Abstract
Objective
The aim of this study is to describe the clinical characteristics and outcome of cancer diagnosed during pregnancy.
Methods
This is a retrospective cohort study of women who were diagnosed with cancer during pregnancy at a tertiary academic hospital between 1995 and 2013. Maternal characteristics, gestational age at diagnosis, and type, stage, symptoms and signs of cancer for each patient were retrieved from the medical records. The cancer treatment, pregnancy management and the subsequent perinatal and maternal outcomes for each cancer were assessed.
Results
A total of 87 women were diagnosed with cancer during pregnancy (172.6 cases per 100,000 deliveries). The most common cancer was breast cancer (n=20), followed by gastrointestinal (n=17), hematologic (n=13), thyroid (n=11), central nervous system (n=7), cervical (n=7), ovarian (n=5), lung (n=3), and other cancers (n=4). Eighteen (20.7%) patients terminated their pregnancies. In the 69 (79.3%) patients who maintained their pregnancies, one patient miscarried and 34 patients delivered preterm. Of the preterm babies, 24 (70.6%) were admitted to the neonatal intensive care unit and 3 (8.8%) of those expired. The maternal mortality rate was 31.0%, with highest rate seen with lung cancers (66.7%), followed by gastrointestinal (50.0%), central nervous system (50.0%), hematologic (30.8%), breast (25.0%), ovarian (20.0%) cervical (14.3%), and thyroid cancers (0%).
Conclusion
The clinical characteristics and outcome of cancer during pregnancy were highly variable depending on the type of cancer. However, timely diagnosis and appropriate management of cancer during pregnancy may improve both maternal and neonatal outcome.
Keywords: Maternal mortality, Neoplasms, Pregnancy, Pregnancy outcome
Introduction
Cancer is the second most common cause of death in women of reproductive age [1,2]. In Korea, the incidence of cancer in reproductive-aged women is reported to be 162 out of 100,000 [3]. Today, the maternal age at birth continues to rise [4]. However, because the incidence of cancer increases significantly with age [1], the risk of cancer during pregnancy may also increase with rising maternal age at birth [5].
Cancer during pregnancy is an important but complex issue requiring consideration of both the mother and the baby [2]. However, cancer diagnosis and management during
pregnancy are not well understood for several reasons. Firstly, although cancer during pregnancy is not rare, it is uncommon, with an incidence rate of approximately 1 in 1,000 pregnancies [6,7]. Therefore, large, prospective studies are not available. Secondly, the type, stage, diagnosis, treatment and prognosis of the cancer vary widely. Thirdly, the diagnosis of cancer during pregnancy is not easy because the symptoms and signs of cancer may mimic the physiologic changes or common complaints reported during pregnancy. Therefore, the symptoms and signs of cancer may be easily ignored by both pregnant women and their doctors, leading to delayed diagnosis and eventual progression to an advanced stage [8].
The type of cancer and outcome during pregnancy may also differ between races and ethnicities [9]. Although the clinical aspects of cancer during pregnancy have been described in a number of studies performed in Western countries, the topic has yet to be studied comprehensively in Korea [6,8,10]. Therefore, this study is aimed at describing the clinical characteristics and outcomes of cancers diagnosed during pregnancy in Korea.
Materials and methods
This is a retrospective cohort study of women who were diagnosed with cancer during pregnancy at a tertiary referral hospital, between the years of 1995 and 2013. Using the in-patient electronic database system, pregnant women who were discharged from the hospital and assigned with the cancer disease code by ICD-10 (International Statistical Classification of Diseases and Related Health Problems 10th revision) were selected as the study population. Women diagnosed with cancer before pregnancy or during the postpartum period were excluded. Also, noninvasive cancers such as carcinoma in situ and borderline ovarian tumors were excluded from the analysis. This study was approved by the institutional review board of our institution.
Maternal age, parity, gestational age at diagnosis, and the type, stage, symptoms and signs of cancer for each patient were reviewed from the medical records. The cancers were grouped into breast, gastrointestinal (GI), hematologic, thyroid, central nervous system, cervical, ovarian, lung and others. Each cancer was staged according to National Comprehensive Cancer Network guidelines, except for the brain tumors and acute leukemia, which do not follow the conventional cancer staging system. The cancer treatment strategy was classified into 4 categories: 1) termination of pregnancy, defined as therapeutic abortion or iatrogenic delivery before fetal viability (24 weeks of gestation) for the purpose of cancer treatment; 2) immediate initiation of cancer treatment during pregnancy; 3) immediate cancer treatment following iatrogenic preterm delivery (PTD); or 4) delay of cancer treatment until completion of obstetrically indicated delivery.
