Abstract
Aims
To evaluate the prevalence of significant aortic valve stenosis (AS) in a randomly selected study population of elderly individuals representing the general population of Iceland. Furthermore, to predict the number of individuals likely to have severe AS in the future.
Methods and results
Echocardiography and computed tomography (CT) data from individuals who participated in the AGES-Reykjavik study were used. Echocardiography data from 685 individuals (58% females) aged 67–95 years were available. In both sexes combined, the prevalence for severe AS, defined as an aortic valve area index of <0.6 cm2/m2, in the age groups <70, 70–79 and ≥80 years was 0.92%, 2.4% and 7.3%, respectively. A ROC analysis on the relation between the echocardiography data and the aortic valve calcium score on CT defined a score ≥500 to be indicative of severe AS. Subsequently, in a CT study cohort of 5256 individuals the prevalence of severe AS in the same age groups was 0.80%, 4.0% and 9.5%, respectively. Overall, the prevalence of severe AS by echocardiography and CT in individuals ≥70 years was 4.3% and 5.9%, respectively. A prediction on the number of elderly ≥70 years for the coming decades demonstrated that patients with severe AS will have increased 2.4 fold by the year 2040 and will more than triple by the year 2060.
Conclusion
This study, in a cohort of elderly individuals representative of the general population in a Nordic country, predicts that AS will be a large health problem in the coming decades.
Keywords: aortic valve stenosis, epidemiology, prevalence, elderly, future prediction
INTRODUCTION
Aortic valve stenosis (AS) is the most prevalent valvular disease in the western hemisphere and the one most often leading to aortic valve implantation (AVI) [1]. The prevalence of AS has been assessed in large-scale surveys and in epidemiological studies in various populations [1–5]. The prevalence of AS increases with age and, in line with the rapidly increasing elderly population in the industrialized countries, the number of patients diagnosed with AS will increase considerably [3, 5]. A proportion of these patients will, if suitable, need AVI either by open surgery or transcatheter aortic valve implantation (TAVI) [6, 7]. Often these elderly patients with AS will have high-risk co-morbidities that contraindicate open surgery but still make them candidates for TAVI. Many, however, will be too ill and frail for either procedure and will need demanding treatment for heart failure, atrial fibrillation and coronary artery disease [8–10].
The increasing number of elderly with AS in the coming decades will be a huge therapeutic challenge and impose a considerable economical burden on the health care systems of the western countries. Attempts have been made to evaluate the future need for open AVI and TAVI. These are based on epidemiological data from different populations that have used various methods and definitions for AS, thus yielding conflicting results [5, 7].
The aim of the current study was to assess the prevalence of severe AS in a well defined and randomly selected elderly population study cohort, representative of the total population of Iceland [11]. Both echocardiography and computed tomography data were used. Official government data predicting the size, sex and age distribution of the national population for the coming decades were also used [12]. By combining these data an evaluation was done to assess the number of patients likely to have severe AS in the future, potentially needing open AVI or TAVI.
METHODS
Study population
The Age, Gene/Environment Susceptibility (AGES)-Reykjavík study was started in 2002 as collaboration between the National Institute on Aging in the United States (NIA) and the Icelandic Heart Association (IHA). Its aim is to examine genetic susceptibility and gene/environment interactions, as these contribute to phenotypes common in old age. The AGES-Reykjavík study is a subset of the larger population based Reykjavík study who’s aim was to prospectively assess risk factors for cardiovascular disease in the Icelandic population [13].
The IHA established the Reykjavik Study in 1967 as a prospective epidemiological survey on cardiovascular disease and its risk factors. The population in Iceland is of Nordic origin and ethnically relatively homogeneous. The population now numbers somewhat less than one third of a million people, of which more than half lives in the Reykjavík capital area. The standard of living is similar to that of the other Scandinavian countries. The study was longitudinal, running from 1967 to 1994 and collected mid-life data on clinical and biochemical cardiovascular traits from a population based sample of 30.795 randomly selected individuals borne in the years 1907–35 (age range 45 to 74 years), residing in the Reykjavík area. A random sample of 27.281 persons was invited to participate during different stages (I–V) and 19.381 individuals entered the study and attended examinations. Overall, the participation rate in the study was 72% for men and 80% for women [13].
The AGES-Reykjavík study randomly selected 8.030 individuals from the surviving 11.459 members of the original Reykjavik Study cohort (now 67 years and older) to assess quantitative traits related to diseases and conditions of old age, and collected genetic and other biologic specimens. To better define phenotypes, molecular markers and modern imaging techniques such as echocardiography, computed tomography (CT) and magnetic resonance, were also used. Incorporation of these methods in conjunction with data already available in the Reykjavik Study greatly improved phenotyping for association studies. Recruitment to the study was finalized in January 2006. A total of 5764 participants (58% women) were recruited to the AGES-Reykjavik Study and the response rate was 72%. The AGES-Reykjavík study has been approved by the National Bioethics Committee in Iceland, in accordance with the Helsinki Declaration and by the National Institute on Aging Intramural Institutional Review Board. Informed consent was obtained for all participants [13]. Official data from the “Statistics Iceland” institution were used to obtain information about the current size, age and sex distribution of the Icelandic population. Prediction for these same factors in to the sixth decade of the 21 century is officially available on-line from the institution [12].
