Abstract
In this issue of Blood, Zhou et al reported the high-resolution structure of the collagen-activated osteoclast-associated receptor (OSCAR) bound to a collagen model peptide. Together with binding studies, the results confirm a novel recognition mechanism for collagen by immunoglobulin-like motifs.1
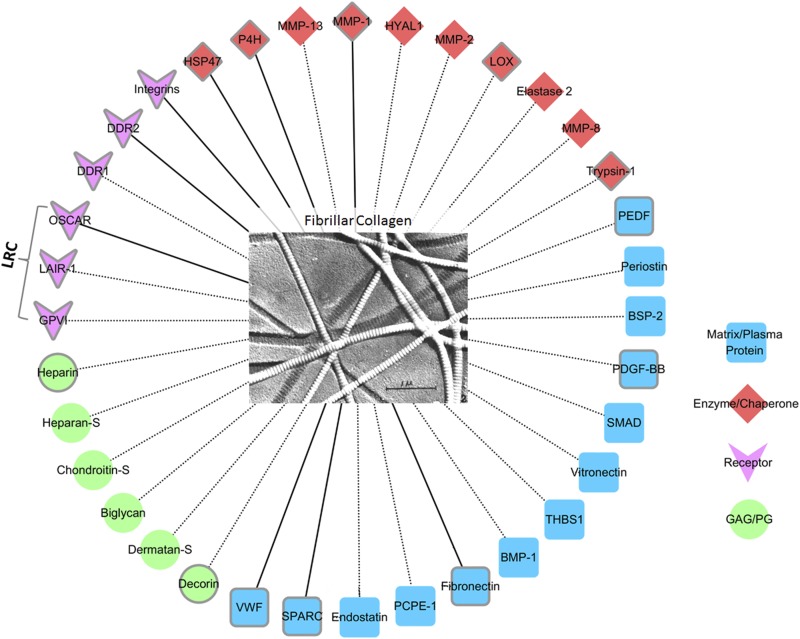
A schematic diagram of the interaction network of major fibrillar collagens showing the classes of molecules that interact with them, together with representative examples of each class (plotted with Cytoscape 3.2.1, based on multiple sources including MatrixDB [http://matrixdb.ibcp.fr/]). A gray outline around the molecule name indicates the crystal structure of the molecule has been determined. Molecules connected to collagen by solid lines indicate that the structures of cocrystals of that molecule (or part of that molecule) with a collagen-like peptide have been solved. BMP, bone morphogenetic protein; BSP, bone sialoprotein; DDR, discoidin domain receptor; GAG, glycoaminoglycan; GPVI, glycoprotein VI; HSP, heat shock protein; HYAL, hyaluronidase; LAIR-1, leukocyte-associated immunoglobulin-like receptor-1; LOX, lysyl oxidase; LRC, leukocyte receptor complex; MMP, matrix metalloproteinase; PCPE, procollagen C-proteinase enhancer; PDGF, platelet-derived grwoth factor; PEDF, pigment epithelium-derived factor; PG, proteoglycan; SPARC, secreted protein acidic and rich in cysteine; THBS, thrombospondins; VWF, von Willebrand factor. The central electron micrograph of collagen fibrils is from Gross and Schmitt.2
Collagen forms rodlike triple-helical molecules which assemble into cross-linked fibrils that provide mechanical strength to tissues. In addition to its structural properties, collagen plays important biological roles in the extracellular matrix (ECM). A schematic diagram of the collagen interactome (see figure2) illustrates the different categories of molecules which bind collagen, including its involvement with other ECM and plasma proteins, its interactions with enzymes during biosynthesis, and its binding to receptors which trigger cell signaling. Among proteins which interact with collagens are 3 members of the immunoglobulin superfamily of receptors: GPVI, LAIR-1, and OSCAR. The genes of these 3 receptors are found in the LRC, and all 3 have extracellular ligand-binding immunoglobulin-like domains which may come in contact with collagen following inflammation or tissue damage. GPVI, expressed at platelet surfaces, is involved in platelet adhesion to subendothelial collagen and subsequent activation.3 LAIR-1, expressed on most immune cells, is an inhibitory receptor involved in reducing signals from activating receptors.4 OSCAR is an osteoclast-associated receptor, and its activation by collagen costimulates the Fc receptor common γ-chain, inducing immunoreceptor tyrosine-based activation motif signaling pathways for osteoclastogenesis.5
The increasing number of receptors and ligands found to bind to collagen during normal biological processes or in pathological conditions raises questions about the specificity and nature of binding to this triple-helical protein. Recent high-resolution structures of cocrystals of triple-helical peptides (THPs) bound to collagen partners have confirmed well-established principles of protein interactions and also shown a diversity of binding modes.6 Some collagen-binding proteins recognize 1 very precise site in the triple helix, or even 1 specific face of the triple helix, for example, VWF or DDR1. Other receptors or ligands bind with less specificity. GPVI, LAIR-1, and OSCAR all fall into the latter category and can bind multiple sites within fibrillar collagens. The high-resolution structures of GPVI and LAIR-1 have been reported, and computational docking simulations, mutation analysis, and nuclear magnetic resonance studies have been used to define the residues in their immunoglobulin-like domains which interact with the collagen triple helix.7,8 The high-resolution x-ray structure of a cocrystal of the extracellular collagen-binding domain of OSCAR with a triple-helix peptide reported by Zhou et al1 provides the first direct and detailed picture of an LRC receptor interaction with collagen.
