckling at the site of the stone on flouroscopy 3d. A ureteric catheter over the wire, without contrast, allowed the wire to be directed towards the edge, rather than the middle of the stone 3e. The wire was passed under fluoroscopic control beyond the stone, and could then be advanced straightforwardly to the level of the kidney, and be secured as a safety guide wire.
If one is unable to pass any wire beyond the obstruction, it is worth performing a retrograde study (via the ureteric catheter in-situ) at the level of the obstruction. This will not only enable the ureter to be opacified to highlight ureteric anatomy, but also open the lumen between the obstructing stone and the ureteric wall. Once contrast is seen passing beyond the stone, one can quickly pass a hydrophilic tip/angled wire via the ureteric catheter to aid the direction of the wire's passage.
If it is still unable to pass the obstruction with a safety wire, then use a semi-rigid ureteroscope, bringing it close to the site of obstruction. The use of contrast and different wires can then be retried as above, with the additional advantage of being able to aim the wire at the gap between the stone and the ureteric wall under endoscopic vision.
Finally, if the obstruction is secondary to an impacted stone and completely impassable with a wire, consider careful stone fragmentation via the ureteroscope until a wire can be passed (best done by an expert). When the proximal ureter can be seen, insert a safety wire and secure this in place. Ureteroscopy can then be continued as planned. Alternatively, once up to the stone a “Billiard Cue” technique can be used, with gentle nudging of the stone to try and dislodge it proximally. This often is useful for an impacted distal, intra-mural stone. A similar technique can be used with a ureteric catheter as well under fluoroscopic screening in impacted distal ureteric stones.
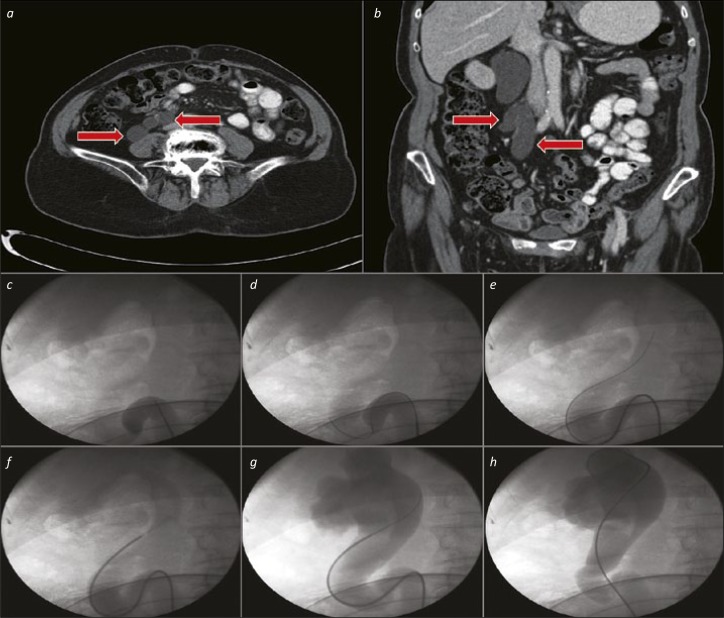
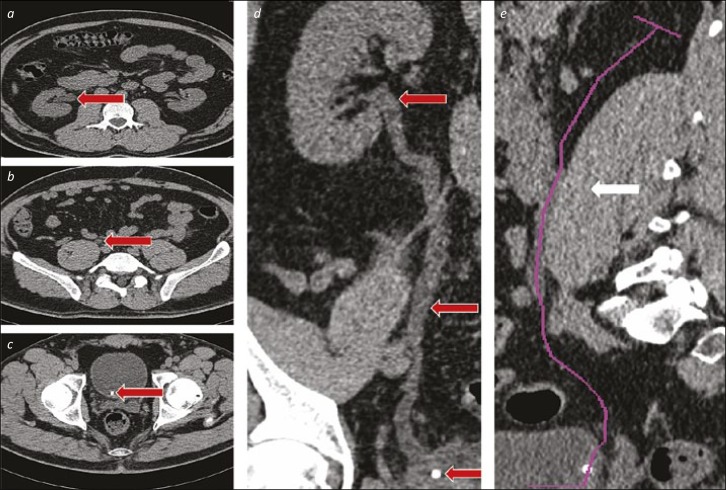
An obstructed stone in the distal ureter can result in proximal ureteric dilatation and a “Z” configuration of the upper ureter. A wire alone will not always pass through both bends of the “Z”, but sequential advancement of the wire within a 5–6Ch ureteric catheter will allow passage of the wire, then catheter, into the upper urinary tract (see Figure 4).
Figure 4.
Ureteric catheter for tortuous upper ureter. 4a and 4b. Axial and Coronal CT images showing a substantially dilated, tortuous proximal ureter (red arrows). 4c. The ureteric catheter is advanced as far as the beginning of the “Z” loop. 4d. A hydrophilic-tipped wire is advanced via the ureteric catheter, and onwards around the “Z” loop. 4e. The ureteric catheter and wire are negotiated upwards towards the kidney in combination. 4f. After removal of the wire, a retrograde study can be performed via the ureteric catheter to define the pelvicalyceal system anatomy (and ensure that the subsequent JJ stent is placed in the correct position). 4g. The wire is replaced via the ureteric catheter – in cases with a particularly tortuous ureter such as this, a “super-stiff” wire is often useful to aid stent placement without buckling or misplacement. 4h. The ureteric catheter is removed over the wire (and replaced with a stent – not shown).