Echocardiography
Overall, 685 participants had suitable echocardiography examinations for assessment of the aortic valve. None of these had previously undergone AVI. Their age ranged from 67 to 95 years (mean 76 ± 6 years) and 58% were women. The participants underwent 2-D and M-mode echocardiography, and pulsed-, continuous wave and colour Doppler, in accordance with a protocol applying with the guidelines of the American Society of Echocardiography, as previously detailed [13]. The examinations were done by a certified echo technician using an Acuson Sequoia echocardiography machine with a 3V2c transducer. The data were digitally stored and the examinations read by a cardiologist experienced in echocardiography. A random selection of examinations was also sent to a reference core laboratory at the National Institute of Health, Bethesda, USA, for quality assessment.
The morphology of the aortic valve was noted and the amount of calcification graded. Both the peak velocity in the left ventricular outflow tract and the maximal velocity across the aortic valve were measured from the apical position, with pulsed and continuous wave Doppler, respectively. The diameter of the left ventricular outflow tract was measured from the parasternal long-axis position. The maximum and mean gradients across the aortic valve were calculated from the maximal velocity spectrum using standard machine software and the aortic valve area (AVA) calculated using the continuity equation. The AVA, indexed for body surface area, was used as a standardized estimate of the AS severity. None/mild, moderate and severe AS was defined as >0.85, 0.6–0.85, and <0.6 cm2/m2, respectively [14].
Computed tomography
Data available for assessment of aortic valve calcification was available from 5262 participants, but six individuals had previously undergone AVI. Although it did not significantly influence prevalence or statistical evaluations, for the sake of clarity, they were excluded from the current study cohort, which consists of 5256 individuals (58% females) whose age ranged from 67 to 96 years (mean 76 ± 6 years). Images for calcium scoring of the aortic valve were obtained using a Siemens Somatom Sensation 4 multi-detector CT scanner with prospective ECG triggering set at 50% of the cardiac R-R interval, as previously described in detail [11]. Calcified lesions were assessed using standard Agatston methods where by three or more contiguous pixels with a brightness of at least 130 Hounsfield units (HU) were taken to indicate the presence of calcium. The Agatston score was then calculated by multiplying the lesion area with the density factor derived from the maximal HU within the area. The density factor was defined as follows: 1 = 130–199, 2 = 200–299, 3 = 300–399 and 4 = >400 HU. A total calcium score was determined by summing the score for each lesion. Lesions were classified as aortic valve calcification if they were within the leaflets or commissures, excluding the aortic annulus, proximal aorta and coronary arteries [11]. The association between the AVA index by echocardiography and the aortic valve calcium score (AVCS) on CT was assessed by ROC analysis to find a score value indicating severe AS [15].
Statistics
Univariate comparison between two groups were done using a two-tailed, unpaired Student’s t-test or Fischers exact test, as appropriate. The association between the AVA index on echocardiography and the AVCS on CT was evaluated by ROC analysis and sensitivity and specificity defined. A two-tailed p value <0.05 was considered statistically significant. The data analysis for this paper was generated using SAS/STAT software, Version 9.2 of the SAS System [15].
RESULTS
Echocardiography
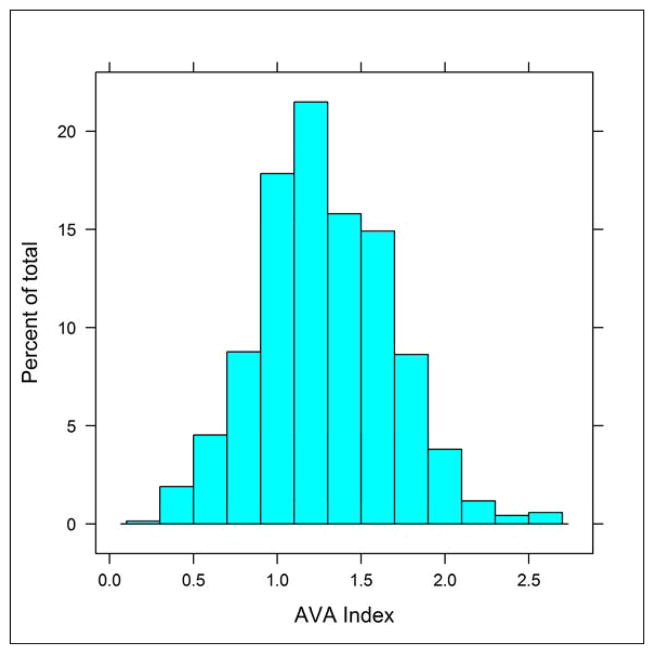
The main descriptive echocardiographic variables are presented in table 1. Of interest, in comparison to individuals with none to moderate AS, those with severe AS (AVA index <0.6 cm2/m2) were older, had a higher left ventricular mass index, a smaller left ventricular outflow tract diameter and a lower stroke volume index. The left ventricular ejection fraction, however, was similar in the groups. The distribution of the AVA index in the total cohort is shown in figure 1. The prevalence for moderate and severe AS in both sexes combined and according to age groups is shown in table 2. Overall, the prevalence of severe AS by echocardiography in individuals ≥70 years was 4.1% in men, 4.5% in women and 4.3% in both sexes combined.
