Abstract
Objective
The aim of this study is to assess the effectiveness of oral progestin treatment in women diagnosed with complex atypical hyperplasia (CAH) or grade 1 endometrial cancer (G1EC), who desire to preserve their fertility, as alternative treatment to a hysterectomy.
Methods
We reviewed the medical records of women younger than 45 years old that had been diagnosed with CAH or G1EC, who expressed a desire to preserve their fertility using alternative treatment at our institution. Women without evidence of myometrial invasion on pelvic magnetic resonance imaging scans were included. The study period was between 2004 and 2014. Endometrial biopsies were taken at follow-up appointments.
Results
We identified 31 young women with CAH or G1EC. The median age was 33 years old (range, 20 to 41), and the median period of time undertaking the treatment was 5 months (range, 1 to 12). Twenty-three patients (74.2%) achieved complete remission (CR; median time to CR was 3 months; range, 1 to 22), 16 patients (88.9%) with CAH and 7 (53.8%) with G1EC achieved CR. 6 patients (26.1%) who had achieved CR, had recurrence of the disease (median time from CR to recurrence was 12.5 months; range, 4 to 18). Eight patients (25.8%) finally underwent a hysterectomy.
Conclusion
Oral progestin therapy is an alternative treatment for women with CAH or G1EC who desire fertility preservation. However, more prospective studies are needed for standard progestin regimen. Also, there still remains a risk of disease progression and recurrence. Therefore, close follow-up is important during treatment and after CR. In addition, a hysterectomy is recommended as a definitive treatment after completion of childbearing.
Keywords: Endometrial hyperplasia, Endometrial cancer, Fertility, Progestins
Introduction
Endometrial adenocarcinoma (EC) is the most common gynecological malignancy in developed countries, and is the third most common cause of gynecological cancer death [1]. Complex atypical hyperplasia (CAH) is an immediate precursor of EC [2]. The majority of women with EC are postmenopausal. However, 15.0% to 25.0% of EC cases occur prior to the menopause [3,4,5,6,7,8,9], 10.0% will be aged <45 years and 4.0% aged <40 years [4,8], of which 70.0% are nulliparous [10]. Most women diagnosed with CAH or grade 1 endometrial cancer (G1EC) have a strong desire to preserve their fertility [11]. In addition, the number of patients with EC who desire fertility preservation will increase in the future, considering social trends such as increasing tendency to marry later in life.
The standard treatment for endometrial cancer is a hysterectomy and bilateral salpingo-oophorectomy. Younger women tend to be diagnosed with low grade, early stage disease and have an excellent prognosis with five- and ten-year diseasefree survival rates of up to 99.2% and 98.0%, respectively [2,12,13,14]. Here, we consider an alternative treatment to a hysterectomy, until the completion of childbearing, for women with CAH or G1EC who desire fertility preservation. The most-studied fertility-preserving treatment for CAH and G1EC in young women is oral progestin. This alternative therapy using progestin should be restricted to patients with disease up to grade I, with no evidence of myometrial invasion or extrauterine disease upon imaging, and motivation to achieve pregnancy soon after disease remission [15].
Various success rates are reported in the literature with the administration of different progestin regimens. However, definitive treatment guidelines do not exist, therefore it remains unclear which type of progestin and duration of therapy is the most effective. In addition, it is unclear which patients have a more effective response to progestin therapy based on their characteristics.
The objective of our study is to assess the efficacy of oral progestin treatment in women with CAH or G1EC as alternative treatment to a hysterectomy. Also, we analyzed various characteristics as predictors of treatment response.
