ABSTRACT
Mother-to-child transmission (MTCT) of HIV provides a setting for studying immune correlates of protection. Neutralizing antibodies (NAbs) are suggested to contribute to a viral bottleneck during MTCT, but their role in blocking transmission is unclear, as studies comparing the NAb sensitivities of maternal viruses have yielded disparate results. We sought to determine whether transmitting mothers differ from nontransmitting mothers in the ability to neutralize individual autologous virus variants present at transmission. Ten transmitting and 10 nontransmitting HIV-infected mothers at high risk of MTCT were included in this study. Full-length HIV envelope genes (n = 100) were cloned from peripheral blood mononuclear cells obtained near transmission from transmitting mothers and at similar time points from nontransmitting mothers. Envelope clones were tested as pseudoviruses against contemporaneous, autologous maternal plasma in neutralization assays. The association between transmission and the log2 50% inhibitory concentration (IC50) for multiple virus variants per mother was estimated by using logistic regression with clustered standard errors. t tests were used to compare proportions of neutralization-resistant viruses. Overall, transmitting mothers had a median IC50 of 317 (interquartile range [IQR], 202 to 521), and nontransmitting mothers had a median IC50 of 243 (IQR, 95 to 594). Transmission risk was not significantly associated with autologous NAb activity (odds ratio, 1.25; P = 0.3). Compared to nontransmitting mothers, transmitting mothers had similar numbers of or fewer neutralization-resistant virus variants, depending on the IC50 neutralization resistance cutoff. In conclusion, HIV-infected mothers harbor mostly neutralization-sensitive viruses, although resistant variants were detected in both transmitting and nontransmitting mothers. These results suggest that MTCT during the breastfeeding period is not driven solely by the presence of maternal neutralization escape variants.
IMPORTANCE
There are limited data demonstrating whether NAbs can prevent HIV transmission and infection in humans, and for this reason, NAbs have been studied in MTCT, where maternal antibodies are present at the time of transmission. Results of these studies have varied, perhaps because of differences in methods. Importantly, studies often used cultured viruses and samples from time points outside the window of transmission, which could confound findings. Here, we considered the role of maternal NAbs against individual maternal virus variants near the time of transmission. We found no evidence that NAbs are associated with protection from infection. In fact, depending on the cutoff used to define neutralization resistance, we found evidence that nontransmitting mothers have more neutralization-resistant virus variants. These results suggest that lack of virus transmission in the early breastfeeding period is not simply due to an absence of maternal neutralization escape variants and likely includes multiple factors.
INTRODUCTION
The development of an effective HIV-specific neutralizing antibody (NAb) response remains a major goal of HIV vaccine research. As a proof of concept, NAbs have been shown to protect nonhuman primates against a simian/human immunodeficiency virus challenge (reviewed in references 1 to 3). In these studies, however, the passively administered antibodies were known to potently neutralize the challenge virus and thus did not take into account whether protection would occur with viruses that exhibit a range of neutralization sensitivities. Additionally, these studies used viruses that were adapted in culture and in animals, which are not representative of infectious viruses circulating in human populations (2). In humans, where HIV antigenic diversity is extensive, it has been challenging to address the role of NAbs in protection and, to date, there is limited direct evidence that NAbs can prevent HIV infection in humans.
Mother-to-child transmission (MTCT) provides another setting in which to examine the humoral immune correlates of protection, as infants receive antibodies from their mothers while in utero. Because HIV-infected mothers and their infants are monitored closely during the pre- and postnatal periods, timing of infant infection can be accurately determined and relevant immune correlates at the time of transmission can be studied in the mother and infant. In the absence of any intervention, only about 30 to 45% of infants become infected despite ample virus exposure (including postpartum through breastfeeding), suggesting that there may be a protective immune factor on the maternal or infant side (reviewed in reference 4).
In MTCT, there is evidence that NAbs contribute to an observed bottleneck for the viruses that are transmitted. Infants are typically infected with a single viral variant, and these variants have been shown to be more resistant to maternal plasma neutralization than maternal virus variants are in a number of studies (5–8). Accordingly, the role of maternal NAbs in preventing virus transmission has been directly assayed but results have varied. While some studies have observed a protective effect of maternal NAbs (7–19), others have seen no association (20–29), and a few have observed an increased risk of transmission in mothers with higher NAb titers (30, 31). The variation in these results may be due to differences in methodology, including the viruses tested (primary versus lab-adapted, heterologous versus autologous), timing of sampling and whether it was near the time of transmission (versus before or after), and availability of key clinical data that could influence NAb responses (e.g., timing of infant infection and maternal viral load) (32).
Several of these divergent studies specifically focused on maternal antibodies and their ability to neutralize autologous virus, which, by analogy to nonhuman primate vaccine protection studies, provides a measure of the protective efficacy of the antibodies against the “challenge” virus. In the MTCT studies, a primary isolate derived from short-term passage of maternal virus in culture was used to measure autologous NAb activity. These studies therefore considered the overall neutralization sensitivity of the maternal virus population but not the range of susceptibilities of individual variants, which may be more relevant if the bottleneck observed during MTCT is driven by selection for an escape variant. If NAbs are selecting for transmission of viruses that cannot be effectively neutralized by maternal antibodies, then nontransmitting mothers may have fewer of these resistant viruses and, hence, have a lower risk of transmission. Neutralization-resistant viruses may be challenging to detect when examining overall virus population sensitivity, and thus, a more relevant measure to consider is the neutralization properties of individual virus variants.
