Abstract
Various new technologies have been applied for developing vaccines against various animal diseases. Virus-like particle (VLP) vaccine technology was used for manufacturing the porcine circovirus type 2 and RNA particle vaccines based on an alphavirus vector for porcine epidemic diarrhea (PED). Although VLP is classified as a killed-virus vaccine, because its structure is similar to the original virus, it can induce long-term and cell-mediated immunity. The RNA particle vaccine used a Venezuela equine encephalitis (VEE) virus gene as a vector. The VEE virus partial gene can be substituted with the PED virus spike gene. Recombinant vaccines can be produced by substitution of the target gene in the VEE vector. Both of these new vaccine technologies made it possible to control the infectious disease efficiently in a relatively short time.
Keywords: Vaccines, Virus-like particle vaccines, Porcine circovirus, Venezuelan equine encephalitis virus, Porcine epidemic diarrhea virus
Introduction
Modern vaccines are based on the research of Edward Jenner and Louis Pasteur. In 1798, Edward Jenner introduced cowpox pustule fluid into people to induce protection against smallpox [1], and Louis Pasteur attenuated the fowl cholera pathogen coincidently. Pasteur realized that the pathogenicity of pathogens reduced with age, and termed the attenuated strain a vaccine [2]. In Latin, vacca means cow, honoring Edward Jenner's experiment. Interestingly, the initial development of both of these human vaccines was deeply related to animals.
Vaccination can provide cost-effective long-term protection against disease by stimulating the natural defense system of the host to generate sufficient immunity. New vaccine technologies are developed globally and are introduced through various journals to determine more efficient ways of inducing immunity. However, only limited numbers of these developments are adopted as actual human vaccines because of the high level of safety required. The most effective example to explain the difficulty of developing human vaccines is adjuvants. There are many possible adjuvants but alum (aluminum compounds) is the most common commercial adjuvant in current killed vaccines [3]. Various new technologies and adjuvants could not be used because of safety concerns. Before human use, vaccines developed with new technologies or new adjuvants should be approved for each national regulatory authority. For authorization, the World Health Organization (WHO) guideline requires detailed, extensive information, specifically for safety, which is only available from vast clinical studies. Therefore, human vaccine development is very expensive and time consuming. These factors are a large obstacle, resulting in a reduction in the vaccine research and development industry. However, for animals, these new technologies and various adjuvants have already been used. Before using new types of vaccines or their components for humans, it is very useful to use them for animals as a transition study. Through animal vaccine trials, we can obtain valuable information that could be applied to human vaccine development [4].
Most of the newly emerging life-threatening infectious diseases (severe acute respiratory syndrome, avian influenza, Ebola, Middle East respiratory syndrome, etc.) are zoonoses, which are transmissible diseases caused by contact with all types of pathogens, including bacteria, viruses, parasites, and fungi. Over 200 diseases are currently classified as zoonoses, which are global or inherent to a specific region of the world (WHO 2015). There are numerous modes of zoonotic disease transmission. Human can be infected through insects such as mosquitoes and ticks, handling or eating undercooked or raw meat, or coming into contact with the blood, urine, or feces of an infected animal. Regarding the progress of zoonoses, human-animal contact plays an important role. Considering the diversity of transmission, the relationship among humans, animals, and the source of the disease, zoonoses exert a tremendous impact on public health. Therefore, the development of animal vaccines has a critical function to prevent humans from infection with pathogens by removing the root cause of the infected animal. New upcoming agents could be prevented with effective animal vaccines developed against such pathogens timeously. Simultaneously, as the frequency and administration of animal vaccines increase, the influence of animal vaccines on public health expands [5].
Considering the diversity of transmission modes, the relationship between human and animals, and source of the disease, we need to focus on animal disease and vaccine technology for public health. In the present paper, two new vaccine technologies in industrial animal vaccines are introduced.
