Highlights
-
•
Shigella sonnei is an emergent and highly drug resistant diarrheal pathogen.
-
•
The half-life of maternal S. sonnei IgG in infants is 43 days.
-
•
Maternal titer, antibody transfer ratio and gestational age influence birth titer.
-
•
Incidence of seroconversion in infants in southern Vietnam is 4/100 infant years.
-
•
Children should be vaccinated after 5 months of age if a candidate is licensed.
Keywords: Shigella, Maternal antibody, Placental transfer, Seroconversion
Abstract
Background
Shigella sonnei is an emergent and major diarrheal pathogen for which there is currently no vaccine. We aimed to quantify duration of maternal antibody against S. sonnei and investigate transplacental IgG transfer in a birth cohort in southern Vietnam.
Methods and results
Over 500-paired maternal/infant plasma samples were evaluated for presence of anti-S. sonnei-O IgG and IgM. Longitudinal plasma samples allowed for the estimation of the median half-life of maternal anti-S. sonnei-O IgG, which was 43 days (95% confidence interval: 41–45 days). Additionally, half of infants lacked a detectable titer by 19 weeks of age. Lower cord titers were associated with greater increases in S. sonnei IgG over the first year of life, and the incidence of S. sonnei seroconversion was estimated to be 4/100 infant years. Maternal IgG titer, the ratio of antibody transfer, the season of birth and gestational age were significantly associated with cord titer.
Conclusions
Maternal anti-S. sonnei-O IgG is efficiently transferred across the placenta and anti-S. sonnei-O maternal IgG declines rapidly after birth and is undetectable after 5 months in the majority of children. Preterm neonates and children born to mothers with low IgG titers have lower cord titers and therefore may be at greater risk of seroconversion in infancy.
1. Introduction
The bacterial genus Shigella is a major contributor to the global burden of diarrheal disease. This genus of enteric pathogens is typically associated with disease in children under 5 years of age in industrializing regions [1], and is estimated to be responsible for 100,000 deaths annually [2]. Shigella infections are characteristically associated with dysentery (blood and mucus in the stool) and can be severe in young children [3], [4]. Of the four Shigella species, Shigella flexneri and Shigella sonnei predominate worldwide [1]. S. flexneri is traditionally associated with disease in industrializing countries, whereas S. sonnei is more commonly isolated in industrialized regions. However, this distribution is changing. S. sonnei is globally emergent and replacing S. flexneri as the most common cause of bacterial dysentery [5], [6]. This trend may be being exacerbated by resistance to common antimicrobials, with several recent reports of S. sonnei exhibiting resistance against fluoroquinolones and 3rd generation cephalosporins in the USA, Vietnam and elsewhere [7], [8], [9]. Improved sanitation and antimicrobial treatment remain the only current tools for prevention and control as there are no licensed Shigella vaccines [10].
Neonates and infants are typically at increased risk from infectious agents such as Shigella due to immaturity of the immune system [11]. While neonates have some capacity for cell-mediated immunity [12], humoral immunity is very limited in early life [13]. Antibody responses in neonates are shorter, delayed in onset and of lower affinity than those observed in healthy adults [14]. The transfer of maternal IgG antibody to the fetus during pregnancy confers short-term passive immunity and represents a primary mechanism for protection against infectious diseases at birth [11]. Transport of maternal antibody across the placenta to fetal capillaries is mediated by the neonatal Fc receptor (FcRn) [15], [16], [17] and can be affected by factors such as gestational age, maternal IgG concentration and infection [18], [19], [20], [21].
Maternally transferred IgG against S. sonnei in infancy has not been substantially investigated. Work conducted in Israel in the mid-1990s found that the concentration of anti-S. sonnei lipopolysaccharide (LPS) IgG present in umbilical cord plasma positively correlated with the concentration in maternal plasma [22]. IgG against LPS, specifically the O-antigen component, is the best described S. sonnei immune marker as it is the major bacterial surface antigen exposed to the immune system during infection. Although anti-S. sonnei-O IgG is not a definitive correlate of protective immunity [23], it is an indicator of some degree of acquired immunity; lack of Shigella serotype specific antibody is associated with an increased risk of symptomatic disease [24], [25]. Furthermore, titers of anti-S. sonnei-O IgG rise significantly after symptomatic infection [22], [26], [27], with titers doubling 10 weeks post-infection [26], [28]. Previous work from Vietnam in the late 1980s showed that anti-S. sonnei-LPS and anti-S. flexneri-LPS IgG rise dramatically from birth, peak at 3–4 years of age and then permanently plateau [29].
An understanding of the nature and duration of maternal antibody protection in infancy is important for determination of an appropriate vaccination schedule when Shigella vaccines eventually become available. Additionally, although IgG titers against S. flexneri and Shigella dysenteriae type I in Vietnam were found to be high in children and adults in the early 1990s [27], [29], exposure to Shigella has not been measured in a contemporary Vietnamese population. As S. sonnei is now the predominant Shigella species in Vietnam [30], we hypothesized there would be substantial evidence of population exposure and S. sonnei maternal antibody transfer in this rapidly industrializing country. Therefore, we aimed to quantify maternal anti-S. sonnei-O antibody decay using the largest sample size to date, with over 500 paired mother and infant plasma samples. We also investigated transplacental IgG transfer and determined the incidence of S. sonnei seroconversion in infancy in southern, urban Vietnam.
2. Methods
2.1. Ethical approval
Written informed consent was required from all enrolled families. Ethical approval was granted from Hung Vuong Hospital, Oxford Tropical Research Committee as well as the London School of Hygiene & Tropical Medicine for the main cohort study. Ethical approval was also granted from the Hospital for Tropical Diseases in HCMC and OxTREC for the studies collecting acute and convalescent plasma samples from culture-positive Shigella and Salmonella cases for ELISA validation.