Materials and methods
We performed a retrospective analysis and identified 31 women with CAH or G1EC, including those younger than 45 years old, who were treated with oral progestin for fertility preservation at Chonnam National University Hospital. The study period included cases from 2004 to 2014. Clinical data was obtained from medical records. Women with a histological diagnosis of CAH or G1EC, without evidence of myometrial invasion on pelvic magnetic resonance imaging scans, were included. Diagnosis and assessment of response to treatment were based on the results of an endometrial biopsy or dilatation and curettage, with hysteroscopy being used for biopsy of focal endometrial lesions or a more accurate endometrial curettage. Follow-up biopsy was performed at 1 month after initiation of progestin treatment, and every 3 months since then. Resulting specimens were reviewed by a gynecological pathologist. Complete remission (CR) was defined as no microscopic evidence of either hyperplasia or cancer cells in the endometrial histopathology. A proliferative phase, secretary phase, or hormonal effect found in the endometrial histopathology were considered as normal. The period of time to CR was measured from the initiation of progestin treatment to the first negative biopsy. Recurrence was defined as either hyperplasia with atypia or cancer cells present in histopathology following CR. The time to recurrence was measured from CR to the first positive biopsy. Progestin therapy consisted of megestrol acetate (MA, 80–160 mg) or medroxyprogesterone acetate (MPA, 40–120 mg).
Continuous variables were described using median values and ranges. Categorical variables were described using frequencies and proportions. A body mass index (BMI) greater than 25 kg/m2 was considered obese. Comparisons were performed using Fisher's exact test, the χ2 test, and the Mann-Whitney test. Statistical significance was set at the standard value of P <0.05. IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Result