Virus-like Particle Vaccine for Porcine Circovirus-Associated Disease
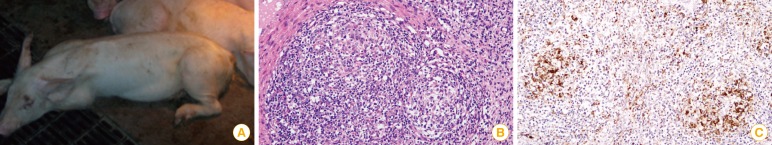
In Saskatchewan, Canada, a new swine disease was reported with clinical signs including progressive weight loss, tachypnea, dyspnea, and jaundice [6]. However, the causative pathogen was initially unknown. However, in 1998, the new virus, only 17 nm in size, was identified, with similar features to the porcine circovirus identified by Tischer in 1982 [7]. Lyoo et al. [8] identified and reported this disease as the first porcine circovirus-associated disease (PCVAD) case in Korea [8]. Porcine circovirus infection rate increased dramatically and its symptoms have become more severe since 2004. The representative clinical signs and histological characteristics are explained in Fig. 1. Conventional farm mortality resulting from PCVAD ranged from 20% to 60%, affecting the Korean swine industry severely.
Fig. 1.
Porcine circovirus type 2 (PCV2) affected pigs. (A) The pigs showed severe wasting symptoms. (B) The lymph node showed lymphoid depletion with histiocytic infiltration in lymphoid follicles (H&E staining, scale bar=50 µm). (C) Many PCV2 antigens (brown diaminobenzidine reaction) within the cytoplasm of histiocytic cells were observed in the lymphoid follicles (immunohistochemisty, scale bar=50 µm).
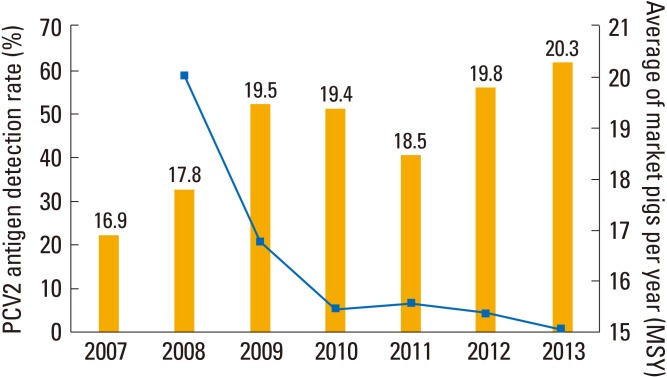
From 2008, porcine circovirus type 2 (PCV2) vaccines from global vaccine manufacturers were administered at swine farms. After beginning PCV2 vaccination, farm swine mortality decreased dramatically. Despite these PCV2 vaccines being killed vaccines, they showed effective disease preventive efficacy. We compared the PCV2 virus detection rate in abortion cases, as a good example of PCV2 vaccine efficacy because the PCV2 virus can infect the fetus during pregnancy [9]. Before PCV2 vaccine inoculation, the detection rate of PCV2 in fetuses was 58.9%. However, in 2012, the detection rate of PCV2 in fetuses decreased to 0.9% (Fig. 2). The killed PCV2 vaccine made this possible because they were made from PCV2 virus-like particles (VLPs). As the PCV2 VLP is able to show similar immunogenicity with the native PCV2 virus, the sera induced by PCV2 VLP can show strong reactions with naïve PCV2 antigens [10].
Fig. 2.
Porcine circovirus type 2 (PCV2) detection rate in aborted fetuses using polymerase chain reaction. PCV2 virus can infect the fetus and cause abortion. After beginning PCV2 vaccine inoculation, PCV2 positive rates decreased from 58.9% to 0.9%.