Pregnancy outcomes included spontaneous abortion, preterm or term delivery, gestational age at delivery, delivery mode, sex and birth weight of the baby, admission to neonatal intensive care unit (NICU), neonatal morbidity and mortality. PTD was defined as delivery before 37 weeks of gestation; this was categorized into spontaneous PTD (due to preterm labor or preterm premature rupture of membranes) and iatrogenic PTD (to treat cancer). Maternal outcome was categorized into 1) complete response with no evidence of disease; 2) partial response; 3) stable disease or progressive disease; 4) recurrence; 5) death; or 6) loss to follow-up with unknown outcome. The definitions of complete response, partial response, stable disease, and progressive disease were determined based on Response Evaluation Criteria in Solid Tumors.
For the comparison of multiple means, analysis of variance or the Kruskal-Wallis test was used, as appropriate. Categorical variables were compared using the chi-square test or Fisher's exact test, as appropriate. A P-value of <0.05 was considered statistically significant.
Results
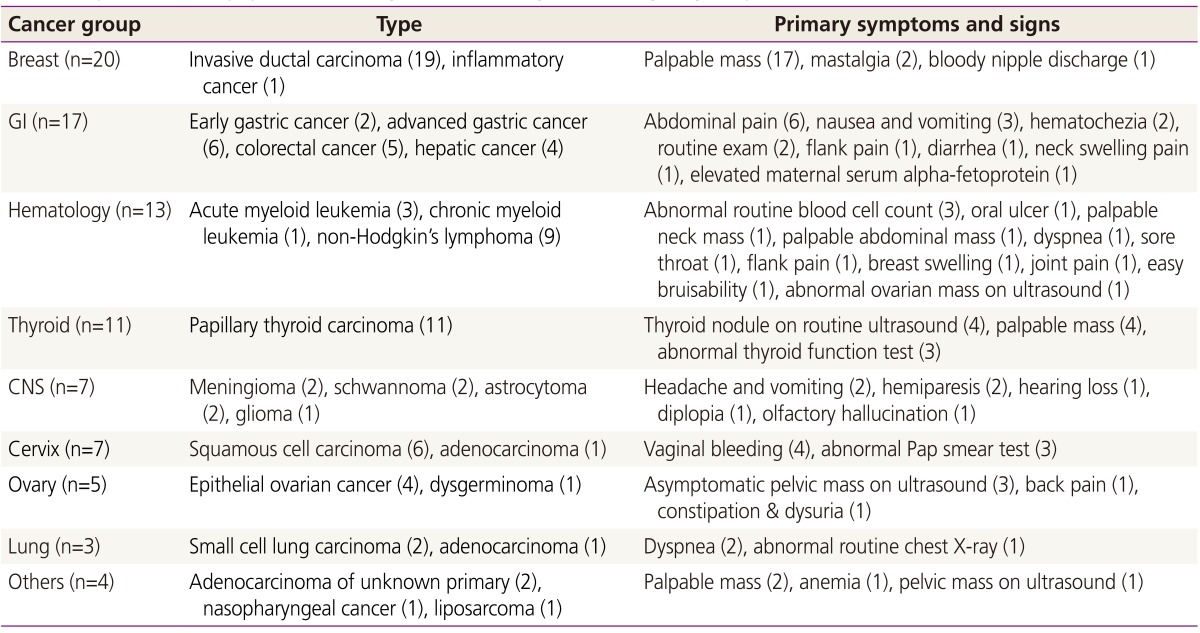
Over the 19-year study period, 98 cases of pregnant women diagnosed with cancer were retrieved from the database. Among them, 9 cases of cervical carcinoma in situ, 1 case of breast ductal carcinoma in situ and 1 case of borderline ovarian tumor were excluded. The remaining 87 patients were diagnosed with invasive cancer during pregnancy. The incidence of cancer diagnosed during pregnancy was 172.6 in 100,000 pregnancies (87 out of 50,412 deliveries over the 19 years). The actual number of cases and the incidence of cancer rose over time, with 17 cases from 1995–2000 (76.5/100,000), 27 cases from 2001–2006 (187.4/100,000), and 43 cases from 2007–2013 (311.9/100,000). Breast cancer (n=20) was most common form of cancer, followed by GI (n=17), hematologic (n=13), thyroid (n=11), central nervous system (n=7), cervical (n=7), ovarian (n=5), lung (n=3), and other cancers (n=4). The type, primary symptoms and signs at diagnosis of each cancer group are described in Table 1.
Table 1. Type and primary symptoms and signs of cancer diagnosed during pregnancy.
GI, gastrointestinal; CNS, central nervous system.
