Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
GPIb-IX signaling cooperates with PAR signaling to promote platelet response to low concentrations of thrombin, which are important in vivo.
Thrombin induces a GPIb-IX–specific signaling pathway that requires the cytoplasmic domain of GPIbα, 14-3-3 protein, Rac1, and LIMK1.
Abstract
Thrombin-induced cellular response in platelets not only requires protease-activated receptors (PARs), but also involves another thrombin receptor, the glycoprotein Ib-IX complex (GPIb-IX). It remains controversial how thrombin binding to GPIb-IX stimulates platelet responses. It was proposed that GPIb-IX serves as a dock that facilitates thrombin cleavage of protease-activated receptors, but there are also reports suggesting that thrombin binding to GPIb-IX induces platelet activation independent of PARs. Here we show that GPIb is neither a passive thrombin dock nor a PAR-independent signaling receptor. We demonstrate a novel signaling-mediated cooperativity between PARs and GPIb-IX. Low-dose thrombin-induced PAR-dependent cell responses require the cooperativity of GPIb-IX signaling, and conversely, thrombin-induced GPIb-IX signaling requires cooperativity of PARs. This mutually dependent cooperativity requires a GPIb-IX–specific 14-3-3-Rac1-LIMK1 signaling pathway, and activation of this pathway also requires PAR signaling. The cooperativity between GPIb-IX signaling and PAR signaling thus drives platelet activation at low concentrations of thrombin, which are important for in vivo thrombosis.
Introduction
Thrombin is a major platelet agonist and plays critical roles in platelet activation, thrombus formation, and inflammation. Thrombin-induced platelet activation requires protease-activated receptors (PARs), which are members of the G protein–coupled receptor family of proteins. PARs are activated when their N-terminal domain is cleaved by thrombin, exposing a tethered ligand sequence that binds intramolecularly to the receptor, causing receptor activation, thereby initiating intracellular signaling through G proteins.1,2 There are species differences in expression of different PAR isoforms in platelets. PAR1 and PAR4 are expressed in human platelets,2 whereas PAR3 and PAR4 are expressed in mouse platelets.3,4 PAR1 and PAR4 similarly signal via the Gq and G13 (and possibly Gi).5,6 PAR3 appears to serve as a facilitator of PAR4 cleavage by thrombin but is unable to signal independent of PAR4.3 Despite the requirement of PARs in thrombin-induced platelet activation, platelets respond poorly to low-dose thrombin in the absence of another membrane receptor, the glycoprotein (GP) Ib-IX-V complex, which has long been known to bind thrombin with high affinity and be required for platelet response to low concentrations of thrombin.7,8
The platelet GPIb-IX-V complex is a multifunctional hetero-oligomeric membrane protein complex that plays important roles in hemostasis and thrombosis, cancer metastasis, and inflammation.9-13 GPIb-IX-V is composed of 4 single-pass transmembrane subunits.14-16 The disulfide-linked GPIbα and GPIbβ subunits are tightly complexed with GPIX.17,18 Complex formation is essential for efficient expression of each subunit.19 GPIb-IX is sufficient to function as a receptor without glycoprotein V (GPV).20,21 Expression of GPV has been suggested to negatively regulate GPIb-IX function, particularly in response to thrombin.22 GPIb-IX is a receptor for von Willebrand factor (VWF), thrombin, P-selectin and β2 integrins, with binding sites for these ligands located in the N-terminal domain of GPIbα.23 The interaction between VWF and GPIb-IX mediates the initial adhesion of platelets to injured or stimulated vessel walls,24 and also elicits intracellular signals, leading to stable platelet adhesion, morphologic shape change, granule secretion, and thrombus formation.23 The cytoplasmic domain of GPIbα contains binding sites for intracellular proteins including filamin25 and ζ form 14-3-3.26 The binding of 14-3-3ζ requires the C-terminal sequence in the cytoplasmic domain of GPIbα, which is important in VWF-induced GPIb-IX signaling and in regulating the VWF-binding function of GPIb.13,27-29
The role of GPIb-IX in thrombin-induced platelet activation has been a subject of debate. Currently, a prevailing theory is that GPIb-IX itself is not sufficient to initiate platelet response to thrombin, but rather serves as a dock for thrombin, thereby facilitating cleavage of PARs.30 However, others suggest that GPIb-IX serves as an independent thrombin receptor, which is mainly supported by the data suggesting that catalytically inactive thrombin can still initiate a platelet response.31,32 In this study, we show that GPIb is neither a passive thrombin dock that only serves to facilitate cleavage of PARs nor a PAR-independent receptor. We show that thrombin induces GPIb-IX–specific signaling, which cooperates with PAR-dependent signaling to promote platelet response. This mutual cooperativity between GPIb-IX and PARs requires both a GPIb-IX–specific 14-3-3–Rac1–LIMK1 signaling pathway and GPIb-IX–independent PAR signaling. In addition, our data suggest that low concentrations of thrombin are important for in vivo thrombosis. These results demonstrate a new concept of signaling-mediated mutual cooperativity between GPIb-IX and PARs in mediating platelet responses to low concentrations of thrombin.
Methods
Preparation of platelets
For human subjects, Institutional Review Board approval was obtained from the University of Illinois at Chicago, and informed consent from volunteers was obtained in accordance with the Declaration of Helsinki. The generation of megakaryocyte lineage-specific Rac1-knockout mice (Rac1−/−) and LIMK1-knockout mice (LIMK1−/−) has been previously described.33-36 Mice were maintained on a mixed SV/129/C57/Bl-6 background, and littermates were used as controls. Human and mouse platelets were prepared as previously described.35,37
PPACK treatment of thrombin
Wild-type human α-thrombin (Enzyme Research Labs) or recombinant human S195A thrombin (Kerafast) was reacted with 15 molar excess of PPACK (D-Phenylalanyl-prolyl-arginyl Chloromethyl Ketone) for 1 hour at room temperature, as previously described.38 Please see supplemental Materials available on the Blood Web site for further details.
Thrombin activity assay
Thrombin activity assays were conducted by measuring the change in fluorescence on cleavage of a fluorogenic thrombin substrate SN-17a (Haematologic Technologies). Except in cases where otherwise specified, human α-thrombin (also referred to as thrombin or wild-type thrombin) purified from human plasma was used (Enzyme Research Labs). Specific activity of α-thrombin is 3078 NIH U/mg. See supplemental Materials for further details.
Thrombin binding assay
Human platelets (1 × 109) were resuspended in a thrombin binding buffer (25 mM Tris, 0.6% PEG-8000, 1% bovine serum albumin, 136 mM NaCl, pH 7.4), as previously described.39 Thrombin binding was detected using flow cytometry as described in supplemental Materials.
Platelet aggregation and secretion
Platelet aggregation and adenosine triphosphate (ATP) secretion were determined simultaneously in a Chronolog lumiaggregometer at 37°C with stirring (1000 rpm), as previously described.35
Calcium mobilization assay
For detailed procedures, see supplemental Materials. Briefly, thrombin-induced calcium mobilization was measured using a fluorescent calcium-sensitive dye, FLIPR Calcium 5 reagent (Molecular Devices; R8142, Explorer kit), and fluorescence was monitored using a FlexStation plate reader (Molecular Devices).
Activation of LIMK and Rac1
For detailed procedures, please see supplemental Materials. Briefly, platelets or Chinese hamster ovary (CHO) cells (in suspension) were stimulated with thrombin for 2 minutes. The reactions were stopped by addition of an equal volume of 2× sodium dodecyl sulfate sample buffer as described previously35,40 and then immunoblotted with the antibodies recognizing LIMK1/2 phosphorylated at threonine 508/505 or total LIMK1 (Cell Signaling, Danvers, MA). Rac1 activation assays were performed, as described previously.41 Anti-Rac1 antibody (No. 61051; BD Biosciences) was used for western blot.
Reconstitution of human GPIb-IX in CHO cells
CHO cells stably expressing wild-type GPIb-IX (1b9) or mutant GPIb-IX that contains a truncation of the 5 C-terminal amino acids in the α-chain of GPIb (Δ605 cells) were previously established.27 For detailed procedures, please see supplemental Materials. Briefly, CHO cell lines expressing GPIbα with a triple tyrosine to phenylalanine mutation (FFF cells), known to abolish the high affinity binding of thrombin to GPIb,42-44 were stably expressed by cotransduction with GPIbβ and GPIX cDNA into CHO cells with a pLenti6/V5-Dest vector encoding wild-type human GPIbα or mutant GPIbα containing Y276,278,279F mutations. Levels of surface GPIb-IX expression were normalized between cell lines to similar levels by fluorescence-activated cell sorting.
Fluorescence intravital microscopy
Laser-induced cremaster muscle arteriolar thrombosis was induced in wild-type mice as described previously.40 Briefly, platelet thrombus formation and thrombin generation were induced by laser injury and visualized after infusion of DyLight 649-labeled anti-mouse CD42c (0.05 µg/g body weight [BW]) and Alexa Fluor (AF) 488–labeled monoclonal antithrombin antibody (2 µg/g BW, ∼170 nM calculated based on estimated blood volume; AHT-5020; Haematologic Technologies) into mice. This antibody selectively recognizes thrombin (Kd = 14 nM) and thrombin–antithrombin complex, but not prothrombin. In some experiments, mice were treated with dabigatran (0.3 mg/kg) or vehicle control prior to laser injury. Standard curves were generated by measuring fluorescence intensities of known concentrations of AF488-conjugated antithrombin antibody on glass slides under the same microscope settings (also see supplemental Data).