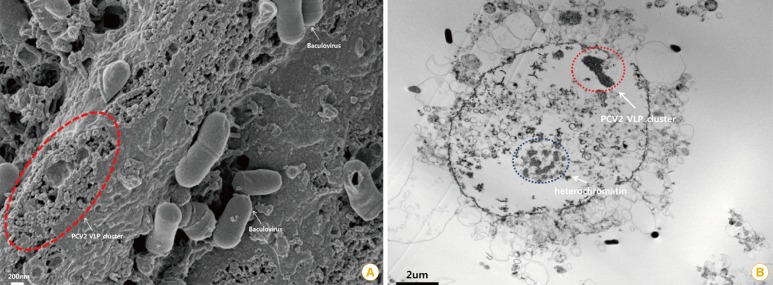
The immunogenic antigen in the VLP vaccine can maintain the native antigenic conformation and mimic the whole structure of the wild virus but as they lack viral nucleic acids, they are not infectious [11]. The VLP vaccine is a category of a subunit vaccine and can be produced using recombinant protein technology without a viral replication system (Fig. 3) [12,13].
Fig. 3.
Characterization of porcine circovirus type 2 (PCV2) virus-like particles (VLPs). (A) Baculovirus and PCV2 VLPs are observed by scanning electron microscopy. Baculovirus and PCV2 VLPs were cultured in the sf9 insect cell line (scale bar=200 nm). The original size of PCV2 is approximately 15-20 nm in diameter [13]. The visible PCV2 VLPs were similar to authentic PCV2 particles in size and morphology. (B) A PCV2 VLP cluster was observed inside a sf9 cell by transmission electron microscopy (scale bar=2 µm).
Subunit vaccines have one important disadvantage. The expressed proteins are not as immunogenic as original viral components. So, more antigens must be used for the same level of protection. For the VLP vaccine, as VLP has similar structure to the original virus, it can elicit stronger humoral immunity with lower quantities of antigen than pre-existing killed or subunit vaccines [14]. Furthermore, as VLP does not contain the any genomic material, it is a very safe vaccine without the risks associated with virus replication of incomplete inactivation.
However, the most important advantage of the VLP vaccine is that it can stimulate B cells and proliferate CD4 and cytotoxic T-lymphocyte responses [15]. But, the stimulation of B cells by VLPs is strong enough to elicit T cell independent IgM antibody induction [16].
The structure of VLP is large and unique to microbes, so mammalian immune systems respond to it vigorously due to antigen arrangements [12]. Why does the antigen arrangement affect the immune system? The influence of antigen epitope density and order on B-cell induction and antibody production was assessed and showed that highly organized antigens can respond promptly [17]. As VLPs are comprised of one or more proteins arranged geometrically into dense, repetitive arrays, it can induce a strong immune response [12]. In addition to inducing strong B-cell responses, VLPs also can induce T-cell responses efficiently through interactions with antigen presenting cells, particularly dendritic cells, and VLPs are processed and presented by MHC II molecules for the activation of T-helper cells [12]. VLP antigen can stimulate dendritic cells to induce more rapid, stronger, and protective cytotoxic T-lymphocyte responses by class I presentation [18].
Recently, various animal vaccines have been developed as VLP forms. In 2012, the foot and mouth disease (FMD) virus serotype O VLP vaccine was developed by scientists in India [19]. As that VLP vaccine was made with a baculovirus expression system in an insect cell, it did not require a bio-safety level three barrier facility for production. The following year, the Pirbright Institute (UK), published research regarding a new FMD VLP vaccine [20]. The neutralization antibody titer greater than 5.5 (Log 2) is considered to be protective, but the new FMD VLP vaccine could maintain neutralization with antibodies greater than 6 (Log 2), up to 22-week post-vaccination [20]. These research results are significant for industrial animals. The new VLP technology makes it possible to manufacture dangerous virus vaccines that are only approved in high-level barrier facilities without infection risks. Therefore, VLP vaccine technology can be a very promising tool for disease prevention in industrial animals.
