Abstract
Resources such as water taken up by plants can be released into soils through hydraulic redistribution and can also be translocated by clonal integration within a plant clonal network. We hypothesized that the resources from one (donor) microsite could be translocated within a clonal network, released into different (recipient) microsites and subsequently used by neighbor plants in the recipient microsite. To test these hypotheses, we conducted two experiments in which connected and disconnected ramet pairs of Potentilla anserina were grown under both homogeneous and heterogeneous water regimes, with seedlings of Artemisia ordosica as neighbors. The isotopes [15N] and deuterium were used to trace the translocation of nitrogen and water, respectively, within the clonal network. The water and nitrogen taken up by P. anserina ramets in the donor microsite were translocated into the connected ramets in the recipient microsites. Most notably, portions of the translocated water and nitrogen were released into the recipient microsite and were used by the neighboring A. ordosica, which increased growth of the neighboring A. ordosica significantly. Therefore, our hypotheses were supported, and plant clonal integration mediated the horizontal hydraulic redistribution of resources, thus benefiting neighboring plants. Such a plant clonal integration-mediated resource redistribution in horizontal space may have substantial effects on the interspecific relations and composition of the community and consequently on ecosystem processes.
Keywords: clonal integration, deuterium, environmental heterogeneity, [15N], hydraulic redistribution, Potentilla anserina, stable isotope
Introduction
The hydraulic redistribution (Burgess et al., 1998) of soil water have been observed to play important roles in many ecosystems, particularly in arid and semiarid ones (Nadezhdina et al., 2010; Neumann and Cardon, 2012; Hao et al., 2013). Though, theoretically speaking, such a hydraulic redistribution can be vertical and/or horizontal, vertical hydraulic redistribution of soil water (or hydraulic lift, Richards and Caldwell, 1987) has received much more attention (Caldwell et al., 1998; Horton and Hart, 1998; Brooks et al., 2006). In the vertical hydraulic redistribution of water, a plant root system takes up water from the soil and translocates it upward before releasing it into an upper layer of the soil (Prieto et al., 2010; Robinson et al., 2012). This vertical hydraulic redistribution of water is a benefit not only to the donor plants (Querejeta et al., 2003) but also to the surrounding biota because it provides an alternative source of water (Ludwig et al., 2004; Brooks et al., 2006). The redistribution of water is also a mechanism through which nutrients in deeper soils are mobilized for uptake by plants (Wang et al., 2009).
Natural environments are often heterogeneous and consist of patches with contrasting levels of resources (García-Palacios et al., 2012). Many clonal plants, particularly stoloniferous or rhizomatous clonal plants, create a clonal network that consists of a large number of interconnected ramets, which may occupy patches that are widely spaced and differ in resource availability (Jońsdóttir and Watson, 1997; Charpentier et al., 2012; Li et al., 2012). A large body of evidence has demonstrated that ramets growing in high resource patches (donor ramets in donor microsites) translocate resources to the interconnected ramets growing in low resource patches (recipient ramets in recipient microsites), and this translocation occurs through horizontal structures such as rhizomes, stolons or roots (Atkinson and Else, 2012; Roiloa and Hutchings, 2013; Luo et al., 2014; Pinno and Wilson, 2014; Roiloa et al., 2014). Such clonal integration greatly improves the performance of the recipient ramets and often also that of the entire clone (Yu et al., 2004; Song et al., 2013).
Thus, soil resources such as water and nutrients are translocated from donor to recipient ramets via clonal integration (Alpert, 1999; Wang et al., 2014; Dong et al., 2015). For some clonal plants with a large clonal network, the distance covered can be large after multiple translocations from donor to recipient ramets. Additionally, the resources are released into the soil through the roots of the recipient ramets, driven hydraulically by a water potential gradient (McCully, 1995; Steudle and Frensch, 1996). Therefore, we hypothesized that soil resources, before being released into recipient microsites, are translocated for relatively long distances among microsites by the horizontal redistribution of soil resources through an integrated clonal network from donor to recipient ramets. Furthermore, we hypothesized that neighboring plants use the release of water and possibly nutrients from the clonal network to improve growth. Although horizontal redistribution of soil resources is vital to understand facilitative effect among plant individuals, especially in dryland ecosystems, so far no study has explicitly tested whether a horizontal redistribution of soil resources can be mediated by clonal integration and whether it can further impact the growth of neighbor plants.
To test these hypotheses, we conducted two greenhouse experiments with the stoloniferous, clonal plant Potentilla anserina. We grew ramets of P. anserina in a high-resource (donor) microsite that were connected to the ramets of P. anserina with a neighbor Artemisia ordosica seedling in a low-resource (recipient) microsite. The isotopes [15N] and deuterium ([D]) were used to trace and quantify the redistribution of nitrogen and water, respectively, from the donor microsite to the recipient microsite via ramets of P. anserina interconnected by stolons. We expected that the tracers added to the donor microsite would be detected in the recipient microsite and in the neighboring plant (A. ordosica) and that the growth of the neighboring plant would be improved by the horizontal hydraulic redistribution of water and nutrients mediated by clonal integration.