Only one study to date has examined individual virus variants to directly address the question of whether or not nontransmitting mothers tend to have fewer viruses resistant to maternal NAbs than transmitting mothers do. In that study, Baan et al. cloned individual virus variants from seven transmitting and four nontransmitting mothers and tested these viruses against autologous maternal plasma (31). The authors reported the somewhat unexpected finding that transmitting mothers tended to have viruses that were more sensitive to neutralization than those that nontransmitting mothers had. That study, however, examined maternal viruses obtained after transmission occurred in four of the seven transmitting mothers. In addition, the maternal plasma samples used for neutralization assays were often not contemporaneous to either the time of transmission or the time the viruses were cloned, adding more complexity to the interpretation of these findings. As antibodies and viruses evolve over the course of infection, the viruses and antibodies present after transmission may not be representative of the maternal viruses and immune responses present at transmission. A more relevant study would be to compare viruses and plasma from the same time point close to the time that transmission is estimated to have occurred.
In this study, we examined the role of maternal NAbs against contemporaneous maternal virus in transmitting and nontransmitting mothers by using samples obtained near the time of transmission (just prior to when infant infection was first detected) from transmitting women and at similar time points from nontransmitting women. We focused on women with high viral loads, who were thus at high risk of HIV transmission, and women whose infants were HIV negative at birth, which allowed us to identify relevant samples near transmission. Overall, maternal autologous NAb responses were not associated with transmission. Similarly, depending on what plasma dilution cutoff was used for analysis, nontransmitting mothers had numbers of neutralization-resistant viruses similar to or greater than those of transmitting mothers. Overall, these results suggest that the presence of virus resistant to antibody neutralization in the mother does not determine the infant infection outcome.
RESULTS
Cohort characteristics.
Ten nontransmitting and 10 transmitting mothers were chosen from the Nairobi Breastfeeding Clinical Trial for this study (Table 1). Mothers were chosen if they had a high viral load and breastfed for at least 3 months and were therefore at risk of transmission during the early breastfeeding period. To ensure that we could sample virus and antibodies near the time of transmission, only mothers whose infants were HIV DNA negative at birth and regularly screened for infection after birth were included in this study. Plasma samples and peripheral blood mononuclear cells (PBMCs) from transmitting women for envelope cloning were chosen from the same visit, just prior to the time that infants were first detected as positive. Similar time points were chosen for nontransmitting mothers.
TABLE 1 .
Characteristics of the mothers included in this studya
| Group and maternal ID no. or parameter |
Infant’s last negative HIV DNA results |
Infant’s first positive HIV DNA result |
Duration of BFb (mo) |
No. of CD4 cells/mm3 |
Maternal log10 plasma VLc |
Env virus subtype |
Env cloning and plasma neutralization time point |
% Maximum pairwise distance |
|---|---|---|---|---|---|---|---|---|
| Nontransmitting | ||||||||
| MA411 | M24 | NAd | 19.5 | 416 | 5.13 | A | P33 | 4.86 |
| MB807 | M24 | NA | 14.5 | 217 | 4.78 (W6) | A | P32 | 3.30 |
| MF074 | M24 | NA | 12.5 | NA | 5.01 | A | W3 | 1.12 |
| MF600 | M24 | NA | 16.5 | 344 | 5.49 | C | W2 | 1.38 |
| MG540 | M24 | NA | 11.5 | 285 | 5.06 | A | P32 | 2.58 |
| ML156 | M9 | NA | 11.23 | 410 | 5 | A/D | P32 | 1.98 |
| MM471 | M24 | NA | 22.5 | 360 | 5.26 | A | P32 | 3.20 |
| MM834 | M24 | NA | 18.5 | 633 | 5.11 | D | P32 | 0.94 |
| MO862 | M24 | NA | 10.5 | 134 | 4.86 | A | P33 | 5.97 |
| MP199 | M24 | NA | 18.5 | 389 | 5.22 (P38) | A | W0 | 5.12 |
| Transmitting | ||||||||
| MB549 | W0 | W6 | 3.47 | 411 | 6.25 | A | P30 | 1.24 |
| MC046 | W0 | W6 | 3 | 255 | 5.05 | A | P35 | 2.55 |
| MF403 | W0 | W6 | 29.5 | 213 | 5.07 | A | W0 | 0.87 |
| MF520 | W1 | W14 | 27.5 | 511 | 5.8 | A | P32 | 1.41 |
| MF535 | W1 | W6 | 19.5 | 690 | 5.53 | A/D | W0 | 6.22 |
| MJ412 | W0 | W6 | 22.5 | 293 | 4.98 | A/C/D | P32 | 5.88 |
| MJ613 | W0 | W6 | 9.67 | 104 | 4.7 | A | P32 | 5.28 |
| MK184 | W0 | W6 | 3.1 | 568 | 4.85 | D | P34 | 3.03 |
| ML035 | W0 | W6 | 7.9 | 249 | 4.26 | A/D | P32 | 3.65 |
| MM596 | W0 | W6 | 3.5 | 392 | 5.65 | A | W0 | 3.66 |
| P value | 0.36 | 0.93 | 0.88 | 0.65 |
P values represent comparisons of nontransmitting and transmitting mother cohort characteristics by Mann-Whitney U tests. Maternal viral loads are from the same visit as envelope cloning, except where indicated in parentheses. W, week after delivery; P, week of pregnancy; M, month after delivery.
BF, breastfeeding.
VL, viral load.
NA, not applicable/available.