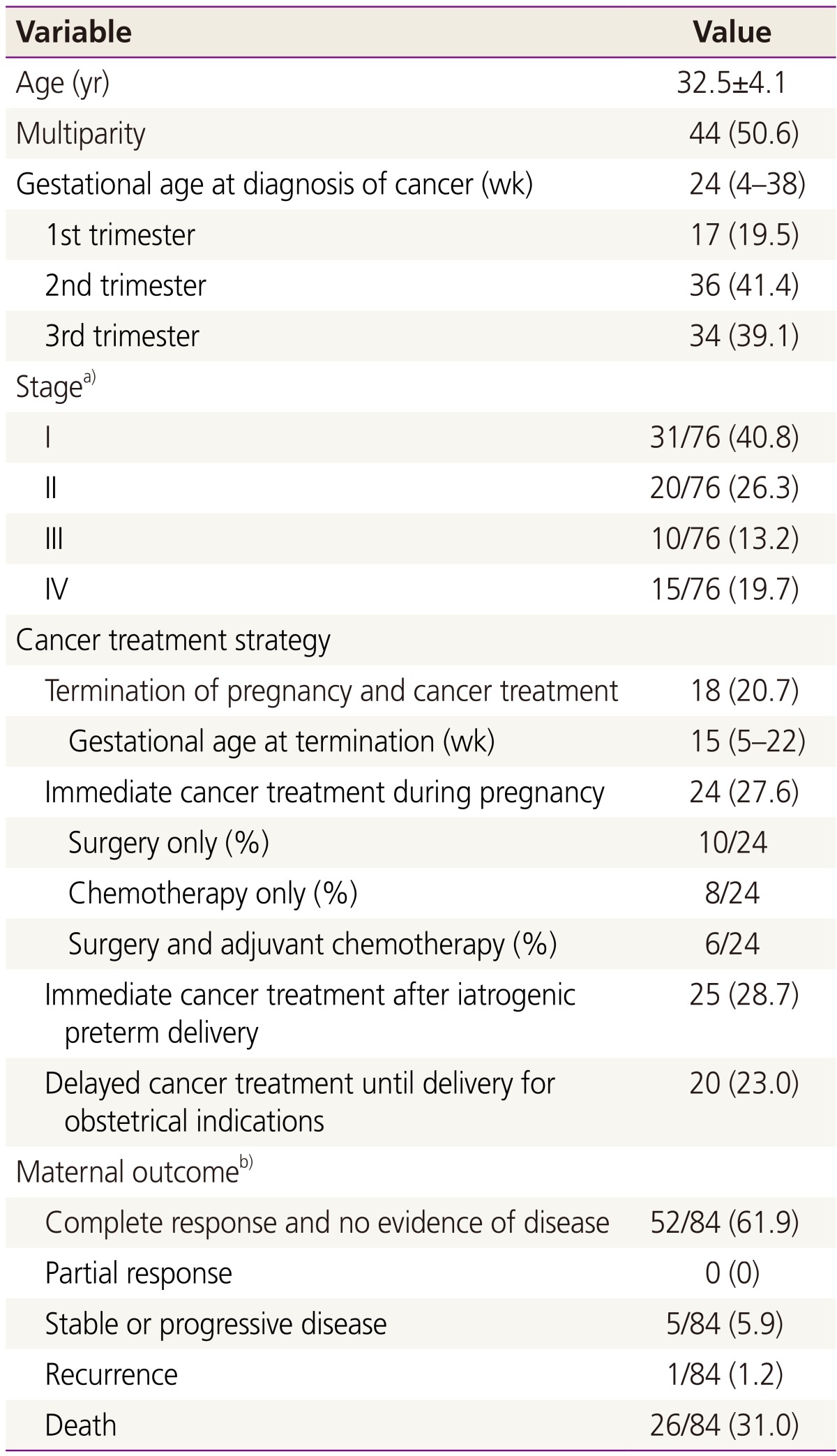
The characteristics, treatment and outcome of all patients diagnosed with cancer during pregnancy are described in Table 2. Eighteen (20.7%) patients opted to terminate their pregnancies due to the cancer; 12 of these patients were diag-nosed during the first trimester and 6 were diagnosed during the second trimester. Twenty-four (27.6%) patients received immediate treatment during the pregnancy. Iatrogenic PTD was performed in 25 (28.7%) patients, for the purpose of initiating cancer treatment. In 20 (23.0%) patients, cancer treatment was delayed until obstetrically indicated delivery was completed; 17 of these patients delivered at term and 3 delivered preterm, with 1 due to spontaneous preterm labor and the other 2 due to preterm premature rupture of membranes.
Table 2. Characteristics, treatment, and outcomes of all diagnosed patients (n=87).
Data are expressed as mean±standard deviation, number (%), or median (range).
a)Stage was unavailable for 11 cases (7 brain tumor cases and 4 leukemia cases); b)Three cases of follow up loss were excluded from the analysis.
Three patients were lost to follow-up after delivery. The total mortality rate of the remaining 84 patients was 31.0% (26/84). The mortality rate varied according to the type of cancer, with the highest rate seen in the lung cancer group (66.7%), followed by GI (50.0%), central nervous system (50.0%), hematologic (30.8%), breast (25.0%), ovarian (20.0%) cervical (14.3%), and thyroid cancers (0%).