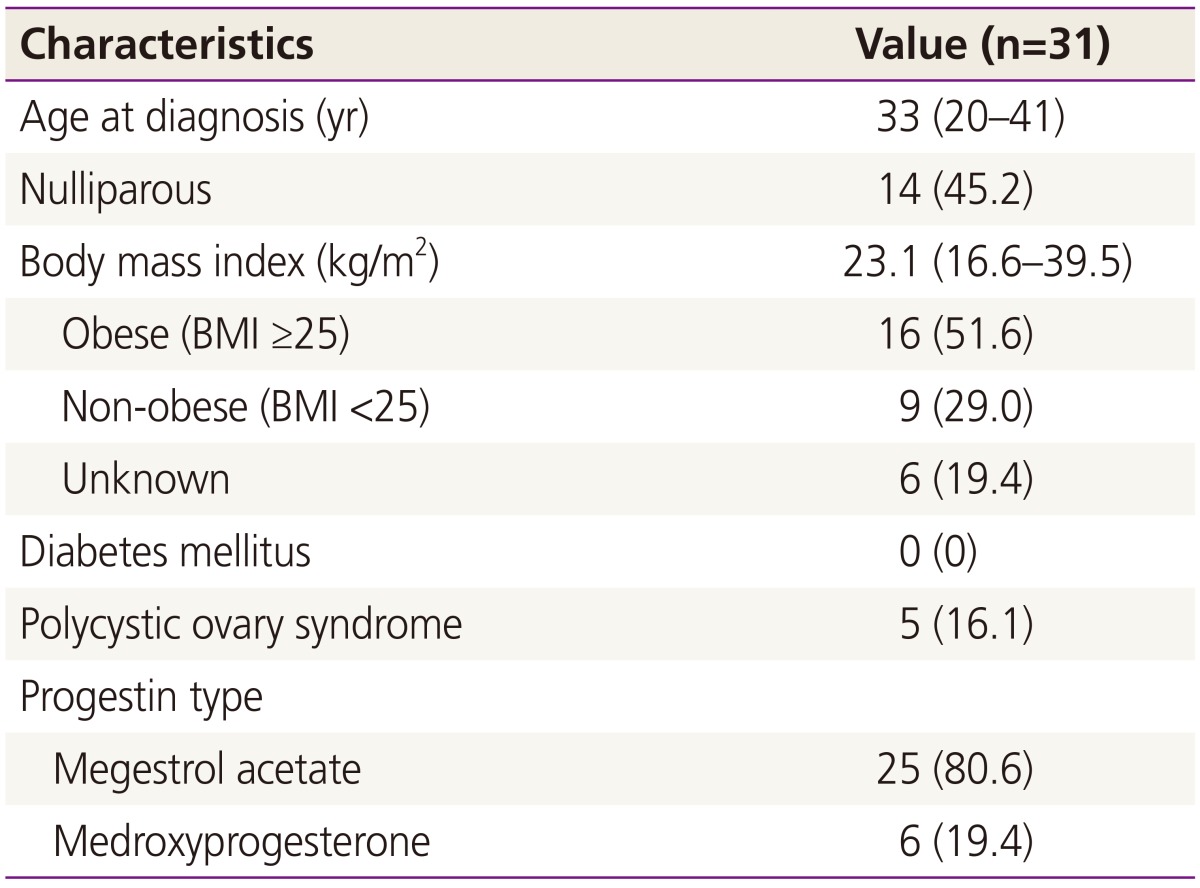
A total of 31 patients met the study criteria. At initial diagnosis, 18 patients (58.1%) had CAH and 13 patients (41.9%) had G1EC. There was no difference between the proportion of women with CAH and G1EC. All patients were evaluated using pelvic magnetic resonance imaging before treatment, and had no evidence of myometrial invasion or extrauterine disease. Population characteristics are described in Table 1. Among these patients, 11 (35.5%) were unmarried and 14 (45.2%) were nulliparous. The median age was 33 years old (range, 20 to 41). Five patients (16.1%) had polycystic ovary syndrome (PCOS) at the time of diagnosis. The median BMI was 23.1 (range, 16.6 to 39.5). No patients in study population had diabetes. The median time receiving treatment was 5 months (range, 1 to 12). Twenty five patients (80.6%) were on MA and 6 patients (19.4%) were on MPA. The median follow-up from the start of progestin treatment was 11.5 months (range, 3 to 29).
Table 1. Patient characteristics.
Data are presented as median (range) or number (%).
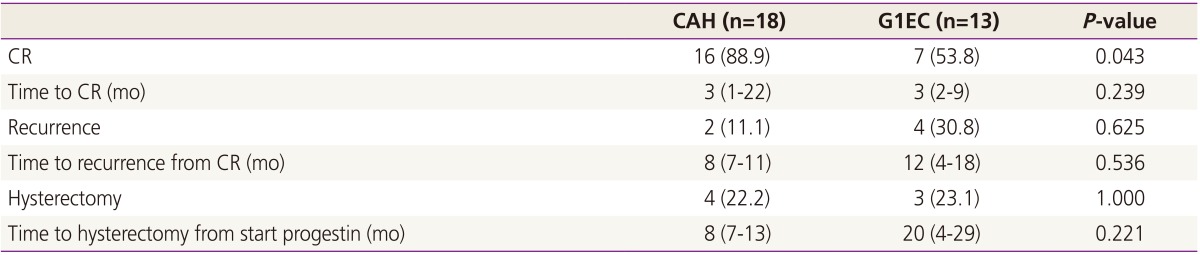
The response to treatment and oncological outcomes as histological diagnoses are presented in Table 2. There was no difference in the period of time to CR, recurrence rates, time to recurrence, hysterectomy rates, and time to hysterectomy between CAH and G1EC.
Table 2. Response to treatment and oncological outcomes as histological diagnoses.
Values are presented as number (%) or median (range).
CAH, complex atypical hyperplasia; G1EC, grade 1 endometrial cancer; CR, complete remission.