Results
Important role of GPIb-IX and intracellular interaction between GPIbα and 14-3-3 in stimulating thrombin-induced cellular signaling in a reconstituted CHO cell model
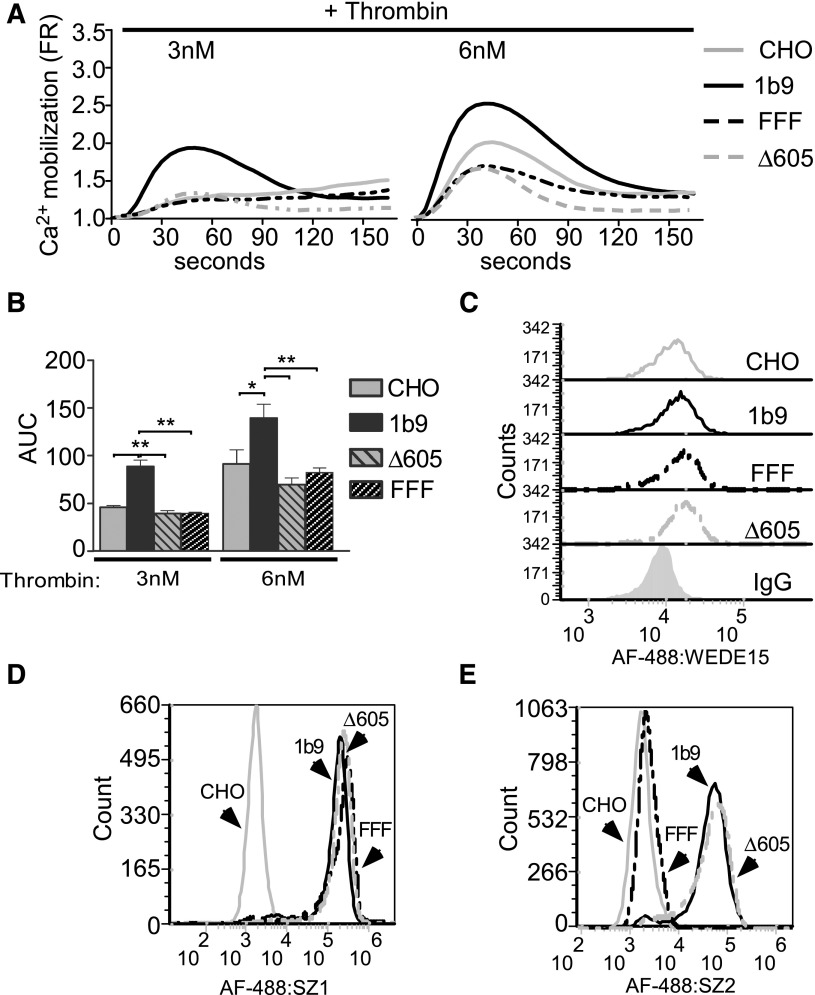
To clearly dissect the role of GPIb in thrombin signaling without complication of secondary or alternative platelet pathways, we reconstituted GPIb-enhanced thrombin signaling in CHO cells expressing endogenous PAR1 and the recombinant wild-type human GPIb-IX complex (1b9 cells). On thrombin stimulation, 1b9 cells displayed enhanced calcium mobilization compared with control CHO cells that express PAR1 but not GPIb-IX (Figure 1A-B). This result is not caused by differences in PAR1 expression as GPIb-IX–expressing CHO cells and control CHO cells expressed similar levels of PAR1 (Figure 1C). This result is similar to previous data comparing normal platelets with Bernard-Soulier syndrome platelets deficient in GPIb-IX,7 supporting the notion that GPIb-IX expression enhances thrombin-induced cellular responses. To verify that the stimulatory role of GPIb-IX requires a GPIb–thrombin interaction, we also tested CHO cell lines expressing similar levels of GPIb-IX with GPIbα having a triple tyrosine to phenylalanine mutation (Y276,278,279 F; FFF) that is known to abolish the high affinity binding of thrombin to GPIb42-44 (Figure 1D). As expected, FFF mutation also abolished the binding of an antibody, SZ2, that blocks the GPIb–thrombin interaction (Figure 1E),45 but did not affect PAR1 expression (Figure 1C). Indeed, the stimulatory role of GPIb-IX on thrombin response was diminished in FFF cells (Figure 1A-B). To investigate whether the stimulatory role of thrombin binding to GPIb requires GPIb-IX signaling, we examined whether and how deletion of the C-terminal 14-3-3 binding site (Δ605) in the cytoplasmic domain of GPIbα27 affects the ability of GPIb-IX to facilitate cellular response to thrombin. The stimulatory effect of GPIb-IX on thrombin-induced calcium mobilization (Figure 1A-B) was abolished in cells expressing the Δ605 mutant of GPIbα, which have comparable levels of GPIb-IX and PAR1 expression as 1b9 cells (Figure 1C-E). These data indicate that GPIb-IX–dependent enhancement of thrombin-induced signaling in this CHO cell model requires interaction of the C-terminal region of GPIbα with the intracellular signaling molecule 14-3-3.
Figure 1.
The importance of the C-terminal 14-3-3-binding site of GPIbα in GPIb-mediated cellular response to thrombin. (A) CHO cells expressing endogenous PAR1 and wild-type human GPIb-IX (1b9), GPIb-IX with a 5-amino acid truncation in the C-terminal 14-3-3-binding site of GPIbα (Δ605), GPIb-IX with a triple tyrosine to phenylalanine mutation (Y276 278,279 F, FFF) in the thrombin binding site of GPIbα, and control CHO cells expressing PAR1 but not GPIb-IX were each loaded with a calcium-sensitive fluorescent dye, FLIPR Calcium 5 reagent, and then stimulated with 3 or 6 nM thrombin. Kinetic changes from baseline calcium fluorescence were recorded and plotted as a fluorescence ratio (FR).4 (B) Quantification of the area under the curves (AUC) shown in A from 4 independent experiments (mean ± standard error of the mean [SEM]; *P < .05 and **P < .01, 1-way analysis of variance [ANOVA]). (C-E) Flow cytometric analysis of the binding of (C) an anti-PAR1 monoclonal antibody WEDE15, (D) an anti-GPIb-IX complex monoclonal antibody SZ1, or (E) an anti-GPIbα monoclonal antibody SZ2 as detected using Alexa Fluor 488–labeled goat anti-mouse IgG to control CHO cells, 1b9, Δ605, and FFF cells.