Materials and Methods
Plant Species
Potentilla anserina L. (Rosaceae) is a clonal, rosette-forming herb with sympodial, plagiotropic stems (stolons; Ma, 1989). Along a stolon, each node produces leaves and roots under moist conditions, forming a potentially independent ramet. In northern China, the stolons wither during autumn and winter, and the ramets become completely independent (Ma, 1989). All ramets of P. anserina used in this study were vegetatively propagated from a single clone collected on 10th August 2008 near the Ordos Sandland Ecological Station (OSES, 39°02′ N; 109°21′ E), which is located in the northeastern section of the Mu Us Sandland in Inner Mongolia, China. The P. anserina clone was cultivated in the greenhouse at OSES to obtain sufficient ramets of similar size and age for the experiments.
The semi-shrub A. ordosica Krasch (Asteraceae) is a dominant species in the Mu Us Sandland. The root system has abundant lateral roots, primarily distributed in the upper, dry sand layer (Li et al., 2010). In sandlands such as Mu Us, P. anserina inhabits the lowlands between dunes and also often spreads into the dunes that are dominated by A. ordosica.
Experiments
Experiment I
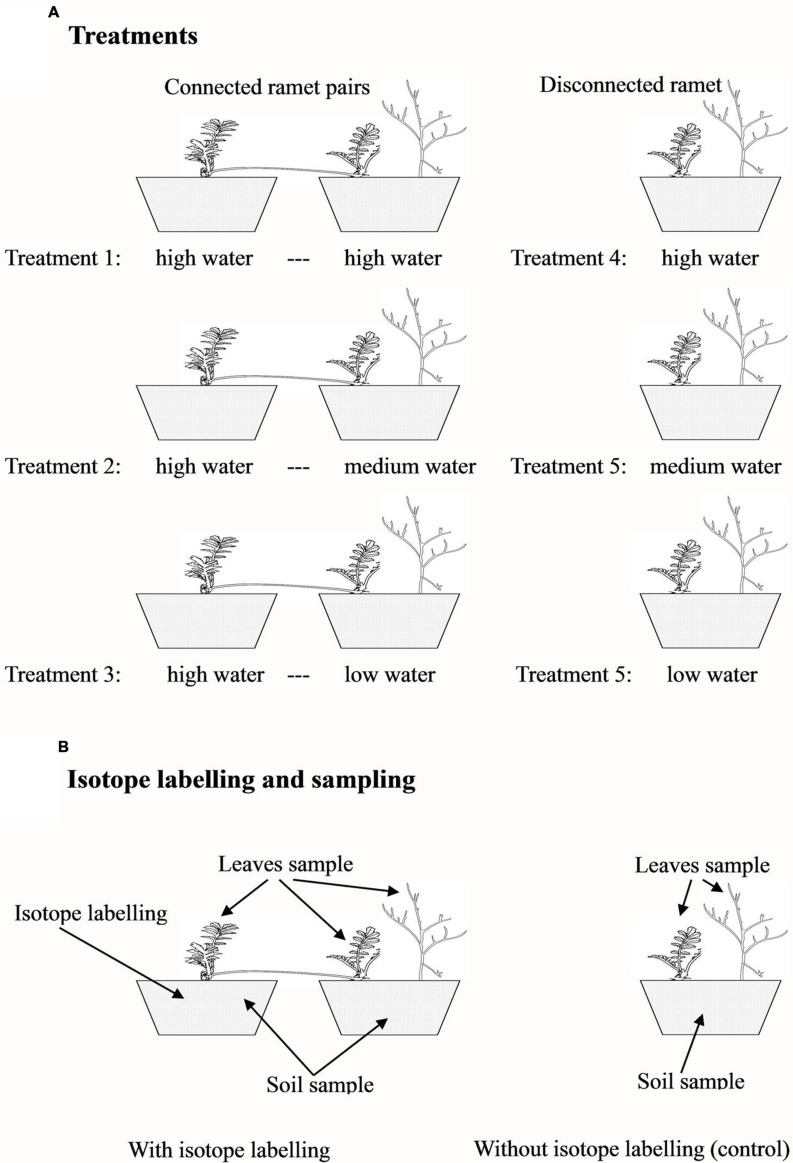
To test whether clonal integration mediated the redistribution of nutrients among microsites, the first experiment was conducted in 2009. We selected 27 pairs of connected ramets and 27 pairs of severed (disconnected) ramets (three leaves per ramet) of P. anserina. Each ramet pair was planted in a pair of cylindrical plastic containers (21 cm in diameter and 21 cm in height) filled with sand collected near the field station. Each severed, developmentally younger ramet was planted in a container of the same size, but all the severed, developmentally older ramets were discarded. Ten days after transplantation, the connected ramet pairs (connected treatment) were randomly assigned to one of three water treatments (Figure 1A): (1) both ramets of a ramet pair were watered with 400 ml every 3 days (high water regime); (2) one ramet was watered with 400 ml every 3 days, and the connected ramet was watered with 300 ml (medium water regime); and (3) one ramet was watered with 400 ml every 3 days, and the connected ramet was watered with 200 ml (low water regime). In this experiment, the developmentally older ramet of each pair was always assigned to the high water treatment (in the potential donor container), and the connected, developmentally younger ramet was randomly assigned to the high, medium, or low water regime and was also grown with one A. ordosica seedling (in the potential recipient container). The different water regimes were used to create different water potential gradients between the two interconnected ramets of each pair to facilitate nutrient translocation through clonal integration (de Kroon et al., 1998). The 27 disconnected, developmentally younger P. anserina ramets (severed treatment in which the stolon internode connecting the two ramets of a pair was cut midway with scissors) were randomly assigned to one of the three water treatments (Figure 1A): (4) high water treatment with A. ordosica, (5) medium water treatment with A. ordosica, and (6) low water treatment with A. ordosica. All seedlings of A. ordosica used in this experiment were approximately 2 cm tall. Nine replicates were used per treatment. The experiment was conducted for 2 months from 15th August 2009 to 15th October 2009. Three days before harvest, three replicates from the three connected treatments were randomly selected, and the older ramets in the high water treatment were labeled with [15N]. For labeling, 400 ml of NH415NO3 solution (containing 0.066 g 15N) was added to the containers with the older ramets to trace the movement of N. All the other containers, including the rest donor containers, all the recipient and disconnected containers with different water treatments, received the identical amount of NH4NO3 but at different concentrations. After harvest, three of the replicates (including all three labeled and three disconnected, unlabeled replicates) were sampled to determine the values of δ15N in the plant leaves and soils (Figure 1B), and the other six replicates were used to measure plant biomass. After oven-drying at 80°C for at least 48 h, the shoot and root biomass was measured for the younger ramet of each pair and the neighbor A. ordosica and for each disconnected ramet of P. anserina and the neighbor A. ordosica.
