ABSTRACT
The ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens cause an increasing number of nosocomial infections worldwide since they escape the inhibitory effect of the available antibiotics and the immune response. Here, we report the broad-spectrum and potent antibacterial activity of Kisameet clay, a natural clay mineral from British Columbia, Canada, against a group of multidrug-resistant ESKAPE strains. The results suggest that this natural clay might be developed as a therapeutic option for the treatment of serious infections caused by these important pathogens.
IMPORTANCE
More than 50 years of misuse and overuse of antibiotics has led to a plague of antibiotic resistance that threatens to reduce the efficacy of antimicrobial agents available for the treatment of infections due to resistant organisms. The main threat is nosocomial infections in which certain pathogens, notably the ESKAPE organisms, are essentially untreatable and contribute to increasing mortality and morbidity in surgical wards. The pipeline of novel antimicrobials in the pharmaceutical industry is essentially empty. Thus, there is a great need to seek for new sources for the treatment of recalcitrant infectious diseases. We describe experiments that demonstrate the efficacy of a “natural” medicine, Kisameet clay, against all of the ESKAPE strains. We suggest that this material is worthy of clinical investigation for the treatment of infections due to multidrug-resistant organisms.
OBSERVATION
The ESKAPE group of bacterial pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) is responsible for a majority of hospital-acquired infections and presents critical threats in nosocomial pathogenesis, transmission, and resistance (1). They are so named since they “escape” the activity of all available antimicrobial agents and cause extensive morbidity and mortality in infected patients (1, 2). They are predicted to be of increasing relevance in infectious disease for the foreseeable future, but the current antibiotic armamentarium has little to offer in terms of treatment, and there are few novel antimicrobial agents under development that show promise in relieving the health crisis caused by these organisms (3).
Clay minerals are naturally occurring layered phyllosilicates with stable crystalline structures, very fine particle sizes (<2.0 µm), and large surface areas (4). Some show antimicrobial or other therapeutic properties, and they have a long history in the treatment of human diseases (5, 6). However, their use is considered to be “naturopathic” medicine, and to date, none have been approved by regulatory agencies for therapeutic applications. On the central coast of British Columbia, Canada, northwest of Vancouver, there exists a clay deposit, Kisameet clay (KC), which has been used by the local First Nations (Heiltsuk) people for several centuries and appears to have excellent therapeutic properties (7). Anecdotal reports indicate the application of KC for a variety of ailments, including ulcerative colitis, duodenal ulcer, arthritis, neuritis, phlebitis, skin irritation, and burns (8, 9). Mineralogical and chemical analyses of KC in the 1940s (7) as well as more recent work (10) indicate that this deposit differs from other clays such as kaolinite or bentonite. X-ray diffraction shows that KC possesses a low clay mineral content (~24% [wt]), dominated by the presence of biotite (S. Behroozian, S. L. Svensson, J. Tang, W. Xu, L. Li, J. Davies, unpublished data). Moreover, as a natural clay deposit, KC has a significant resident microbial community (1,000 to 3,000 taxa), which includes Actinobacteria, which are known to make bioactive small molecules and may contribute to KC activity by the production of antimicrobials (S. L. Svensson, S. Behroozian, W. Xu, M. G. Surette, L. Li, J. Davies, unpublished data). More recently, the antibacterial activities and physicochemical characteristics of other therapeutic clay minerals have been investigated in the laboratory (11, 12). Haydel et al. reported on the broad-spectrum in vitro antibacterial activities of a natural iron-rich clay (CsAgO2) that was used to treat patients with Buruli ulcer (12).
Our studies have shown that KC has potent broad-spectrum antibacterial activity in both stationary and logarithmic phases of growth in vitro (Behroozian et al., unpublished), and to investigate the activity of KC against the ESKAPE pathogens, we assembled and characterized the antibiotic resistance profiles of a collection of 16 strains from a number of sources in Vancouver, including Vancouver General Hospital (VGH), St. Paul’s Hospital (SPH), and the University of British Columbia (UBC) wastewater treatment pilot plant (WWTP) (Table 1). E. faecium was grown in Mueller-Hinton (MH) broth or on MH agar, and S. aureus was grown in Trypticase soy (TS) broth or on TS agar plates. All other strains were cultured in Luria-Bertani (LB) broth or on LB agar. Standard disk diffusion tests using a panel of 36 antibiotics showed widespread, although variable, multidrug resistance among these strains (Table 1). The E. faecium and S. aureus strains exhibited resistance to carbapenems, third-generation cephalosporins, and penicillins, while methicillin-resistant S. aureus (MRSA) was also resistant to first-generation cephalosporins, quinolones, tetracyclines, nitrofurantoin, clindamycin, and erythromycin. All Gram-negative strains were resistant to first- and second-generation cephalosporins and penicillins. In addition, K. pneumoniae, A. baumannii, and P. aeruginosa strains exhibited resistance to third-generation cephalosporins and trimethoprim.