Table 1.
Descriptive echocardiographic variables.
| Variable | AVA index ≥0.6 cm2/m2 n = 659 |
AVA index <0.6 cm2/m2 n = 26 |
P-value |
|---|---|---|---|
| Males (%) | 42.0 | 42.3 | 1.0 |
| Age (years) | 75.8 (5.7) | 79.7 (4.7) | 0.0006 |
| BSA (m2) | 1.84 (0.20) | 1.84 (0.17) | 1.0 |
| LV end-diastolic diameter (cm) | 4.42 (0.65) | 4.35 (0.44) | 0.59 |
| LV end-systolic diameter (cm) | 2.96 (0.66) | 2.98 (0.44) | 0.88 |
| LV mass index (g/m2) | 89.8 (25.0) | 100.7 (20.3) | 0.029 |
| LV ejection fraction (%) | 62.4 (7.0) | 62.0 (3.8) | 0.77 |
| LVOT diameter (cm) | 2.03 (0.28) | 1.78 (0.25) | <0.0001 |
| LVOT peak velocity (cm/s) | 97.5 (19.2) | 91.4 (18.6) | 0.11 |
| LVOT velocity integral (cm) | 20.8 (5.5) | 20.9 (5.3) | 0.85 |
| Stroke volume index (ml/m2) | 36.4 (10.0) | 28.8 (9.7) | 0.0002 |
| Aortic valve calcification grade (0–3) | 0.85 (0.74) | 1.52 (0.98) | <0.0001 |
| Peak aortic valve velocity (cm/s) | 134.3 (34.3) | 263.0 (76.6) | <0.0001 |
| Aortic valve velocity integral (cm) | 28.3 (7.8) | 57.8 (20.7) | <0.0001 |
| Peak aortic valve gradient (mmHg) | 7.7 (4.5) | 29.9 (15.4) | <0.0001 |
| Mean aortic valve gradient (mmHg) | 3.5 (2.1) | 14.1 (7.7) | <0.0001 |
| Aortic valve area (cm2) | 2.43 (0.74) | 0.89 (0.16) | <0.0001 |
| AVA index (cm2/m2) | 1.32 (0.36) | 0.49 (0.09) | <0.0001 |
AVA = aortic valve area, BSA = body surface area, LV = left ventricular, LVOT = left ventricular outflow tract.
Figure 1.
The distribution of the aortic valve area (AVA) index (cm2/m2) in the echocardiography study cohort.
Table 2.
The prevalence (%) of moderate and severe aortic stenosis by echocardiography in both sexes combined.
| Age group | Moderate | Severe |
|---|---|---|
| <70 years | 6.42 | 0.92 |
| 70–79 years | 8.94 | 2.44 |
| ≥80 years | 8.70 | 7.33 |
Moderate and sever aortic stenosis defined as 0.6–0.85 and <0.6 cm2/m2, respectively.
Computed tomography
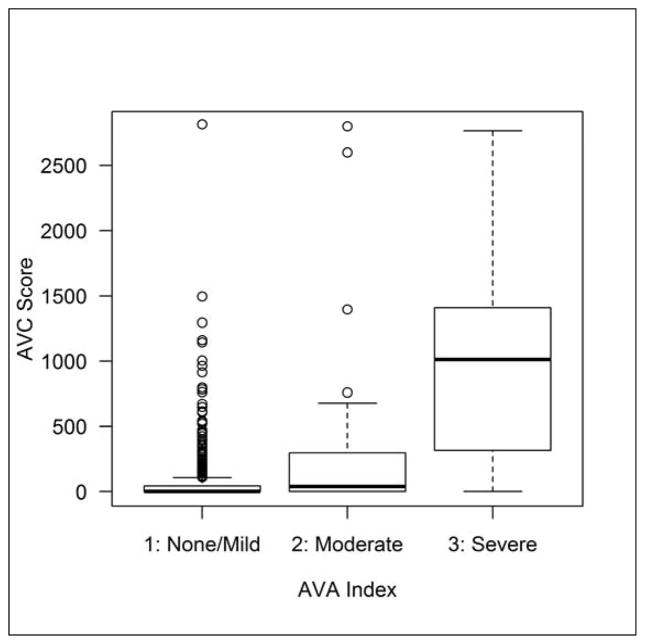
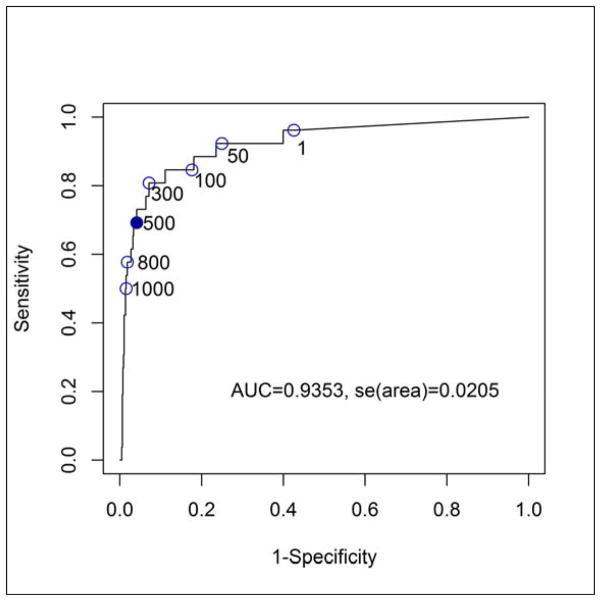
The relationship between the categorically graded AVA index by echocardiography and the AVCS on CT is shown in figure 2. A ROC analysis to establish the AVCS signifying severe AS (AVA index <0.6 cm2/m2) defined a score of ≥500 with a sensitivity and specificity of 70% and 95%, respectively (Fig. 3). A comparison of the prevalence for severe AS determined by echocardiography and by CT is shown in table 3. Overall, in individuals ≥70 years, the prevalence was estimated to be 8.5 % in men, 4.0% in women and 5.9% in both genders.
