Abstract
Pyoderma gangrenosum is an extremely rare chronic cutaneous disease causing severe ulceration. It can be developed after minor trauma or surgical procedure. The typical features mimic acute infection site, however the treatment methods are opposing since pyoderma gangrenosum is improved with the use of corticosteroids, not antibiotic therapy. We here report a patient who had been diagnosed for acute infection after cesarean delivery in 2011 and treated with a number of antibiotics, but failed to recover. The patient had suffered from pain of the disease and also renal failure caused by antibiotics. Ultimately she had been diagnosed as pyoderma gangrenosum and managed successfully with steroids. For her next pregnancy in 2013, we tried vaginal delivery after prior cesarean section and it was uneventful during and after delivery.
Keywords: Cesarean section, Pyoderma gangrenosum, Vaginal birth after cesarean, Wound infection
Introduction
Pyoderma gangrenosum is a rare chronic skin disease resulting in extensive ulceration and extremely uncommon in pregnancy. It is typically known to affect the lower limbs, but several cases have been reported occurring after minor trauma or surgical procedure. Numerous systemic disorders have been described to be associated, including inflammatory bower disease, rheumatologic arthritis, antiphospholipid antibody syndrome, or hematological malignancy [1]. Pyoderma gangrenosum is characterized by extensive pustular lesion that rapidly progresses to painful ulcers with undermined violaceous borders. The diagnosis is mainly based on the clinical manifestation and is confirmed through exclusion of other causes of cutaneous diseases.
We present a challenging case of successful vaginal delivery with pyoderma gangrenosum which developed after prior cesarean section and failed to improve with subsequent antibiotic therapy.
Case Report
A 33-year-old woman with parity 1-0-2-1, was referred to obstetric department for early pregnancy in October 2013. She has been diagnosed as pyoderma gangrenosum and had a history of admission to dermatology department of Seoul National University hospital from July 1st to August 3rd in 2012. Her dermatologist sent her to obstetric department for consultation of antenatal management immediately after recognition of current pregnancy. At her first office based visit, transvaginal ultrasound examination was performed and normal intrauterine pregnancy was confirmed. Estimated gestation age was about 7 weeks.
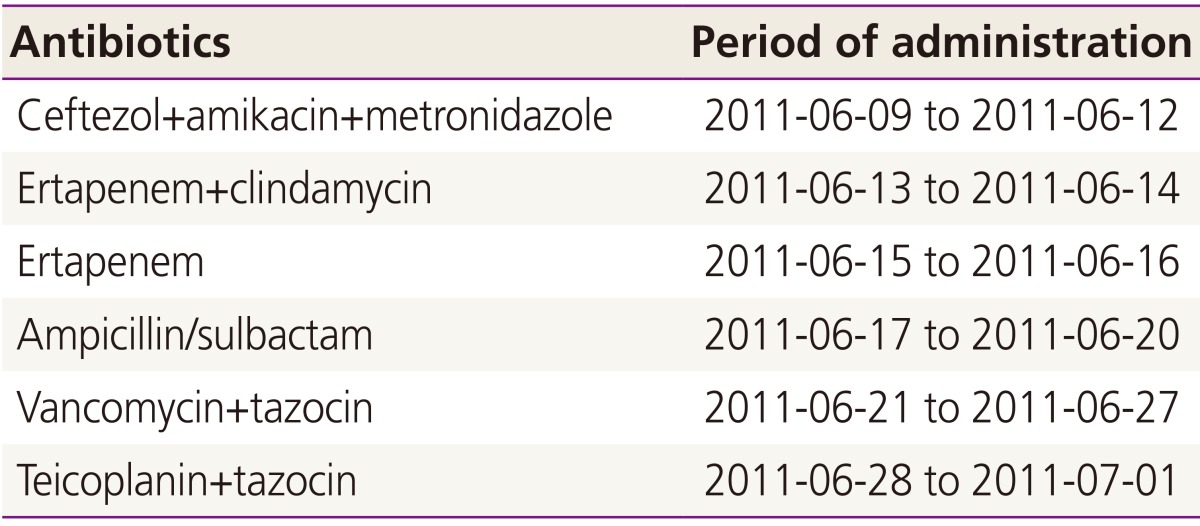
Thorough history taking and record review were followed. In June of 2011, she delivered a healthy female child at term by emergency cesarean section with lower uterine segment transverse incision due to premature rupture of membrane and alleged placenta previa totalis at a local clinic. The surgical procedure was uneventful. After 1 week, skin wound showed dehiscence with purulent discharge and painful induration. Wound culture was done and antibiotics (ceftezol, amikacin, and metronidazole) were given. Careful wound dressing was performed twice daily. On postoperative day 9, debridement and closure of wound were done, however wound dehiscence was found again and pain was aggravated. The previous wound culture showed no growth of bacteria, but the clinician changed antibiotics to ertapenem and clindamycin.