RNA Particle Vaccine for Porcine Epidemic Diarrhea
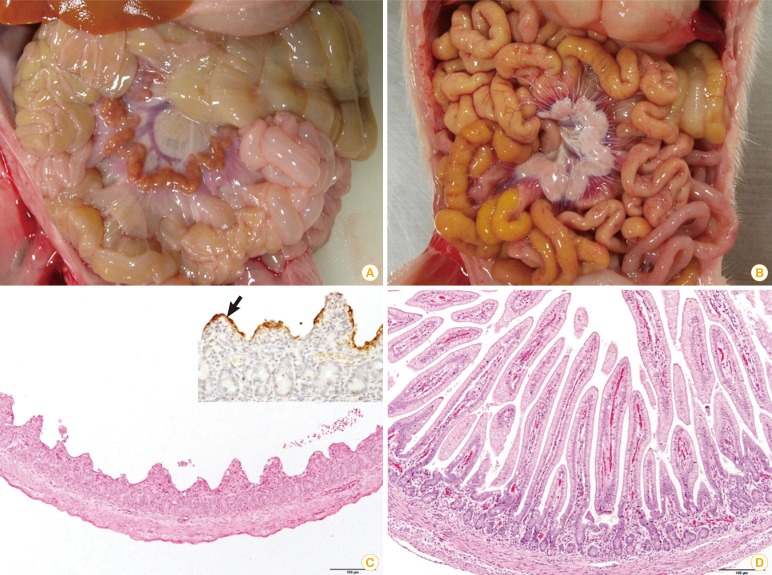
In May 2013, porcine epidemic diarrhea (PED) case was reported in the United States for the first time [21]. As PED is an acute and very contagious disease, it caused significant economic loss to the swine industry [22]. Pregnant sows infected with the PED virus during the first 30 days of gestation had a 12.6% decrease of farrow rate (from 91.1% to 78.5%). The number of piglets born alive decreased from 10.7 to 8.5 (piglets/litter) in gilts' litters [23]. The clinical signs and histological characteristics of PED are explained in Fig. 4. As PED had not broken out in North America before, unfortunately there were no vaccines available in the US animal medicine market.
Fig. 4.
Comparison of porcine epidemic diarrhea (PED) affected (A, C) and non-porcine epidemic diarrhea virus infected (B, D) piglets. (A) The walls of the small intestine were thin and transparent. (B) No remarkable changes were found in the small intestine. (C) Severe villous atrophy and degeneration of epithelial cells were observed in the small intestine (H&E staining, scale bar=100 µm). Insert: PED antigens showed the apical portion of epithelial cells of atrophic villi (arrow, immunohistochemistry). (D) The villous height/crypt depth ratio was the range of normal sucking piglets (H&E staining, scale bar=100 µm).
One US venture company, Harris Vaccine Inc., employed alphavirus vector technology to produce a PED vaccine. In June 2014, a new PED vaccine became the first PED virus vaccine to receive a conditional US Department of Agriculture license since the first PED outbreak (Fig. 5). Alphavirus vector technology was used for cluster IV H3N2 influenza vaccines without adjuvants [24].
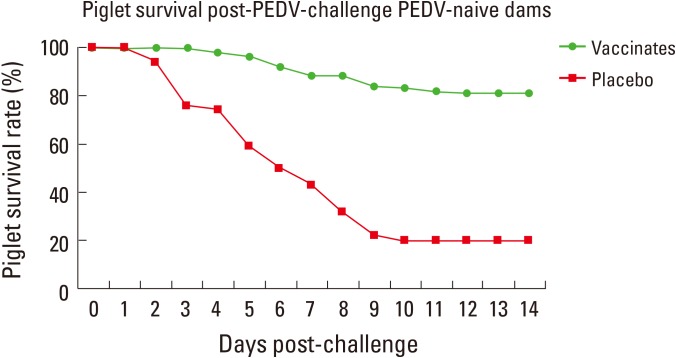
Fig. 5.
Piglet survival rate post–porcine epidemic diarrhea (PED) virus (PEDV) challenges in PEDV naive dams with or without alphavirus vector PED vaccine inoculation. The no vaccinated group showed 80% mortality but the vaccinated group showed 20% mortality (courtesy of Harris Vaccine Inc., Ames, IW, USA [not published]).