Figure 2.
The relationship between categorically graded aortic valve area (AVA) index by echocardiography and the aortic valve calcium (AVC) score by computed tomography.
Figure 3.
A ROC analysis to establish the aortic valve calcium score on computed tomography that signifies severe aortic stenosis by echocardiography (AVA index <0.6 cm2/m2) defines a score of ≥500 with a sensitivity of 70% and specificity of 95%.
Table 3.
Prevalence (%) of severe aortic stenosis assessed by echocardiography (Echo) and computed tomography (CT) in both sexes combined.
| Echo | CT | |
|---|---|---|
| 67–69 years | 0.92 | 0.80 |
| 70–79 years | 2.44 | 4.00 |
| ≥80 years | 7.33 | 9.50 |
Severe aortic stenosis defined by echocardiography as an aortic valve area index <0.6 cm2/m2 and by computed tomography as an aortic valve calcium score ≥500.
Baseline characteristics of participant groups with an AVCS <500 and ≥500 are shown in table 4. Participants with a score ≥500 were more often men, older, higher, heavier and thus had a larger body surface are. Their systolic blood pressure was higher, they were more likely to be taking anti-hypertensive medication and more often tended to have diabetes. These subjects also had higher cholesterol levels and were more often on statins. Clinically, compared to the group with a score <500 those with a score ≥500 more frequently had angina pectoris and a history of heart failure, while the presence of atrial fibrillation was similar. Furthermore, the latter group more often had a history of coronary artery bypass surgery or any prior coronary event and also had slightly worse renal function.
Table 4.
Baseline characteristics of study subjects.
| Variable | Aortic calcium score <500 n = 4919 |
Aortic calcium ≥500 n = 282 |
P-value |
|---|---|---|---|
| Males (%) | 41.5 | 62.1 | <0.0001 |
| Age (years) | 76.4 (5.5) | 79.8 (5.4) | <0.0001 |
| Height (cm) | 166.9 (9.4) | 168.9 (9.6) | <0.0001 |
| Weight (Kg) | 75.5 (14.5) | 77.4 (15.3) | 0.0362 |
| BSA (m2) | 1.84 (0.20) | 1.87 (0.21) | 0.0146 |
| BMI (kg/m2) | 27.0 (4.4) | 27.1 (4.5) | 0.97 |
| Systolic BP (mmHg) | 142.3 (20.3) | 145.5 (21.2) | 0.0104 |
| Diastolic BP (mmHg) | 73.9 (9.7) | 74.7 (9.8) | 0.1471 |
| On anti-hypertensive drugs (%) | 63.0 | 74.5 | <0.0001 |
| Known diabetes (%) | 11.7 | 15.7 | 0.0582 |
| Smoker (%) | 12.2 | 12.4 | 0.8519 |
| Se-Total cholesterol (mmol/l) | 5.6 (1.2) | 5.4 (1.2) | 0.0032 |
| Se-Triglycerides (mmol/l ) | 1.20 (0.66) | 1.18 (0.64) | 0.62 |
| On statins (%) | 22.2 | 29.8 | 0.0042 |
| History of angina pectoris (%) | 25.6 | 28 | 0.3631 |
| Has angina pectoris (Rose) (%) | 2.3 | 4.3 | 0.0447 |
| History of heart failure (%) | 3.1 | 5.7 | 0.0235 |
| In atrial fibrillation (%) | 7.1 | 8.2 | 0.4763 |
| CABG before (%) | 6.3 | 10.6 | 0.0086 |
| PCI before (%) | 6.8 | 7.4 | 0.6279 |
| MI before (%) | 7.7 | 11 | 0.0528 |
| Any prior coronary event (%) | 14.9 | 20.9 | 0.0081 |
| GFR (mL/min/1.73m2) | 64.3 (16.5) | 62.2 (14.6) | 0.0304 |
Any prior coronary event = MI, PCI, CABG. BMI = body mass index, BSA = body surface area, CABG = coronary artery bypass graft., ECG= electrocardiogram, GFR = glomerular filtration rate, MI = myocardial infarct, PCI = percutaneous coronary intervention.