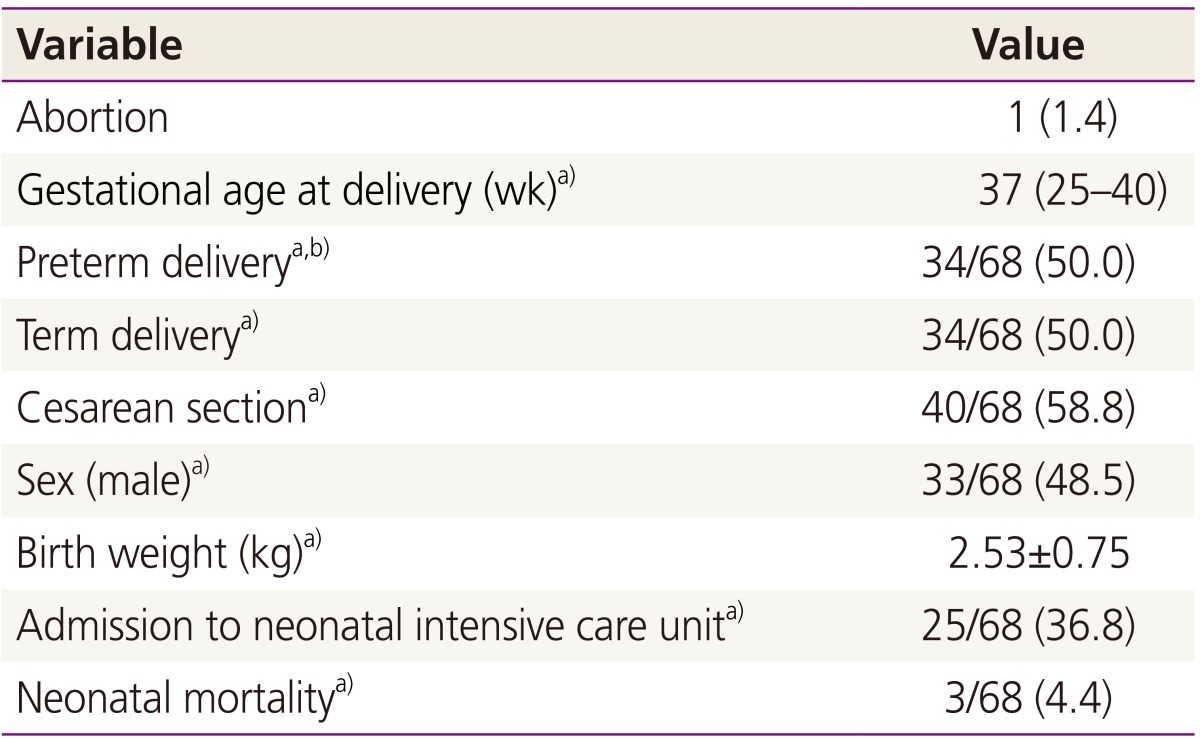
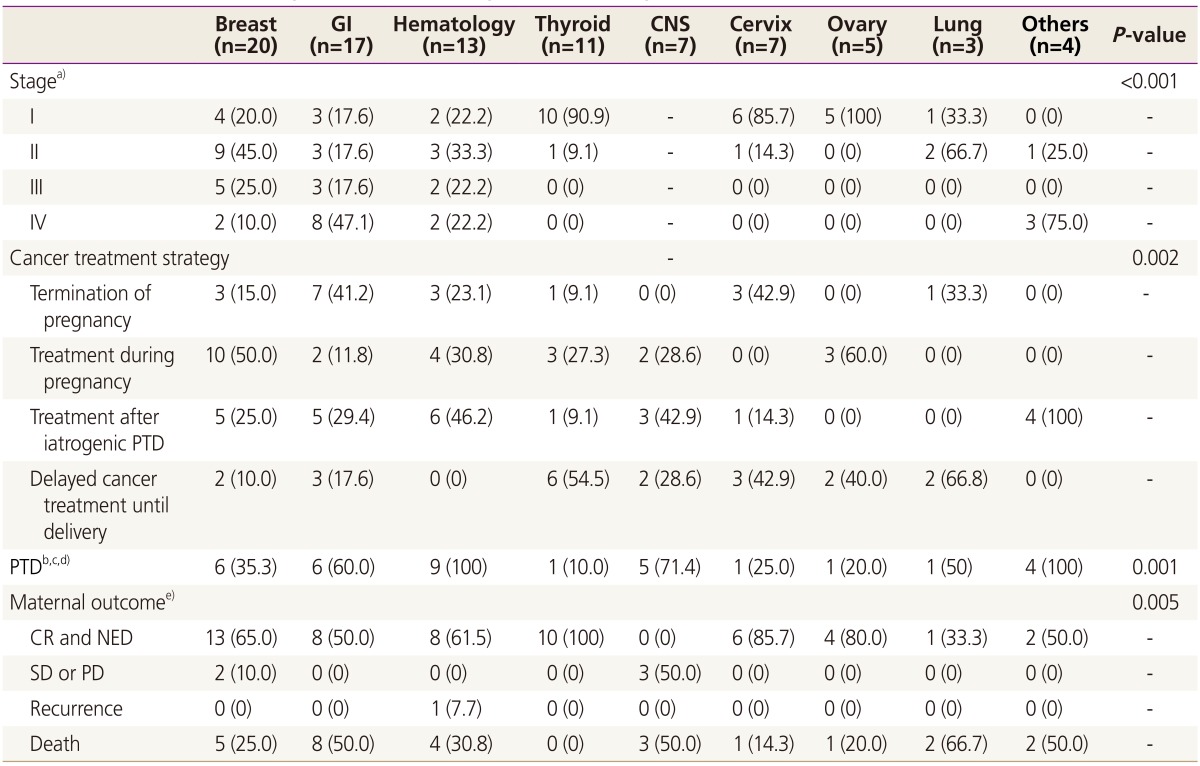
The pregnancy outcomes of the 69 patients who maintained their pregnancies are shown in Table 3. One patient with acute myelocytic leukemia died due to intracranial hemorrhage during chemotherapy at 15 weeks of gestation and the fetus was aborted. Among the remaining 68 patients, 34 (50%) delivered at term, of which one (2.9%) baby was admitted to the NICU due to meconium aspiration syndrome. Of the 34 preterm babies, 24 (70.6%) were admitted to the NICU, of which 13 had respiratory distress syndrome. The NICU admission rate was significantly higher for preterm babies born before 34 weeks compared to those born at 34 to 36 weeks gestation (18/18 [100%] vs. 6/16 [37.5%], P <0.05). Three babies born at 28, 29, and 29 weeks of gestation expired during the neonatal period. The clinical characteristics and outcomes of each cancer are described in Table 4. Maternal age, parity and gestational age at diagnosis were not significantly different between cancer groups (data not shown). However, the cancer stage at diagnosis, treatment strategy, PTD rate and maternal outcome were differed significantly by cancer type.
Table 3. Pregnancy outcome of patients who maintained the pregnancy (n=69).
Data are expressed as number (%), median (range), or mean±standard deviation.
a)One case of abortion was excluded from the analysis; b)Both iatrogenic and spontaneous preterm delivery.
Table 4. Comparisons of the stage and outcome among different cancer groups.
Data are expressed in number (%).
GI, gastrointestinal; CNS, central nervous system; PTD, preterm delivery; CR, complete response; NED, no evidence of disease; SD, stable disease; PD, progressive disease.
a)Stage was unavailable for 11 cases (7 brain tumor cases and 4 leukemia cases); b)Only the patients who maintained the pregnancy were included in the analysis; c)Both iatrogenic and spontaneous preterm delivery; d)One case of abortion was excluded from the analysis; e)Three cases who were lost to follow up was excluded from the analysis.