FIGURE 1.
Experimental design. Three water treatments for connected ramet pairs or for disconnected, single ramets (A); and the sampling design with or without isotope labeling (B).
Experiment II
To exclude the effect of nutrients and to verify the effect of water on plant growth, the second experiment was conducted in 2011 to test whether the redistribution of water mediated by clonal integration benefited neighboring plants. We selected 30 pairs of connected ramets and 30 pairs of severed (disconnected) ramets (three leaves per ramet) of P. anserina. Each ramet pair was planted into a pair of cylindrical plastic containers (21 cm in diameter and 21 cm in height) filled with sand, and each disconnected, developmentally younger ramet was planted in a similar container (all the severed, developmentally older ramets were discarded). The experimental design was identical to that for Experiment I, with the exception that all A. ordosica seedlings were 40 days-old (approximately 15 cm tall) and that D2O was used for labeling 3 days before harvest (Figure 1A). The experiment began on 31st August 2011 and ended on 30th September 2011. Ten replicates were used per treatment. Three days before harvest, three replicates in the connected treatments were randomly selected, and the older ramets in the high water treatment were labeled with D2O. For the labeling, a 300 ml solution of 1/400 D2O solution (containing 99.8% [D] in D2O) was added to the containers to trace the movement of water, whereas the other containers not used for labeling received 300 ml of double distilled water. Three replicates (including the three labeled and the three disconnected, unlabeled replicates) were sampled to determine the values of δD in the leaves and soils (Figure 1B), and the other seven replicates were used to measure plant biomass, as in Experiment I.
Isotope Measurements
We measured the [15N] or [D] concentrations (1) in the soils and in the P. anserina leaves in the labeled (donor) containers; (2) in the soils, P. anserina leaves and A. ordosica leaves in the unlabeled, paired (recipient) containers (growing with the connected P. anserina ramets); and (3) in the soils, P. anserina leaves and A. ordosica leaves in the unlabeled, control containers (growing with the single, severed P. anserina ramets) using a MAT253 Isotope Ratio Mass Spectrometer (Thermo Fisher Scientific, Inc., USA) coupled with an Elemental Analyser (Flash EA1112 HT, Thermo Finnigan, USA). The abundance [15N] isotope ratio (δ15N, ‰) and the [D] isotope ratio (δD, ‰) were calculated as follows:
| (1) |
where Rsample and Rstandard are the 15N/14N ratio or the D/1H ratio in a sample and the standard, respectively. The standards were international standard atmospheric air N2 for nitrogen and standard mean ocean water for deuterium.
We calculated the ratios of RN and RD from the δ15N and δD values in the soils, P. anserina leaves or A. ordosica leaves in the labeled (donor) containers or unlabeled, paired (recipient) containers and those in the unlabeled, control containers as follows:
| (2) |
| (3) |
where δ15NExp is the δ15N in the soils, P. anserina leaves or A. ordosica leaves in the labeled, donor containers or unlabeled, recipient containers; δ15NNat is the δ15N in the soils, P. anserina leaves or A. ordosica leaves in the corresponding unlabeled, control containers; RN is the ratio of δ15N in experimental materials to that of the controls; δDExp is the δD in the soils, P. anserina leaves or A. ordosica leaves in the labeled, donor containers or unlabeled, recipient containers; δDNat is the δD in the soils, P. anserina leaves or A. ordosica leaves in the corresponding unlabeled, control containers; and RD is the ratio of δD in the experimental materials to that in the controls. When RN > 1 or RD > 1, [15N] or [D] was translocated into the soil and plant, and high values of RN or RD indicated that more [15N] or [D] was translocated.
Data Analyses
We used one-sample t-tests to determine whether the RN and RD in soils and in plant leaves in each water treatment in the unlabeled, recipient containers were significantly larger than 1 (one-tailed tests). One-way ANOVAs were used to test the effects of water treatment (high, medium, or low water supply) on the RN and RD in soils and plant leaves. When a significant effect was detected, Tukey’s HSD tests were used for multiple comparisons. We used two-way ANOVAs to analyze the effects of clonal integration (stolon severing or not) and water treatments (high, medium, or low water supply) on the biomass of P. anserina and A. ordosica. The data for the RN and the RD were log-transformed to meet the assumption of normality and homogeneity of variance. All statistical analyses were performed using the SPSS17.0 statistical software package (SPSS, Chicago, IL, USA).