Predicting the future
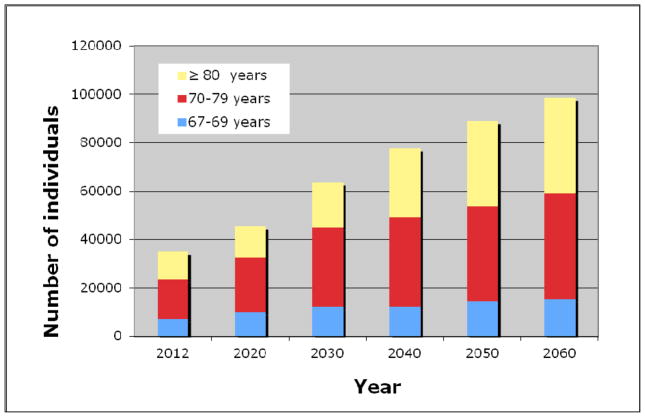
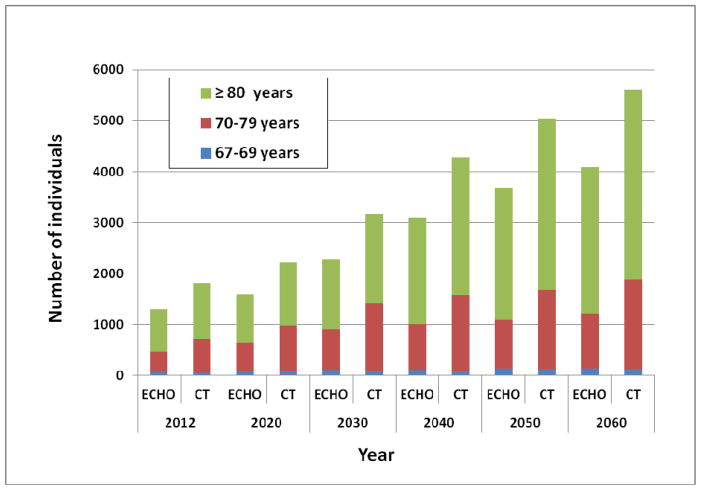
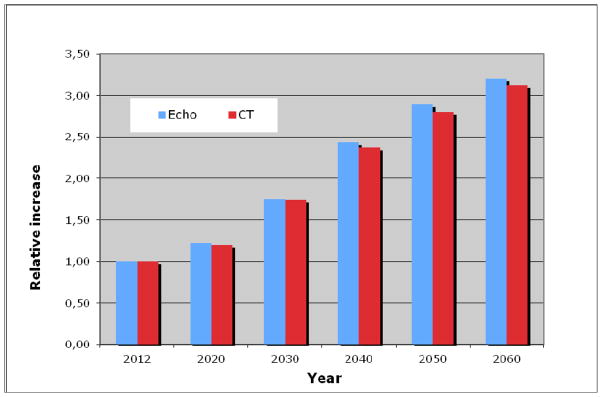
Based on data from the “Statistics Icelandic” institution, the number of elderly according to age groups was estimated for the coming decades, until 2060 (Fig. 4). The largest increase will be in the age groups 70–79 years and 80 years and older. The number of elderly individuals ≥70 years predicted to have severe AS based on the prevalence found by echocardiography will increase rapidly, and even more so if a prediction by CT data is used (Fig. 5). The number of elderly individuals ≥70 years predicted to have severe AS by echocardiography will increase from 1230 in the year 2012 to 2989 and 3954 in the years 2040 and 2060, respectively. By CT assessment in the same age category, 1762 individuals had severe AS in 2012, and their number is predicted to increase to 4184 and 5495 in the years 2040 and 2060, respectively. The relative increase in the number of individuals with severs AS, estimated by both imaging modalities for the coming decades is further shown in figure 6. Thus, the number of individuals with severe AS is predicted to increase 2.4 fold by the year 2040 and will likely more than triple by the year 2060.
Figure 4.
The predicted number of elderly in Iceland in the coming decades according to age groups.
Figure 5.
The number of individuals 67 years and older in the sexes combined predicted to have severe aortic stenosis based on the prevalence found by echocardiography (ECHO) and computed tomography (CT).
Figure 6.
The relative increase in the coming decades in the number of individuals of both sexes combined with severe aortic stenosis, defined by echocardiography (ECHO) as an aortic valve area index <0.6 cm2/m2 and by computed tomography (CT) as an aortic valve calcium score ≥500. Baseline in the year 2012 is set as 1, when 1230 individuals by ECHO and 1762 by CT were estimated to have severe aortic stenosis.
DISCUSSION
The main strength of the current study is that it was conducted in a randomly selected study cohort representative of the Icelandic people, who are a well a defined, homogeneous white population of Nordic European origin. The participation rate in this study is high and the recruitment process minimizes the likelihood of selection bias [13]. Previous studies on the prevalence of AS have been done by large scale surveys and epidemiological studies in various populations [1–5]. Many of these studies are conducted on a selected patient population and do not represent the overall spectrum of the disease. Some cross sectional studies have been carried out in defined populations, but also in these studies a certain selection process has occurred [2–4]. A recent meta-analysis has pointed out these huge discrepancies in the estimated prevalence of AS in various studies [5].