OSCAR contains 2 immunoglobulin-like domains, D1 and D2, and their interdomain orientation differs significantly from other LRC receptors, with a suggestion of flexibility.1 The triple-helix peptide containing the consensus sequence GPOGPAGFO (where O represents hydroxyproline) is seen to bind to the N-terminal D1 domain of 1 OSCAR molecule and to the C-terminal D2 domain of the other molecule in the asymmetric unit. Direct binding assays on OSCAR molecules with mutations conclusively showed that D2, but not D1, is critical for collagen binding. This was a surprising result because collagen binds to the D1 domain of GPVI and LAIR-1, and this unusual binding may relate to the unique interdomain structure of OSCAR. The triple-helix bound to a groove on the β-sheet surface of D2 in OSCAR. Some backbone carbonyls of the triple helix are hydrogen bonded to D2 Tyr and Arg side chains, whereas the Phe is located in a D2 hydrophobic pocket, a feature seen for other collagen receptors. The D2 domain interacted with 2 of the 3 chains within the triple helix (middle and trailing chains), consistent with the requirement for the triple-helix structure for binding. Given the differences in specificity and binding for OSCAR compared with GPVI and LAIR-1, it will be exciting to see cocrystals of these other 2 LRC receptors with THPs as well.
Advances in our understanding of collagen-ligand interactions, such as that provided through high-resolution structures of complexes of receptors with collagen model peptides, offer the potential for the discovery and design of novel therapeutic agents that can target specific collagen interactions. Such directed binding could either suppress interactions between collagen and its ligand or promote lost collagen interactions in pathological conditions where normal collagen function is impaired. OSCAR may contribute to the pathogenesis and severity of a number of diseases including osteoporosis, atherosclerosis, chronic obstructive pulmonary disease, and rheumatoid arthritis.9-11 For example, OSCAR expression by monocytes is inversely correlated with disease activity in rheumatoid arthritis.9 OSCAR expression is highly specific to osteoclasts and their precursors, compared with other immune system modulators such as receptor activator of nuclear factor κB, triggering receptor expressed on myeloid cells 2, and DNAX-activating protein of 12 kDa, making OSCAR a promising therapeutic target for common diseases with elevated osteoclast bone resorption activity such as osteoporosis and rheumatoid arthritis. Therapies that target osteoclast maturation and inhibit inflammatory osteoclastogenesis may be possible through regulation of OSCAR function, using collagen-like peptides, anti-OSCAR antibodies, or recombinant soluble OSCAR. The findings by Zhou et al1 provide important new insights into the molecular mechanism of OSCAR-collagen interactions and create a foundation for potential therapies.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Zhou L, Hinerman JM, Blaszczyk M, et al. Structural basis for collagen recognition by the immune receptor OSCAR. Blood. 2016 doi: 10.1182/blood-2015-08-667055. 127(5):529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross J, Schmitt FO. The structure of human skin collagen as studied with the electron microscope. J Exp Med. 1948;88(5):555–568. doi: 10.1084/jem.88.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibbins JM, Okuma M, Farndale R, Barnes M, Watson SP. Glycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor gamma-chain. FEBS Lett. 1997;413(2):255–259. doi: 10.1016/s0014-5793(97)00926-5. [DOI] [PubMed] [Google Scholar]
- 4.Meyaard L, Adema GJ, Chang C, et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity. 1997;7(2):283–290. doi: 10.1016/s1074-7613(00)80530-0. [DOI] [PubMed] [Google Scholar]
- 5.Barrow AD, Raynal N, Andersen TL, et al. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J Clin Invest. 2011;121(9):3505–3516. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An B, Lin YS, Brodsky B. Collagen interactions: drug design and delivery [published online ahead of print November 26, 2015]. Adv Drug Deliv Rev. doi: 10.1016/j.addr.2015.11.013. doi:10.1016/j.addr.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Horii K, Kahn ML, Herr AB. Structural basis for platelet collagen responses by the immune-type receptor glycoprotein VI. Blood. 2006;108(3):936–942. doi: 10.1182/blood-2006-01-010215. [DOI] [PubMed] [Google Scholar]
- 8.Brondijk TH, de Ruiter T, Ballering J, et al. Crystal structure and collagen-binding site of immune inhibitory receptor LAIR-1: unexpected implications for collagen binding by platelet receptor GPVI. Blood. 2010;115(7):1364–1373. doi: 10.1182/blood-2009-10-246322. [DOI] [PubMed] [Google Scholar]
- 9.Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195(2):201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth K, Schoppet M, Al-Fakhri N, et al. The role of osteoclast-associated receptor in osteoimmunology. J Immunol. 2011;186(1):13–18. doi: 10.4049/jimmunol.1002483. [DOI] [PubMed] [Google Scholar]
- 11.Barrow AD, Palarasah Y, Bugatti M, et al. OSCAR is a receptor for surfactant protein D that activates TNF-α release from human CCR2+ inflammatory monocytes. J Immunol. 2015;194(7):3317–3326. doi: 10.4049/jimmunol.1402289. [DOI] [PMC free article] [PubMed] [Google Scholar]