2.2. Study population
The birth cohort population and methodology has been described previously in detail [31]. Briefly, mothers delivering at Hung Vuong obstetric hospital in Ho Chi Minh City (HCMC) were invited to enroll during either an antenatal visit in the final month of pregnancy or at the time of hospital admission for delivery. Children born between January and December 2013 in HCMC were included in the analysis presented here. Pregnant women were eligible if they lived in district 8 of HCMC (a previously identified endemic hotspot for Shigella [30]), were aged 16 years or older and were HIV seronegative at the time of birth. Mothers answered a baseline questionnaire and blood (umbilical cord and venous) samples were collected in EDTA tubes. After delivery, infants were recalled regularly for routine follow up visits. A 1 ml EDTA blood sample was collected at the 4, 9 and 12 month visits. All blood samples were separated into cells and plasma and stored at −20 °C until required.
2.3. S. sonnei anti-O antigen ELISA
Antibody (IgG and IgM) against S. sonnei O-antigen were measured using an enzyme-linked immunosorbent assay (ELISA) in maternal, umbilical and longitudinally collected infant plasma samples. Purified S. sonnei O-antigen was extracted as previously described [32]. For the ELISA assays, 96-well microtiter plates (Maxisorb; NUNC) were coated overnight with 0.5 g/ml S. sonnei O-antigen in PBS pH 7.0 at 4 °C, plates were then washed and blocked in PBS containing 5% skimmed milk powder for 2 h. After washing, 100 μl of each plasma sample (diluted at 1:200 in PBS containing 1% skimmed milk) were added and plates were incubated for 2 h at room temperature. IgG and IgM against S. sonnei O-antigen were detected by incubation with alkaline phosphatase directly conjugated anti-human IgG/IgM for 1 h. Plates were developed by p-nitrophenyl-phosphate solution (Sigma) and were read at absorbance 405 nm and 490 nm by an ELISA platereader (Microplate reader, Biorad). Each plate contained a 2-fold serially diluted pool of anti-S. sonnei-O antigen human plasma (primary concentration 1:200). A standard curve was generated from the corresponding optical density (OD) and ELISA units using a 4-parameter logistic regression fit. One ELISA unit (EU) was defined as the reciprocal dilution of the standard plasma that gave an absorbance value equal to 1 in this assay. The ELISAs were done in duplicate. Antibody (IgG and IgM) units in the cohort members’ plasma were calculated relative to this standard each time the assay was performed. Acute and convalescent plasma samples for the ELISA validation were derived from pediatric culture-positive S. sonnei and Salmonella dysentery cases presenting to either the Hospital for Tropical Disease in HCMC as part of another ongoing study.
2.4. Statistical analyses
Geometric mean titers (GMT) were calculated to summarize anti-S. sonnei-O IgG in maternal and cord plasma. Paired t-tests were used to compare log10 titers between paired maternal/cord samples. Analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons was used to compare maternal and cord log-transformed antibody titers within categorical groups. The ratio of maternal transfer was compared across groups using the Kruskal–Wallis (KW) test with Dunn's test for multiple comparisons [33]. Linear mixed effects modeling was used to assess the trajectory of infant log10 titers from birth to 20 weeks to account for within-participant association over time. The half-life of IgG titer was calculated as the time at which the predicted IgG titer would decrease by 50% from the cord blood titer. The population half-life was derived using the formula:
with b1 equal to the slope of the fixed effect. The 95% confidence interval (CI) for the population level half-life was derived from the CI of the slope of the fixed effect. Children with a 4-fold rise between serial titers or those who were aged <6 months without a decrease in IgG titer were censored after the time point prior to the increase or no decrease, respectively [34].
A Kaplan Meier survival curve was generated to investigate the time taken for titers to fall below a detectable threshold of 10.3 EU. This threshold value was determined by calculating the mean titer value of the observation preceding a 4-fold rise in titer in infants that had a 4-fold rise with a gap between pre and post-seroconversion samples no greater than 24 weeks (n = 15). None of the cord plasma titers and less than 1% (2/502) of the maternal plasma samples had titers that fell below 10.3 EU. For the Kaplan Meier estimation, infants were censored either when they (1) dropped below 10.3 EU (2) had any rise in IgG titer or (3) were lost to follow up. Finally, linear regression was used to evaluate the effect of covariates on anti-S. sonnei-O IgG cord titer as well as the relationship between log10 cord titer and log10 increase in titer between serial follow up visits. All analyses were performed in STATA v13 (TX, USA) with the exception of the mixed effects modeling which was performed in R (version 3.0.2) using the lme4 package [35]. Plots were made in R using the ggplot package v1.0.1 [36].
3. Results
3.1. ELISA validation
We firstly validated the anti-S. sonnei-O ELISA in a population of Vietnamese children hospitalized with dysentery with acute and convalescent plasma samples. All tested (7/7; 100%) stool culture-positive S. sonnei cases presenting to hospital had >4 fold rise (median: 104-fold; range: 22–410) in IgG titer regardless of the number of days between the acute and convalescent samples (median: 116 days; range: 13–202). The IgM titers against S. sonnei O-antigen of the seven responding children also increased dramatically (median: 9-fold, range:3–64). Twenty culture positive Salmonella cases from the same study did not generate an S. sonnei O-antigen IgG response (median fold titer increase: 1.1, range: 0–2.0), with limited IgM response as well (median: 1.4-fold, range: 0–58) (data not shown).
3.2. Cohort baseline characteristics
Of the 503 infants enrolled into the birth cohort in 2013, 52% (260/503) were male, 4% (21/503) were born preterm (<37 weeks of gestation) and 5% (23/503) were of low birth weight (<2.5 kg) as shown in Table 1. The median maternal age was 28 years (interquartile range (IQR): 25–31), with just under half of all mothers (244/503; 49%) reporting at least a higher secondary education. The median maternal gravidity was 2 (IQR: 1–3) and the mean duration of infant follow up was 337 days (range: 1–399 days). A total of 58% (292/503) infants enrolled returned for all three follow up appointments where a blood sample was collected (Fig. 1A). A further 78% (393/503) returned for at least two blood-draw appointments, and 86% (432/503) for at least one follow up blood-draw appointment. There were no major demographic or socioeconomic differences between the families of infants who did not return for all four follow up visits (211/503; 42%) and those that did return for all four visits (292/503; 58%) (Table 2).