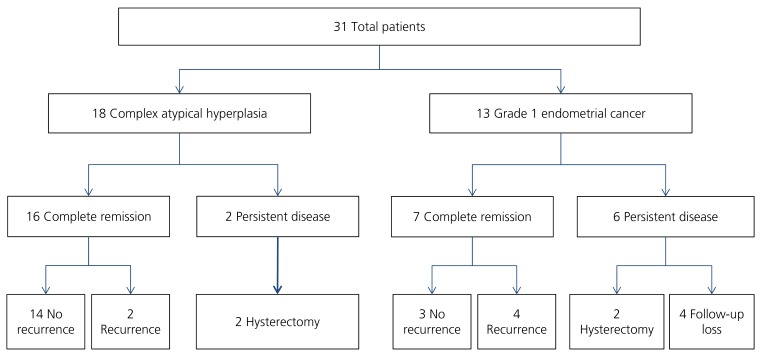
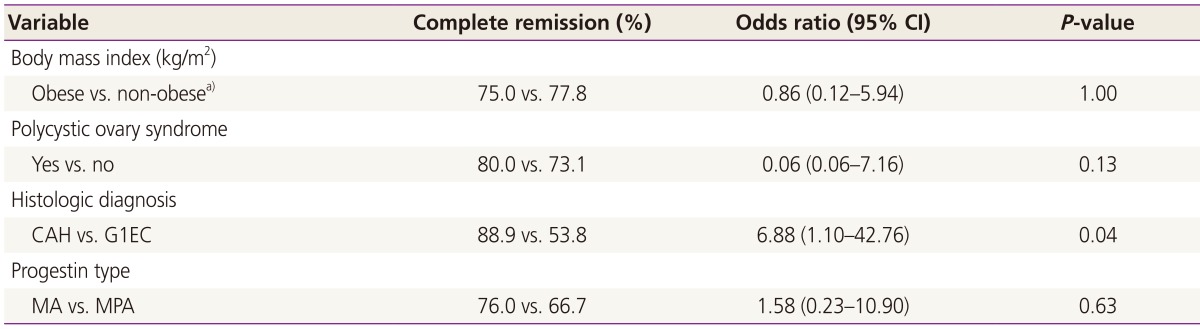
The distribution of evaluated patients is presented in Fig. 1. Twenty three patients (74.2%) achieved CR; 16 (88.9%) with CAH and 7 (53.8%) with G1EC. Of the patients who did not achieve CR, 4 underwent a hysterectomy due to persistent disease and 4 were a follow-up loss. CAH at initial diagnosis was associated with more likelihood of CR (P =0.04; odd ratio, 6.88; 95% confidence interval, 1.10 to 42.76). No other variables were found to be associated with CR (Table 3). The women who achieved CR were similarly proportioned between non-obese (BMI <25) and obese (BMI ≥25) (75.0% vs. 77.8%). In addition, the probability of CR was not significantly different between the patients with or without PCOS (80.0% vs. 73.1%). In addition, progestin type was not associated with CR (P =0.63). The median time to CR was 3 months (range, 1 to 22). Only 8 patients received maintenance therapy with low-dose cyclic progestin after CR (duration 3 to 6 months).
Fig. 1. The distribution of evaluated patients.
Table 3. Variables associated with complete remission.
CI, confidence interval; CAH, complex atypical hyperplasia; G1EC, grade 1 endometrial cancer; MA, megestrol acetate; MPA, medroxyprogesterone acetate.
a)Obese, BMI ≥25; non-obese, BMI <25.
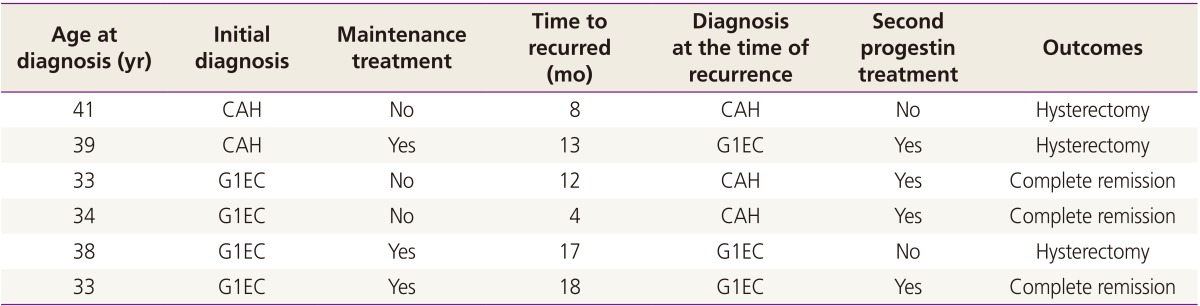
Six patients (26.1%) who had achieved CR recurred (Table 4). Of these patients, 2 patients had CAH and 4 patients had G1EC at initial diagnosis. Maintenance treatment was administered in 3 of 6 patients but not in the other 3 patients. Four of the patients with recurrent disease started a second course of progestin therapy. Three of these (75.0%) responded to treatment; all of which had G1EC at initial diagnosis, however 2 had CAH at recurrence. The median time from CR to recurrence was 12.5 months (range, 4 to 18).