Results
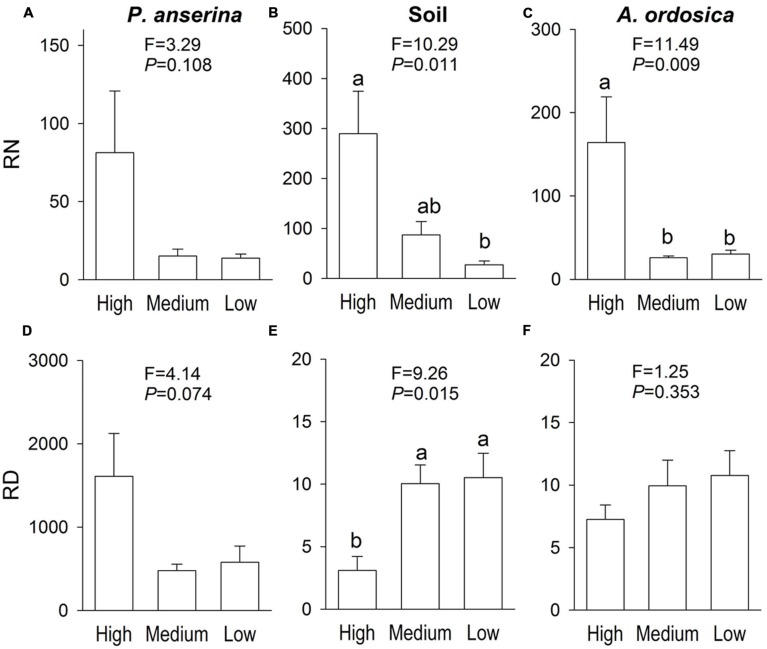
In our experiment, the δ15N in control soils, P. anserine leaves and A. ordosica leaves was 7.691 ± 0.23‰, 29.45 ± 1.14‰, and 3.278 ± 0.21‰, respectively; and δD was -39.91 ± 0.97‰, -1.80 ± 0.92‰, and -44.29 ± 2.43‰, respectively. The RN and the RD, the ratios of δ15N and δD in labeled materials to those in control materials, respectively, were used to show the effects of isotope labeling and translocation. Higher values of RN or RD indicated that more of the isotope was in the experimental materials than in the control materials. In the labeled containers, the RN and RD in soils and P. anserina leaves were much greater than 1 (Supplementary Figure S1), which indicated that the isotopes were successfully added to the soil and absorbed by the plant.
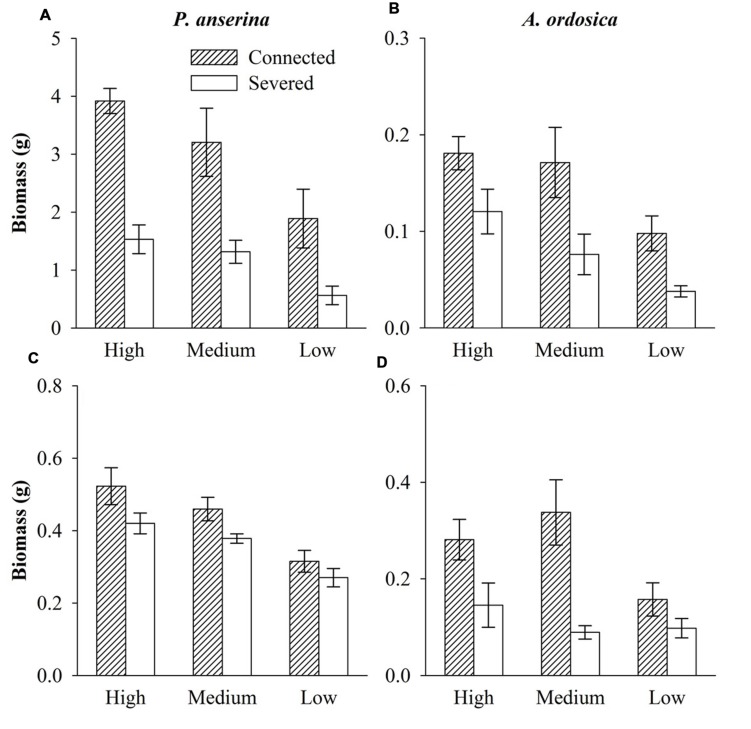
In the unlabeled, recipient containers, both the RN and RD in P. anserina leaves were significantly greater than 1 (P < 0.05; Figures 2A,D), and the clonal integration significantly increased the biomass of P. anserina (Table 1; Figures 2A,C). These results demonstrated that [15N] and [D] were translocated from the donor ramet of P. anserina to the interconnected, recipient ramet, and through clonal integration, the growth of the recipient ramet increased. In the unlabeled, recipient containers, the RN and RD in soil and A. ordosica leaves were significantly larger than 1 (P < 0.05; Figures 2B,C,E,F), and through clonal integration, the biomass of A. ordosica increased significantly (Table 1; Figures 3B,D). Therefore, the increase in the growth of A. ordosica was probably attributable to the uptake of the translocated [15N] and [D] that were released by the recipient ramets into the soil.