Another problem with assessing the prevalence of AS is the method used and the way significant AS is defined. Many previous echocardiography studies have used different measures to detect AS; the velocity across the valve, maximum or mean gradients and calculations of the aortic valve area, most often by the continuity equation [2, 3, 5]. Many of these previous studies included all patients with mild to severe AS; a few have attempted to define severe AS more strictly, but still using different criteria even when applying to current guidelines for defining the severity of AS [14]. There are several pitfalls in using only velocities or gradients across the aortic valve to define the severity of AS. A low gradient can be found in patients with impaired left ventricular function and also in those with paradoxical low flow low gradients in spite of a normal ejection fraction [7, 16]. This is in fact apparent in the current study in the group with severe AS. Using the aortic valve area is a more strict way of assessing the severity of AS, although the continuity equation most often used also has intrinsic errors [18, 19]. The velocity across the valve can differ depending on from which thoracic window the aortic valve jet is scrutinized and this may cause the gradient to be underestimated. Errors in measuring the diameter of the left ventricular outflow tract are well known and it has been suggested that using three-dimensional echo can give a better assessment of the area of the left ventricular outflow tract used in the continuity equation [20]. Problems with pressure recovery and it’s relation to the diameter of the proximal aorta has led to the suggestion that in some selected cases calculating the AVA by using the energy loss index can be a more accurate approach [19]. It also has to be kept in mind that assessment of the AVA from the Gorlin formula by heart catheterization, previously used as the gold standard for echo and Doppler studies, also has several pitfalls [18, 19]. Irrespective of how the AVA is calculated it has been proposed that to index the valve area for body surface area is the most accurate method to standardize assessment of the severity of the aortic valve stenosis [14]. In the current study we therefore decided to use the AVA index in the echocardiography part of the study to assess the severity of AS. In this study we focus mainly on severe AS in patients that will most likely need AVI, either by surgery or the transcatheter approach.
In the current study we first looked at the prevalence of severe AS in an echocardiography subgroup. In both sexes combined the prevalence in individuals 70 years and older was found to be 4.3%. The prevalence of severe AS in this age group reported in previous studies has a wide range. Thus, in a recent meta-analysis in patients over 75 years old the average prevalence of severe AS was found to be 3.4%, but the range was wide from 1.2% to 6.1 % [5]. The definitions for severe AS included in this meta-analysis, however, varied widely. None of the studies used the AVA index, which is a more strict way of assessing the severity of AS and therefore could give a higher prevalence rate.
The current AGES-Reykjavik study includes diverse data obtained by CT in elderly individuals, including aortic valve calcium quantification [11]. To further explore these data a comparison was made between echocardiography and CT data in an unselected population to determine an AVCS that would indicate severe AS. Because the current study population is randomly selected and has a wide spectrum of aortic valve sclerosis and stenosis, a relatively low AVCS was found to indicate significant AS in comparison to some previous studies [21, 22]. These studies, on the other hand, have done a comparison between assessment of the haemodynamic severity of AS and the grade of calcification in the aortic valve by CT on relatively small selected populations, most of whom had severe AS. The finding of much higher calcium score values, usually over 1600, that indicate significant AS in these studies is therefore to be expected. In the current study the AVCS on CT gave a higher prevalence of severe AS than found by echocardiography, in particular in individuals 80 years or older. It can be argued that using a relative low calcium score can lead to an overestimation of the prevalence. On the other hand, using too high a calcium score probably leads to an underestimations. Furthermore, a sensitivity of 70% in the current study for the AVCS value on CT defined to signify severe AS may possibly also lead to an underestimation of the prevalence of AS.
By CT in the current study, more calcium was usually found in the aortic valve in men than in women, especially in those 80 years and older. Furthermore, by CT the prevalence of severe AS was higher in men than in women. This is reflected in the comparison of the groups with a calcium score of less and of more than 500. The latter group is dominated by men; the individuals are on the average older and have more cardiovascular risk factors and co-morbidities. In another AGES-Reykjavik study on largely the same population, assessment of the coronary artery calcium distribution by CT also found higher calcium scores in men than in women [11].
Echocardiography is the first imaging modality to be used when suspecting AS in a patient and gives the best overall evaluation of the aortic valve disease, left ventricular function and other coexisting cardiac abnormalities [14]. In selected cases, such as in patients with paradoxical low flow AS or low gradients due to a poor left ventricular function, the use of CT for assessment of the calcium in the aortic valve can be of help in judging the severity of the aortic valve disease [22]. CT is also helpful in assessing patients who are potential candidates for TAVI, both in assessing the valve, the annulus and the descending part of aorta. Routine use of CT to assess patients suspected of AS is, however, not recommended [14]. With the increasing use of CT to evaluate coronary arteries and other intrathoracic diseases calcium in the aortic valve will be noticed [23]. The current study suggest that a calcium score of 500 or higher should be an indication for further assessment by echocardiography, in contrast to the much higher scores found in other studies.