Intracellular interaction between GPIbα and 14-3-3 is important for low-dose thrombin-induced platelet aggregation, ATP release, and calcium mobilization
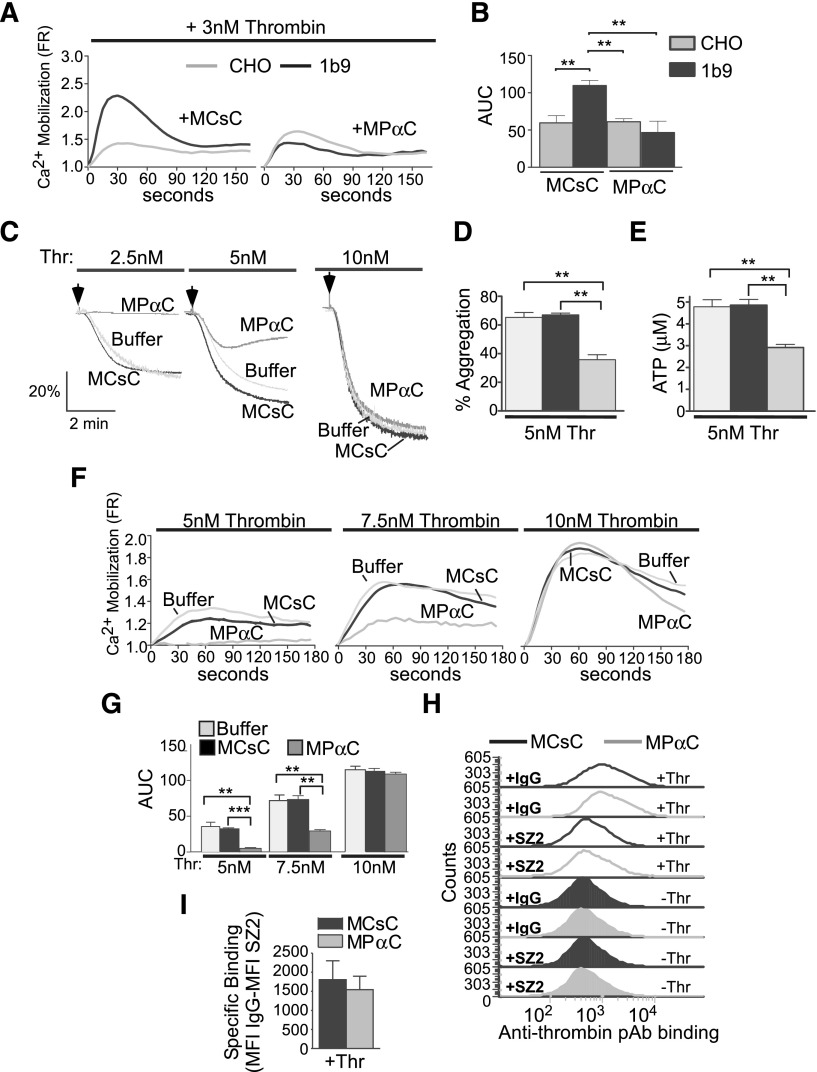
We previously developed a membrane-permeable inhibitor peptide, MPαC, derived from the phosphorylated C-terminal 14-3-3-binding sequence of GPIbα, which abolishes 14-3-3 binding and selectively inhibits VWF/GPIb-IX-dependent platelet response.29 MPαC selectively inhibited thrombin-induced calcium response of GPIb-expressing CHO cells but not control CHO cells, further validating its specificity for GPIb-IX–dependent cell response to thrombin (Figure 2A-B). To investigate the role of the 14-3-3-GPIb interaction in GPIb-IX–dependent promotion of human platelet response to thrombin, we determined the effect of MPαC, or a scrambled control peptide, MCsC, on thrombin-induced human platelet aggregation. MPαC inhibited low-dose thrombin-induced platelet aggregation (Figure 2C-D), suggesting that the 14-3-3–GPIb interaction is important for enhancing thrombin-induced platelet aggregation. Thrombin-stimulated platelet responses include receptor-coupled calcium mobilization and granule secretion.46,47 The observed calcium mobilization induced by low-dose thrombin was not affected by the integrin blocker integrilin, indicating that it is an early response independent of integrin outside-in signaling (supplemental Figure 1A-B). MPαC treatment inhibited low-dose thrombin-induced ATP release (Figure 2E) and calcium mobilization (Figure 2F-G), which is similar to the response of GPIb-IX–deficient platelets from Bernard-Soulier syndrome patients or GPIb blocking antibody-treated platelets.7,48 These data reveal the essential function of the GPIb–14-3-3 interaction in platelet response to low doses of thrombin.
Figure 2.
The effect of an inhibitor of 14-3-3–GPIb interaction on thrombin-induced cellular responses in GPIb-expressing CHO cells and platelets. (A) CHO cells or CHO cells expressing GPIb-IX were loaded with a calcium-sensitive dye (FLIPR Calcium 5) and then treated with MPαC or scrambled control peptide prior to stimulation with 3 nM thrombin. Thrombin-induced calcium responses were determined as in Figure 1. (B) Quantification of data shown in A (mean ± SEM, 3 experiments; **P < .01, 1-way ANOVA). (C) Thrombin-stimulated aggregation of human platelets pretreated with the MCsC (control), MPαC micellar peptide, or buffer alone (no peptide treatment). (D) Quantitative data of 4 aggregation experiments as shown in C (mean ± SEM). (E) Thrombin-stimulated ATP release (mean ± SEM, 4 experiments; *P < .05 and **P < .01, t test). (F) Thrombin-induced calcium mobilization in human platelets pretreated with MCsC, MPαC, or buffer alone. (G) Quantification of the area under the curves (AUC) as shown in F (mean ± SEM, 3 experiments; **P < .01, and ***P < .001, 1-way ANOVA). (H) Stacked fluorescence histograms of 3 nM S195A thrombin binding to washed human platelets pretreated with 10 μM MPαC or control peptide MCsC as analyzed with flow cytometry using a goat antithrombin antibody and an Alexafluor 488–labeled anti-goat IgG antibody. Prior to thrombin addition, samples were preincubated with IgG or SZ2 to verify GPIb-dependent thrombin binding. Thr, 3 nM S195A thrombin. (I) Quantification of specific binding of thrombin to MCsC or MPαC-treated platelets (mean ± SEM, 3 experiments; P = .6847). Specific binding is calculated by subtracting thrombin binding mean fluorescence intensity of SZ-2–treated platelets from IgG-treated platelets.
Intracellular interaction between GPIbα and 14-3-3 is not important for thrombin binding to GPIb
It is known that GPIb contains a high-affinity thrombin binding site,42-44,49 but whether the cytoplasmic 14-3-3 binding region is important for regulating thrombin binding to GPIb is unknown. To exclude the possibility that interfering with the GPIb-14-3-3 interaction also affects thrombin binding to GPIb-IX, we measured thrombin binding to human platelets pretreated with MPαC. MPαC pretreatment did not affect low-dose thrombin binding to GPIb (Figure 2H-I). In contrast, thrombin binding was inhibited by the GPIb thrombin binding site-blocking antibody SZ2 (Figure 2H). These data indicate that the 14-3-3–GPIb-IX interaction is not important for thrombin binding to GPIb-IX.
Role of Rac1 in mediating thrombin-induced GPIb-IX signaling
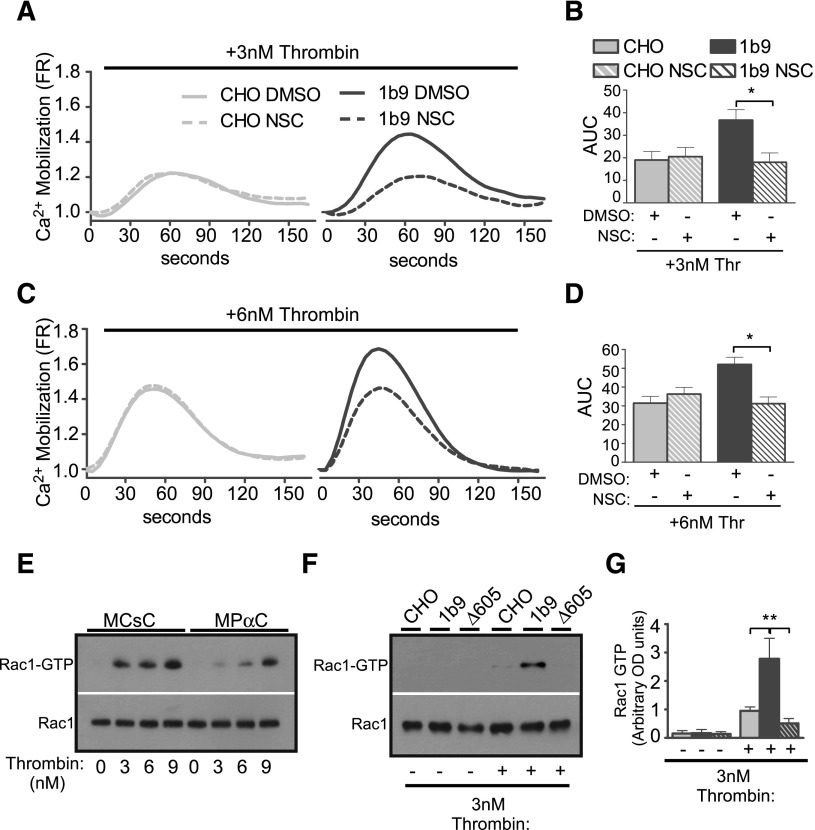
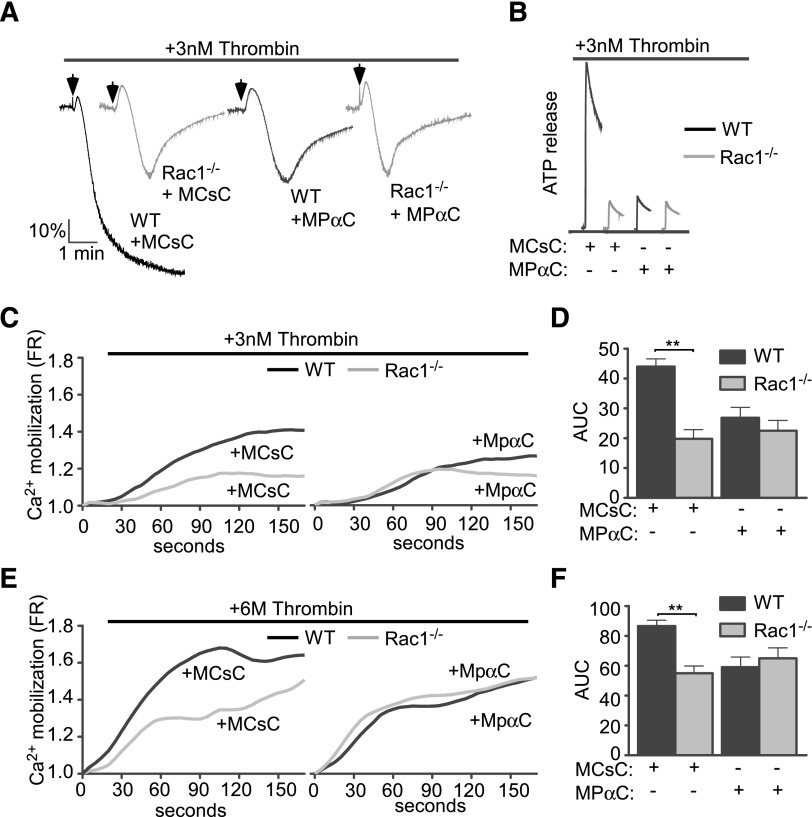
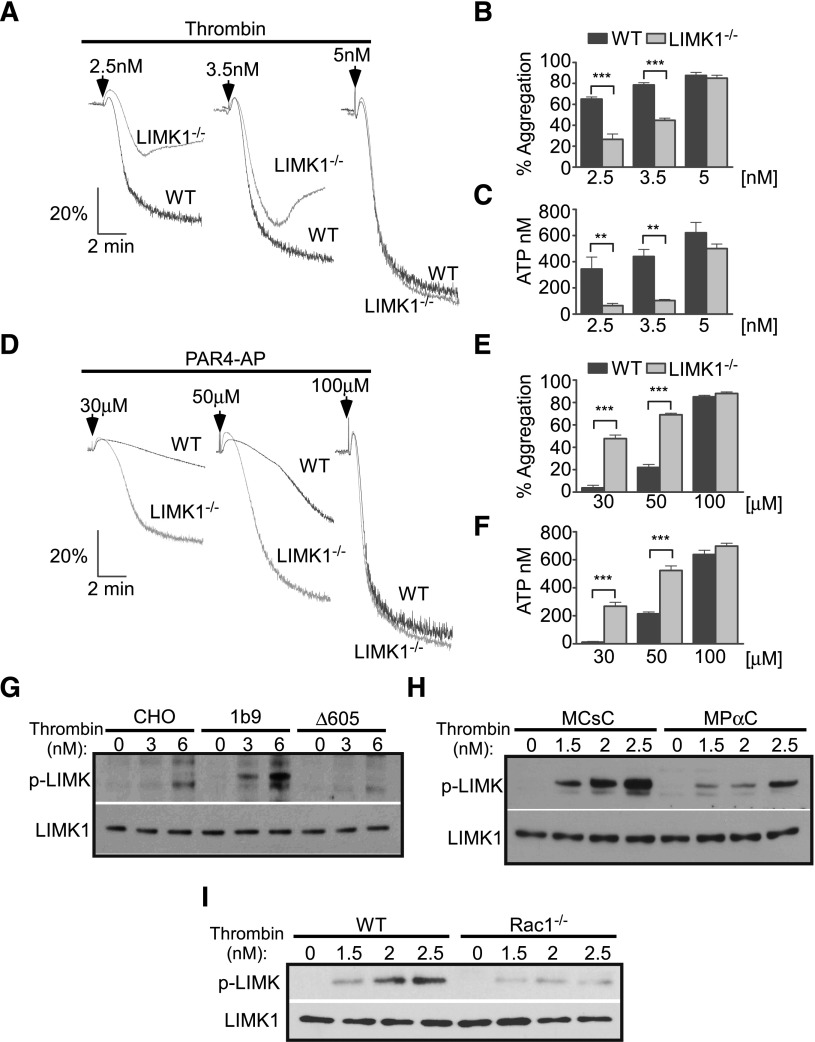
To further identify the thrombin-induced GPIb-IX signaling pathway involved in stimulating platelet response, we tested the hypothesis that the thrombin-induced GPIb-IX signaling pathway is similar to the VWF-induced platelet activation pathway, in which a Lyn/Rac signaling pathway plays an important role.33 A Rac1 inhibitor, NSC23766, diminished low-dose thrombin-induced calcium mobilization only in wild-type GPIb-IX–expressing CHO cells (1b9), but not in control CHO cells (Figure 3A-D), indicating that Rac1 is required for GPIb-dependent thrombin signaling leading to calcium mobilization. Importantly, on low-dose thrombin stimulation, 1b9 cells displayed enhanced Rac1 activity, which was absent in Δ605 cells expressing the 14-3-3 binding deficient mutant GPIbα (Figure 3F-G). In human platelets, low-dose thrombin similarly induced activation