The condition of wound did not appear to be improved and serial follow-ups of wound culture resulted in no bacterial growth. Her vital signs including body temperature were normal. Wound dressing was done twice daily with saline irrigation. Wound tissue was obtained and the result of biopsy was supprative inflammation with fibrinoid vascular necrosis, consistent with abscess. The clinician changed antibiotics three times more and all the antibiotics that she had been received are listed in Table 1. On postoperative day 24, urine output started to decrease remarkably and creatinine level was reached to 4.9 mg/dL. Finally she was referred to Seoul National University Hospital for the management of acute renal failure.
Table 1. Antibiotics administered at the prior hospital.
On July 1st of 2011, she was admitted to dermatology department. Extensive painful ulcerative open wound with erythematous plaques and violaceous colored border involved the whole lower abdomen (Fig. 1A, B). Tenderness was found around wound margins. Vital signs of the patient including body temperature were normal. Her blood test revealed remarkable increase in creatinine (5.3 mg/dL) and C-reactive protein (16.97 mg/dL). To check urine output effectively, Foley catheter was inserted and enough hydration through intravenous line was followed. Nephrologists recommended to use ceftriaxone only for antibiotics and stop all other medications that could cause nephrotoxicity. Kidney ultrasound showed no hydronephrosis, but increased echogenicity and decreased vascularity for both sides. Acute kidney injury caused by several antibiotics was highly suspected.
Fig. 1. (A,B) Cesarean delivery wound before treatment for pyoderma gangrenosum on 1st day of admission. (C) Appearance of wound on 13th day of hospitalization. (D) Appearance of wound after complete healing.
Dermatologists suspected pyoderma gangrenosum for the first site since there had been no improvement on several antibiotics used in previous hospital and biopsy result had confirmed chronic granulation tissue rather than acute infection site. They decided to use prednisolone with careful attention on renal function, starting at 70 mg daily. Cyclosporin was not given because of nephrotoxicity. In addition, evaluation for other systemic diseases such as antiphospholipid syndrome, rheumatologic diseases, and inflammatory bowel disorders was performed. Lupus erythematous cell antibody, anti-double-stranded DNA, rheumatoid factor and fluorescent antinuclear antibody were all negative, although antiphospholipid antigen was found to be weakly positive. Endoscopic gastroduodenoscopy and colonoscopy showed no sign of inflammatory bowel disorder, but mild erosion only.
Consultation to plastic surgery department was done during admission for consideration of skin graft. After thorough discussion, doctors determined not to perform skin graft operation for several reasons. First of all, there was strong possibility that the graft donor site might develop pyoderma gangrenosum as well. Continued use of steroid was another risk of vulnerability to acute infection or other complications of surgery. Therefore, only silver foam dressing and methylprednisolone ointment were applied without any surgical intervention.
On her hospital day 5, creatinine fell down to 3.51 mg/dL and C-reactive protein was 1.93 mg/dL. From 16th day of hospitalization, her creatinine was under 1.00 mg/dL and C-reactive protein was checked under 0.5 mg/dL. The wound responded dramatically to prednisolone. No more discharge came out of the wound and healing started from recession of erythermatous margin (Fig. 1C). She was discharged from hospital with oral antibiotics in addition to prednisolone tapered to 40 mg and office based follow-ups with regular dressing were done with tapering steroid. After one month, synergistic cyclosporine was started at 150 mg daily, increasing to 200 mg daily 24 days later. At the same time, prednisolone was lowered to 7.5 mg. She took 100 mg of cyclosporine only since January 2012 until she decided to prepare for next pregnancy.
For current pregnancy, she regularly visited obstetric department and routine antenatal check-ups were performed. She presented with gross rupture of membranes at 35 weeks and 5 days gestational age. Cervical dilatation was 1 cm and uterine contraction was irregular. The previous wound from pyoderma gangrenosum was found to be perfectly healed with fibrinotic skin formation (Fig. 1D). After observation at ward for one week with intravenous antibiotics, labor pain developed at 36 weeks and 4 days of gestation and augmentation with careful use of intravenous oxytocin was applied. A healthy female baby of 2,300 g was delivered through vaginal vacuum extracted delivery with an Apgar score of 9. Without any complication, she was discharged with her baby on postpartum day 2.