The 20 women had a median plasma HIV load of 5.07 log10 copies/ml, a median CD4 cell count of 360/mm3, and a median duration of breastfeeding of 13.5 months. Viral loads did not differ between nontransmitting women and transmitting women (5.09 log10 copies/ml versus 5.06 log10 copies/ml; P = 0.88). Duration of breastfeeding (15.5 months versus 8.79 months; P = 0.36) and CD4 cell count (360/mm3 versus 342.5/mm3; P = 0.93) also did not differ between nontransmitting and transmitting mothers. All of the infants of the 10 transmitting mothers were HIV DNA negative at birth and first detected as HIV infected at either 6 (n = 9) or 14 (n = 1) weeks of age. For nine of the infected infants, RNA samples were also available from birth and were negative for HIV RNA, suggesting that transmission occurred very late in gestation, during delivery, or very early in the breastfeeding period. Thus, the maternal time point analyzed, which was typically at 32 weeks of gestation or at birth (range, 30th week of gestation to 3 weeks after birth) was within approximately a month of when transmission occurred.
Envelope clones.
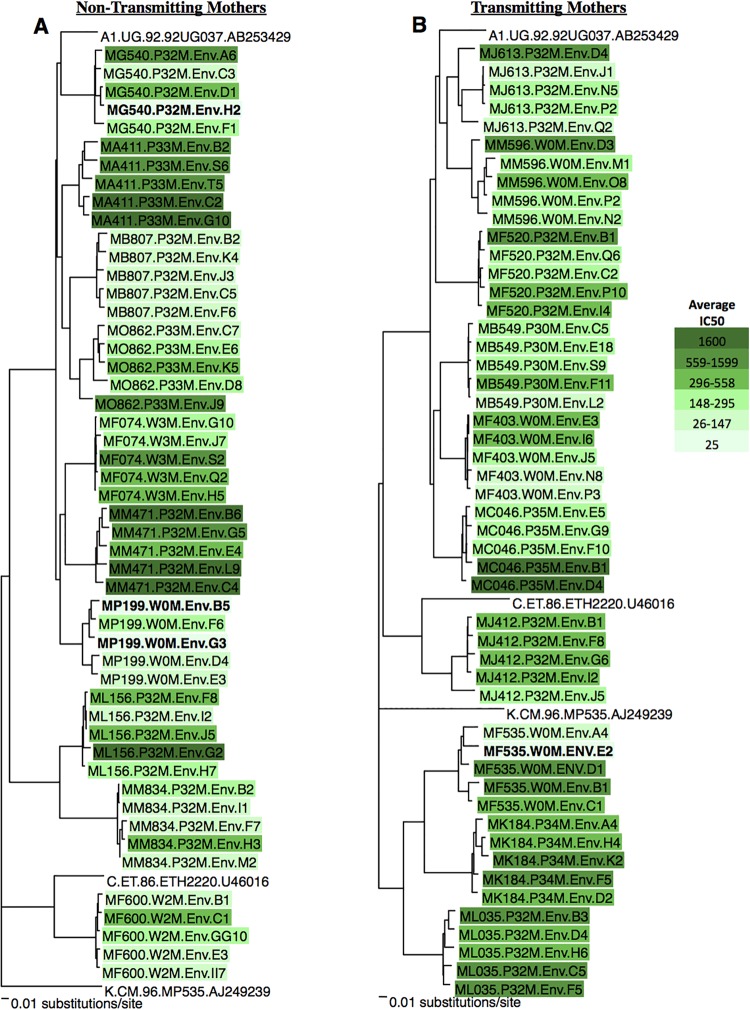
From each mother, five functional full-length gp160 envelope clones were obtained and are shown in neighbor-joining trees for nontransmitting (Fig. 1A) and transmitting (Fig. 1B) mothers. For each mother, the HIV envelope sequence diversity was calculated by using the maximum pairwise distance (Table 1). The maximum pairwise distance ranged from 0.87 to 6.22%. There was no difference in the median maximum pairwise distance between nontransmitting and transmitting women (2.89 versus 3.34%; P = 0.65).
FIG 1 .
Neighbor-joining trees of maternal HIV envelope genes. Envelope clones from nontransmitting (A) and transmitting (B) mothers. Each envelope sequence is identified by a maternal ID number followed by the timing of isolation and envelope ID. P number, pregnancy week; W number, week after delivery. Samples are color coded on the basis of average IC50s, as shown at the right, when tested against contemporaneous maternal plasma. Viruses resistant to neutralization at a 1:50 dilution of maternal plasma (IC50, 25) are in bold. Reference sequences are not colored.
Maternal virus subtypes were determined from full-length envelope sequences. Seven nontransmitting mothers and six transmitting mothers were infected with subtype A viruses (13/20 mothers; 65%). The remaining mothers were infected with subtype C (n = 1) subtype D (n = 2), or recombinant forms (n = 4).
Neutralization assay results.