FIGURE 2.
The ratio of δ15N (RN, above, in Experiment I) and the ratio of δD (RD, below, in Experiment II) in Potentilla anserina leaves (A,D), soils (B,E), and A. ordocia leaves (C,F) in the unlabeled, recipient containers with the high, medium and low water treatments that were connected to the P. anserina ramets with the high water supply. All means are significantly greater than 1 (one-sample t-tests). The F and P-values of the one-way ANOVA are also provided, and different letters indicate significant differences in means among treatments.
Table 1.
Effects of severing stolons and water treatment on biomass and shoot to root ratio of Potentilla anserina and Artemisia ordosica in (A) Experiment I and (B) Experiment II.
| Effect |
P. anserina |
A. ordosica |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomass |
Root/shoot |
Biomass |
Root/shoot |
||||||
| DF | F | P | F | P | F | P | F | P | |
| (A) Experiment I | |||||||||
| Severing (S) | 1, 31 | 30.9 | <0.001 | 26.2 | <0.001 | 10.4 | 0.003 | 13.5 | 0.001 |
| Water (W) | 2, 31 | 5.2 | 0.011 | 0.6 | 0.556 | 4.1 | 0.027 | 15.6 | <0.001 |
| S × W | 2, 31 | 0.6 | 0.574 | 6.8 | 0.004 | 0.4 | 0.662 | 3.8 | 0.032 |
| (B) Experiment II | |||||||||
| Severing (S) | 1, 29 | 5.3 | 0.029 | 1.3 | 0.272 | 15.1 | 0.001 | 0.2 | 0.652 |
| Water (W) | 2, 29 | 14.4 | <0.001 | 2.4 | 0.106 | 2.3 | 0.118 | 1.7 | 0.200 |
| S × W | 2, 29 | 0.2 | 0.856 | 3.6 | 0.040 | 2.2 | 0.132 | 1.2 | 0.303 |
Values in bold indicate significance at P < 0.05.
FIGURE 3.
Biomass of P. anserina (A,C) and Artemisia ordosica (B,D) in the unlabeled containers with the high, medium, and low water treatments that were connected with or severed from the P. anserina ramets grown in the high water treatment in Experiment I (A,B) and Experiment II (C,D).
The RN in soils and in A. ordosica leaves in the unlabeled, recipient containers was significantly higher in the high water supply treatments than in the low water supply treatment (Figures 2B,C); however, the RD in soils was significantly lower (Figure 2E), possibly because more of the water supply was diluted under high water treatment. The RN and RD in P. anserina leaves and the RD in A. ordosica leaves did not differ significantly among the three water treatments (Figures 2A,D,F). Furthermore, the effect of clonal integration on the growth of P. anserina and A. ordosica were also not dependent on the water regime (Table 1; Figure 3).
Severing stolon had significant effects on both root/shoot ratio of P. anserina and A. ordosica, and water treatment significantly affected root/shoot ratio of A. ordosica in Experiment I; while in Experiment II, neither severing stolon nor water treatment had significant effects on root/shoot ratio of P. anserina and A. ordosica (Table 1).
Discussion
Our hypotheses were supported: via a clonal network, clonal integration mediated the horizontal redistribution of soil resources essential for plant growth and reproduction, between different microsites. Moreover, the redistribution of water and nitrogen increased the growth of neighboring plants.
The horizontal redistribution of soil resources is limited by the abilities to translocate resources and to release resources into the soil (Nadezhdina et al., 2010; Neumann and Cardon, 2012). Numerous studies have confirmed that resources are transported from one ramet to another via horizontal clonal organs, including stolons, rhizomes and roots, through clonal integration (Alpert, 1999; D’Hertefeldt and Jónsdóttir, 1999; van Kleunen et al., 2000; Roiloa and Hutchings, 2013). A large body of evidence has also indicated that plant roots release resources into the soil through hydraulic redistribution (Moreira et al., 2003; Leffler et al., 2005; Smart et al., 2005; Brooks et al., 2006). Our results show that water and nitrogen were translocated from a donor to a recipient ramet of P. anserina through clonal integration and then released by the roots of the recipient ramet into the soil, most likely through hydraulic redistribution. In a previous study based on deuterium labeling, (de Kroon et al. (1998) have also noted that soil water is translocated through a clonal network and then released into soil. However, with a model, Magyar et al. (2004) have shown that clonal integration may also mediate soil nutrient redistribution through decomposition; i.e., nutrients taken up by a donor ramet in the donor microsite are translocated to the recipient ramet in the recipient microsite and then released into the soil in the recipient microsite with the decomposition of the dead, recipient ramet. Although we could not fully eliminate the effect of root decomposition, we presumed that this effect released much less water and nitrogen than that of hydraulic redistribution because the duration of the experiment was short (not more than 2 months), and the isotopes were added only 3 days before harvest. Thus, the release of nutrients through the decomposition of roots was expected to be minimal. In cases in the field in which clonal plants form large, interconnected clonal networks, clonal integration mediates the long-distance translocation of resources (D’Hertefeldt and Jónsdóttir, 1999; Li et al., 2012). Therefore, for the species with large clonal networks that include a few dozen or 100s of ramets (Li et al., 2012; Song et al., 2013), we propose that the extent of resource redistribution in the field is substantial when resources are available and that the long-distance redistribution of resources may be possible through clonal integration. This area of research has promise for future study.