Alphaviruses belong to the family Togaviridae. The members of Alphavirus possess a single-stranded RNA with the nucleocapsid surrounded by a membrane protein [25]. Among 26 currently recognized members, Venezuelan equine encephalitis (VEE) virus, which causes epidemics in horses and humans, was chosen as the vaccine vector [26]. Attenuated VEE strains were used for high-level heterologous gene expression vectors as a naked RNA vector, replication deficient recombinant particles, and layered DNA vectors [27]. For an emergency PED vaccine, replication deficient recombinant particles were used resembling the VLP form.
Various viral structural proteins including the influenza virus are targeted for vaccine development with alphavirus vectors [28,29].
The alphavirus vector vaccine has shown its efficacy in protection against challenges with the H5N1 virus in chickens [29]. The alphavirus vector has shown cellular or humoral responses and protection against lethal dose virus challenges, non-viral pathogens (like bacteria), and even cancers [27]. Additionally characteristics of RNA particle vaccines based on the alphavirus vector were explained in Table 1.
Table 1.
Comparison of the RNA particle vaccine to traditional vaccines
| Variable | Traditional vaccine | RNA particle vaccine | ||
|---|---|---|---|---|
| Killed virus | Modified live | Extract antigen | RNA particle | |
| Humoral immunity | + | + | + | + |
| Cellular immunity | - | + | - | + |
| May cause disease | - | + | - | - |
| Grow agent | + | + | + | - |
| Adjuvant required | + | - | + | - |
| Emergency vaccine | - | - | - | + |
Primarily, the alphavirus vector vaccine can make it possible for vaccinations in emergency situations. From US PED cases, it was possible to produce the PED vaccine in 13 months by substitution of the interest gene into the alphavirus vector. For a highly pathogenic influenza outbreak, to produce an emergency vaccine is indispensable, but, with traditional vaccine manufacturing methods like virus isolation and inactivation, it is too time-consuming. Therefore, recombinant vaccine technology, like the RNA particle vaccine, can be helpful tools for the prevention of various infectious diseases.
Conclusion
Historically, the early version of human vaccines and technologies were developed or discovered based on animals, for example, the smallpox and fowl cholera vaccines. Recent animal vaccine markets can be a good test market to evaluate the new technologies before adopting those new technologies directly to humans. In actual cases, many new vaccine technologies were used for animal vaccines. The most dramatic changes in recent animal vaccines were the usage of VLP-form vaccines for PCV2 and RNA particle vaccines that are produced from gene combinations of vectors and pathogens. As these vaccines are made from the baculovirus in insect cells and the VEE virus, respectively, they can reduce the time for development and make it possible to produce the highly pathogenic vaccine antigens, like the FMD virus, without high-level barrier facilities.
The most important advantage of these vaccine technologies allow us to confront viral diseases, like the influenza virus that is hyper-variable, in a relatively short time. Based on these experiences and technologies for animals, effective preventive tools that are beneficial for human disease can be developed.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jenner E. An inquiry into the causes and effects of the variolae vaccinae. London: Dawsons of Pall Mall; 1966. [Google Scholar]
- 2.Goldsby RA, Kindt TJ, Osborne BA. Kuby immunology. 4th ed. New York: W. H. Freeman and Company; 2000. [Google Scholar]
- 3.Tomljenovic L, Shaw CA. Answers to common misconceptions regarding the toxicity of aluminum adjuvants in vaccines. In: Shoenfeld Y, Agmon-Levin N, Tomljenovic L, editors. Vaccines and autoimmunity. Hoboken: John Wiley and Sons Inc.; 2015. pp. 43–56. [Google Scholar]
- 4.Williamson D. Approaches to modelling the human immune response in transition of candidates from research to development. J Immunol Res. 2014;2014:395302. doi: 10.1155/2014/395302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkelman RL. Human illness associated with use of veterinary vaccines. Clin Infect Dis. 2003;37:407–414. doi: 10.1086/375595. [DOI] [PubMed] [Google Scholar]