Table 1.
Baseline characteristics of 503 Vietnamese infants enrolled in the birth cohort in 2013.
| Characteristic | n (%), Median (IQR) |
|---|---|
| Male sex | 260 (51.7) |
| Gestational age (weeks) | 39 (38–40) |
| Preterm (<37 weeks) | 21 (4.2) |
| Birth weight (kg) | 3.15 (2.9–3.4) |
| Low birth weight (<2.5 kg) | 23 (4.6) |
| Vaginal delivery | 288 (57.3) |
| Breastfed during month 1 | |
| Exclusively | 215 (43.0) |
| Plus formula | 243 (48.6) |
| No, only formula | 42 (8.4) |
| Gravidity | 2 (1–3) |
| Maternal education | |
| Lower secondary or below | 255 (50.7) |
| Higher secondary or above | 248 (49.3) |
| Maternal age (years) | 28 (25–31) |
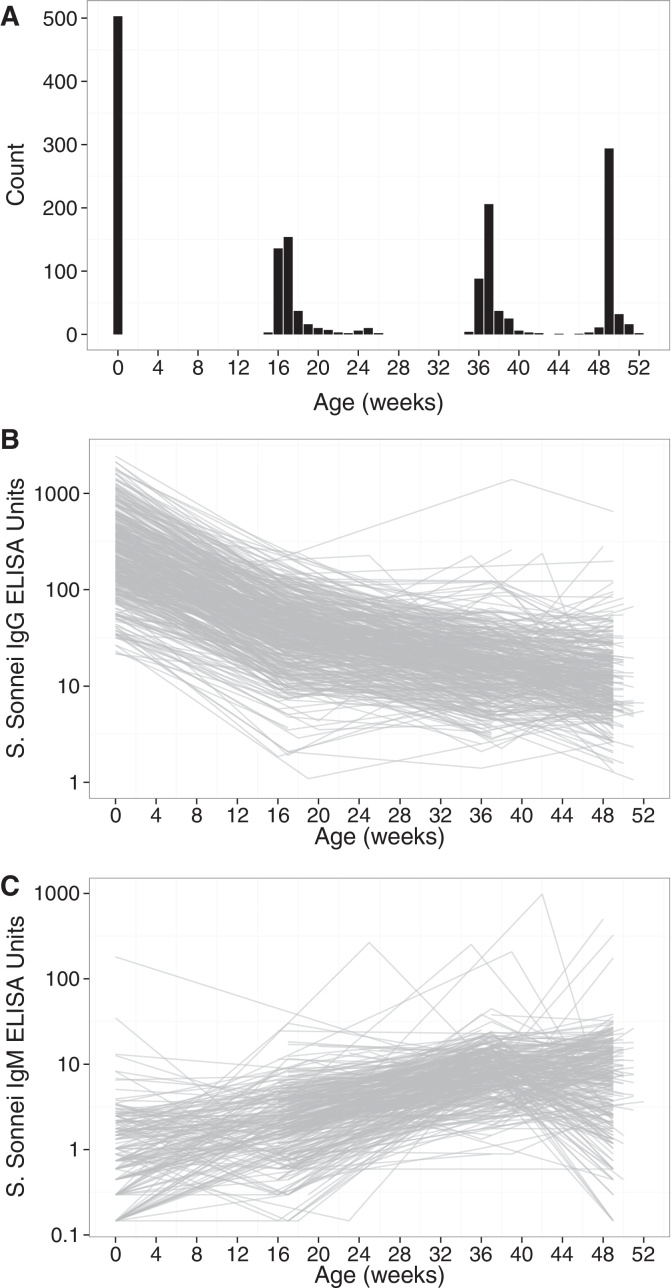
Fig. 1.
Anti-S. sonnei-O antibody levels in the first year of life in a cohort of 503 Vietnamese children. (A) Count of the number of assayed infant plasma samples at different ages in the first year after birth. Anti-S. sonnei-O IgG (B) and IgM (C) titers shown over time for each individual in the cohort on a log10 scale.
Table 2.
Demographic and socioeconomic characteristics of infants with plasma samples available from four follow up visits (0, 4, 9 and 12 months of age) and those who attended less than four visits, n (%).
| Characteristic | <4 visits | 4 visits | pa |
|---|---|---|---|
| n = 211 | n = 292 | ||
| Vaginal birth | 111 (52.6) | 177 (60.6) | 0.073 |
| Male infant | 113 (53.6) | 147 (50.3) | 0.477 |
| Infant low birthweight | 9 (4.3) | 14 (4.8) | 0.779 |
| Any previous children | 128 (60.7) | 185 (63.4) | 0.539 |
| Maternal age ≥28 years | 109 (51.7) | 150 (51.4) | 0.949 |
| Low maternal education | 106 (50.2) | 142 (48.6) | 0.722 |
| Household crowding | 116 (55) | 185 (63.4) | 0.059 |
| Infant cord log10 titer > 2.3b | 122 (57.8) | 151 (51.7) | 0.175 |
| Preterm (<37 weeks) | 9 (4.3) | 12 (4.1) | 1.00 |
| Breastfed during month 1 | |||
| Exclusively | 89 (42.6) | 126 (43.3) | 0.726 |
| Plus formula | 100 (47.8) | 143 (49.1) | |
| Formula + food | 20 (9.6) | 22 (7.6) | |
| Mother ethnic minority | 21 (10) | 21 (7.2) | 0.269 |
| Father ethnic minority | 26 (12.3) | 24 (8.2) | 0.129 |
| Watersource | |||
| Piped home | 144 (68.2) | 208 (71.2) | 0.73 |
| Bottled | 63 (29.9) | 80 (27.4) | |
| Other | 4 (1.9) | 4 (1.4) | |
p-value derived from chi-square or Fisher's exact test.
Median.