of Rac1, which was inhibited by the inhibitor of the GPIb-14-3-3 interaction, MPαC, but not by the control peptides (Figure 3E). Furthermore, whereas thrombin responses of wild-type mouse platelets, as indicated by platelet aggregation, secretion, and calcium mobilization, were inhibited by MPαC, suggesting the importance of the GPIb-IX–14-3-3 interaction also in mouse platelets, Rac1−/− mouse platelets, which already showed reduced platelet response to low-dose thrombin, could not be further inhibited by MPαC (Figure 4). These data suggest that the GPIb-IX–14-3-3 interaction and Rac1 mediate the same signaling pathway (Figure 4A-F). Together these data show that thrombin-induced GPIb signaling mediates Rac1 activation via a 14-3-3–dependent mechanism and that Rac1 is required for the GPIb-IX signaling that stimulates low-dose thrombin-induced cellular response.
Figure 3.
The GPIb-IX–dependent effect of Rac1 inhibitor NSC23766 on thrombin-induced calcium elevation. Control CHO cells and 1b9 cells were loaded with a fluorescent calcium-sensitive dye and then treated with either 0.1% dimethylsulfoxide (DMSO) or 100 μM NSC23766 (NSC). Calcium fluorescence signals of these cells were then recorded following stimulation with (A-B) 3 nM or (C-D) 6 nM thrombin. (A,C) Typical graphs of calcium elevation. (B,D) Quantitative data of A and C (mean ± SEM, 3 experiments; 1-way ANOVA). *P < .05. (E-F) Western blot analysis of levels of GTP-bound active Rac1 in thrombin-stimulated human platelets (E) pretreated with either MPαC or MCsC or in (F) control CHO cells or 1b9 cells. The amount of GTP-bound Rac1 was determined using a PAK-GST pull-down assay, followed by western blotting with an anti-Rac1 antibody. Total levels of Rac1 in platelet lysates were also determined by western blot using anti-Rac1 antibody. (G) Densitometric analysis of western blots as shown in F (mean ± SEM, 3 experiments; **P < .01, 1-way ANOVA) using National Institutes of Health Image J. The results were expressed as arbitrary units of uncalibrated optical density.
Figure 4.
Comparison of thrombin-induced aggregation, ATP secretion, and calcium elevation and the inhibitory effect of MPαC between wild-type and Rac1−/− platelets. Washed wild-type or Rac1−/− platelets were pretreated with MPαC or control peptide MCsC and then analyzed for (A) aggregation, (B) ATP release, and (C-F) calcium mobilization induced by thrombin. (C,E) Typical plots of calcium mobilization. (D,F) Quantitative data from 3 experiments (mean ± SEM). **P < .01 (1-way ANOVA).
Rac1/LIMK1 pathway that mediates thrombin-induced GPIb-IX signaling
LIMK1 can be activated by Rac1 signaling50 and is involved in the amplification of VWF-induced platelet activation.35 To investigate whether LIMK1 is important in thrombin-induced GPIb-IX signaling, we determined the effects of LIMK1 knockout on platelet activation induced by thrombin. LIMK1−/− platelets displayed significantly reduced aggregation and ATP release in response to low doses of thrombin (Figure 5A-C), indicating a stimulatory role for LIMK1 in thrombin-induced platelet activation. The stimulatory role of LIMK1 in thrombin-induced platelet response is independent of PAR signaling, because LIMK1−/− platelets displayed significantly enhanced aggregation and ATP release in response to PAR4-activating peptide (Figure 5D-F). These data suggest that LIMK1 plays a stimulatory role in platelet response to thrombin but a negative regulatory role in PAR signaling. To determine whether the thrombin-induced GPIb-IX signaling activates LIMK1, we assessed LIMK1 activation as indicated by its phosphorylation in 1b9, Δ605, and control CHO cells stimulated with thrombin. Thrombin induced some degree of LIMK1 phosphorylation in CHO cells lacking GPIb-IX expression, but this was detectable only at a higher concentration of thrombin (Figure 5G). In contrast, thrombin stimulation of 1b9 cells induces much greater LIMK1 phosphorylation, indicating the role of GPIb-IX signaling in promoting LIMK1 phosphorylation. However, in Δ605 cells lacking the 14-3-3 binding site of GPIbα, the enhanced LIMK1 phosphorylation was absent. These data indicate that thrombin-induced GPIb-IX signaling mediates 14-3-3–dependent LIMK1 activation. In mouse platelets, MPαC treatment inhibited thrombin-induced LIMK1 phosphorylation, suggesting that the 14-3-3–GPIb interaction is important in thrombin-induced LIMK1 activation in platelets (Figure 5H). In contrast to the inhibitory effect of MPαC, inhibition of adenosine diphosphate (ADP) signaling using ADP receptor antagonists did not affect thrombin-induced LIMK1 phosphorylation, suggesting that thrombin-induced GPIb-IX–dependent LIMK1 phosphorylation does not require subsequent secretion of ADP (supplemental Figure 1D). In other cell types, it is known that LIMK1 is activated downstream of Rac1.50 In platelets, however, whether thrombin signaling activates LIMK1 in a Rac1-dependent manner is unknown. Thus, to determine whether LIMK1 lies downstream of Rac1, we assessed LIMK1 phosphorylation in thrombin-stimulated Rac1−/− platelets. Thrombin-stimulated Rac1−/− platelets displayed defective LIMK1 phosphorylation (Figure 5I). Together, these data indicate a GPIb-IX–14-3-3–Rac1–LIMK1 signaling pathway that is important in thrombin-induced GPIb-IX signaling and GPIb-IX signaling–dependent platelet response.