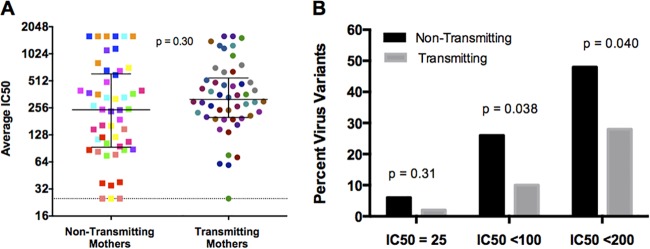
To determine the impact of maternal autologous NAbs near the time of transmission on transmission risk, the envelope clones were tested against contemporaneous plasma in a TZM-bl neutralization assay. Transmitting and nontransmitting maternal viruses displayed a range of neutralization sensitivities (50% inhibitory concentration [IC50] range, 25 to 1,600; Fig. 2A). Overall, the median IC50 for nontransmitting mothers was 243 (interquartile range [IQR], 95 to 594), which was lower than the median IC50 for transmitting mothers of 317 (IQR, 202 to 521). In a logistic regression analysis with clustered standard errors to account for intrawoman correlation, autologous NAb activity was not associated with transmission (odds ratio, 1.25; P = 0.30). When tested against autologous plasma at a 1:50 starting dilution, nontransmitting and transmitting mothers had similar numbers of neutralization-resistant viruses (estimated IC50 = 25) (6 versus 2%; P = 0.31; Fig. 2B). As the NAb concentration required to protect against infection has not been clearly established, and as plasma dilutions as low as approximately 1:100 to 1:200 have been associated with protection in nonhuman primate studies (33, 34), we also considered the percentage of neutralization-resistant viruses with average IC50s of <100 and <200. At these cutoffs, nontransmitting mothers had significantly higher percentages of neutralization-resistant virus variants than transmitting mothers did (P = 0.038 for IC50s of <100 and P = 0.040 for IC50s of <200; Fig. 2B).
FIG 2 .
Autologous NAb responses of nontransmitting and transmitting mothers. (A) IC50s of nontransmitting and transmitting mothers are color coded by mother. Each square or dot represents the IC50 of an individual envelope variant tested against contemporaneous autologous plasma. Data are shown as raw IC50s on a log2 scale; black bars represent medians and IQRs, and the dotted line represents an IC50 of 25 (resistant to neutralization at a 1:50 dilution). The P value is based on logistic regression with clustered standard errors using log2-transformed data. (B) Percentages of virus variants with IC50s below the detection limit, which we defined as the midpoint between 0 and the lowest dilution tested (IC50, 25); IC50s of <100; and IC50s of <200 for nontransmitting (black) and transmitting (gray) mothers. The P values were determined by t tests comparing the proportions of neutralization-resistant viruses of transmitting and nontransmitting mothers at the IC50s shown.
DISCUSSION
In this study, we compared the autologous NAb responses of transmitting and nontransmitting mothers to maternal envelope variants. We specifically focused on testing contemporaneous antibody and envelope variants just prior to when HIV DNA and RNA were first detected in infected infants to ensure that the measured responses were the most relevant NAb responses at the time of transmission. Importantly, we also included only breastfeeding mothers with high viral loads whose infants were HIV negative at birth. By restricting our analyses to these mothers, we focused on the women at highest risk of transmission for whom we could accurately estimate the timing of transmission and thus analyzed samples that were most relevant to the immune responses at transmission. Overall, we observed that there was no statistically significant difference in maternal autologous NAb responses between transmitting and nontransmitting mothers and that transmitting mothers had numbers of neutralization-resistant viruses similar to or lower than those of nontransmitting mothers.
In total, 100 functional envelope variants were cloned by single-copy PCR from the 20 women (five envelope variants per woman). These envelope clones displayed a range of neutralization sensitivities against contemporaneous autologous plasma. Transmitting women had slightly higher IC50s, suggesting that their viruses were more sensitive overall than those of nontransmitting mothers; however, this difference was not statistically significant. These data suggest that autologous NAbs are not a major correlate of protection from transmission. With only 10 women in each group, our power to detect a true difference may have been limited, despite our testing of five virus variants from each mother. However, the observation of higher IC50s in transmitting mothers than in nontransmitting mothers makes our hypothesis that transmitting mothers have weaker NAb responses (more resistant viruses) less likely. Similar to our results, a number of older studies using primary isolates to assess the overall neutralization sensitivity of the virus population observed no difference in the titers of NAbs against autologous virus and the risk of transmission (10, 13, 21–24, 27). However, other studies using similar methods did report higher levels of autologous NAbs in nontransmitting mothers (7, 8, 11, 12). It is important to note that such studies give an overall view of neutralization sensitivity or resistance of all viruses in the population, but they do not directly address the question of whether transmitting mothers have more individual neutralization-resistant variants that could escape NAb pressure.
Given that a number of studies (including one from the Nairobi Breastfeeding Clinical Trial) have suggested that NAbs contribute to the virus bottleneck observed in MTCT (5–8), we originally hypothesized that nontransmitting mothers may harbor mostly neutralization-sensitive variants and that resistant variants would be rarer in these mothers than in transmitting mothers. However, we observed similar numbers of viruses that were resistant to neutralization at the highest plasma concentration tested (1:50 dilution) in both transmitters and nontransmitters. Surprisingly, nontransmitting mothers had more viruses that were resistant to neutralization at a range of plasma dilutions (1:100 and 1:200) than did transmitting mothers, with a significant difference observed at both cutoffs. These observations, coupled with the findings of slightly higher IC50s in the transmitting women than in the nontransmitting women, do not support our original hypothesis that nontransmitting mothers harbor fewer neutralization-resistant viruses. Indeed, they are consistent with the one prior small study (11 women, 23 viruses) that also examined individual viral variants from transmitting and nontransmitting women and observed significantly stronger NAb responses in transmitting mothers (31). The basis for the differences in these findings, suggesting high NAb levels in transmitting mothers when examining individual viral variants cloned directly from mothers compared to findings suggesting the opposite from studies with cultured virus populations, is unclear. It is possible that culturing perturbed the virus population. While short-term culture retains most of the dominant variants (35), the precise culturing methods were often not fully described and longer-term culture could distort the virus population. Given the results presented here, these discrepant findings highlight the need for larger studies that include analyses of both individual virus variants and virus populations using well-timed samples near transmission, especially given the interest in the use of monoclonal NAbs for MTCT.