3.3. The decay of maternal anti-S. sonnei-O IgG and incidence of seroconversion
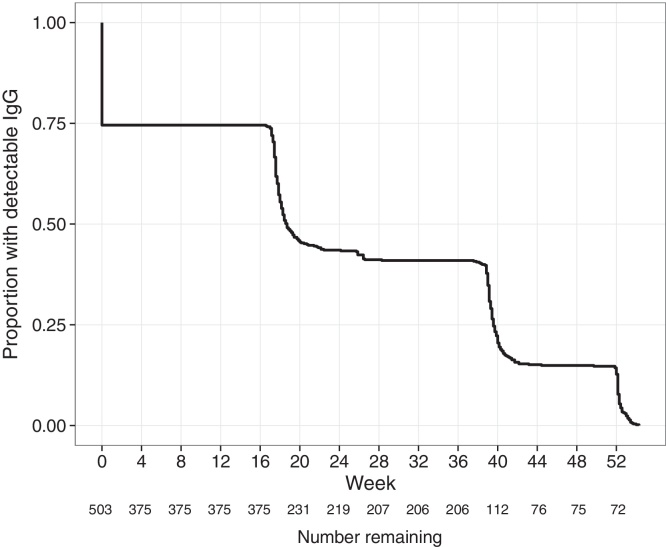
The anti-S. sonnei-O IgG and IgM titers in infants over the first 12 months of life are shown in Figs. 1B and C, respectively. Using samples collected within 20 weeks of birth, we estimated the median half-life of anti-S. sonnei-O IgG to be 43.2 days (95%CI: 41.9–44.5 days). As shown in Fig. 2, by 18.7 weeks (95%CI: 18.1–20.1 weeks) 50% of infants had undetectable levels of anti-S. sonnei-O IgG. A total of 16 children had a >4-fold rise in anti-S. sonnei-O IgG titer in the first year after birth (3.2%), the majority of which occurred between 4 and 9 months (8/16, 50%), or 9 and 12 months (6/16, 38%) after birth (Fig. 2). Critically, a higher fold-rise in anti-S. sonnei-O IgG over the first 12 months of life was associated with a lower cord titer (p < 0.001; linear regression). There were 463.5 infant years of follow up in this cohort, leading to a seroconversion rate (defined by >4-fold rise in titer) of 3.5/100 years of follow up in the first 12 months of life. Two children did not have a detectable decrease in IgG titer between birth and 20 weeks of life (0.4%). Furthermore, 49/503 infants (10%) had a 2-fold rise in anti-S. sonnei-O IgG over the course of the first 12 months of life, the majority of which (25/49, 51%) occurred between 9 and 12 months of age. Out of the 503 infants enrolled in the cohort, 162 (32%) had a rise (any) in titer over the first year of life, which were more commonly detected between 9 and 12 months of age (84/162; 52%).
Fig. 2.
Kaplan Meier curve showing the proportion of infants with detectable anti-S. sonnei-O IgG in the first year after birth. The proportion of infants with detectable anti-S. sonnei-O IgG censored by (1) when their titer dropped below 10.3 EU (see methods), (2) had any detectable increase in IgG titer or, (3) lost to follow up. The number of infants with detectable antibody at each time point are shown below the x-axis.
3.4. Maternal antibody transfer
The geometric mean titers of anti-S. sonnei-O IgG in cord plasma and maternal plasma were 234.1 EU (range: 21.6–3687.6 EU) and 167.4 EU (range: 3.75–2553 EU), respectively (Table 2). The median ratio of cord:maternal plasma anti-S. sonnei-O IgG was 1.32 (range: 0.3–12.4) (Table 2). Anti-S. sonnei-O IgG titers in cord plasma were consistently and significantly higher than those in maternal plasma (Table 2), with the exception of babies born preterm (p = 0.71, paired t-test of log10 titers). The ratio of maternal transfer in preterm babies (median: 1.13) was significantly lower than in babies born 37–40 weeks (median: 1.35) (p = 0.02; KW). Furthermore, the transplacental transfer ratio in first-pregnancy mothers (median: 1.38) was moderately higher than in mothers that had had previous pregnancies (median: 1.30, p = 0.066; KW). The maternal transfer ratio was also slightly greater in younger mothers (<28 years) than in mothers ≥28 years of age (median: 1.36 versus 1.29, respectively; p = 0.065; KW). Finally, the transplacental transfer ratio was significantly higher in infants born in January–March (median: 1.70) and in April–June (median: 1.90) compared to those born in July–September (median: 1.14) and October–December (median: 1.12) (Table 3).
Table 3.
Geometric mean titers (GMT) of anti-S. sonnei-O IgG in maternal and cord plasma and the ratio of cord:maternal IgG titer.