Rarely, if everything else fails one might have to resort to a percutaneous nephrostomy and subsequent management by antegrade stenting and subsequent RIRS, or even an antegrade ureteroscopy.
Negotiating the ureter
Once inside the ureter, advancement of a semi-rigid ureteroscope is often limited to the lower and mid-ureter below the pelvic brim, particularly in men. Be careful not to bend your semi-rigid ureteroscope if trying to advance above the pelvic brim – the ureter is not a straight tube, with curves both lateral to medial, as well as posterior to anterior, as the scope moves from VUJ to PUJ (see Figure 5). Gentle advancement of the ureteroscope with hand pressure is advisable, rather than performing a “pole vaulting” movement (leaning on the ureteroscope with shoulder pressure).
Figure 5.
The curve of the ureter. 5a, b and c. The ureter (highlighted with red arrows) is shown at the PUJ (a), in its middle third (b) and with a stone at the VUJ (c). In this series, the arrows show the movement needed from medial (at the VUJ) to lateral (at the PUJ) required for ureteroscope advancement. 5d. This full-length coronal reconstruction shows the initial course of the lower third of the ureter passes laterally, before moving medially in the middle and into the proximal ureter, before curving laterally towards the renal pelvis. 5e. The purple line on this sagittal reconstruction demonstrates the initial posterolateral direction of the ureter, and the substantial anterior displacement needed to traverse the middle third, particularly in patients with a well-developed psoas muscle (highlighted with a white arrow).
Negotiating the natural curvature of the ureter in this area can be assisted by using a second guidewire via the ureteroscope, leaving it protruding a few centimeters beyond the end of the ureteroscope as a “proboscis” (Figure 2). This will often aid passage through more tricky areas of the ureter. Simple abdominal pressure can sometimes aid ureteric straightening and scope passage. If unable to advance the scope, consider performing a contrast study, plus pulsed fluoroscopy, via the ureteroscope to highlight the path ahead.
If a tight UO has been passed successfully and the procedure completed, be careful on withdrawing the ureteroscope. The shaft of the ureteroscope can “hug” the lower ureter/UO area; therefore remove the ureteroscope slowly, avoiding ureteric avulsion or injury. Rotating the ureteroscope on its axis a few degrees either way continuously, whilst withdrawing it from the ureter is a safe manoeuvre. Occasionally the ureter can even go into spasm and in these cases very slow withdrawal should be performed. Whilst withdrawing the ureteroscope, it is imperative to inspect for possible ureteric injury or stones, which may have been missed on the way up. If one has chosen not to use a safety guidewire, then it is worth considering having a wire pre-loaded in the ureteroscope on withdrawal in case a ureteric injury is noted.
Rigid ureteroscopy and fragmentation/basket extraction
When approaching any ureteric stone, one has to be aware of its position and the possible need for adjuvant flexible ureterorenoscopy. If this is planned for preoperatively then any retro-pulsed stone fragments into the kidney can be treated simultaneously. It is important to ensure that the desired fragmentation device (commonly Holmium:YAG laser), appropriate size laser fibre, access sheath and baskets are ready and easily accessible before the start of the case.
It is perhaps sensible to try and use one standard basket size for the ureter or kidney. If performing simultaneous ureteric and kidney stone, try to use one basket for both to reduce costs. Never attempt to remove any ureteric stone without visualisation of the stone (blind basketing) [1]. Consider using a smaller calibre laser fibre (200 µm or 272 µm) if you are likely to do simultaneous flexible ureterorenoscopy. Choose your laser settings according to stone characteristics and your desired laser effect. The most effective lithotripsy system is the holmium laser, which is accepted as the gold standard for rigid and flexible ureteroscopy (see Figure 6) [1]. Pneumatic/ballistic and ultrasonic systems can be used during rigid ureteroscopy, but stone migration is a common problem, therefore basketing the stone before fragmentation with such devices may help prevent this from occuring [1].
Figure 6.
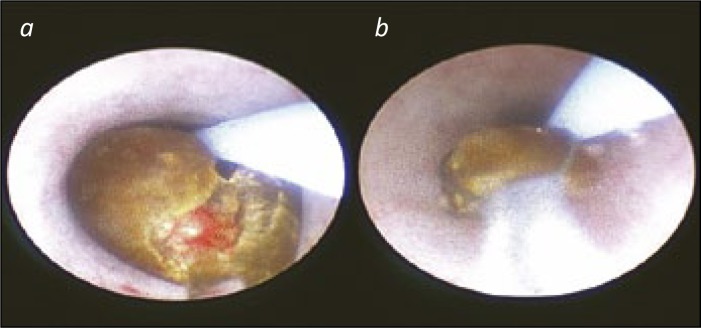
A stone being lasered and then basketed. 6a. A stone being fragmented by laser energy. The stone is being “carved into two” to allow the pieces to be retrieved with a basket. 6b. A stone fragment in a tip-less nitinol basket for extraction and biochemical analysis. The stone is close enough to be seen, whilst allowing the ureteric mucosa to be observed at all times that the ureteroscope is being withdrawn.