Most notably, the clonal integration in P. anserina significantly increased the biomass of A. ordosica grown as a neighbor to the recipient ramet of P. anserina. Thus, the neighbor A. ordosica must have absorbed a substantial amount of the water and nitrogen released by P. anserina into the soil. This interpretation was confirmed by isotope labeling; [15N] and [D] of the neighbor A. ordosica grown with an unlabeled, P. anserina ramet that was connected to a labeled ramet were much higher than those of A. ordosica grown with an unlabeled, disconnected ramet of P. anserina. Whereas previous studies show that resources released by a target plant through hydraulic lift can be used by neighboring plants and microbes (Burgess, 2011; Sekiya et al., 2011; Pang et al., 2013), we are the first to demonstrate that the resource redistribution mediated by clonal integration affects the growth of neighboring plants. Horizontal hydraulic redistribution of soil resources also significantly affected root/shoot ratio of neighboring plant A. ordosica in Experiment I, but had no significant effects on it in Experiment II. It may because of different neighboring plant size in two experiments. Larger plant may need greater water gradient and greater ability of hydraulic redistribution to affect plant biomass allocation. The quantitative study on plant hydraulic redistribution ability was necessary.
The horizontal hydraulic redistribution of soil resources not only benefits the donor plants and their neighboring plants and soil microbes (Caldwell et al., 1998) but also may influence plant interactions, community structure and ecosystem processes, such as the cycling of water, carbon, and other nutrients (Horton and Hart, 1998; Leffler et al., 2005). Because the distance that resources are redistributed increases with a large, integrated clonal network, the effect of horizontal hydraulic distribution at the community and ecosystem levels can be strong, particularly in drylands in which both hydraulic redistribution and large clonal networks are common. Our results also suggest that plant clonality may have a profound effect at the ecosystem level because clonal plants mediate the redistribution of soil resources. However, the effects of plant clonality in communities and ecosystems require further study (Wilsey, 2002; Yu et al., 2009; Cornelissen et al., 2014), and future studies should be designed to test for these effects in the field.
We expected that an increase in the water gradient between the donor and the recipient ramets of P. anserina would increase the translocation of both water and nitrogen and, as a consequence, increase the beneficial effects of clonal integration on P. anserina and also possibly on A. ordosica. However, with an increase in the water gradient, only the RD in soils increased, and the RN decreased in soils and in A. ordosica. High water treatment may also dilute the D concentration in soils under high water treatment, to further decrease RD in soil under high water treatment. Additionally, the increase in the water gradient did not significantly affect the RN or the RD in P. anserina or the RD in A. ordosica and did not increase the effect of clonal integration on the biomass of P. anserina or A. ordosica. Possible explanations may be that the low water supply treatment decreased nitrogen availability, and the ability of A. ordosica to uptake water and translocate nitrogen were not fully coupled (de Kroon et al., 1998).
Notably, our results were based on a single clone of P. anserina, and therefore, the extrapolation of the results to other systems might be limited. In future research, multiple genotypes and also multiple species should be examined for integration-mediated resource distribution. Additionally, we did not calculate water or nitrogen fluxes between plant-soil-plant systems, and to quantify these fluxes, fine-resolution experiments are required. Despite these limitations, our findings confirmed that the long-distance redistribution of resources through integrated clonal networks is possible.
Conclusion
Clonal integration can mediate the redistribution of resources among different microsites and benefit neighboring plants. Therefore, clonal integration may be a previously undescribed mechanism facilitating plant water and nutrient cycling in ecosystems. In heterogeneous environments, clonal integration may affect the ecosystem at two levels: as a response trait that helps clonal plants perform better in heterogeneous habitats and as an effect trait that modifies habitat heterogeneity and affects interspecific interactions and possibly the community structure and ecosystem processes (Magyar et al., 2004; Cornelissen et al., 2014). We propose that future studies should focus on the effects of plant clonality on communities and ecosystems.