| Category | n Pairs | Maternal IgG | Cord IgG | Median ratio | Comparisona |
|
|---|---|---|---|---|---|---|
| GMT (range) | GMT (range) | (range) | p value | Group | ||
| Total | 503 | 167.4 (3.75–2553.7) | 230.8 (0.22–3687.6) | 1.33 (0–12.4) | ||
| Gestational age | ||||||
| <37 weeks (1) | 21 | 190.8 (48.5–545.8) | 197.4 (49.5–546.8) | 1.13 (0.4–2.6) | 0.019 | 1:2 |
| 37–40 weeks (2) | 549 | 166.5 (3.7–2553.7) | 232.1 (0.22–3687.6) | 1.35 (0–12.4) | 0.130 | 1:3 |
| >40 weeks (3) | 23 | 165.7 (22.3–1175.0) | 237.7 (68.8–1163.3) | 1.33 (0.6–6.2) | 1.000 | 2:3 |
| Sexb | ||||||
| Female | 243 | 149.2 (8.2–2553.7) | 209.9 (0.22–2140.9) | 1.37 (0–12.4) | 0.177 | |
| Male | 260 | 186.4 (3.7–2524.2) | 252.1 (23.1–3687.6) | 1.28 (0.3–11.2) | ||
| Birthweight | ||||||
| <2500 g | 23 | 150.8 (26.1–1280.1) | 195.9 (42.4–2140.9) | 1.25 (0.5–3.8) | 0.515 | |
| ≥2500 g | 480 | 168.3 (3.7–2553.7) | 232.6 (0.22–3687.8) | 1.33 (0–12.4) | ||
| Gravidity | ||||||
| 1 | 190 | 150.5 (3.7–2553.7) | 216.7 (0.2–2824.8) | 1.39 (0–12.4) | 0.066 | |
| >1 | 313 | 178.6 (15.7–2524.2) | 239.7 (23.1–3687.6) | 1.3 (0.3–11.2) | ||
| Maternal ageb, c | ||||||
| <28 years | 244 | 138.7 (3.7–1530.9) | 204.5 (1.1–2824.8) | 1.36 (0.3–12.4) | 0.065 | |
| ≥28 years | 259 | 199.9 (20.6–2553.7) | 258.6 (0.22–3687.6) | 1.29 (0–8.3) | ||
| Maternal education | ||||||
| Lower secondary or below | 255 | 179.9 (3.7–2553.7) | 250 (26.7–3687.6) | 1.29 (0.3–12.4) | 0.449 | |
| Higher secondary or above | 248 | 155.5 (15.7–2524.2) | 212.5 (0.22–3206.5) | 1.35 (0–11.2) | ||
| Maternal IgMc | ||||||
| ≤1.37 | 253 | 158 (3.7–2553.7) | 268.4 (22.1–2824.8) | 1.66 (0.37–12.4) | <0.001 | |
| >1.37 | 250 | 177.5 (15.7–2524.2) | 203.8 (21.6–3687.6) | 1.16 (0.3–11.2) | ||
| Seasonc | ||||||
| January–March (1) | 89 | 148.2 (3.7–2553.7) | 244.4 (34.1–2824.8) | 1.7 (0.6–11.8) | 0.024 | 1:2 |
| April–June (2) | 133 | 174.3 (8.2–1596.0) | 358.3 (65.3–3687.6) | 1.9 (0.4–12.4) | <0.001 | 1:3 |
| July–September (3) | 161 | 162.6 (15.7–2524.2) | 181.8 (22.1–3206.5) | 1.14 (0.3–11.2) | <0.001 | 1:4 |
| October–December (4) | 120 | 182.3 (33.5–1969.7) | 187 (0.22–2123.2) | 1.12 (0–5.8) | <0.001 | 2:3 |
| <0.001 | 2:4 | |||||
| 1.000 | 3:4 | |||||
p-values comparing ratio of transfer between categories of each characteristic, p-values corrected for multiple comparisons are shown with groups indicated in parentheses next to the group name.
Significant difference in log10 titers of maternal plasma per category.
Significant difference between log10 titers of cord samples per category; titers are shown in ELISA Units (EU).
3.5. Factors influencing anti-S. sonnei-O cord blood antibody titers
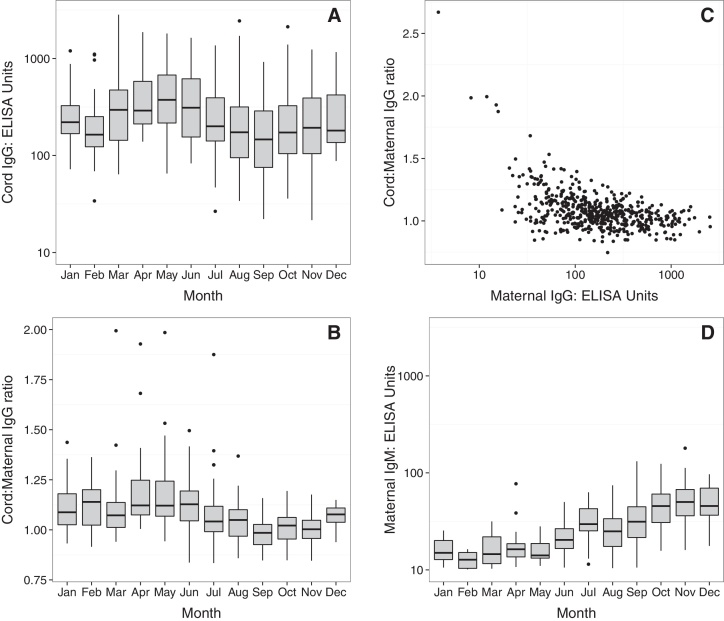
Anti-S. sonnei-O cord IgG titer was associated with several covariates in a univariate analysis (Table 3). However, after controlling for the effects of confounding in an adjusted analysis the only covariates that remained significantly associated with anti-S. sonnei-O cord IgG titer were ratio of transplacental IgG transer, maternal IgG titer and the season of birth. As shown in Fig. 3A, anti-S. sonnei-O cord IgG titers in babies born in the first half of the year were higher than those born in the second half of the year. The ratio of anti-S. sonnei-O IgG maternal transfer was also elevated in the first half of the year compared to the later months (Fig. 3B). Significantly, the ratio of transplacental transfer was higher in mothers with low IgG at the time of birth (Fig. 3C) (p < 0.001; linear regression of log10 maternal titers) (Table 4).
Fig. 3.
Temporal anti-S. sonnei-O antibody cord titers and transplacental transfer dynamics. (A) Anti-S. sonnei-O IgG cord plasma titers shown by month of birth on a log10 scale. (B) The ratio of cord:matneral anti-S. sonnei-O IgG titer by month of birth. (C) Scatterplot showing the relationship between maternal anti-S. sonnei-O IgG titers and the ratio of cord:maternal plasma transfer. (D) Maternal anti-S. sonnei-O IgM titers shown by month of birth on a log10 scale.
Table 4.
Univariate and multiple linear regression measuring the effect of different covariates on the outcome of log10 cord anti-S. sonnei-O IgG titer.
| Characteristic | Cord blood IgG titer |
|||
|---|---|---|---|---|
| Univariate |
Adjusted |
|||
| Beta | p | Beta | p | |
| Cord:maternal IgG ratio | 0.595 | 1.36 | <0.001 | |
| Infant | ||||
| Male sex | 0.07 | 0.067 | 0.00 | 0.850 |
| Gestational age | 0.04 | 0.006 | 0.01 | 0.549 |
| Birthweight | 0.08 | 0.102 | −0.01 | 0.504 |
| Maternal | ||||
| Age | 0.01 | 0.001 | 0.00 | 0.246 |
| Low education | −0.06 | 0.123 | 0.01 | 0.410 |
| Gravidity | 0.03 | 0.088 | 0.00 | 0.921 |
| Log10 IgM | −0.14 | 0.006 | −0.01 | 0.798 |
| Log10 IgG | 0.76 | <0.001 | 1.01 | <0.001 |
| Season | ||||
| January–March | 0.13 | 0.013 | 0.02 | 0.170 |
| April–June | 0.29 | <0.001 | 0.08 | <0.001 |
| July–September | 1.00 | – | 1.00 | – |
| October–December | 0.04 | 0.417 | 0.01 | 0.637 |
Beta values represent the slope of the linear association and p-values demonstrate whether the slope is significantly different from the null hypothesis of 0.