While this study is the largest analysis to date of contemporaneous autologous NAb responses in transmitting and nontransmitting mothers against individual maternal virus variants, it does present some potential limitations. First, the timing and method of infant infection are estimated from the regular sampling and testing of infant samples. Of the 10 infected infants, 9 were HIV RNA negative at birth (the 10th infant did not have a plasma sample from birth available to test), suggesting that infection occurred late in utero, during delivery, or in the early breastfeeding period. On the basis of results from the original Nairobi Breastfeeding Clinical Trial cohort, which compared breastfeeding and formula-feeding mothers, there was a great increase in infections during the first 6 weeks after birth in the breastfeeding arm but not in the formula-feeding arm (36), suggesting that the majority of transmissions in the 10 mother-infant pairs here occurred via breastfeeding. Therefore, choosing maternal samples from late pregnancy or the early breastfeeding period before the infant was detected as HIV positive is most relevant to when infant infection occurred. Additionally, because breastfeeding samples were limited, we cloned from PBMC samples. The most comprehensive studies comparing breast milk- and PBMC-derived viruses suggest that there is intermingling of viruses in blood and breast milk (37, 38). Thus, the viruses cloned from blood are likely representative of those found and transmitted via breast milk. Finally, the HIV quasispecies is quite large and diverse in any given individual, making it difficult to clone and test all of the functional variants for each mother. The choice of five variants per mother used here is a greater number than what previous studies have used when looking at individual variants (31). Furthermore, the fact that we could detect functional neutralization-resistant virus variants in nontransmitting mothers within this sampling supports our conclusion that the presence of neutralization-resistant variants is not what drives transmission.
Overall, we present here the largest study to date of contemporaneous autologous NAb responses against individual maternal virus variants in transmitting and nontransmitting mothers. An important aspect of this study was our focus on the virus variants and antibodies present close to the time of transmission. Our results show that transmitting and nontransmitting mothers harbor a mixture of mostly neutralization-sensitive viruses and have similar overall neutralization IC50s. Remarkably, even women at high risk of transmission (based on high viral loads) had relatively neutralization-resistant variants within their virus population and yet did not transmit the virus to their infants. These results suggest that the presence of neutralization escape variants does not fully predict MTCT and that transmission risk likely includes multiple factors, perhaps including selective pressure to transmit escape variants when other conditions are favorable for infection.
MATERIALS AND METHODS
Study design.
Samples for this study were from the Nairobi Breastfeeding Clinical Trial, which was conducted in the mid-1990s in Kenya (36). For the study presented here, mothers were included on the basis of the following criteria: (i) a high plasma viral load of >4.6 log10 copies/ml (the overall cohort median [39]), (ii) breastfeeding for ≥3 months, (iii) an infant that was HIV DNA negative at birth, and (iv) a maternal sample that was available near the time of transmission (or at a similar time point for the nontransmitting mothers). On the basis of these criteria, 10 nontransmitting mothers and 9 transmitting mothers were chosen for this study. One transmitting mother (ML035) with a viral load slightly lighter than the cohort median (4.26 log10 copies/ml) was also included, for a total of 10 transmitting mothers. The ethical review committee of the Kenyatta National Hospital Institutional Review Board, the Institutional Review Board of the University of Washington, and the Institutional Review Board of the Fred Hutchinson Cancer Research Center gave permission to conduct the Nairobi Breastfeeding Clinical Trial.
Envelope cloning and pseudovirus generation.
Envelope clones were already available for MF535 from a previous study (5). In the other 19 mothers, full-length (gp160) envelope genes were cloned from DNA isolated from uncultured PBMCs. DNA was extracted with Qiagen’s QIAamp Blood minikit according to the manufacturer’s protocol. The number of HIV copies present in the PBMC DNA was determined with a pol real-time PCR assay (40). DNA templates were diluted to approximately single copy on the basis of the real-time PCR quantification, and envelope genes were amplified with a nested PCR outlined elsewhere (5). The following primers were used. For the first round, we used vpr1 (5′ GATAGATGGAACAAGCCCCAG 3′) and nef24 (5′ TACTTGTGATTGCTCCAT GT 3′) mixed in an equal molar ratio with nef34 (5′ TACTTGTGACTGCTCCATGT 3′). For the second round, we used primers vpr21a1 (5′ TAACCTAGACGCGTGGAATCACCCGGGAAGTCAGCCTACAACACCTTGTA 3′) and vpr21a2 (5′ TAACCTAGACGCGTGGAATCACCCGGGAAGCCGGCCTACAACACCTTGTA 3′) and primers nef60a1 (5′CTTGTGGCGGCCGCATGTTTATCTAAATCTCGAGATACTGCTCCTACTCCTGGTGCTG 3′) and nef60a2 (5′ CTTGTGGCGGCCGCATGTTTAGCTAAATCTCGAGATACTGCTCCTACTCCTGGTGCT 3′) in equal molar ratios. PCR products were cloned into either pcDNA3.1/V5-His-Topo or pCI-Neo (Invitrogen).
To generate pseudoviruses capable of one round of infection, plasmids containing the maternal HIV envelopes were cotransfected with a second plasmid containing a subtype A env-deficient HIV genome (Q23Δenv) (41). The infectivity of pseudotyped viruses was screened by single-round infection of TZM-bl cells. Functional, infectious envelopes obtained from separate PCRs (five per woman) were sequenced and used for neutralization studies.