Table 4. Patients who recurred after achievement of complete remission.
CAH, complex atypical hyperplasia; G1EC, grade 1 endometrial cancer.
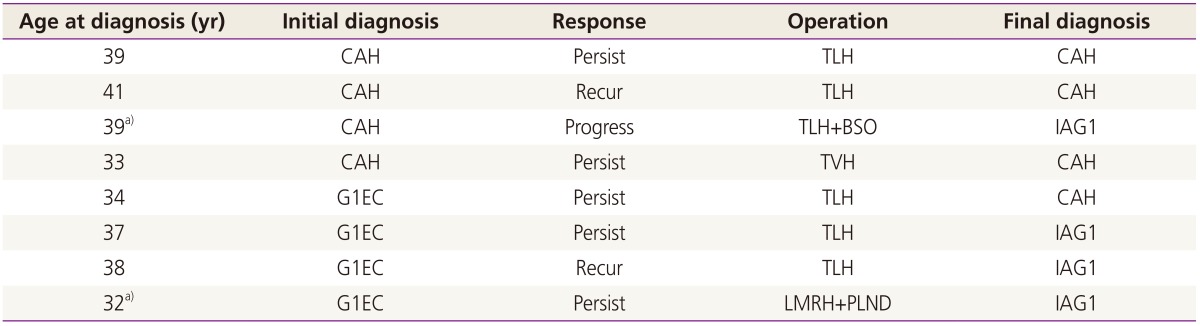
Only 8 patients (25.8%) finally underwent a hysterectomy; 2 (25.0%) had recurrent disease, 5 (65.2%) had persistent disease, and 1 (12.5%) had disease progression (Table 5). Five patients (62.5%) had CAH and 3 patients (37.5%) had G1EC at initial diagnosis. One patient had disease progression, who had had CAH on initial diagnosis. She had been receiving MA for 3 months, and changed to MPA due to weight gain. After 7 months of treatment, the results of the follow-up endometrial biopsy showed the presence of G1EC. Thus, she underwent a hysterectomy and the final diagnosis was G1EC, FIGO (International Federation of Gynecology and Obstetrics) stage IA. There was no significant difference between CAH and G1EC at the time of the first diagnosis on the need to undergo a hysterectomy. The median time from the start of the treatment to a hysterectomy was 10 months (range, 4 to 29).
Table 5. Patients who underwent a hysterectomy.
CAH, complex atypical hyperplasia; TLH, total laparoscopic hysterectomy; BSO, bilateral salpingo-oophorectomy; IAG1, endometrial cancer stage IA grade1; TVH, transvaginal hysterectomy; G1EC, grade 1 endometrial cancer; LMRH+PLND, laparoscopic modified radical hysterectomy and pelvic lymph node dissection.
a)Two patients underwent a hysterectomy after completion of childbirth.