Figure 5.
GPIb-IX– and Rac-1–dependent activation of LIMK1 and the roles of LIMK1 in promoting thrombin-induced platelet activation but negatively regulating PAR4-activating peptide-induced platelet activation. Aggregation and ATP release of washed wild-type (WT) or LIMK1−/− mouse platelets in response to increasing doses of (A-C) thrombin or (D-F) PAR4-activating peptide–induced (PAR4-AP) stimulation. (A,D) Typical aggregation traces. (B,E) Quantification of the aggregation (mean ± SEM, 4 experiments). (C,F) ATP release (mean ± SEM, 4 experiments). **P < .01 and ***P < .001, 1-way ANOVA. Western blot analysis of LIMK1 phosphorylation using a phospho-Thr505/508–dependent anti-LIMK1 antibody in (G) control CHO cells, 1b9, and Δ605 cells; (H) washed mouse platelets pretreated with either MPαC or MCsC; and (I) wild-type or Rac1−/− mouse platelets, which were stimulated with low doses of thrombin. Loading levels were determined using a phosphorylation-independent LIMK1 antibody.
Cooperativity between GPIb-IX signaling and PARs in mediating thrombin-induced cellular response
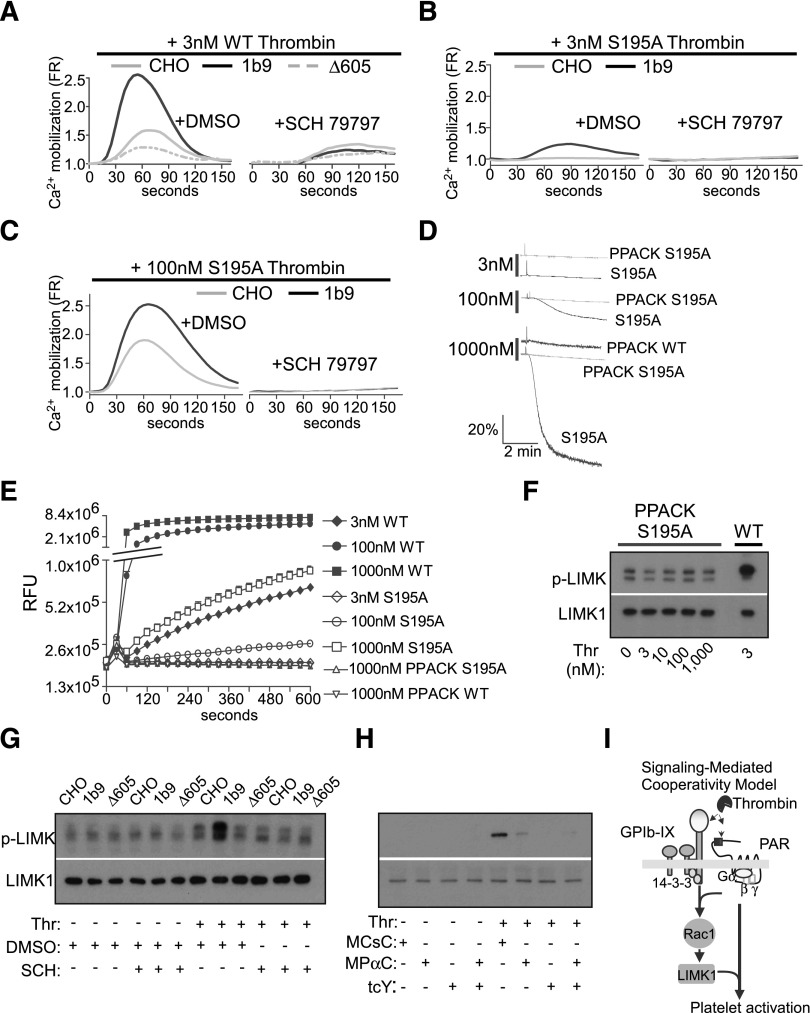
Demonstration of a role for GPIb-IX signaling in stimulating platelet response to thrombin reignites the debate with regard to whether GPIb-IX mediates PAR-independent signaling that is sufficient to initiate cellular response. To determine whether thrombin induces GPIb-dependent cellular response independent of PAR signaling, we treated GPIb-expressing cells with a PAR1 antagonist, SCH 79797. SCH 79797 pretreatment abolished thrombin-induced calcium mobilization in 1b9 cells expressing wild-type human GPIb-IX and in control CHO cells and Δ605 cells (Figure 6A), suggesting that thrombin-induced calcium mobilization requires PAR1 signaling in the presence or absence of GPIb-IX signaling. These data suggest that GPIb-IX signaling is not sufficient to elicit detectable calcium mobilization in response to thrombin in the absence of PAR1 signaling in this reconstituted CHO cell model. This result is consistent with a previous study showing that PAR4 knockout mouse platelets are unable to respond to thrombin.4 However, PAR signaling requires catalytic activity of thrombin, and a major piece of previous data suggesting PAR-independent GPIb-IX signaling was that S195A mutant thrombin that reportedly lost enzymatic activity or PPACK-inhibited thrombin can still stimulate platelet activation in the absence of GPV.32 We did not observe cell response induced by PPACK-treated wild-type thrombin (Figure 6D). As PPACK treatment carries the possibility of incomplete inhibition resulting in residual enzymatic activity that may vary between different laboratories, we further tested whether S195A mutant thrombin is able to induce PAR-independent GPIb-IX signaling in the reconstituted CHO cell model and in platelets. Indeed, S195A mutant thrombin induced calcium mobilization, which was significantly enhanced in 1b9 cells expressing wild-type GPIb-IX in a manner similar to wild-type thrombin (Figure 6B). However, the S195A mutant thrombin-induced calcium mobilization was abolished by PAR1 antagonist SCH79797 (Figure 6C). These data suggest the requirement for PAR1 in the GPIb-IX–dependent cell response to either S195A or wild-type thrombin in the reconstituted CHO cell model. In platelets, S195A mutant thrombin was unable to induce platelet aggregation at low doses, which is consistent with a previous report.51 However, S195A thrombin induced platelet aggregation at doses of 100 nM or higher (Figure 6D), and this effect of S195A thrombin was abolished by its treatment with PPACK (Figure 6D), suggesting that S195A thrombin contains residual enzymatic activity that is required for its activation of platelets. Indeed, the enzymatic activity of S195A thrombin at high concentrations was detectable using a fluorogenic thrombin substrate (Figure 6E), and this activity was abolished by further treatment with PPACK. Similarly, S195A thrombin induced mouse platelet aggregation at high concentrations, which was also abolished by PPACK treatment (data not shown). These data suggest that thrombin-induced GPIb-IX–dependent cellular response requires the catalytic activity of thrombin and PAR-dependent signaling. Furthermore, these data also suggest a mutually dependent cooperativity between GPIb-IX signaling and PAR signaling that is important for platelet response to low-dose thrombin.
Figure 6.
Thrombin-induced GPIb signaling requires cooperation of PARs. (A-D) Calcium mobilization in control CHO cells, 1b9, and Δ605 CHO cells pretreated with DMSO control or PAR1 inhibitor SCH79797 (20 µM). Cells were stimulated with (S) wild-type (WT) thrombin or (B) low or (C) high concentrations of S195A mutant thrombin and then recorded for calcium mobilization as described in the Methods. (D) Aggregation of human platelets stimulated with increasing doses of S195A mutant thrombin, PPACK-treated S195A thrombin, and 1000 nM PPACK-treated WT thrombin. (E) In vitro enzymatic activity of WT, S195A mutant thrombin, PPACK-treated S195A mutant thrombin, and PPACK-treated WT thrombin. The y-axis is shown as a log scale of relative fluorescence units (RFUs). (F) Western blot analysis of LIMK1 phosphorylation in human platelets stimulated with increasing doses of PPACK-treated S195A mutant thrombin or wild-type thrombin (Thr). (G) LIMK1 phosphorylation in control CHO, 1b9, and Δ605 cells preincubated with vehicle control (0.1% DMSO) or 20 μM PAR1 antagonist SCH 79797 and stimulated with WT thrombin. (H) LIMK1 phosphorylation in mouse platelets preincubated with 10 μM MCsC (control peptide), 10 µM MPαC, and/or 2 mM PAR4 antagonist tcY-NH2 and then stimulated with 3 nM WT thrombin. LIMK1 phosphorylation was detected using an anti-LIMK1 phospho-Thr505/508 antibody. Loading was determined using a phosphorylation-independent anti-LIMK1 antibody. (I) A schematic of signaling-mediated cooperativity between GPIb-IX and PARs. This cooperativity requires a unique GPIb-IX–dependent signaling pathway involving 14-3-3. Rac1 and LIMK1 and activation of this pathway requires thrombin binding to GPIb-IX and stimulation of PARs.