- 6.Harding JC. Post-weaning multisystemic wasting syndrome (PMWS): preliminary epidemiology and clinical presentation. Proc Am Assoc Swine Pract. 1997;28:503. [Google Scholar]
- 7.Ellis J, Hassard L, Clark E, et al. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can Vet J. 1998;39:44–51. [PMC free article] [PubMed] [Google Scholar]
- 8.Lyoo YS, Kim JH, Park CK. Identification of porcine circoviruses with genetic variation from lymph nodes collected in pigs with PMWS. Korean J Vet Res. 1999;39:353–358. [Google Scholar]
- 9.Meehan BM, McNeilly F, McNair I, et al. Isolation and characterization of porcine circovirus 2 from cases of sow abortion and porcine dermatitis and nephropathy syndrome. Arch Virol. 2001;146:835–842. doi: 10.1007/s007050170152. [DOI] [PubMed] [Google Scholar]
- 10.Liu LJ, Suzuki T, Tsunemitsu H, et al. Efficient production of type 2 porcine circovirus-like particles by a recombinant baculovirus. Arch Virol. 2008;153:2291–2295. doi: 10.1007/s00705-008-0248-x. [DOI] [PubMed] [Google Scholar]
- 11.Roy P, Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin. 2008;4:5–12. doi: 10.4161/hv.4.1.5559. [DOI] [PubMed] [Google Scholar]
- 12.Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007;6:381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- 13.Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ. Diseases of swine. 9th ed. Ames: Blackwell Publishing; 2006. [Google Scholar]
- 14.Buonaguro L, Tornesello ML, Buonaguro FM. Virus-like particles as particulate vaccines. Curr HIV Res. 2010;8:299–309. doi: 10.2174/157016210791208659. [DOI] [PubMed] [Google Scholar]
- 15.Schirmbeck R, Bohm W, Reimann J. Virus-like particles induce MHC class I-restricted T-cell responses. Lessons learned from the hepatitis B small surface antigen. Intervirology. 1996;39:111–119. doi: 10.1159/000150482. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Ge S, Li L, Wu X, Liu Z, Wang Z. Virus-like particles: potential veterinary vaccine immunogens. Res Vet Sci. 2012;93:553–559. doi: 10.1016/j.rvsc.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 18.Bachmann MF, Lutz MB, Layton GT, et al. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26:2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 19.Mohana Subramanian B, Madhanmohan M, Sriraman R, et al. Development of foot-and-mouth disease virus (FMDV) serotype O virus-like-particles (VLPs) vaccine and evaluation of its potency. Antiviral Res. 2012;96:288–295. doi: 10.1016/j.antiviral.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Porta C, Kotecha A, Burman A, et al. Rational engineering of recombinant picornavirus capsids to produce safe, protective vaccine antigen. PLoS Pathog. 2013;9:e1003255. doi: 10.1371/journal.ppat.1003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson GW, Hoang H, Schwartz KJ, et al. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Li G, Stasko J, et al. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol. 2014;52:234–243. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison B, Goede D. Epidemiology an economic impact of the PED; Documento Procedente de AASV 45th Annual Meeting; 2014 Mar 1-4; Dallas, TX, USA. [Google Scholar]
- 24.Sandbulte MR, Spickler AR, Zaabel PK, Roth JA. Optimal use of vaccines for control of influenza A virus in swine. Vaccines (Basel) 2015;3:22–73. doi: 10.3390/vaccines3010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver SC, Salas R, Rico-Hesse R, et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet. 1996;348:436–440. doi: 10.1016/s0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]
- 27.Lundstrom K. Alphavirus vectors in vaccine development. J Vaccines Vaccin. 2012;3:139. [Google Scholar]
- 28.Malone JG, Bergland PJ, Liljestrom P, Rhodes GH, Malone RW. Mucosal immune responses associated with polynucleotide vaccination. Behring Inst Mitt. 1997;(98):63–72. [PubMed] [Google Scholar]
- 29.Schultz-Cherry S, Dybing JK, Davis NL, et al. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology. 2000;278:55–59. doi: 10.1006/viro.2000.0635. [DOI] [PubMed] [Google Scholar]