On additional analysis we found that maternal IgM increased (Fig. 3D) from May to July, plateauing in the later months of the year, suggesting that some mothers were likely exposed to S. sonnei at the time of birth during April-June. Maternal IgG levels did not significantly change throughout the year, although the titers were generally high, suggesting previous and potentially sustained exposure. The combination of low existing maternal IgG in some mothers during April–June and the increased the ratio of transplacental transfer during this period lead to an overall elevated anti-S. sonnei-O IgG in babies born during this period, which may be during a period of increased seasonal S. sonnei transmission in HCMC.
4. Discussion
S. sonnei is an emergent and increasingly antimicrobial resistant diarrheal pathogen. As such S. sonnei is a growing challenge in Vietnam and other similarly industrializing countries [5], [6], [37], [38], [39], [40], [41], [42]. The aims of this study were: (1) to quantify the duration of maternal IgG in infants, (2) to measure incidence of S. sonnei seroconversion in the first year of life and (3) to examine transplacental IgG transfer during pregnancy. As S. sonnei vaccines are in development [23], understanding the potential of maternal immunity in infants will be critical for evaluating future vaccine efficacy and identifying the infant groups that are most at risk of S. sonnei seroconversion [4].
The estimated half-life of maternal anti-S. sonnei-O IgG (43 days, 95%CI: 42–45 days) is similar to that of Haemophilus influenzae (33 days), pertussis (36–40 days) and S. pneuomoniae (35 days) [43], [44], [45]. However, as the sampling was infrequent in the early weeks after birth these data should be interpreted with caution. Nevertheless, it is apparent that maternal antibody wanes rapidly and by 5 months of age the majority of infants had no circulating maternal antibody and are likely at increased risk of infection. Correspondingly, evidence of S. sonnei exposure in infants in our cohort suggests an incidence of seroconversion of approximately 4/100 infant years of follow up in HCMC. Yet given the known lack of general humoral immune responses against polysaccharides during infancy [13], in addition to loss to follow up, this seroconversion incidence is likely an underestimate.
We found that lower cord titers were associated with higher fold-increases in anti-S. sonnei-O IgG titer in the first year of life in our cohort, suggesting that neonates born with lower cord titers are at increased risk of seroconversion during infancy. The most important influences on anti-S. sonnei-O cord titer were maternal IgG titer and the ratio of transplacental transfer, which were inversely correlated. Such a relationship is due in part to saturation of the Fc receptor, as IgG that is not bound is digested by lysosomal enzymes inside the syncytiotrophoblast [11], [46]. The negative relationship between maternal IgG concentration and transplacental transfer ratio has been suggested to demonstrate the existence of a mechanism to protect the newborn through strengthening the transfer of antibody when maternal levels are not optimally protective [47], [48]. Furthermore, it has been found that a higher total maternal IgG concentration may lead to reduced transfer efficiency of both total and specific IgG [21], with some suggestion of receptor competition among antigen-specific IgG for the limited number of placental Fc receptors available [20].
Neonates tended to have elevated anti-S. sonnei-O IgG titers compared to mothers in our cohort. Such a phenomenon has been reported for a variety of pathogens including Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa [18], [47], [49]. However, neonates born preterm in this cohort did not have an increased anti-S. sonnei-O titer relative to their mothers. As the majority of IgG is acquired by the fetus during the last 4 weeks of pregnancy [50], it follows that preterm neonates would lack maternal immunity and are potentially at increased risk for infections in the first few months of life. Furthermore, we found that children born to mothers with lower IgG titers had lower cord titers themselves and are at increased risk of exposure.
Interestingly, we noted a seasonal pattern to both cord plasma titers as well as the ratio of transplacental transfer in our cohort. Cord titers and the transplacental transfer ratio were higher in the second quarter of the year. Given the inverse relationship between maternal IgG titer and transfer ratio, we propose that this period may represent a time of increased transmission and, therefore, exposure to S. sonnei in HCMC. This hypothesis was supported by the observed increase in maternal IgM titer between May and July (representing acute infection), suggesting that mothers’ existing immune response may be naturally boosted during this time. If S. sonnei transmission in HCMC is more common between April and June then infants born in during this time are likely better equipped against S. sonnei exposure at birth as the cord titers are highest during this season. However, annual trends are difficult to evaluate from our yearlong dataset.
There were several limitations with this study. Firstly, the infrequent early blood samples from infants prevented high-resolution temporal analyses regarding maternal half-life duration and survival analysis of the waning of maternal IgG. Next, the lack of a similar cohort from a non-endemic area limits our ability to fully interpret the serology data in an epidemiological context. Furthermore, a lack of disease data prohibits an analysis of the protective effect of presence of antibody as well as a more detailed analysis of anti-S. sonnei-O IgG and IgM response in infants after infection. However, the major strength of this study is the cohort design and relatively limited loss to follow up which enables us to generalize our conclusions to Vietnamese infants in urban HCMC. In the future, investigations into additional protective factors against Shigella, such as breastfeeding, may be warranted [51].
In summary, S. sonnei exposure is common in HCMC and maternal IgG is readily transferred across the placenta, waning by 5 months of age in the majority of infants. In the event of licensure of a sufficiently safe and immunogenic S. sonnei vaccine, it would be prudent to vaccinate after the waning of maternal IgG in settings such as HCMC. Furthermore, we found that neonates have a higher concentration of IgG compared to mothers in most cases, and the ratio of transplacental transfer is inversely related to the maternal anti-S. sonnei-O IgG titer. Finally, we identified those likely to be more at risk of S. sonnei exposure in infancy to include preterm neonates and those born to mothers with lower IgG titers. Therefore, appropriate monitoring and prevention strategies can be targeted to such groups.