Neutralization assays.
Functional pseudoviruses were tested in neutralization assays against contemporaneous autologous maternal plasma as previously described (5, 42). Briefly, pseudovirus titers were first determined with a single-round infection of TZM-bl cells by directly counting β-galactosidase-positive “blue” cells at 48 h postinfection. Serial dilutions of heat-inactivated maternal plasma were incubated with 500 infectious pseudovirus particles in a total volume of 50 µl at 37°C for 60 min. Maternal plasma dilutions started at 1:50. Subsequently, TZM-bl cells (1 × 104 in 100 µl of Dulbecco’s modified Eagle’s medium) were added to virus-antibody mixtures. Forty-eight hours postinfection, β-galactosidase levels were measured with the Galacto-Light system (Applied Biosystems). Percent neutralization was calculated as the percent reduction of β-galactosidase activity compared to virus without any patient plasma. The reciprocal plasma dilution that resulted in 50% inhibition of virus infection was determined from a dose-response curve. Each virus was run twice in duplicate with the corresponding maternal plasma. Additionally, each maternal plasma sample was tested against simian immunodeficiency virus to ensure that background neutralization was below the limit of detection. If the IC50s from the two runs differed by more than 2-fold, a third assay was run (in duplicate) and all of the results were averaged. Neutralization IC50s less than the lowest dilution tested (1:50) were set at the midpoint between 0 and the lowest dilution (25). Viruses that did not display 50% inhibition at the highest dilution tested (1:1,600) were assigned an IC50 of the highest dilution (1,600).
Phylogenetic analysis.
The nucleotide sequences of all infectious envelopes were determined by Sanger sequencing. Full-length maternal envelope sequences were aligned with MacClade version 4.01. Alignments were manually edited to remove variable regions that could not be unambiguously aligned. Neighbor-joining phylogenetic trees using pairwise distance, based on a general time-reversible model, were made with PAUP* 4.0b10. Reference sequences for subtypes A, C, and K were accessed from the Los Alamos National Laboratory HIV database (http://www.hiv.lanl.gov), and the unrelated subtype K sequence was used as an outgroup. Virus subtypes, based on full-length envelope sequences, were determined by the phylogenetic trees and the NCBI genotyping tool (http://www.ncbi.nlm.nih.gov/projects/genotyping/formpage.cgi). For each mother, an alignment of the five envelope clones was made to determine the percent maximum pairwise distance in PAUP.
Statistical analyses.
Clinical characteristics (viral load, CD4 cell count, duration of breastfeeding) of transmitting and nontransmitting mothers were compared by Mann-Whitney U tests. Percent maximum pairwise distances of envelope clones for transmitting and nontransmitting mothers were also compared by a Mann-Whitney U test. For analyses, IC50s were log2 transformed, as plasma samples were diluted 2-fold in neutralization assays. The log2 IC50s of nontransmitting and transmitting mothers were compared by using a logistic regression with clustered standard errors to account for intrawoman correlation. A two-tailed t test with Welch’s correction was used to compare the proportions of neutralization-resistant viruses in transmitting and nontransmitting women.
ACKNOWLEDGMENTS
We thank the women and infants who participated in the Nairobi Breastfeeding Clinical Trail. We also thank Stephanie Rainwater for her cloning assistance.
M.M.O., C.M., and J.O. designed experiments. M.M.O., V.C., and C.M. conducted experiments. K.O.-D., B.A.R., and C.M. conducted all statistical analysis. R.N. oversaw the original Nairobi Breastfeeding Clinical Trial and the collection of the samples used in this study. C.M. and J.O. wrote the paper with input from all of the coauthors.
This article is a direct contribution from a Fellow of the American Academy of Microbiology.
Funding Statement
This project was supported by NIH grant R01 AI076105. CM was supported by NIH training grant T32 AI083203 and fellowship F30 AI112385. MMO was supported by NIH training grant D43 TW000007. The Funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Milligan C, Omenda MM, Chohan V, Odem-Davis K, Richardson BA, Nduati R, Overbaugh J. 2016. Maternal neutralization-resistant virus variants do not predict infant HIV infection risk. mBio 7(1):e02221-15. doi:10.1128/mBio.02221-15.