At the final follow-up appointment, 4 patients were a follow-up loss. Three patients were referred to an endocrinologist for PCOS treatment after the termination of progestin therapy. According to our medical records, 4 women tried to conceive using assisted reproductive technologies. Two out of the 4 women were unsuccessful in conceiving, 1 tried in vitro fertilization/embryo transfer eight times to become pregnant, but finally ceased trying. The other 2 women underwent a hysterectomy following childbirth. The first of these women had progestin treatment for 3 months, subsequently tried in vitro fertilization/embryo transfer, and had a successful pregnancy on the third attempt. Finally, she underwent a hysterectomy after delivery. The second of these women also had progestin treatment for 3 months and tried intrauterine insemination. She became pregnant on the first attempt, but terminated the pregnancy at 23 weeks due to incompetent internal os of cervix. The second attempt yielded a successful delivery, however due to the fact that she wanted more children, she started progestin therapy for persisting G1EC. Nevertheless, she underwent a hysterectomy due to persisting G1EC found in a follow-up biopsy.
Discussion
Fertility preservation in young women diagnosed with CAH or G1EC is important. Although fertility-sparing treatment is not the standard, progestin therapy may be considered for women with CAH or G1EC who wish to preserve their reproductive potential. Therefore, our study suggests that oral progestin therapy is an effective alternative treatment, with over 70.0% of our patients achieving a CR.
We identified 31 young women with CAH or G1EC confined to endometrium as seen by magnetic resonance imaging, treated them with oral progestin, and monitored their disease every 3 months through endometrial biopsy. The overall CR rate of 72.4% in our study is relatively similar to the results seen in the majority of other studies [16,17,18,19], however, a slightly lower rate has been reported by others 62.0% to 63.0% [20,21,22]. The number of our patients who had comorbidities was smaller than that seen in other studies, and there were no concurrent malignancies such as ovarian cancer seen here.
A multicenter retrospective study showed that patients with G1EC had a CR rate of 77.7% [23], however we observed a lower CR rate of 53.8% in patients with G1EC, respectively. The difference in our study results was possibly due to the shorter treatment duration and lower progestin dose, as compared to the previous study. We observed a CR rate of 88.9% and 53.8% in patients with CAH and G1EC. CAH was associated with a higher rate of CR than was G1EC. In contrast to our study, a different study found no difference in the rate of CR between CAH and G1EC [18]. Other studies demonstrated that a BMI >25 kg/m2 was significantly associated with failure to achieve a CR [23], or that older age at diagnosis was associated with a lower incidence of CR [11]. Further studies have shown no correlation with age [24], BMI, diabetes mellitus [24,25], hypertension, or history of PCOS [24], progestin dose and type [22]. In our study, no other variables were associated with the incidence of CR except for histological diagnosis. Prior studies have reported that progesterone receptor (PR) expression predicts response to progestin therapy in young women with CAH or G1EC [26]. Conversely, Gunderson et al. [27] reported that the pre-treatment and post-treatment status of the estrogen receptor (ER) or the PR did not predict the response to progestin therapy. In their relatively large series of 46 patients, achievement of CR was not associated with pretreatment ER percentage, intensity or PR expression. In addition, posttreatment ER and PR status were found to be unrelated to achievement of CR. In our study, immunohistochemical analysis of ER and PR were not carried out. As a result of the limitation of retrospective study, it is difficult to evaluate the association between achievement of CR and hormone receptors (ER and PR). Due to the fact that many studies have shown very different results, it is difficult to predict a response to treatment based on several characteristics. More research is needed in this area including immunohistochemical markers to predict which patients will have a positive response to treatment.
MA and MPA are the most commonly used progestins for fertility-sparing treatment in early endometrial cancer. In our study, progestin type was not associated with CR. Progestin dose varied among patients due to the physician's decision. Also, only 8 patients received maintenance treatment with low-dose cyclic progestin. Three of these patients were recurred after CR. However, there was no association between recurrence and maintenance treatment because of the small size of our study population. Patients who did not receive maintenance therapy wanted to get pregnant immediately or did not want to take more drugs after CR. Because our study was retrospective, there were no standard regimens for progestin treatment and definitive criteria for maintenance treatment. Therefore, more prospective studies are needed for standard progestin treatment regimen.