Discussion
In this cohort study, we described 87 cases of cancer diagnosed during pregnancy at a single tertiary center in Korea over a period of 19 years. We found that the incidence of cancer diagnosed during pregnancy steadily increased over the 19-year study period. Our data shows that the clinical characteristics of cancer diagnosed during pregnancy are highly variable and depend on the type of cancer. The cancer treatments, pregnancy management and the subsequent perinatal and maternal outcomes were varied and complex.
The incidence and type of cancer during pregnancy may differ between races and ethnicities. In the Western countries, breast cancer, melanoma, cervical cancer and hematologic cancers are the most commonly reported cancers during pregnancy [5,7,10,11,12,13]. However, in our study, no cases of melanoma were diagnosed during pregnancy. The incidence of melanoma is much lower in Asian countries compared to Western countries [14]. In our study, the most common cancer during pregnancy was breast cancer, followed by GI, hematologic and thyroid cancers, similar to the standard cancer incidence for the female population in Korea (thyroid, breast, colorectal, and stomach) [3]. Thyroid cancer is the most commonly diagnosed female cancer in Korea [15], mainly due to early detection using ultrasound for routine health care examination [16,17]. However, thyroid ultrasonography is not included in routine prenatal tests. Only 4 of the 11 thyroid cancers in our study were detected using thyroid ultrasound during routine health screening exams. Thyroid cancer was ranked as the fourth most common cancer diagnosed during pregnancy in our study. However, when GI cancer and hematologic cancer are divided into the subgroups of stomach (n=8), colorectal (n=5), liver (n=4), leukemia (n=4), and lymphoma (n=9), respectively, thyroid cancer (n=11) becomes the second most common cancer, after breast cancer.