TABLE 1 .
Resistance patterns of ESKAPE strains for different classes of antibioticsa
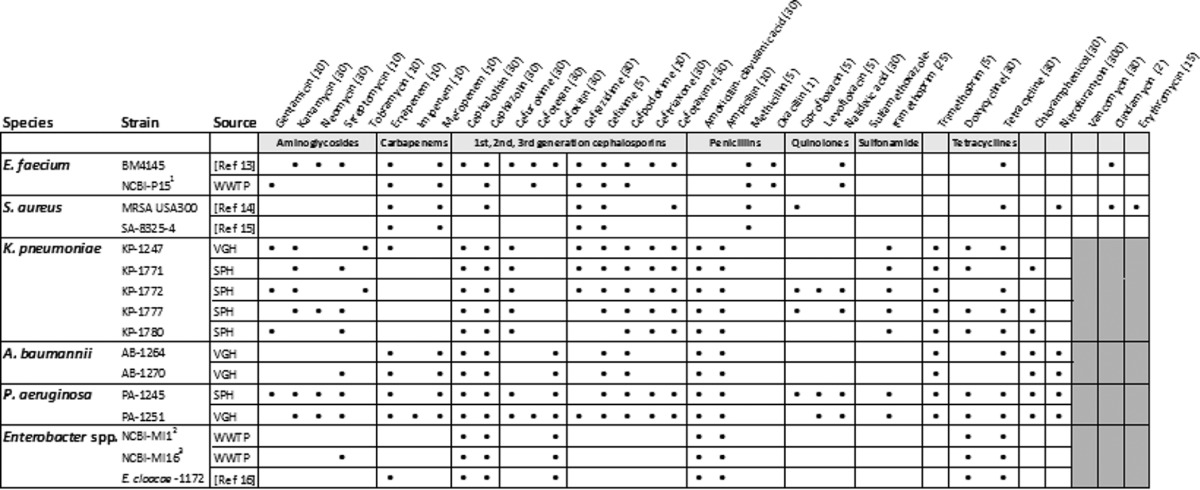
Filled black circles indicate resistance (zone of inhibition of ≤1 mm from the edge of disk of antibiotics); the absence of a mark indicates a wider zone of inhibition. Gray boxes indicate that no test was conducted. The amount (micrograms) per disk of antibiotics (Oxoid, BBL) is indicated in parentheses. Colistin and polymyxin B, polypeptides active against only Gram-negative bacteria, were also tested on K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter spp.; no resistance was observed. Superscript numerals indicate strains identified by 16S rRNA gene sequences (accession numbers are KT827400, KT827398, and KT827399 for strains marked by numerals 1, 2, and 3, respectively).