In women who achieve CR, recurrence rates are generally high at 26.0% to 40.0% [28]. In our study, the recurrence rate was 26.1% and similar to the other studies. Two of 6 women with recurring disease underwent a hysterectomy without a second course of progestin therapy, and the remaining women started a second course. Three of these 4 patients achieved CR, and 1 patient underwent a hysterectomy without more courses of progestin therapy. A retrospective study of 33 patients with recurring disease showed an 85% CR rate after a second course of progestin therapy [28], however our CR rate following a second course of progestin therapy is lower, likely due to the small population in the study.
A systematic review of 34 observational studies reported a live birth rate of 26.0% to 28.0%. It showed that the use of assisted reproductive technology achieved more live births compared with natural spontaneous conception (39.0% vs. 14.0%) [28]. Gunderson et al. [27] reported that 36.0% of their 315 patients achieved pregnancy at least once, with similar rates among patients with hyperplasia and those with cancer. In our study, only 2 women achieved live births and underwent a hysterectomy after completion of childbirth. One women achieved pregnancy spontaneously, however she was a follow-up loss. Due to the limitations of retrospective study, it is unclear how many women in this study population were actively trying to conceive.
The limitations of our study include its retrospective nature, lack of randomization, small sample size, and limited followup. In addition, patients did not routinely undergo fertility intervention as part of the treatment. Therefore, prospective clinical trials with a standardized evaluation and treatment protocol are needed in order to provide more information on oncological and reproductive outcomes in this diseases.
In conculsion, we can consider that oral progestin therapy is an alternative treatment to hysterectomy for women with CAH or G1EC who wish to preserve their fertility. However, we observed a lower CR rate in women with G1EC than the rates found in previous multicenter study. This is because of the shorter treatment duration and lower progestin dose in our study. Therefore, we expect that the higher progestin dose and the longer treatment duration are needed for effective treatment in women with G1EC, and more prospective studies are needed for standard progestin treatment duration and dose. Also, there still remains a risk of recurrence after CR. It is important that the patient is well informed and carefully counseled regarding the risks of this conservative treatment. Close follow-up is essential, and a hysterectomy after completion of childbirth is recommended as a definitive treatment.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417–420. [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Odicino F, Maisonneuve P, Beller U, Benedet JL, Heintz AP, et al. Carcinoma of the corpus uteri. J Epidemiol Biostat. 2001;6:47–86. [PubMed] [Google Scholar]
- 5.Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS Gynecologic Oncology Group Study. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:587–592. doi: 10.1200/JCO.2005.02.8464. [DOI] [PubMed] [Google Scholar]
- 6.Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001;83:388–393. doi: 10.1006/gyno.2001.6434. [DOI] [PubMed] [Google Scholar]
- 7.Gitsch G, Hanzal E, Jensen D, Hacker NF. Endometrial cancer in premenopausal women 45 years and younger. Obstet Gynecol. 1995;85:504–508. doi: 10.1016/0029-7844(95)00001-8. [DOI] [PubMed] [Google Scholar]
- 8.Lee NK, Cheung MK, Shin JY, Husain A, Teng NN, Berek JS, et al. Prognostic factors for uterine cancer in reproductive-aged women. Obstet Gynecol. 2007;109:655–662. doi: 10.1097/01.AOG.0000255980.88205.15. [DOI] [PubMed] [Google Scholar]