The diagnosis of cancer during pregnancy presents challenges for several reasons. Firstly, the detection of cancers in their early stages is difficult because they are generally asymptomatic [18,19]. And even if symptoms or signs develop, they may be similar to the symptoms of a normal pregnancy. For example, nausea and vomiting may be confused with morning sickness and small breast masses may not be palpable due to breast engorgement during pregnancy. Also, headache and abdominal pain or discomfort are very common complaints during pregnancy. Therefore, it is difficult to differentiate these cancer symptoms from those of normal pregnancy, leading to delayed diagnosis. In our study, more than half of the GI cancers were diagnosed in the advanced stages and their prognosis was extremely poor. In contrast, all cervical and ovarian cancers were diagnosed in the early stages and their prognosis was good. This is thought to be due to the routine cervicovaginal cytology test and pelvic ultrasound included in the prenatal exams. Therefore, a physician should always be aware of the possibility of cancer in pregnant woman and initiate appropriate evaluations to diagnose the cancer in a timely manner.
Secondly, diagnostic tools available to detect cancer during pregnancy are limited and furthermore, both pregnant women and physicians are, in many cases, reluctant to carry out such diagnostic tests. Ultrasound is considered safe for both mother and fetus [20], and it is the most commonly used test during pregnancy for diagnosing cancers such as breast and thyroid cancers. Magnetic resonance imaging is also safe for the fetus, but it is generally avoided during the first trimester [21]. X-ray exposure from a single diagnostic procedure, especially exposure to less than 5 rad (50 mGy), does not result in any harm to the fetus [22,23]. However, high-dose ionizing radiation tests, such as abdominal and pelvic computed tomography, should be avoided, especially during early pregnancy [24]. Limited information is available regarding the fetal safety of nuclear medicine such as bone scans, thyroid scans and positron emission tomography scans. Upper GI endoscopy and sigmoidoscopy are sensitive for detecting GI cancers and are considered safe for both the fetus and mother, but should only be performed in the presence of strong cancer indications during pregnancy [25,26,27]. Fine needle aspiration and excisional biopsy to confirm cancer are feasible and safe during pregnancy [6].
Once cancer is diagnosed during pregnancy, a multidisciplinary approach and patient counseling regarding the therapeutic strategy are needed. The major issue regarding cancer treatment during pregnancy is deciding whether to continue or terminate the pregnancy, which depends on the gestational age, the type and stage of the cancer, the treatment options, and the woman's desire to continue with the pregnancy. In our study, 20% of patients terminated their pregnancies. All of these patients were diagnosed with cancer during the first or early second trimesters, and termination was performed before the fetus reached viability. However, there is no guideline delineating the benefits of termination in terms of maternal outcome. Therapeutic abortion during the first trimester may be indicated in patients with advanced cancer, in patients who already have children and thus have no desire to continue pregnancy, and in patients who may have a chance for future pregnancy after complete remission of the disease [6,28].
Over 80% of patients maintained their pregnancies in our study population, and about one third of these received treatments that included surgery, chemotherapy and/or radiation therapy during pregnancy. Treatment selection should be individualized depending on the gestational age and the nature and stage of the cancer, and the risks of modified or delayed treatment should be considered [26,29]. Surgery can be performed safely after 12 to 14 weeks of gestation with minimal risk of abortion, but should be performed regardless of gestational age if delayed treatment will potentially jeopardize maternal health. Radiation therapy is generally avoided during pregnancy because of the potential adverse effects on the fetus [30]. However, radiation therapy for head and neck cancers can be performed with relative safety using appropriate abdomen shielding.
Chemotherapy during the first trimester can induce harmful effects on the fetus, including spontaneous abortion, malformations, growth restriction, mental retardation, sterility and even risk of potential future malignancy [6,28]. However, after the first trimester, chemotherapy can be administered without obvious risk of fetal anomaly or fetal loss [31,32]. In our study, 14 patients received chemotherapy during pregnancy, with no cases of fetal or neonatal complications directly associated with the chemotherapy, although long-term follow-up data for these babies were not available. Amant et al. [33] conducted a multicentre observational cohort study of 70 children who were prenatally exposed to chemotherapy and found that fetal exposure to chemotherapy was not associated with long-term adverse cognitive or cardiac outcomes. However, impaired cognitive development was associated with prematurity. In our study, we also found that prematurity was the main determinant of adverse neonatal outcome. Half of the patients who maintained their pregnancies delivered preterm, mostly due to iatrogenic PTD for cancer treatment. These preterm neonates, especially those born before 34 weeks, had poor perinatal outcomes in terms of higher rate of NICU admission, increased risk of respiratory distress syndrome and higher mortality rate. Therefore, iatrogenic PTD should be avoided if possible and every effort should be made to prolong the pregnancy until 34 weeks of gestation.