Author Contributions
MD and X-HY directed and coordinated this study; X-HY, Y-LZ, Z-LL, S-QG, and Y-BS carried out the fieldwork and lab analyses; X-HY, Y-LZ, F-HL, and MD did the data analysis and wrote the first manuscript draft. All authors commented on the manuscript and consent with the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30900179, 31470032, 31100300, and 39825106).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00077
References
- Alpert P. (1999). Clonal integration in Fragaria chiloensis differs between populations: ramets from grassland are selfish. Oecologia 120 69–76. 10.1007/s004420050834 [DOI] [PubMed] [Google Scholar]
- Atkinson C. J., Else M. A. (2012). Hydraulic conductivity and PAT determine hierarchical resource partitioning and ramet development along Fragaria stolons. J. Exp. Bot. 63 5093–5104. 10.1093/jxb/ers155 [DOI] [PubMed] [Google Scholar]
- Brooks J. R., Meinzer F. C., Warren J. M., Domec J. C., Coulombe R. (2006). Hydraulic redistribution in a Douglas-fir forest: lessons from system manipulations. Plant Cell Environ. 29 138–150. 10.1111/j.1365-3040.2005.01409.x [DOI] [PubMed] [Google Scholar]
- Burgess S. S. O. (2011). Can hydraulic redistribution put bread on our table? Plant Soil 341 25–29. 10.1007/s11104-010-0638-1 [DOI] [Google Scholar]
- Burgess S. S. O., Adams M. A., Turner N. C., Ong C. K. (1998). The redistribution of soil water by tree root systems. Oecologia 115 306–311. 10.1007/s004420050521 [DOI] [PubMed] [Google Scholar]
- Caldwell M. M., Dawson T. E., Richards J. H. (1998). Hydraulic lift, consequences of water efflux from the roots of plants. Oecologia 113 151–161. 10.1007/s004420050363 [DOI] [PubMed] [Google Scholar]
- Charpentier A., Anand M., Bauch C. T. (2012). Variable offspring size as an adaptation to environmental heterogeneity in a clonal plant species, integrating experimental and modelling approaches. J. Ecol. 100 184–195. 10.1111/j.1365-2745.2011.01899.x [DOI] [Google Scholar]
- Cornelissen J. H. C., Song Y. B., Yu F. H., Dong M. (2014). Plant traits and ecosystem effects of clonality, a new research agenda. Ann. Bot. 114 369–376. 10.1093/aob/mcu113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kroon H., van der Zalm E., van Rheenen J. W. A., van Dijk A., Kreulen R. (1998). The interaction between water and nitrogen translocation in a rhizomatous sedge (Carex flacca). Oecologia 116 38–49. 10.1007/s004420050561 [DOI] [PubMed] [Google Scholar]
- D’Hertefeldt T., Jónsdóttir I. S. (1999). Extensive physiological integration in intact clonal systems of Carex arenaria. J. Ecol. 87 258–264. 10.1046/j.1365-2745.1999.00345.x [DOI] [Google Scholar]
- Dong B. C., Alpert P., Zhang Q., Yu F. H. (2015). Clonal integration in homogeneous environments increases performance of Alternanthera philoxeroides. Oecologia 179 393–403. 10.1007/s00442-015-3338-y [DOI] [PubMed] [Google Scholar]
- García-Palacios P., Maestre F. T., Bardgett R. D., de Kroon H. (2012). Plant responses to soil heterogeneity and global environmental change. J. Ecol. 100 1303–1314. 10.1111/j.1365-2745.2012.02014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X. M., Li W. H., Guo B., Ma J. X. (2013). Simulation of the effect of root distribution on hydraulic redistribution in a desert riparian forest. Ecol. Res. 28 653–662. 10.1007/s11284-013-1058-5 [DOI] [Google Scholar]
- Horton J. L., Hart S. C. (1998). Hydraulic lift, a potentially important ecosystem process. Trends Ecol. Evol. 13 232–235. 10.1016/S0169-5347(98)01328-7 [DOI] [PubMed] [Google Scholar]
- Jońsdóttir I. S., Watson M. A. (1997). “Extensive physiological integration: an adaptive trait in resource-poor environments?,” in The Ecology and Evolution of Clonal Plants, eds de Kroon H., van Groenendal J. (Leiden: Backhuys Publishing; ), 109–136. [Google Scholar]
- Leffler A. J., Peek M. S., Ryel R. J., Ivans C. Y., Caldwell M. M. (2005). Hydraulic redistribution through the root systems of senesced plants. Ecology 86 633–642. 10.1890/04-0854 [DOI] [Google Scholar]
- Li J. M., Alpert P., Yu F. H. (2012). Multiclonal tussocks in the grass Achnatherum splendens (Trinius) Nevskia (Poaceae). Flora 207 581–585. 10.1016/j.flora.2012.06.010 [DOI] [Google Scholar]
- Li S. L., Pieter A. Z., Yu F. H., Marinus J. A. W., Dong M. (2010). Effects of denudation and burial on growth and reproduction of Artemisia ordosica in Mu Us Sandland. Ecol. Res. 25 655–661. 10.1007/s11284-010-0699-x [DOI] [Google Scholar]
- Ludwig F., Dawson T. E., Prins H. H. T., Berendse F., de Kroon H. (2004). Below-ground competition between trees and grasses may overwhelm the facilitative effects of hydraulic lift. Ecol. Lett. 7 623–631. 10.1111/j.1461-0248.2004.00615.x [DOI] [Google Scholar]
- Luo F. L., Chen Y., Huang L., Wang A., Zhang M. X., Yu F. H. (2014). Shifting effects of physiological integration on performance of a clonal plant during submergence and de-submergence. Ann. Bot. 113 1265–1274. 10.1093/aob/mcu057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. (1989). Flora of Inner Mongolia. Hohhut: Inner Mongolia People Press. [Google Scholar]
- Magyar G., Kertesz M., Oborny B. (2004). Resource transport between ramets alters soil resource pattern: a simulation study on clonal growth. Evol. Ecol. 18 469–492. 10.1007/s10682-004-5140-x [DOI] [Google Scholar]