This study, whether using echocardiography or CT data, highlights the increasing number of elderly that are expected to have significant AS in the coming decades. Some studies already indicate that a relatively large number of patients are undertreated as regards AVI by surgery or TAVI [5, 8]. In Iceland the only hospital doing valve implantations is Landspitali, the University Hospital in Reykjavík. During the year 2012 a total of 36 patients older than 67 years had an aortic valve implanted, 21 by surgery and 15 patients by TAVI. This number of operations is strikingly low in comparisons to the estimated number in the current study of patients with severe AS. Several epidemiological studies and surveys clearly indicate that many elderly patients with symptomatic significant AS are not even evaluated for AVI by their attending general practitioner or cardiologist [1, 5, 8]. Clinical symptoms in the elderly with AS are often not specific and do not always correlate well with the severity of AS [24]. With the rapid increase of TAVI procedures doctors may now be more aware of the improved possibility of AVI, even in those previously considered surgically inoperable. Currently the TAVI procedure is mainly indicated in patients with severe AS considered at too high a risk to undergo a surgical implantation. There is, however, already a trend to lower the threshold for TAVI to patients with low and intermediate surgical risk [25]. Thus, it is to be expected that there will be a large increase in the number of patients in the coming decades that will be referred for this procedure [5, 7].
As shown in the current study, elderly patients with significant AS suitable for AVI often have several cardiovascular risk factors, a history of previous cardiac events and several co-morbidities. Even asymptomatic aortic valve sclerosis is associated with increased morbidity and mortality after controlling for concomitant risk factors. Thus, the risk of myocardial infarction and cardiovascular death is increased 40% and 50%, respectively [26]. The large number of the elderly in the coming decades will be a great therapeutic challenge and have huge health-economical implications in the western societies. Currently there is no known medical treatment to halt or prevent the devolvement of aortic valve calcification that may evolve into significant AS [27]. Calcification of the aortic valve is an active disease process, in some ways similar to atherosclerosis. This process involves a complex deposition of lipoproteins, chronic inflammation, bone formation and mineralization. There are also some indications that the renin-angiotensin system and endothelial dysfunction may also be involved in the calcification process of the aortic valve [27, 28]. In addition, similar clinical factors are associated with aortic valve sclerosis and atherosclerosis and include older age, hyperlipidemia, smoking, male gender, hypertension and diabetes [26, 29]. Vitamin D status, bone remodeling and secondary hyperparathyroidism has also been implicated in AS progression [30]. Furthermore, genetic factors are known to predispose to the development of AS [31]. Although only a small percentage of individuals with aortic valve sclerosis progress to clinically significant AS, given the foreseen increase in the elderly population, there has been some hope that the process might be susceptible to drug intervention. Due to the similarity between the development of aortic valve calcification and atherosclerosis some studies have evaluated the use of statins. The randomized, placebo controlled SEAS study, were simvastatin was used in patients with AS, however, demonstrated no effect [32]. Although ACE inhibitors may have potentially favorable effects on the remodeling and hypertrophic changes in the myocardium of patients with AS, currently no studies have shown an effect on the calcification progress [27]. It is therefore likely that inescapably there will be a large increase in the number of TAVI procedures in the elderly in the future.
Conclusions
The number of elderly with severe AS will greatly increase in the coming decades. The current study shows that the largest increase will be in the age population 70 years and older, both in men and in women. Similar development is foreseen in other western countries. How to deal with and treat age related diseases in the large elderly population in the future will be a huge health-economical challenge. Although no medical treatment is currently effective in slowing down the development of AS the outlook for elderly patients that undergo AVI, either by surgery or TAVI, has fortunately greatly improved [33].
Limitations of this study
Although this study was conducted in a randomly selected study cohort from a well defined population the echo subgroup is relatively small. An attempt was made to compensate for this by using a larger date set of CT result on aortic valve calcium. Although this study uses a well defined variable to define the severity of AS, the AVA index, assessing this variable is not without errors. The velocity across the aortic valve was only measured by Doppler from the apical position. This may lead to an underestimation of gradients. However, as both the peak velocity in the left ventricular outflow tract and across the aortic valve are measured from the same position, calculating the AVA from these velocities may in fact give a more accurate assessment of the severity of AS than the use of velocities and gradients alone. Furthermore, extrapolating from echocardiography assessment of the severity of AS to an AVCS by CT to define severe AS will always depend greatly on the selection of the study population and the sensitivity of the AVCS values that are used.
Acknowledgments
Source of funding
The AGES Reykjavik study was funded by NIH contract N01-AG-1-2100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament).
References
- 1.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 2.Eveborn GW, Chirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. The Tromsö study. Heart. 2013;99:396–400. doi: 10.1136/heartjnl-2012-302265. [DOI] [PubMed] [Google Scholar]
- 3.Berry C, Lloyd SM, Wang Y, MacDonald A. The changing course of aortic valve disease in Scotland: temporal trends in hospitalizations and mortality and prognostic importance of aortic stenosis. Euro Heart J. 2013;34:1538–1547. doi: 10.1093/eurheartj/ehs339. [DOI] [PubMed] [Google Scholar]
- 4.Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 5.Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly. Disease prevalence and number of candidates for transcatheter aortic valve replacement: A Meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Leon MB, Smith CR, Mack M, et al. PARTNER Trial Investigators. Transcatheter aortic valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 7.Osnabrugge RL, Head SJ, Bogers AJ, Kappetein P. Patient selection for transcatheter aortic valve replacement: what does the future hold? Expert Rev Cardiovac Ther. 2012;10:679–681. doi: 10.1586/erc.12.54. [DOI] [PubMed] [Google Scholar]
- 8.Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J. 2005;26:2714–2720. doi: 10.1093/eurheartj/ehi471. [DOI] [PubMed] [Google Scholar]
- 9.Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–1117. doi: 10.1093/europace/eur132. [DOI] [PubMed] [Google Scholar]
- 10.Rosenhek R, Zilberszac R, Schemper M, et al. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. doi: 10.1161/CIRCULATIONAHA.109.894170. [DOI] [PubMed] [Google Scholar]
- 11.Gudmundsson EF, Gudnason V, Sigurdsson S, Launer LJ, Harris TB, Aspelund Coronary artery calcium distribution in older persons in the AGES-Reykjavik study. Eur J Epidemiol. 2012;27:673–687. doi: 10.1007/s10654-012-9730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statistics Iceland. www.statice.is.