Discussion
Negative wound cultures and the worsening of condition despite of antibiotics indicated an inflammatory process, not an acute infection by microbiologic causes. The diagnosis of pyoderma gangrenosum could be supported by rapid response to steroid therapy. For an obstetrician, who rarely experiences this disease, the diagnosis could be mistaken for an acute wound infection and easily missed to be properly managed. Even though pyoderma gangrenosum is an extremely rare disease, suspicion of the diagnosis in cases of vigorous and extensive wound infection unresponsive to antibiotics should be considered since appropriate therapy including corticosteroids or cyclosporine usually improves the wound dramatically. To avoid another disasterous wound, we performed vaginal delivery after cesarean section for above patient and it ended successfully. This is a unique case for pregnancy with pyoderma gangrenosum.
Pyoderma gangrenosum is extremely uncommon noninfectious neutrophilic dermatoses. Majority of cases develop for the age group of 20 to 50 years, with women being more frequently affected than men [1]. Lesions can develop after surgery or even minor trauma, sometimes even spontaneously. The typical presentation is the wide extension of wound with an erythematous papule or pustule that forms purulent discharge as well as protruding borders spreading peripherally. The etiology is not fully understood and the literatures published about its occurrence during pregnancy are extremely rare. Because it has been known to be commonly associated with systemic diseases, pregnancy as a state of immunosuppression state may contribute to the manifestation of pyoderma gangrenosum.
In addition to correct diagnosis and treatment, investigation for other associated systemic diseases such as systemic lupus erhythematous, inflammatory bowel disease, rheumatoid arthritis, antiphospholipid antibody syndrome, and hematologic malignancies is essential [2]. Some expertss also recommend screening for human immunodeficiency virus since there have been more than five cases reporting pyoderma gangrenosum related to human immunodeficiency virus infection [3]. This patient underwent above evaluation and showed none of them was associated.
For wound management, local therapy with gentle saline dressings and steroid ointment is adequate. Unlike wound infection, systemic treatment for pyoderma gangrenosum mainly consists of immune modulation and the use of corticosteroids. Corticosteroids, such as prednisolone, 1 to 2 mg per kg daily, are commonly used for initial therapy. Immunosuppressive therapy with cyclosporine has become an accepted adjuvant treatment [4]. Dapsone (diaminodiphenylsulfone), a drug for leprosy, is another commonly used medication with combination of steroids [5].
There are several alternative treatment options studied. Infliximab in a single dose of 5 mg/kg given daily has been recently shown to be effective. Intralesional cyclosporine and azathioprine have been studied, however the results revealed various range of improvement [6]. Autologous skin graft can be considered for wide lesion, but it may flare up the disease at the donor site. Use of bioengineered skin is still experimental [7].
Making no traumatic lesion is the best method of prevention and therefore, trial of vaginal delivery should be considered for patients with alleged pyoderma gangrenosum in pregnancy if there is no absolute indication for cesarean section.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Amin SV, Bajapai N, Pai A, Bharatnur S, Hebbar S. Pyoderma gangrenosum in two successive pregnancies complicating caesarean wound. Case Rep Obstet Gynecol. 2014;2014:654843. doi: 10.1155/2014/654843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23:1008–1017. doi: 10.1111/j.1468-3083.2009.03199.x. [DOI] [PubMed] [Google Scholar]
- 3.Clark HH, Cohen PR. Pyoderma gangrenosum in an HIV-infected patient. J Am Acad Dermatol. 1995;32(5 Pt 2):912–914. doi: 10.1016/0190-9622(95)91561-3. [DOI] [PubMed] [Google Scholar]
- 4.Callen JP, Jackson JM. Pyoderma gangrenosum: an update. Rheum Dis Clin North Am. 2007;33:787–802. doi: 10.1016/j.rdc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273–283. doi: 10.1016/j.jaad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Bhat RM. Management of pyoderma gangrenosum: an update. Indian J Dermatol Venereol Leprol. 2004;70:329–335. [PubMed] [Google Scholar]
- 7.Poucke SV, Jorens PG, Peeters R, Jacobs W, de Beeck BO, Lambert J, et al. Pyoderma gangrenosum: a challenging complication of bilateral mastopexy. Int Wound J. 2004;1:207–213. doi: 10.1111/j.1742-4801.2004.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]