- McCully M. E. (1995). Water efflux from the surface of field-grown grass roots, observations by cryo-scanning electron microscopy. Physiol. Plant 95 217–224. 10.1034/j.1399-3054.1995.950207.x [DOI] [Google Scholar]
- Moreira M. Z., Scholz F. G., Bucci S. J., Sternberg L. S., Goldstein G., Meinzer F. C., et al. (2003). Hydraulic lift in a neotropical savanna. Funct. Ecol. 17 573–581. 10.1046/j.1365-2435.2003.00770.x [DOI] [Google Scholar]
- Nadezhdina N., David T. S., David J. S., Ferreira M. I., Dohnal M., Tesař M., et al. (2010). Trees never rest, the multiple facets of hydraulic redistribution. Ecohydrology 3 431–444. 10.1002/eco.148 [DOI] [Google Scholar]
- Neumann R. B., Cardon Z. G. (2012). The magnitude of hydraulic redistribution by plant roots, a review and synthesis of empirical and modeling studies. New Phytol. 194 337–352. 10.1111/j.1469-8137.2012.04088.x [DOI] [PubMed] [Google Scholar]
- Pang J. Y., Wang Y. M., Lambers H., Tibbett M., Siddique K. H. M., Ryan M. H. (2013). Commensalism in an agroecosystem, hydraulic redistribution by deep-rooted legumes improves survival of a droughted shallow-rooted legume companion. Physiol. Plant. 149 79–90. 10.1111/ppl.12020 [DOI] [PubMed] [Google Scholar]
- Pinno B. D., Wilson S. D. (2014). Nitrogen translocation between clonal mother and daughter trees at a grassland-forest boundary. Plant Ecol. 215 347–354. 10.1007/s11258-014-0305-3 [DOI] [Google Scholar]
- Prieto I. K., Martinez-Tilleria K., Martinez-Manchego L., Montecinos S., Pugnaire F. I., Squeo F. A. (2010). Hydraulic lift through transpiration suppression in shrubs from two arid ecosystems, patterns and control mechanisms. Oecologia 163 855–865. 10.1007/s00442-010-1615-3 [DOI] [PubMed] [Google Scholar]
- Querejeta J. I., Egerton-Warburton L. M., Allen M. F. (2003). Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia 134 55–64. 10.1007/s00442-002-1111-5 [DOI] [PubMed] [Google Scholar]
- Richards J. H., Caldwell M. M. (1987). Hydraulic lift - substantial nocturnal water transport between soil layers by Artemisia tridentata Roots. Oecologia 73 486–489. 10.1007/BF00379405 [DOI] [PubMed] [Google Scholar]
- Robinson J. L., Slater L. D., Schafer K. V. R. (2012). Evidence for spatial variability in hydraulic redistribution within an oak-pine forest from resistivity imaging. J. Hydrol. 430 69–79. 10.1016/j.jhydrol.2012.02.002 [DOI] [Google Scholar]
- Roiloa S. R., Antelo B., Retuerto R. (2014). Physiological integration modifies δ15N in the clonal plant Fragaria vesca, suggesting preferential transport of nitrogen to water-stressed offspring. Ann. Bot. 114 399–411. 10.1093/aob/mcu064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiloa S. R., Hutchings M. J. (2013). The effects of physiological integration on biomass partitioning in plant modules, an experimental study with the stoloniferous herb Glechoma hederacea. Plant Ecol. 214 521–530. 10.1007/s11258-013-0186-x [DOI] [Google Scholar]
- Sekiya N., Araki H., Yano K. (2011). Applying hydraulic lift in an agroecosystem, forage plants with shoots removed supply water to neighboring vegetable crops. Plant Soil 341 39–50. 10.1007/s11104-010-20581-1 [DOI] [Google Scholar]
- Smart D. R., Carlisle E., Goebel M., Nunez B. A. (2005). Transverse hydraulic redistribution by a grapevine. Plant Cell Environ. 28 157–166. 10.1111/j.1365-3040.2004.01254.x [DOI] [Google Scholar]
- Song Y. B., Yu F. H., Keser L. H., Dawson W., Fischer M., Dong M., et al. (2013). United we stand, divided we fall, a meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia 171 317–327. 10.1007/s00442-012-2430-9 [DOI] [PubMed] [Google Scholar]
- Steudle E., Frensch J. (1996). Water transport in plants, role of the apoplast. Plant Soil 187 67–79. 10.1007/BF00011658 [DOI] [Google Scholar]
- van Kleunen M., Fischer M., Schmid B. (2000). Clonal integration in Ranunculus reptans, by-product or adaption? J. Evol. Biol. 13 237–249. 10.1046/j.1420-9101.2000.00161.x [DOI] [Google Scholar]
- Wang P., Xu Y. S., Dong B. C., Xue W., Yu F. H. (2014). Effects of clonal fragmentation on intraspecific competition of a stoloniferous floating plant. Plant Biol. 16 1121–1126. 10.1111/plb.12170 [DOI] [PubMed] [Google Scholar]
- Wang X., Tang C., Guppy C. N., Sale P. W. G. (2009). The role of hydraulic lift and subsoil P placement in P uptake of cotton Gossypium hirsutum L. Plant Soil 325 263–275. 10.1007/s11104-009-9977-1 [DOI] [Google Scholar]
- Wilsey B. (2002). Clonal plants in a spatially heterogeneous environment, effects of integration on Serengeti grassland response to defoliation and urine-hits from grazing mammals. Plant Ecol. 159 15–22. 10.1023/A:1015556816307 [DOI] [Google Scholar]
- Yu F. H., Dong M., Krusi B. (2004). Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytol. 162 697–704. 10.1111/j.1469-8137.2004.01073.x [DOI] [PubMed] [Google Scholar]
- Yu F. H., Wang N., Alpert P., He W. M., Dong M. (2009). Physiological integration in an introduced, invasive plant increases its spread into experimental communities and modifies their structure. Am. J. Bot. 96 1983–1989. 10.3732/ajb.0800426 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.