- 13.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Rekjavik study: Multidisiplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The joint task force on the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 15.SAS Institute Inc. User’s Guide. Cary, NC: SAS Institute Inc; 2008. SAS/STAT 9.2. [Google Scholar]
- 16.Danielsen R, Nordrehaug JE, Vik-Mo H. Limitations in assessing the severity of aortic stenosis by Doppler gradients. Br Heart J. 1988;59:551–555. doi: 10.1136/hrt.59.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Sullivan CJ, Stortecky S, Heg D, et al. Clinical outcomes of patients with low-flow, low-gradient sever aortic stenosis and either preserved or reduced ejection fraction undergoing transcatheter aortic valve implantation. Eur Heart J. 2013;34:3437–3450. doi: 10.1093/eurheartj/eht408. [DOI] [PubMed] [Google Scholar]
- 18.Danielsen R, Nordrehaug JE, Vik-Mo H. Factors affecting Doppler echocardiographic valve area assessment in aortic stenosis. Am J Cardiol. 1989;63:1107–1111. doi: 10.1016/0002-9149(89)90087-8. [DOI] [PubMed] [Google Scholar]
- 19.Bahlmann E, Gerdts E, Cramariuc D, et al. Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation. 2013;127:1149–1156. doi: 10.1161/CIRCULATIONAHA.112.078857. [DOI] [PubMed] [Google Scholar]
- 20.Muraru D, Badano LP, Vannan M, Iliceto S. Assessment of aortic valve complex by three-dimensional echocardiography: a framework for effective application in clinical practice. Eur Heart J-Cardiovasc Imaging. 2012;13:541–555. doi: 10.1093/ehjci/jes075. [DOI] [PubMed] [Google Scholar]
- 21.Koos R, Mahnken AH, Sinha AM, Wildberger JE, Hoffmann R, Kühl HP. Aortic valve calcification as a marker for aortic stenosis severity: Assessment on 16-MDCT. AJR. 2004;183:1813–1818. doi: 10.2214/ajr.183.6.01831813. [DOI] [PubMed] [Google Scholar]
- 22.Cueff C, Serfaty J-M, Cimadevilla C, et al. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implications for patients with low ejection fraction. Heart. 2011;97:721–726. doi: 10.1136/hrt.2010.198853. [DOI] [PubMed] [Google Scholar]
- 23.Koos R, Kühl HP, Mühlenbruch G, Wilderberger JE, Günther RW, Mahnken AH. Radiology. 2006;241:76–82. doi: 10.1148/radiol.2411051163. [DOI] [PubMed] [Google Scholar]
- 24.Danielsen R, Nordrehaug JE, Vik-Mo H. Clinical and haemodynamic features in relation to severity of aortic stenosis in adults. Eur Heart J. 1991;12:791–795. doi: 10.1093/eurheartj/12.7.791. [DOI] [PubMed] [Google Scholar]
- 25.Wenaweser P, Stortecky S, Schwander S, et al. Clinical outcomes of patients with estimated low or intermediate surgical risk undergoing transcatheter aortic valve implantation. Eur Heart J. 2013;34:1894–1905. doi: 10.1093/eurheartj/eht086. [DOI] [PubMed] [Google Scholar]
- 26.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcified aortic valve disease. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 27.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 28.Sverdlov AL, Ngo DTM, Chan WPA, et al. Determinants of aortic sclerosis progression: Implications regarding impairment of nitric oxide signaling and potential therapeutics. Eur Heart J. 2012;33:2419–2425. doi: 10.1093/eurheartj/ehs171. [DOI] [PubMed] [Google Scholar]
- 29.Katz R, Wong ND, Kronmal R, et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the multi-ethnic study of atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 30.Hekimian G, Boutten A, Flamant M, et al. Progression of aortic valve stenosis is associated with bone remodeling and secondary hyperparathyroidism in elderly patients-the COFRASA study. Eur Heart J. 2013;34:1915–1922. doi: 10.1093/eurheartj/ehs450. [DOI] [PubMed] [Google Scholar]
- 31.Thanasoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 33.Bonow RO. Improving outlook for elderly patients with aortic stenosis. JAMA. 2013;310:2045–2047. doi: 10.1001/jama.2013.281825. [DOI] [PubMed] [Google Scholar]