To further investigate the mechanism of this cooperativity between GPIb-IX signaling and PAR signaling, we examined whether GPIb-IX–dependent activation of the Rac1-LIMK1 pathway requires cooperativity of PAR signaling. Pretreatment with a PAR1 antagonist in CHO cells or PAR4 antagonist in mouse platelets abolished the thrombin-induced GPIb-IX–dependent stimulation of LIMK1 activation (Figure 6G-H). Furthermore, the PPACK-treated S195A mutant thrombin failed to induce a dose-dependent increase in LIMK1 phosphorylation in human platelets (Figure 6F). These data suggest that thrombin-induced GPIb-IX signaling requires cooperativity of PAR signaling. Taken together, our data indicate that there is a mutual cooperativity between GPIb-IX signaling and PAR signaling during thrombin-induced platelet activation. PAR-dependent signaling is important for thrombin-induced GPIb-IX signaling, and thrombin-induced GPIb-IX signaling is important for stimulating PAR signaling, particularly at low thrombin concentrations. This signaling-mediated cooperativity provides a novel mechanism for the requirement of dual thrombin receptors (PARs and GPIb-IX) in mediating platelet response (Figure 6I).
Low concentrations of thrombin are generated in vivo and are important for thrombus formation
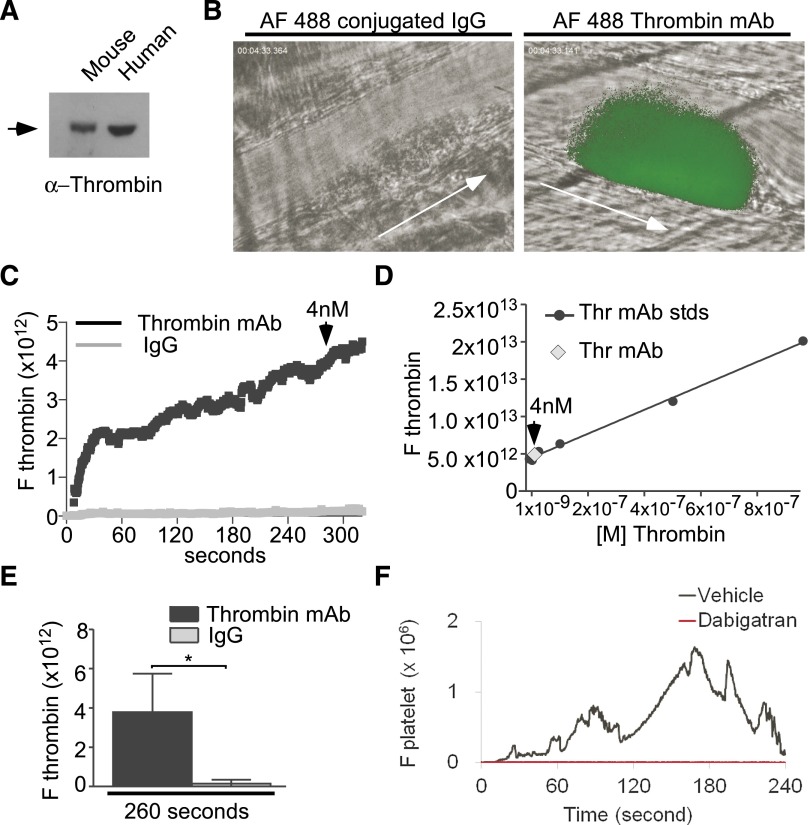
To investigate whether low concentrations of thrombin are important during thrombosis in vivo, we used a laser-induced cremaster arteriolar thrombosis model that has previously been shown to require thrombin activity.40,52,53 We estimated the approximate concentration of thrombin generated after laser injury using a saturating concentration of an Alexa Fluor 488 (AF 488)–labeled monoclonal antithrombin antibody, which is verified to selectively recognize both mouse and human α-thrombin (Figure 7A), but not prothrombin (as indicated by the manufacturer, also data not shown). We detected significant thrombin after laser injury by comparison with an AF 488–labeled immunoglobulin (Ig)G control antibody with identical fluorophore to antibody ratio (Figure 7B). Importantly, injection of the AF 488–labeled thrombin-specific antibody did not inhibit thrombus formation compared with IgG control (supplemental Figure 2; supplemental Movies 1 and 2). To quantify thrombin concentration, a standard curve of fluorescence intensity vs thrombin antibody concentration was generated under the same microscope settings. The kinetics of thrombin generation showed rapid increase after vessel injury and then plateaued around 5 minutes after injury (supplemental Figure 2; supplemental Movies 1 and 2). By comparing with the standard curve of known concentrations of labeled monoclonal thrombin antibody, we were able to approximate that thrombin concentrations present at the site of injury were <10 nM even at its plateau and is <3 nM during early platelet thrombus formation (Figure 7C-E). These findings are consistent with other methods used to estimate the concentration of thrombin generated in vivo.54 To verify the importance of these concentrations of thrombin in this thrombosis model, we further tested the effect of a direct thrombin inhibitor, dabigatran. This thrombin inhibitor nearly abolished platelet thrombus formation after the injury (Figure 7F; supplemental Figure 3). Taken together, these data suggest that low concentrations of thrombin are generated at the site of laser-induced arteriolar injury and are important for arterial thrombus formation in this mouse model of thrombosis.
Figure 7.
Low concentrations of thrombin are generated after vascular injury and are important for thrombosis in vivo. (A) Western blot detection of 0.2 mg/mL purified human and mouse α−thrombin protein using an antithrombin monoclonal antibody. (B) In vivo imaging of thrombin generation (green) during thrombosis captured using intravital microscopy after laser-induced cremaster arteriolar wall injury in wild-type mice injected with 2 mg/kg Alexa Fluor 488–conjugated antithrombin monoclonal antibody or an Alexa Fluor 488–conjugated control IgG. Arrows indicate the direction of blood flow. Also provided are movies of thrombin generation and platelet thrombus formation (supplemental Movies 1 and 2) and representative images of time-dependent changes in thrombin concentration and platelet thrombus formation (supplemental Figure 2). (C) Median integrated fluorescence intensity over time for Alexa Fluor 488–conjugated (AF 488) antithrombin antibodies and control IgGs. Based on the standard curve (D), 4 nM denotes the approximate concentration of thrombin generated after 260 seconds of laser injury. (D) A standard curve was computed using imaging of fluorescence intensities of known concentrations of AF 488–conjugated thrombin monoclonal antibody under equivalent intravital instrument settings as in A. (E) Quantification of integrated fluorescence intensity at 260 seconds (Mann Whitney U test,*P < .05; 30 thrombi in 4 mice per group). (F) Median integrated fluorescence intensity over time for wild-type mice infused with 0.3 mg/kg BW of dabigatran or vehicle control. The kinetics of platelet accumulation were plotted as the median fluorescence intensity as a function of time in 30 thrombi in 3 to 4 mice per group. For images, also see supplemental Figure 3.
Discussion
The seminal work of discovery and cloning of PARs established the knowledge that thrombin-induced cellular response requires thrombin-catalyzed cleavage and activation of PARs.1 This important advance also ignited debates about the role of the most abundant thrombin-binding membrane protein in platelets (and the “old” thrombin receptor), GPIb-IX, in thrombin-induced platelet activation. As several previous reports indicate that GPIb-IX is important in platelet response to thrombin,7,30,42,48 the debate is mainly about whether both GPIb-IX and PARs signal independently or GPIb-IX serves as a dock for thrombin by facilitating access and cleavage of PARs. Our data indicate that thrombin binding to GPIb-IX induces GPIb-IX–specific signaling that is important for the PAR-dependent platelet response to low-dose thrombin. However, our data also indicate that the thrombin-induced GPIb-IX signaling requires cooperativity of PAR signaling, and thus is not PAR independent. Therefore, we introduce a novel concept of signal-mediated cooperativity between GPIb-IX and PARs that drives platelet response to low-dose thrombin.