Authors’ contributions
CNT, KLA, LTQN, CS and SB designed and set up the cohort study. LTPT performed the ELISAs. CT carried out the analysis. NTH, LLV, NVVC, VTD, NNMC, TTHC, HHT, TVTN, PVM and TDHN were involved in the laboratory and clinical management of the cohort study. AS, LBM, AP and CG provided S. sonnei antigen and protocols for the ELISA. CT, GT and SB wrote the manuscript.
Acknowledgements
We would like to thank the Hung Vuong Hospital study nurses whose tireless efforts have made this study possible (Nguyen Thi En, Le Thi Hanh, Hoang Thi Sen, Nguyen Thi Hong Nhat and Nguyen Thi Tuyet Hanh). Thanks also go to Dr. Phung Khanh Lam and Dr. Marcel Wolbers for statistical advice. Finally, we thank the families who participated in the study. The Wellcome Trust of Great Britain funded this work. SB is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (100087/Z/12/Z). The funders had no role in the study design, data collection or interpretation or the decision to submit the work for publication.
Conflict of interest: AS, LBM and AP are employed. All other authors declare no conflict of interest.
References
- 1.Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case–control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennish M.L., Harris J.R., Wojtyniak B.J., Struelens M. Death in shigellosis: incidence and risk factors in hospitalized patients. J Infect Dis. 1990;161:500–506. doi: 10.1093/infdis/161.3.500. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi S. Shigella infections in children: new insights. Semin Pediatr Infect Dis. 2004;15:246–252. doi: 10.1053/j.spid.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Thompson C.N., Thanh D.P., Baker S. The rising dominance of Shigella sonnei: an intercontinental shift in the etiology of bacillary dysentery. PLoS Negl Trop Dis. 2015;9:e0003708. doi: 10.1371/journal.pntd.0003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt K.E., Baker S., Weill F.-X., Holmes E.C., Kitchen A., Yu J. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat Genet. 2012;44:1056–1059. doi: 10.1038/ng.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen A., Hurd J., Hoover C., Khachadourian Y., Traphagen E., Harvey E. Importation and domestic transmission of Shigella sonnei resistant to ciprofloxacin – United States, May 2014–February 2015. Morb Mortal Wkly Rep. 2015;64:318–320. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J.S., Kim J.J., Kim S.J., Jeon S., Seo K.Y., Choi J. Shigella sonnei associated with travel to Vietnam, Republic of Korea. Emerg Infect Dis. 2015;21:1247–1250. doi: 10.3201/eid2107.150363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lappe N., Connor J.O., Garvey P., Mckeown P., Cormican M. Ciprofloxacin-resistant Shigella sonnei associated with travel to India. Emerg Infect Dis. 2015;21:894–895. doi: 10.3201/eid2105.141184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine M.M., Kotloff K.L., Barry E.M., Pasetti M.F., Sztein M.B. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmeira P., Quinello C., Silveira-Lessa A.L., Zago C.A., Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall-Clarke S., Reen D., Tasker L., Hassan J. Neonatal immunity: how well has it grown up? Immunol Today. 2000;21:35–41. doi: 10.1016/s0167-5699(99)01548-0. [DOI] [PubMed] [Google Scholar]
- 13.PrabhuDas M., Adkins B., Gans H., King C., Levy O., Ramilo O. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 14.Siegrist C.-A., Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 15.Roopenian D.C., Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 16.Leach J.L., Sedmak D.D., Osborne J.M., Rahill B., Lairmore M.D., Anderson C. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal–fetal antibody transport. J Immunol. 1996;157:3317–3322. [PubMed] [Google Scholar]
- 17.Simister N.E., Story C.M., Chen H.L., Hunt J.S. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–1531. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 18.De Moraes-Pinto M.I., Almeida A.C., Kenj G., Filgueiras T.E., Tobias W., Santos A. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173:1077–1084. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 19.Okoko B.J., Wesumperuma L.H., Ota M.O., Pinder M., Banya W., Gomez S.F. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J Infect Dis. 2001;184:627–632. doi: 10.1086/322808. [DOI] [PubMed] [Google Scholar]
- 20.Englund J. The influence of maternal immunization on infant immune responses. J Comp Pathol. 2007;137:16–19. doi: 10.1016/j.jcpa.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Hartter H.K., Oyedele O.I., Dietz K., Kreis S., Hoffman J.P., Muller C.P. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J. 2000;19:635–641. doi: 10.1097/00006454-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Passwell J.H., Freier S., Shor R., Farzam N., Block C., Lison M. Shigella lipopolysaccharide antibodies in pediatric populations. Pediatr Infect Dis J. 1995;14:859–865. doi: 10.1097/00006454-199510000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Camacho A.I., Irache J.M., Gamazo C. Recent progress towards development of a Shigella vaccine. Expert Rev Vaccines. 2013;12:43–55. doi: 10.1586/erv.12.135. [DOI] [PubMed] [Google Scholar]
- 24.Cohen D., Green M.S., Block C., Slepon R., Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen D., Green M.S., Block C., Rouach T., Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israli military population. J Infect Dis. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 26.Cohen D., Block C., Green M., Lowell G., Ofek I. Immunoglobulin M, A, and G antibody response to lipopolysaccharide O antigen in symptomatic and asymptomatic Shigella infections. J Clin Microbiol. 1989;27:162–167. doi: 10.1128/jcm.27.1.162-167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cam P., Pál T., Lindberg A. Immune response against lipopolysaccharide and invasion plasmid-coded antigens of Shigellae in Vietnamese and Swedish dysenteric patients. J Clin Microbiol. 1993;31:454–457. doi: 10.1128/jcm.31.2.454-457.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayani K., Guerrero M., Ruiz-Palacios G., Gomez H., Cleary T. Evidence for long-term memory of the mucosal immune system: milk secretory immunoglobulin A against Shigella lipopolysaccharides. J Clin Microbiol. 1991;29:2599–2603. doi: 10.1128/jcm.29.11.2599-2603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ekwall E., Cam P.D., Tracht D.D., Taubef A., Alf A. Shigella flexneri O-antigen-specific enzyme immunoassay class-specific antibody titres against lipopolysaccharide antigens in healthy Vietnamese and Swedish populations. Serodiagn Immunother. 1988;2:47–61. [Google Scholar]