This study has several limitations. Firstly, because of its retrospective nature, this study is prone to information bias. Data from pregnant women who were diagnosed with cancer and underwent therapeutic or spontaneous abortion in the outpatient department may have been lost and thus unintentionally excluded from this study. Secondly, although our study is the largest study to investigate the clinical aspects of cancer during pregnancy in Korea, the statistical power was insufficient to assess the prognostic factors associated with maternal and neonatal outcomes due to the small number of patients in each cancer groups. Also, there was considerably wide variation regarding the stage of cancer, gestational age and treatment between cancer types.
In conclusion, timely diagnosis and appropriate management of cancer during pregnancy is important for improving maternal and neonatal outcomes. A high level of suspicion is necessary when pregnant women present with clinically significant symptoms and signs that could possibly indicate the presence of cancer. Therapeutic abortion or termination of pregnancy is not necessary for all patients diagnosed with cancer during pregnancy. Appropriate treatment should be individualized and selected depending on gestational age, the type and stage of the cancer, the woman's desire to continue the pregnancy, and the risks of modifying or delaying treatment. Overall, however, the most important principle to follow in the treatment of cancer during pregnancy is that "a woman should never be penalized because she is pregnant [34]."
Acknowledgement
This study was supported in part by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HI14C0306).
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Korean Statistical Information Service. Cause of death statistics: 1995-2013 web-based report [Internet] Daejeon: Korean Statistical Information Service; c2014. [cited 2014 Nov 3]. Available from: http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B34E02&vw_cd=&list_id=&scrId=&seqNo=&lang_mode=ko&obj_var_id=&itm_id=&conn_path=K1&path=#. [Google Scholar]
- 2.Weisz B, Meirow D, Schiff E, Lishner M. Impact and treatment of cancer during pregnancy. Expert Rev Anticancer Ther. 2004;4:889–902. doi: 10.1586/14737140.4.5.889. [DOI] [PubMed] [Google Scholar]
- 3.Korean Statistical Information Service. Cancer incidence rate: 2012 web-based report [Internet] Daejeon: Korean Statistical Information Service; c2015. [cited 2015 Jan 12]. Available from: http://kosis.kr/statHtml/statHtml.do?orgId=117&tblId=DT_117N_A00023&vw_cd=MT_ZTITLE&list_id=101_11744&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=E1. [Google Scholar]
- 4.Korean Statistical Information Service. Simulated average childbearing age: 1993-2013 web-based report [Internet] Daejeon: Korean Statistical Information Service; c2014. [cited 2014 Aug 26]. http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B81A20&vw_cd=MT_ZTITLE&list_id=A21_6&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=E1#. [Google Scholar]
- 5.Van Calsteren K, Heyns L, De Smet F, Van Eycken L, Gziri MM, Van Gemert W, et al. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol. 2010;28:683–689. doi: 10.1200/JCO.2009.23.2801. [DOI] [PubMed] [Google Scholar]
- 6.Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist. 2002;7:279–287. [PubMed] [Google Scholar]
- 7.Weisz B, Schiff E, Lishner M. Cancer in pregnancy: maternal and fetal implications. Hum Reprod Update. 2001;7:384–393. doi: 10.1093/humupd/7.4.384. [DOI] [PubMed] [Google Scholar]
- 8.Dunkelberg JC, Barakat J, Deutsch J. Gastrointestinal, pancreatic, and hepatic cancer during pregnancy. Obstet Gynecol Clin North Am. 2005;32:641–660. doi: 10.1016/j.ogc.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.US Cancer Statistics Working Group. United States cancer statistics: 1999-2011 incidence and mortality web-based report [Internet] Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; c2014. [cited 2014 Dec 24]. http://www.cdc.gov/cancer/npcr/pdf/USCS_FactSheet.pdf. [Google Scholar]
- 10.Antonelli NM, Dotters DJ, Katz VL, Kuller JA. Cancer in pregnancy: a review of the literature. Part I. Obstet Gynecol Surv. 1996;51:125–134. doi: 10.1097/00006254-199602000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Andersson TM, Johansson AL, Fredriksson I, Lambe M. Cancer during pregnancy and the postpartum period: a population-based study. Cancer. 2015;121:2072–2077. doi: 10.1002/cncr.29325. [DOI] [PubMed] [Google Scholar]