Studies on platelet response to thrombin are complicated by genetic differences between humans and mice. Human platelets express both PAR1 and PAR4, whereas mouse platelets express PAR3 and PAR4.2 Thus, the convincing evidence for the requirement of PAR4 in mouse platelet activation cannot be applied to human platelets in which PAR1 signals play an important role. To understand the relationship between human GPIb-IX and PAR1, we successfully reconstituted GPIb-IX/PAR1 cooperativity in response to thrombin in a CHO cell line expressing recombinant human GPIb-IX and PAR1 but not PAR4, which allowed us to study the mechanisms of cooperativity between GPIb and PAR1 using molecular biology. With this model, we were able to provide the first specific evidence of cooperativity between GPIb-IX and PAR1 in mediating calcium signaling induced by low-dose thrombin. Importantly, we show that the role of GPIb-IX in stimulating thrombin response not only requires the thrombin binding site of GPIbα, but also the C-terminal 14-3-3 binding site in the cytoplasmic domain of GPIbα that is important for activating the GPIb-IX–dependent Rac1/LIMK1 signaling pathway. Deletion of the C-terminal 14-3-3 binding site in GPIbα or blocking 14-3-3 binding using a specific membrane-permeable inhibitor based on the 14-3-3 binding sequence of GPIbα diminished the response to low-dose thrombin. Thus, the role of GPIb-IX in stimulating cellular response to thrombin is not due to its function as a passive dock (although we do not exclude that it may also function as a dock), but requires thrombin-induced GPIb-IX signaling. Importantly, an inhibitor of the GPIb–14-3-3 interaction similarly diminished platelet response to low-dose thrombin both in human and mouse platelets, indicating that a similar mechanism is involved in the cooperativity of GPIb-IX with PAR1 and PAR4 in response to thrombin both in human and mouse platelets. Thus, the different PAR combinations in different species does not appear to affect GPIb cooperativity with PARs. To examine the possible in vivo relevance of this cooperativity, we detected low concentrations of thrombin (<10 nM at peak, <3 nM at earlier stages) following laser-induced arteriolar injury and demonstrated its important role in laser-induced arteriolar thrombosis (Figure 7). Others54-56 suggested that low concentrations of thrombin were generated during systemic inflammation. However, we wish to point out that our estimation of thrombin levels is only limited to this particular model of thrombosis, and thrombin concentrations may vary in different thrombotic conditions depending on the extent of coagulation. Also, we do not claim that our estimation is exactly accurate due to the possibility of blockage of antibody access to a population of thrombin by competing molecules. However, as thrombus formation was not affected by this high affinity antibody (Figure 7), it is unlikely to competitively interact with important functional sites of thrombin. Thus, these data support the notion that the role of GPIb-IX in low-dose thrombin-induced platelet activation is relevant to certain types of in vivo thrombosis such as arterial thrombosis and systemic vascular inflammation.
In elucidating the importance of GPIb-IX signaling, we have not only demonstrated the importance of 14-3-3–dependent GPIb-IX signaling function, but also for the first time identified an important role for the GPIb-IX–dependent Rac1/LIMK1-mediated signaling pathway in thrombin-induced cell response. Although Rac1 and its downstream effector kinase LIMK1 can be activated by several different platelet agonists, including PAR agonist peptides,57 our data indicate that low-dose thrombin-induced activation of the Rac1-LIMK1 pathway is GPIb-IX dependent and GPIb-IX specific. First, we have shown that low-dose thrombin-induced activation of Rac1 and LIMK1 is diminished in the absence of GPIb-IX expression and in cells expressing mutant GPIbα with a 14-3-3 binding site deleted. Second, inhibition of 14-3-3 binding to GPIbα in either human or mouse platelets resulted in diminished activation of Rac1 and LIMK1. Importantly, the LIMK1 knockout platelets showed reduced response to low-dose thrombin, but showed enhanced response to PAR4 agonist peptide, excluding the stimulatory role of LIMK1 in PAR4-dependent platelet response. It is important to note that the GPIb-IX–dependent activation of Rac1 and LIMK1 can be induced by the binding of adhesive protein VWF to GPIb-IX independent of other agonist receptors.33,35 In contrast, thrombin is insufficient to induce significant activation of the Rac1-LIMK1 pathway without the cooperativity of PAR signaling.
The conclusion that thrombin-induced GPIb-IX signaling requires cooperativity of PAR signaling is supported by data that inhibition of either PAR1 in the reconstituted CHO cell model or PAR4 in platelets abolished thrombin-induced response and is also consistent with previous data that PAR4 knockout platelets do not respond to thrombin.4 The major evidence supporting thrombin-induced PAR-independent GPIb-IX signaling comes from data that S195A thrombin or PPACK-treated thrombin-induced platelet activation.31,32 However, we show that whereas S195A thrombin indeed induces platelet response, this effect is dependent on residual catalytic activity of S195A thrombin and is dependent on PARs. Also, in a previous report,31 the PPACK concentration used for thrombin treatment was lower than described to be needed for complete inhibition of thrombin activity.38 In our experiments, when either wild-type or S195A thrombin was treated with PPACK as previously described, platelet response was completely inhibited. Thus, our data, consistent with the data obtained with PAR4 knockout,4 suggests that thrombin-induced GPIb-IX–dependent platelet response requires PAR signaling. Together, our data indicate that GPIb-IX and PAR are mutually dependent to induce optimal platelet response to low concentrations of thrombin, which are important during in vivo thrombosis. This cooperativity requires a unique GPIb-IX–dependent signaling pathway involving Rac1 and LIMK1 and also requires PAR signaling. This novel finding not only provides a conceptual advance in our understanding of the mechanisms of cellular response to the important prothrombotic and proinflammatory protease, thrombin, but may also serve as the basis for developing new platelet selective antithrombotic and anti-inflammatory therapies targeting GPIb and PAR cooperativity.
Acknowledgments
The authors thank Drs Tatyana A. Voyno-Yasenetskaya and Zhengping Jia for providing the LIMK1 mice and Dr Radek Skoda (University Hospital Basel) and Dr Mark Ginsberg (University of California, San Diego) for providing Pf4-Cre mice.
This work was supported by National Institutes of Health, National Heart, Lung and Blood Institute grants HL062350, HL080264, HL125356 (to X.D.), and HL123319 (to B.E.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.E. performed experiments, created figures, analyzed data, and wrote the paper; K.K. and J.C. performed experiments, created figures, analyzed data, and provided valuable suggestions; A.S.-T., and M.K.D. performed experiments and provided valuable suggestions; B.S. provided valuable suggestions; Z.M.R. and C.R. provided reagents and valuable discussions and helped edit the paper; and X.D. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests. University of Illinois holds a patent relevant to the study (inventor, X.D.).
Correspondence: Xiaoping Du, Department of Pharmacology, University of Illinois College of Medicine, 835 South Wolcott Ave, Chicago IL 60612; e-mail: xdu@uic.edu.
References
- 1.Vu T-KH, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 2.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103(6):879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakanishi-Matsui M, Zheng Y-W, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404(6778):609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 4.Sambrano GR, Weiss EJ, Zheng Y-W, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413(6851):74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3(8):1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim S, Foster C, Lecchi A, et al. Protease-activated receptors 1 and 4 do not stimulate G(i) signaling pathways in the absence of secreted ADP and cause human platelet aggregation independently of G(i) signaling. Blood. 2002;99(10):3629–3636. doi: 10.1182/blood.v99.10.3629. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson GA, Okumura T. Reduced thrombin binding and aggregation in Bernard-Soulier platelets. J Clin Invest. 1978;61(3):861–864. doi: 10.1172/JCI109000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggeri ZM, Zarpellon A, Roberts JR, Mc Clintock RA, Jing H, Mendolicchio GL. Unravelling the mechanism and significance of thrombin binding to platelet glycoprotein Ib. Thromb Haemost. 2010;104(5):894–902. doi: 10.1160/TH10-09-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc Natl Acad Sci USA. 2000;97(6):2803–2808. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmeier W, Piffath CL, Goerge T, et al. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci USA. 2006;103(45):16900–16905. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S, Zuka M, Liu J, et al. Platelet glycoprotein Ib α supports experimental lung metastasis. Proc Natl Acad Sci USA. 2007;104(21):9024–9028. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corken A, Russell S, Dent J, Post SR, Ware J. Platelet glycoprotein Ib-IX as a regulator of systemic inflammation. Arterioscler Thromb Vasc Biol. 2014;34(5):996–1001. doi: 10.1161/ATVBAHA.113.303113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin H, Stojanovic-Terpo A, Xu W, et al. Role for platelet glycoprotein Ib-IX and effects of its inhibition in endotoxemia-induced thrombosis, thrombocytopenia, and mortality. Arterioscler Thromb Vasc Biol. 2013;33(11):2529–2537. doi: 10.1161/ATVBAHA.113.302339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez JA, Chung DW, Fujikawa K, Hagen FS, Davie EW, Roth GJ. The alpha and beta chains of human platelet glycoprotein Ib are both transmembrane proteins containing a leucine-rich amino acid sequence. Proc Natl Acad Sci USA. 1988;85(7):2135–2139. doi: 10.1073/pnas.85.7.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickey MJ, Williams SA, Roth GJ. Human platelet glycoprotein IX: an adhesive prototype of leucine-rich glycoproteins with flank-center-flank structures. Proc Natl Acad Sci USA. 1989;86(17):6773–6777. doi: 10.1073/pnas.86.17.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanza F, Morales M, de La Salle C, et al. Cloning and characterization of the gene encoding the human platelet glycoprotein V. A member of the leucine-rich glycoprotein family cleaved during thrombin-induced platelet activation. J Biol Chem. 1993;268(28):20801–20807. [PubMed] [Google Scholar]