- 30.Vinh H., Nhu N.T.K., Nga T.V.T., Duy P.T., Campbell J.I., Hoang N.V.M. A changing picture of shigellosis in southern Vietnam: shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC Infect Dis. 2009;9:204–216. doi: 10.1186/1471-2334-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anders K.L., Nguyen N.M., Van Thuy N.T., Hieu N.T., Nguyen H.L., Hong Tham N.T. A birth cohort study of viral infections in Vietnamese infants and children: study design, methods and characteristics of the cohort. BMC Public Health. 2013;13:937–946. doi: 10.1186/1471-2458-13-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caboni M., Pédron T., Rossi O., Goulding D., Pickard D., Citiulo F. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 2015;11:e1004749. doi: 10.1371/journal.ppat.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunn O. Multiple comparisons using rank sums. Technometrics. 1964;6:241–252. [Google Scholar]
- 34.Brandenburg A.H., Groen J., Steensel-Moll H.A., Claas E.C.J., Rothbarth P.H., Neijens H.J. Respiratory syncytial virus specific serum antibodies in infants under six months of age limited serological response upon infection. J Med Virol. 1997;52:97–104. doi: 10.1002/(sici)1096-9071(199705)52:1<97::aid-jmv16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 35.Bates D., Maechler M., Bolker B., Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4, R package version 1; pp. 1–7. [Google Scholar]
- 36.Wickham H. Springer; New York: 2009. ggplot2: Elegant graphics for data analysis. [Google Scholar]
- 37.Holt K., Thieu Nga T., Thanh D., Vinh H., Kim D., Vu Tra M. Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc Natl Acad Sci U S A. 2013;110:17522–17527. doi: 10.1073/pnas.1308632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu F., Bao C., Chen S., Cui E., Guo T., Wang H. Genotypes and antimicrobial profiles of Shigella sonnei isolates from diarrheal patients circulating in Beijing between 2002 and 2007. Diagn Microbiol Infect Dis. 2012;74:166–170. doi: 10.1016/j.diagmicrobio.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fullá N., Prado V., Durán C., Lagos R., Levine M.M. Surveillance for antimicrobial resistance profiles among Shigella species isolated from a semirural community in the northern administrative area of Santiago, Chile. Am J Trop Med Hyg. 2005;72:851–854. [PubMed] [Google Scholar]
- 40.Sousa MÂB, Mendes E.N., Collares G.B., Péret-Filho L.A., Penna F.J., Magalhães P.P. Shigella in Brazilian children with acute diarrhoea: prevalence, antimicrobial resistance and virulence genes. Mem Inst Oswaldo Cruz. 2013;108:30–35. doi: 10.1590/S0074-02762013000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tajbakhsh M., García Migura L., Rahbar M., Svendsen C.A., Mohammadzadeh M., Zali M.R. Antimicrobial-resistant Shigella infections from Iran: an overlooked problem? J Antimicrob Chemother. 2012;67:1128–1133. doi: 10.1093/jac/dks023. [DOI] [PubMed] [Google Scholar]
- 42.Koh X.P., Chiou C.S., Ajam N., Watanabe H., Ahmad N., Thong K.L. Characterization of Shigella sonnei in Malaysia, an increasingly prevalent etiologic agent of local shigellosis cases. BMC Infect Dis. 2012:12. doi: 10.1186/1471-2334-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulholland K., Suara R., Siber G., Roberton D., Jaffar S., N’Jie J. Maternal immunization with Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine in The Gambia. J Am Med Assoc. 1996;275:1182–1188. [PubMed] [Google Scholar]
- 44.Healy C.M., Munoz F.M., Rench M.A., Halasa N.B., Edwards K.M., Baker C.J. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. J Infect Dis. 2004;190:335–340. doi: 10.1086/421033. [DOI] [PubMed] [Google Scholar]
- 45.Shahid N.S., Steinhoff M.C., Hoque S.S., Begum T., Thompson C., Siber G.R. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252–1257. doi: 10.1016/s0140-6736(95)91861-2. [DOI] [PubMed] [Google Scholar]
- 46.Saji F., Koyama M., Matsuzaki N. Human placental Fc receptors. Placenta. 1994;15:453–466. doi: 10.1016/s0143-4004(05)80415-1. [DOI] [PubMed] [Google Scholar]
- 47.Silveira Lessa A.L., Krebs V.L.J., Brasil T.B., Pontes G.N., Carneiro-Sampaio M., Palmeira P. Preterm and term neonates transplacentally acquire IgG antibodies specific to LPS from Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2011;62:236–243. doi: 10.1111/j.1574-695X.2011.00807.x. [DOI] [PubMed] [Google Scholar]
- 48.Kohler P.F., Farr R.S. Elevation of cord over maternal IgG immunoglobulin: evidence for an active placental IgG transport. Nature. 1966;210:1070–1071. doi: 10.1038/2101070a0. [DOI] [PubMed] [Google Scholar]
- 49.Gonçalves G., Cutts F.T., Hills M., Rebelo-Andrade H., Trigo F.A., Barros H. Transplacental transfer of measles and total IgG. Epidemiol Infect. 1999;122:273–279. doi: 10.1017/s0950268899002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saji F., Samejima Y., Kamiura S., Koyama M. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reprod. 1999;4:81–89. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 51.Clemens J., Stanton B., Stoll B., Shahid N.S., Banu H., Chowdhury A.A. Breastfeeding as a determinant of severity in shigellosis. Am J Epidemiol. 1986;123:710–720. doi: 10.1093/oxfordjournals.aje.a114291. [DOI] [PubMed] [Google Scholar]