REFERENCES
- 1.Evans DT, Silvestri G. 2013. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS 8:255–261. doi: 10.1097/COH.0b013e328361cee8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A, Boyd DF, Overbaugh J. 2015. Development of SHIVs with circulating, transmitted HIV-1 variants. J Med Primatol 44:296–300. doi: 10.1111/jmp.12179. [DOI] [PubMed] [Google Scholar]
- 3.Hatziioannou T, Evans DT. 2012. Animal models for HIV/AIDS research. Nat Rev Microbiol 10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehman DA, Farquhar C. 2007. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Rev Med Virol 17:381–403. doi: 10.1002/rmv.543. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Parast AB, Richardson BA, Nduati R, John-Stewart G, Mbori-Ngacha D, Rainwater SM, Overbaugh J. 2006. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol 80:835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Rola M, West JT, Tully DC, Kubis P, He J, Kankasa C, Wood C. 2010. Functional properties of the HIV-1 subtype C envelope glycoprotein associated with mother-to-child transmission. Virology 400:164–174. doi: 10.1016/j.virol.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickover R, Garratty E, Yusim K, Miller C, Korber B, Bryson Y. 2006. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J Virol 80:6525–6533. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliks SC, Wara DW, Landers DV, Levy JA. 1994. Features of HIV-1 that could influence maternal-child transmission. JAMA 272:467–474. [PubMed] [Google Scholar]
- 9.Ugen KE, Goedert JJ, Boyer J, Refaeli Y, Frank I, Williams WV, Willoughby A, Landesman S, Mendez H, Rubinstein A. 1992. Vertical transmission of human immunodeficiency virus (HIV) infection. Reactivity of maternal sera with glycoprotein 120 and 41 peptides from HIV type 1. J Clin Invest 89:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarlatti G, Albert J, Rossi P, Hodara V, Biraghi P, Muggiasca L, Fenyö EM. 1993. Mother-to-child transmission of human immunodeficiency virus type 1: correlation with neutralizing antibodies against primary isolates. J Infect Dis 168:207–210. doi: 10.1093/infdis/168.1.207. [DOI] [PubMed] [Google Scholar]
- 11.Scarlatti G, Leitner T, Hodara V, Halapi E, Rossi P, Albert J, Fenyö EM. 1993. Neutralizing antibodies and viral characteristics in mother-to-child transmission of HIV-1. AIDS 7(Suppl 2):S45–S48. doi: 10.1097/00002030-199311002-00010. [DOI] [PubMed] [Google Scholar]
- 12.Lathey JL, Tsou J, Brinker K, Hsia K, Meyer WA, Spector SA. 1999. Lack of autologous neutralizing antibody to human immunodeficiency virus type 1 (HIV-1) and macrophage tropism are associated with mother-to-infant transmission. J Infect Dis 180:344–350. [DOI] [PubMed] [Google Scholar]
- 13.Bongertz V, Costa CI, Veloso VG, Grinsztejn B, João Filho EC, Calvet G, Pilotto JH, Guimarães ML, Morgado MG. 2001. Vertical HIV-1 transmission: importance of neutralizing antibody titer and specificity. Scand J Immunol 53:302–309. doi: 10.1046/j.1365-3083.2001.00866.x. [DOI] [PubMed] [Google Scholar]
- 14.Bongertz V, Costa CI, Veloso VG, Grinsztejn B, Filho EC, Calvet G, Pilotto JH. 2002. Neutralization titres and vertical HIV-1 transmission. Scand J Immunol 56:642–644. doi: 10.1046/j.1365-3083.2002.01174.x. [DOI] [PubMed] [Google Scholar]
- 15.Barin F, Jourdain G, Brunet S, Ngo-Giang-Huong N, Weerawatgoompa S, Karnchanamayul W, Ariyadej S, Hansudewechakul R, Achalapong J, Yuthavisuthi P, Ngampiyaskul C, Bhakeecheep S, Hemwutthiphan C, Lallemant M, Perinatal HIV Prevention Trial Group . 2006. Revisiting the role of neutralizing antibodies in mother-to-child transmission of HIV-1. J Infect Dis 193:1504–1511. doi: 10.1086/503778. [DOI] [PubMed] [Google Scholar]
- 16.Samleerat T, Thenin S, Jourdain G, Ngo-Giang-Huong N, Moreau A, Leechanachai P, Ithisuknanth J, Pagdi K, Wannarit P, Sangsawang S, Lallemant M, Barin F, Braibant M. 2009. Maternal neutralizing antibodies against a CRF01_AE primary isolate are associated with a low rate of intrapartum HIV-1 transmission. Virology 387:388–394. doi: 10.1016/j.virol.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diomede L, Nyoka S, Pastori C, Scotti L, Zambon A, Sherman G, Gray CM, Sarzotti-Kelsoe M, Lopalco L. 2012. Passively transmitted gp41 antibodies in babies born from HIV-1 subtype C-seropositive women: correlation between fine specificity and protection. J Virol 86:4129–4138. doi: 10.1128/JVI.06359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaillon A, Wack T, Braibant M, Mandelbrot L, Blanche S, Warszawski J, Barin F. 2012. The breadth and titer of maternal HIV-1-specific heterologous neutralizing antibodies are not associated with a lower rate of mother-to-child transmission of HIV-1. J Virol 86:10540–10546. doi: 10.1128/JVI.00518-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Permar SR, Fong Y, Vandergrift N, Fouda GG, Gilbert P, Parks R, Jaeger FH, Pollara J, Martelli A, Liebl BE, Lloyd K, Yates NL, Overman RG, Shen X, Whitaker K, Chen H, Pritchett J, Solomon E, Friberg E, Marshall DJ, Whitesides JF, Gurley TC, Holle Von T, Martinez DR, Cai F, Kumar A, Xia S-M, Lu X, Louzao R, Wilkes S, Datta S, Sarzotti-Kelsoe M, Liao H-X, Ferrari G, Alam SM, Montefiori DC, Denny TN, Moody MA, Tomaras GD, Gao F, Haynes BF. 2015. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J Clin Invest 125:2702–2706. doi: 10.1172/JCI81593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broliden K, Sievers E, Tovo PA, Moschese V, Scarlatti G, Broliden PA, Fundaro C, Rossi P. 1993. Antibody-dependent cellular cytotoxicity and neutralizing activity in sera of HIV-1-infected mothers and their children. Clin Exp Immunol 93:56–64. doi: 10.1111/j.1365-2249.1993.tb06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Husson RN, Lan Y, Kojima E, Venzon D, Mitsuya H, McIntosh K. 1995. Vertical transmission of human immunodeficiency virus type 1: autologous neutralizing antibody, virus load, and virus phenotype. J Pediatr 126:865–871. doi: 10.1016/S0022-3476(95)70198-2. [DOI] [PubMed] [Google Scholar]