- 17.Phillips DR, Agin PP. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977;252(6):2121–2126. [PubMed] [Google Scholar]
- 18.Du X, Beutler L, Ruan C, Castaldi PA, Berndt MC. Glycoprotein Ib and glycoprotein IX are fully complexed in the intact platelet membrane. Blood. 1987;69(5):1524–1527. [PubMed] [Google Scholar]
- 19.Modderman PW, Admiraal LG, Sonnenberg A, von dem Borne AE. Glycoproteins V and Ib-IX form a noncovalent complex in the platelet membrane. J Biol Chem. 1992;267(1):364–369. [PubMed] [Google Scholar]
- 20.Dong JF, Gao S, López JA. Synthesis, assembly, and intracellular transport of the platelet glycoprotein Ib-IX-V complex. J Biol Chem. 1998;273(47):31449–31454. doi: 10.1074/jbc.273.47.31449. [DOI] [PubMed] [Google Scholar]
- 21.Gu M, Xi X, Englund GD, Berndt MC, Du X. Analysis of the roles of 14-3-3 in the platelet glycoprotein Ib-IX-mediated activation of integrin alpha(IIb)beta(3) using a reconstituted mammalian cell expression model. J Cell Biol. 1999;147(5):1085–1096. doi: 10.1083/jcb.147.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramakrishnan V, Reeves PS, DeGuzman F, et al. Increased thrombin responsiveness in platelets from mice lacking glycoprotein V. Proc Natl Acad Sci USA. 1999;96(23):13336–13341. doi: 10.1073/pnas.96.23.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X. Signaling and regulation of the platelet glycoprotein Ib-IX-V complex. Curr Opin Hematol. 2007;14(3):262–269. doi: 10.1097/MOH.0b013e3280dce51a. [DOI] [PubMed] [Google Scholar]
- 24.Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84(2):289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 25.Andrews RK, Fox JE. Interaction of purified actin-binding protein with the platelet membrane glycoprotein Ib-IX complex. J Biol Chem. 1991;266(11):7144–7147. [PubMed] [Google Scholar]
- 26.Du X, Harris SJ, Tetaz TJ, Ginsberg MH, Berndt MC. Association of a phospholipase A2 (14-3-3 protein) with the platelet glycoprotein Ib-IX complex. J Biol Chem. 1994;269(28):18287–18290. [PubMed] [Google Scholar]
- 27.Du X, Fox JE, Pei S. Identification of a binding sequence for the 14-3-3 protein within the cytoplasmic domain of the adhesion receptor, platelet glycoprotein Ib alpha. J Biol Chem. 1996;271(13):7362–7367. doi: 10.1074/jbc.271.13.7362. [DOI] [PubMed] [Google Scholar]
- 28.Bodnar RJ, Gu M, Li Z, Englund GD, Du X. The cytoplasmic domain of the platelet glycoprotein Ibalpha is phosphorylated at serine 609. J Biol Chem. 1999;274(47):33474–33479. doi: 10.1074/jbc.274.47.33474. [DOI] [PubMed] [Google Scholar]
- 29.Dai K, Bodnar R, Berndt MC, Du X. A critical role for 14-3-3zeta protein in regulating the VWF binding function of platelet glycoprotein Ib-IX and its therapeutic implications. Blood. 2005;106(6):1975–1981. doi: 10.1182/blood-2005-01-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J Biol Chem. 2001;276(7):4692–4698. doi: 10.1074/jbc.M008160200. [DOI] [PubMed] [Google Scholar]
- 31.Adam F, Guillin M-C, Jandrot-Perrus M. Glycoprotein Ib-mediated platelet activation. A signalling pathway triggered by thrombin. Eur J Biochem. 2003;270(14):2959–2970. doi: 10.1046/j.1432-1033.2003.03670.x. [DOI] [PubMed] [Google Scholar]
- 32.Ramakrishnan V, DeGuzman F, Bao M, Hall SW, Leung LL, Phillips DR. A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V. Proc Natl Acad Sci USA. 2001;98(4):1823–1828. doi: 10.1073/pnas.98.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delaney MK, Liu J, Zheng Y, Berndt MC, Du X. The role of Rac1 in glycoprotein Ib-IX-mediated signal transduction and integrin activation. Arterioscler Thromb Vasc Biol. 2012;32(11):2761–2768. doi: 10.1161/ATVBAHA.112.254920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng Y, Zhang Y, Tregoubov V, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35(1):121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 35.Estevez B, Stojanovic-Terpo A, Delaney MK, et al. LIM kinase-1 selectively promotes glycoprotein Ib-IX-mediated TXA2 synthesis, platelet activation, and thrombosis. Blood. 2013;121(22):4586–4594. doi: 10.1182/blood-2012-12-470765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delaney MK, Liu J, Kim K, et al. Agonist-induced platelet procoagulant activity requires shear and a Rac1-dependent signaling mechanism. Blood. 2014;124(12):1957–1967. doi: 10.1182/blood-2014-03-560821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen B, Zhao X, O’Brien KA, et al. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature. 2013;503(7474):131–135. doi: 10.1038/nature12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greco NJ, Tenner TE, Jr, Tandon NN, Jamieson GA. PPACK-thrombin inhibits thrombin-induced platelet aggregation and cytoplasmic acidification but does not inhibit platelet shape change. Blood. 1990;75(10):1989–1990. [PubMed] [Google Scholar]
- 39.Harmon JT, Jamieson GA. Thrombin binds to a high-affinity approximately 900 000-dalton site on human platelets. Biochemistry. 1985;24(1):58–64. doi: 10.1021/bi00322a010. [DOI] [PubMed] [Google Scholar]
- 40.Shen B, Estevez B, Xu Z, et al. The interaction of Gα13 with integrin β1 mediates cell migration by dynamic regulation of RhoA. Mol Biol Cell. 2015;26(20):3658–3670. doi: 10.1091/mbc.E15-05-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akbar H, Kim J, Funk K, et al. Genetic and pharmacologic evidence that Rac1 GTPase is involved in regulation of platelet secretion and aggregation. J Thromb Haemost. 2007;5(8):1747–1755. doi: 10.1111/j.1538-7836.2007.02646.x. [DOI] [PubMed] [Google Scholar]
- 42.De Marco L, Mazzucato M, Masotti A, Ruggeri ZM. Localization and characterization of an alpha-thrombin-binding site on platelet glycoprotein Ib alpha. J Biol Chem. 1994;269(9):6478–6484. [PubMed] [Google Scholar]
- 43.Marchese P, Murata M, Mazzucato M, et al. Identification of three tyrosine residues of glycoprotein Ib alpha with distinct roles in von Willebrand factor and alpha-thrombin binding. J Biol Chem. 1995;270(16):9571–9578. doi: 10.1074/jbc.270.16.9571. [DOI] [PubMed] [Google Scholar]
- 44.Zarpellon A, Celikel R, Roberts JR, et al. Binding of alpha-thrombin to surface-anchored platelet glycoprotein Ib(alpha) sulfotyrosines through a two-site mechanism involving exosite I. Proc Natl Acad Sci USA. 2011;108(21):8628–8633. doi: 10.1073/pnas.1017042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward CM, Andrews RK, Smith AI, Berndt MC. Mocarhagin, a novel cobra venom metalloproteinase, cleaves the platelet von Willebrand factor receptor glycoprotein Ibalpha. Identification of the sulfated tyrosine/anionic sequence Tyr-276-Glu-282 of glycoprotein Ibalpha as a binding site for von Willebrand factor and alpha-thrombin. Biochemistry. 1996;35(15):4929–4938. doi: 10.1021/bi952456c. [DOI] [PubMed] [Google Scholar]
- 46.Cattaneo M, Canciani MT, Lecchi A, et al. Released adenosine diphosphate stabilizes thrombin-induced human platelet aggregates. Blood. 1990;75(5):1081–1086. [PubMed] [Google Scholar]
- 47.Brass LF, Joseph SK. A role for inositol triphosphate in intracellular Ca2+ mobilization and granule secretion in platelets. J Biol Chem. 1985;260(28):15172–15179. [PubMed] [Google Scholar]
- 48.Greco NJ, Tandon NN, Jones GD, et al. Contributions of glycoprotein Ib and the seven transmembrane domain receptor to increases in platelet cytoplasmic [Ca2+] induced by alpha-thrombin. Biochemistry. 1996;35(3):906–914. doi: 10.1021/bi951503y. [DOI] [PubMed] [Google Scholar]
- 49.Gralnick HR, Williams S, McKeown LP, Hansmann K, Fenton JW, II, Krutzsch H. High-affinity alpha-thrombin binding to platelet glycoprotein Ib alpha: identification of two binding domains. Proc Natl Acad Sci USA. 1994;91(14):6334–6338. doi: 10.1073/pnas.91.14.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang N, Higuchi O, Ohashi K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393(6687):809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 51.Wu QY, Sheehan JP, Tsiang M, Lentz SR, Birktoft JJ, Sadler JE. Single amino acid substitutions dissociate fibrinogen-clotting and thrombomodulin-binding activities of human thrombin. Proc Natl Acad Sci USA. 1991;88(15):6775–6779. doi: 10.1073/pnas.88.15.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 2006;107(10):3902–3906. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117(4):953–960. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoldhelyi P, Chesebro JH, Owen WG. Hirudin as a molecular probe for thrombin in vitro and during systemic coagulation in the pig. Proc Natl Acad Sci USA. 1993;90(5):1819–1823. doi: 10.1073/pnas.90.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14(1):55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21(3):131–142. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Hartwig JH, Bokoch GM, Carpenter CL, et al. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82(4):643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]