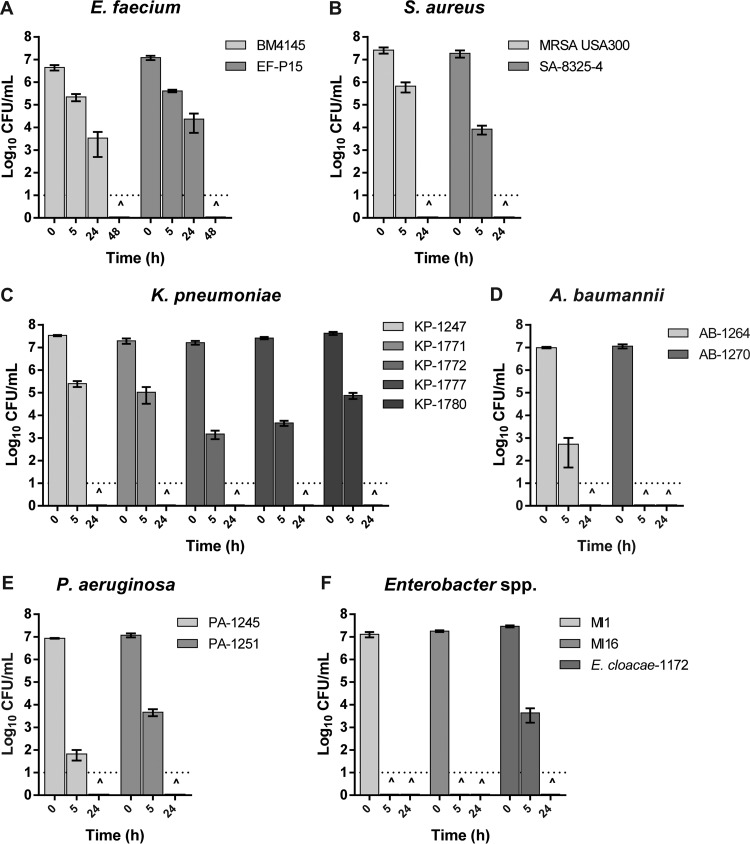
An in vitro assay was used to examine the effect of KC on the ESKAPE strains. Overnight cultures were diluted into the appropriate growth medium to ~107 CFU/ml and incubated at 37°C with gentle rotary mixing (200 rpm) to the mid-logarithmic phase of growth. Cells were collected by centrifugation, washed with sterile phosphate-buffered saline, and resuspended at ~107 CFU/ml either in 1% (wt/vol) KC suspension (10 mg vacuum-desiccated, ground, autoclaved clay in 1 ml deionized distilled water) or in water only (as control) and then incubated with gentle shaking at 37°C. Viability was determined by serial dilution plating of aliquots removed at 0, 5, and 24 h of incubation. The presence of KC dramatically reduced the viability of all strains tested (Fig. 1). For example, after a 5-h exposure to KC, no viable cells of A. baumannii AB-1270, Enterobacter sp. strain MI1, or Enterobacter sp. strain MI16 could be recovered, indicating potent activity against these strains. S. aureus, K. pneumoniae, P. aeruginosa, A. baumannii AB-1264, and Enterobacter cloacae 1172 lost viability completely after 24 h, and the same killing took 48 h for E. faecium strains. In contrast, in water-only controls without KC, the decline in CFU during the same period of incubation was ≤1 log10 for all Gram-negative strains and ~1 to 3 log10 for E. faecium and S. aureus strains, respectively.
FIG 1 .
Effect of 1% (wt/vol) aqueous suspensions of KC on the viability of various ESKAPE strains. The dotted line at log10 −1 of the y axis represents the limit of detection for CFU. CFU have been determined at 0, 5, and 24 h of incubation for all strains and also at 48 h for E. faecium strains. ^ indicates that no viable cell could be recovered at that time point. Error bars represent the standard errors of the means from at least three independent replicates of each strain in these six groups.
Conclusion.
The ESKAPE pathogens are responsible for the majority of recalcitrant bacterial outbreaks in nosocomial settings, but the therapeutic choices are extremely limited. Despite intensive searches for new antimicrobial agents, there are few active candidates in the pipeline. Here, we show the bactericidal effect of KC, a natural mineral clay, against a panel of both Gram-positive and Gram-negative multidrug-resistant ESKAPE strains. In addition to its antibacterial activity, KC has an antifungal property. It also effectively disperses biofilms of S. aureus and P. aeruginosa and prevents their formation (Behroozian et al., unpublished). Moreover, aqueous extracts of KC (without mineral particles) demonstrate the same broad-spectrum antibacterial activity, suggesting that the active component(s) can be extracted and used in defined preparations. Although there are differences in susceptibility between isolates of the same species, to date no resistance to KC has been observed. The Heiltsuk nation employ KC in geophagia for a variety of internal ailments, suggesting that this natural mineral might be an option for treatment of intractable infections such as Clostridium difficile.
We suggest that the broad-spectrum antibacterial activity of KC may be a valuable option for the treatment of ESKAPE infections, especially in last-resort situations. It remains to be seen if KC acts synergistically with common antimicrobials to potentiate their activity against resistant pathogens. To date, no toxic side effects have been reported in the human use of KC (9), and detailed in vivo studies in animal models and cytotoxicity investigations remain to be carried out. Nonetheless, such ancient medicinals and other natural mineral-based agents may provide new weapons in the battle against multidrug-resistant pathogens. Harrison et al. described a successful application of an ancient natural medicine (17). Therefore, reassessment of the potency and mechanisms of action of other natural agents deserves more attention.