- 9.Tran BN, Connell PP, Waggoner S, Rotmensch J, Mundt AJ. Characteristics and outcome of endometrial carcinoma patients age 45 years and younger. Am J Clin Oncol. 2000;23:476–480. doi: 10.1097/00000421-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005;105:575–580. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- 11.Simpson AN, Feigenberg T, Clarke BA, Gien LT, Ismiil N, Laframboise S, et al. Fertility sparing treatment of complex atypical hyperplasia and low grade endometrial cancer using oral progestin. Gynecol Oncol. 2014;133:229–233. doi: 10.1016/j.ygyno.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Crissman JD, Azoury RS, Barnes AE, Schellhas HF. Endometrial carcinoma in women 40 years of age or younger. Obstet Gynecol. 1981;57:699–704. [PubMed] [Google Scholar]
- 13.Lajer H, Elnegaard S, Christensen RD, Ortoft G, Schledermann DE, Mogensen O. Survival after stage IA endometrial cancer: can follow-up be altered? A prospective nationwide Danish survey. Acta Obstet Gynecol Scand. 2012;91:976–982. doi: 10.1111/j.1600-0412.2012.01438.x. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg SG, Makowski EL, Roche WD. Endometrial carcinoma in women under 40 years of age: comparison of cases in oral contraceptive users and non-users. Cancer. 1977;39:592–598. doi: 10.1002/1097-0142(197702)39:2<592::aid-cncr2820390233>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Dorais J, Dodson M, Calvert J, Mize B, Travarelli JM, Jasperson K, et al. Fertility-sparing management of endometrial adenocarcinoma. Obstet Gynecol Surv. 2011;66:443–451. doi: 10.1097/OGX.0b013e31822f8f66. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95:133–138. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 17.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–440. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 18.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125:477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Koskas M, Azria E, Walker F, Luton D, Madelenat P, Yazbeck C. Progestin treatment of atypical hyperplasia and well-differentiated adenocarcinoma of the endometrium to preserve fertility. Anticancer Res. 2012;32:1037–1043. [PubMed] [Google Scholar]
- 20.Kim YB, Holschneider CH, Ghosh K, Nieberg RK, Montz FJ. Progestin alone as primary treatment of endometrial carcinoma in premenopausal women: report of seven cases and review of the literature. Cancer. 1997;79:320–327. doi: 10.1002/(sici)1097-0142(19970115)79:2<320::aid-cncr15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Shirali E, Yarandi F, Eftekhar Z, Shojaei H, Khazaeipour Z. Pregnancy outcome in patients with stage 1a endometrial adenocarcinoma, who conservatively treated with megestrol acetate. Arch Gynecol Obstet. 2012;285:791–795. doi: 10.1007/s00404-011-2021-8. [DOI] [PubMed] [Google Scholar]
- 22.Cade TJ, Quinn MA, Rome RM, Neesham D. Progestogen treatment options for early endometrial cancer. BJOG. 2010;117:879–884. doi: 10.1111/j.1471-0528.2010.02552.x. [DOI] [PubMed] [Google Scholar]
- 23.Park JY, Kim DY, Kim JH, Kim YM, Kim KR, Kim YT, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002) Eur J Cancer. 2013;49:868–874. doi: 10.1016/j.ejca.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Penner KR, Dorigo O, Aoyama C, Ostrzega N, Balzer BL, Rao J, et al. Predictors of resolution of complex atypical hyperplasia or grade 1 endometrial adenocarcinoma in premenopausal women treated with progestin therapy. Gynecol Oncol. 2012;124:542–548. doi: 10.1016/j.ygyno.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Eftekhar Z, Izadi-Mood N, Yarandi F, Shojaei H, Rezaei Z, Mohagheghi S. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: our experiences with 21 patients. Int J Gynecol Cancer. 2009;19:249–252. doi: 10.1111/IGC.0b013e31819c5372. [DOI] [PubMed] [Google Scholar]
- 26.Vereide AB, Kaino T, Sager G, Arnes M, Orbo A. Effect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-alpha and ER-beta) in human endometrial hyperplasia. Gynecol Oncol. 2006;101:214–223. doi: 10.1016/j.ygyno.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Gunderson CC, Dutta S, Fader AN, Maniar KP, Nasseri-Nik N, Bristow RE, et al. Pathologic features associated with resolution of complex atypical hyperplasia and grade 1 endometrial adenocarcinoma after progestin therapy. Gynecol Oncol. 2014;132:33–37. doi: 10.1016/j.ygyno.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 28.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207:266.e1–266.e12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]