- 12.Eibye S, Kjaer SK, Mellemkjaer L. Incidence of pregnancyassociated cancer in Denmark, 1977-2006. Obstet Gynecol. 2013;122:608–617. doi: 10.1097/AOG.0b013e3182a057a2. [DOI] [PubMed] [Google Scholar]
- 13.Cardonick E. Pregnancy-associated breast cancer: optimal treatment options. Int J Womens Health. 2014;6:935–943. doi: 10.2147/IJWH.S52381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HY, Chay WY, Tang MB, Chio MT, Tan SH. Melanoma: differences between Asian and Caucasian patients. Ann Acad Med Singapore. 2012;41:17–20. [PubMed] [Google Scholar]
- 15.National Cancer Information Center. Cancer prevalence by generation: 2012 web-based report [Internet] Goyang: National Cancer Information Center; c2014. [cited 2014 Dec 24]. http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040103000000. [Google Scholar]
- 16.Chung JH. Cost-of-illness trend of thyroid gland disease in Korea. Endocrinol Metab (Seoul) 2014;29:248–250. doi: 10.3803/EnM.2014.29.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han MA, Choi KS, Lee HY, Kim Y, Jun JK, Park EC. Current status of thyroid cancer screening in Korea: results from a nationwide interview survey. Asian Pac J Cancer Prev. 2011;12:1657–1663. [PubMed] [Google Scholar]
- 18.Lishner M. Cancer in pregnancy. Ann Oncol. 2003;14(Suppl 3):iii31–iii36. doi: 10.1093/annonc/mdg745. [DOI] [PubMed] [Google Scholar]
- 19.Oh SE, Kim HJ, Choi SJ, Oh SY, Roh CR, Kim JH. A case of huge retroperitoneal liposarcoma in pregnancy. Obstet Gynecol Sci. 2014;57:236–239. doi: 10.5468/ogs.2014.57.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abramowicz JS. Benefits and risks of ultrasound in pregnancy. Semin Perinatol. 2013;37:295–300. doi: 10.1053/j.semperi.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Bulas D, Egloff A. Benefits and risks of MRI in pregnancy. Semin Perinatol. 2013;37:301–304. doi: 10.1053/j.semperi.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 22.ACOG Committee on Obstetric Practice. ACOG Committee Opinion. Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet Gynecol. 2004;104:647–651. doi: 10.1097/00006250-200409000-00053. [DOI] [PubMed] [Google Scholar]
- 23.Fenig E, Mishaeli M, Kalish Y, Lishner M. Pregnancy and radiation. Cancer Treat Rev. 2001;27:1–7. doi: 10.1053/ctrv.2000.0193. [DOI] [PubMed] [Google Scholar]
- 24.Brent RL. The effect of embryonic and fetal exposure to Xray, microwaves, and ultrasound: counseling the pregnant and nonpregnant patient about these risks. Semin Oncol. 1989;16:347–368. [PubMed] [Google Scholar]
- 25.Savas N. Gastrointestinal endoscopy in pregnancy. World J Gastroenterol. 2014;20:15241–15252. doi: 10.3748/wjg.v20.i41.15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cappell MS. The fetal safety and clinical efficacy of gastrointestinal endoscopy during pregnancy. Gastroenterol Clin North Am. 2003;32:123–179. doi: 10.1016/s0889-8553(02)00137-1. [DOI] [PubMed] [Google Scholar]
- 27.Jaspers VK, Gillessen A, Quakernack K. Gastric cancer in pregnancy: do pregnancy, age or female sex alter the prognosis? Case reports and review. Eur J Obstet Gynecol Reprod Biol. 1999;87:13–22. doi: 10.1016/s0301-2115(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 28.Pereg D, Koren G, Lishner M. Cancer in pregnancy: gaps, challenges and solutions. Cancer Treat Rev. 2008;34:302–312. doi: 10.1016/j.ctrv.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Malangoni MA. Gastrointestinal surgery and pregnancy. Gastroenterol Clin North Am. 2003;32:181–200. doi: 10.1016/s0889-8553(02)00072-9. [DOI] [PubMed] [Google Scholar]
- 30.Amant F, Han SN, Gziri MM, Vandenbroucke T, Verheecke M, Van Calsteren K. Management of cancer in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29:741–753. doi: 10.1016/j.bpobgyn.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Aviles A, Diaz-Maqueo JC, Talavera A, Guzman R, Garcia EL. Growth and development of children of mothers treated with chemotherapy during pregnancy: current status of 43 children. Am J Hematol. 1991;36:243–248. doi: 10.1002/ajh.2830360404. [DOI] [PubMed] [Google Scholar]
- 32.Lishner M, Koren G. Cancer chemotherapy during pregnancy: consortium of cancer in pregnancy evidence. Can Fam Physician. 2001;47:41–42. [PMC free article] [PubMed] [Google Scholar]
- 33.Amant F, Van Calsteren K, Halaska MJ, Gziri MM, Hui W, Lagae L, et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol. 2012;13:256–264. doi: 10.1016/S1470-2045(11)70363-1. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham FG, editor. Williams obstetrics. 24th ed. New York (NY): McGraw-Hill; 2014. [Google Scholar]