ACKNOWLEDGMENTS
We acknowledge the Heiltsuk First Nation and Kisameet Glacial Inc. for providing the clay samples. We thank L. Li for mineralogical and chemical analyses of KC; D. Roscoe, M. Romney, M. Mohammadali, and R. Robillo for providing bacterial isolates; and V. Miao for helpful discussions in editing the manuscript.
Funding Statement
Support for these studies comes from a MITACS (UBC) student fellowship (grant 22R07416), Kisameet Glacial Clay Inc., and the Tally fund (J.D.).
Footnotes
Citation Behroozian S, Svensson SL, Davies J. 2016. Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. mBio 7(1):e01842-15. doi:10.1128/mBio.01842-15.
REFERENCES
- 1.Rice LB. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D Jr, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Pendleton JN, Gorman SP, Gilmore BF. 2013. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 4.Brigatti MF, Galán E, Theng BKG. 2006. Structure and mineralogy of clay minerals, p 21–81. In Bergaya F, Theng BKG, Lagaly G (ed), Handbook of clay science. Developments in clay science, vol 1 Elsevier Ltd, Amsterdam, Netherlands. [Google Scholar]
- 5.Carretero MI. 2002. Clay minerals and their beneficial effect upon human health. A review. Appl Clay Sci 21:155–163. doi: 10.1016/S0169-1317(01)00085-0. [DOI] [Google Scholar]
- 6.Carretero MI, Gomes CSF, Tateo F. 2006. Clays and human health, p 717–741. In Bergaya F, Theng BKG, Lagaly G (ed), Handbook of clay science. Developments in clay science, vol 1 Elsevier Ltd, Amsterdam, Netherlands. [Google Scholar]
- 7.Hauser EA. 1952. Kisameet Bay clay deposit, p 178–190. In Problems of Clay and Laterite Genesis Symposium at Annual Meeting of the American Institute of Mining and Metallurgical Engineers, St. Louis, MO. [Google Scholar]
- 8.Hauser EA. 1950. Canamin clay and its properties. Can Chem Process Ind 34:979. [Google Scholar]
- 9.Ure W, Harris JA. 1946. Curative properties of rare earths found in B.C. peloid deposits. Bull Vanc Med Assoc 22:230–237. [PubMed] [Google Scholar]
- 10.Williams LB, Metge DW, Eberl DD, Harvey RW, Turner AG, Prapaipong P, Poret-Peterson AT. 2011. What makes a natural clay antibacterial? Environ Sci Technol 45:3768–3773. doi: 10.1021/es1040688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams LB, Haydel SE, Giese RF, Eberl DD. 2008. Chemical and mineralogical characteristics of French green clays used for healing. Clays Clay Miner 56:437–452. doi: 10.1346/CCMN.2008.0560405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haydel SE, Remenih CM, Williams LB. 2008. Broad-spectrum in vitro antibacterial activities of clay minerals against antibiotic-susceptible and antibiotic-resistant bacterial pathogen. J Antimicrob Chemother 61:353–361. doi: 10.1093/jac/dkm468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rende-Fournier R, Leclercq R, Galimand M, Duval J, Courvalin P. 1993. Identification of the satA gene encoding a streptogramin A acetyltransferase in Enterococcus faecium BM4145. Antimicrob Agents Chemother 37:2119–2125. doi: 10.1128/AAC.37.10.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenover FC, Goering RV. 2009. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother 64:441–446. doi: 10.1093/jac/dkp241. [DOI] [PubMed] [Google Scholar]
- 15.Lin AE, Davies JE. 2007. Occurrence of highly fluoroquinolone-resistant and methicillin-resistant Staphylococcus aureus in domestic animals. Can J Microbiol 53:925–929. doi: 10.1139/W07-062. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Miao V, Kwong W, Xia R, Davies J. 2011. Identification of a novel fosfomycin resistance (fosA2) gene in Enterobacter cloacae from Salmon River, Canada. Lett Appl Microbiol 52:427–429. doi: 10.1111/j.1472-765X.2011.03016.x. [DOI] [PubMed] [Google Scholar]
- 17.Harrison F, Roberts AEL, Gabrilska R, Rumbaugh KP, Lee C, Diggle SP. 2015. A 1,000-year-old antimicrobial remedy with antistaphylococcal activity. mBio 6:e01129-15. doi: 10.1128/mBio.01129-15. [DOI] [PMC free article] [PubMed] [Google Scholar]