For stones protruding from the VUJ, consider picking them out with simple graspers. If feasible, the protruding VUJ stone can be fragmented (laser or lithoclast). An alternative is to use a Collings knife, insert adjacent to stone in ureter and try to flick the stone out (no electric current used). Avoid incising the UO to extract the stone as this might result in life-long reflux.
For distal or lower third ureteric stones, basket extraction can be performed if it appears that the stone is small enough to pass through the UO atraumatically (approx. 2–6 mm). Consider performing rigid ureteroscopy beyond the stone and into the upper ureter, enabling the UO and/or intra-mural ureter to be dilated by the ureteroscope. Then, using one's preferred ureteric basket, open the basket in the upper ureter and ”trawl” back down the ureter with the basket open. This will enable the stone to be captured in the basket. When positioning the stone in the basket, make sure that the stone is in the most favourable orientation to aid intra-luminal passage. When withdrawing the ureteroscope with the stone, keep the basket/stone close to the tip of the ureteroscope enabling easier extraction, but far enough to aid vision (see Figure 6B). If the fragment is too large to extract, reposition proximally and further fragment it within the basket until small enough to remove. Alternatively, three pronged baskets are particularly safe as they do not grip the stone strongly and will release fragments automatically if they are too large to pass through the lumen. Don't be too greedy!
Proximal ureteric stones can be trickier to manage and they have a higher risk of retropulsion. Anti-retropulsion devices can be considered in the upper ureter, but this is a surgeon specific choice. Once the stone is visualised and a safety wire is in place, consider passing the stone with a closed ureteric basket. This can be placed proximally and opened. Fragmentation can then be performed. If the stone retropulses back towards the kidney, it should be caught in the basket and this can be repositioned back into the ureter for further fragmentation or for removal with the basket, preventing the need for flexible ureterorenoscopy.
The decision to place a ureteric stent after removal of the stone is controversial [6–8]. Current guidelines suggest that in an “uncomplicated ureteroscopy” a ureteric stent need not be placed [1]. An individual's definition of ”uncomplicated” can vary, with the guidelines not offering a standard definition. But it is worth remembering that, in general, patients do not like ureteric stents!
Ureteric stenting following ureteroscopy should be considered in those who are at increased risk of complications, those with residual fragments, bleeding, perforation/s, UTIs, pregnancy and to avoid potential emergency situations [1]. Consider using a “tethered” stent if required, as they are tolerated equally by patients and are easily removed in the outpatient setting [9]. Tethered stents offer short indwelling time and can be removed easily postoperatively, thus reducing stent related morbidity.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Türk C, Petřík A, Sarica K. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.07.041. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Turney BW, Reynard JM, Noble JG, Keoghane SR. Trends in urological stone disease. BJU Int. 2012;109:1082–1087. doi: 10.1111/j.1464-410X.2011.10495.x. [DOI] [PubMed] [Google Scholar]
- 3.Somani BK, Aboumarzouk O, Srivastava A, Traxer O. Flexible ureterorenoscopy: Tips and tricks. Urol Ann. 2013;5:1–6. doi: 10.4103/0974-7796.106869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulvik Ø, Rennesund K, Gjengstø P, Wentzel-Larsen T, Ulvik NM. Ureteroscopy with and without safety guide wire: should the safety wire still be mandatory? J Endourol. 2013;27:1197–1202. doi: 10.1089/end.2013.0248. [DOI] [PubMed] [Google Scholar]
- 5.Dickstein RJ, Kreshover JE, Babayan RK, Wang DS. Is a safety wire necessary during routine flexible ureteroscopy? J Endourol. 2010;24:1589–1592. doi: 10.1089/end.2010.0145. [DOI] [PubMed] [Google Scholar]
- 6.Song T, Liao B, Zheng S, Wei Q. Meta-analysis of postoperatively stenting or not in patients underwent ureteroscopic lithotripsy. Urol Res. 2012;40:67–77. doi: 10.1007/s00240-011-0385-7. [DOI] [PubMed] [Google Scholar]
- 7.Haleblian G, Kijvikai K, de la Rosette J, Preminger G. Ureteral stenting and urinary stone management: a systematic review. J Urol. 2008;179:424–430. doi: 10.1016/j.juro.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Nabi G, Cook J, N'Dow J, McClinton S. Outcomes of stenting after uncomplicated ureteroscopy: systematic review and meta-analysis. BMJ. 2007;334:e572. doi: 10.1136/bmj.39119.595081.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes KT, Bing MT, Tracy CR. Do ureteric stent extraction strings affect stent-related quality of life or complications after ureteroscopy for urolithiasis: a prospective randomised control trial. BJU Int. 2014;113:605–609. doi: 10.1111/bju.12541. [DOI] [PubMed] [Google Scholar]