- 22.Hengel RL, Kennedy MS, Steketee RW, Thea DM, Abrams EJ, Lambert G, McDougal JS. 1998. Neutralizing antibody and perinatal transmission of human immunodeficiency virus type 1. New York City Perinatal HIV Transmission Collaborative Study Group. AIDS Res Hum Retroviruses 14:475–481. doi: 10.1089/aid.1998.14.475. [DOI] [PubMed] [Google Scholar]
- 23.Mabondzo A, Rouvier P, Raoul H, Le Naour R, Courpotin C, Hervé F, Parnet-Mathieu F, Lasfargues G, Dormont D. 1995. Relationships between humoral factors in HIV-1-infected mothers and the occurrence of HIV infection in their infants. Clin Exp Immunol 102:476–480. doi: 10.1111/j.1365-2249.1995.tb03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mabondzo A, Narwa R, Roques P, Gras GS, Hervé F, Parnet-Mathieu F, Lasfargues G, Courpotin C, Dormont D. 1998. Lack of correlation between vertical transmission of HIV-1 and maternal antibody titers against autologous virus in human monocyte-derived macrophages. J Acquir Immune Defic Syndr Hum Retrovirol 17:92–94. doi: 10.1097/00042560-199801010-00015. [DOI] [PubMed] [Google Scholar]
- 25.Bal AK, Miller G, Viscarello R, Andiman WA. 1996. Syncytium-inhibiting and neutralizing activity in maternal sera fail to prevent vertical transmission of human immunodeficiency virus type 1. Pediatr Infect Dis J 15:315–320. doi: 10.1097/00006454-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Louisirirotchanakul S, Beddows S, Cheingsong R, Shaffer N, Mastro TD, Likanonsakul S, Wasi C, Taylor GP, Weber JN. 1999. Role of maternal humoral immunity in vertical transmission of HIV-1 subtype E in Thailand. J Acquir Immune Defic Syndr 21:259–265. [DOI] [PubMed] [Google Scholar]
- 27.Kittinunvorakoon C, Morris MK, Neeyapun K, Jetsawang B, Buehring GC, Hanson CV. 2009. Mother to child transmission of HIV-1 in a Thai population: role of virus characteristics and maternal humoral immune response. J Med Virol 81:768–778. doi: 10.1002/jmv.21465. [DOI] [PubMed] [Google Scholar]
- 28.Russell ES, Kwiek JJ, Keys J, Barton K, Mwapasa V, Montefiori DC, Meshnick SR, Swanstrom R. 2011. The genetic bottleneck in vertical transmission of subtype C HIV-1 is not driven by selection of especially neutralization-resistant virus from the maternal viral population. J Virol 85:8253–8262. doi: 10.1128/JVI.00197-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omenda MM, Milligan C, Odem-Davis K, Nduati R, Richardson BA, Lynch J, John-Stewart G, Overbaugh J. 2013. Evidence for efficient vertical transfer of maternal HIV-1 envelope-specific neutralizing antibodies but no association of such antibodies with reduced infant infection. J Acquir Immune Defic Syndr 64:163–166. doi: 10.1097/QAI.0b013e31829f6e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guevara H, Casseb J, Zijenah LS, Mbizvo M, Oceguera LF III, Hanson CV, Katzenstein DA, Hendry RM. 2002. Maternal HIV-1 antibody and vertical transmission in subtype C virus infection. J Acquir Immune Defic Syndr 29:435–440. doi: 10.1097/00042560-200204150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Baan E, de Ronde A, Stax M, Sanders RW, Luchters S, Vyankandondera J, Lange JM, Pollakis G, Paxton WA. 2013. HIV-1 autologous antibody neutralization associates with mother to child transmission. PLoS One 8:e69274. doi: 10.1371/journal.pone.0069274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overbaugh J. 2014. Mother-infant HIV transmission: do maternal HIV-specific antibodies protect the infant? PLoS Pathog 10:e1004283. doi: 10.1371/journal.ppat.1004283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Cetrulo Lorenzi JC, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA, Nishimura Y. 2014. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med 211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voronin Y, Chohan B, Emerman M, Overbaugh J. 2007. Primary isolates of human immunodeficiency virus type 1 are usually dominated by the major variants found in blood. J Virol 81:10232–10241. doi: 10.1128/JVI.01035-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, Kreiss J. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 37.Heath L, Conway S, Jones L, Semrau K, Nakamura K, Walter J, Decker WD, Hong J, Chen T, Heil M, Sinkala M, Kankasa C, Thea DM, Kuhn L, Mullins JI, Aldrovandi GM. 2010. Restriction of HIV-1 genotypes in breast milk does not account for the population transmission genetic bottleneck that occurs following transmission. PLoS One 5:e10213. doi: 10.1371/journal.pone.0010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar-Gonzalez JF, Salazar MG, Learn GH, Fouda GG, Kang HH, Mahlokozera T, Wilks AB, Lovingood RV, Stacey A, Kalilani L, Meshnick SR, Borrow P, Montefiori DC, Denny TN, Letvin NL, Shaw GM, Hahn BH, Permar SR, Center for HIV/AIDS Vaccine Immunology A0167854 . 2011. Origin and evolution of HIV-1 in breast milk determined by single-genome amplification and sequencing. J Virol 85:2751–2763. doi: 10.1128/JVI.02316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, Overbaugh J, Bwayo J, Ndinya-Achola JO, Kreiss JK. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis 183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 40.Rousseau CM, Nduati RW, Richardson BA, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. 2004. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis 190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long EM, Rainwater SM, Lavreys L, Mandaliya K, Overbaugh J. 2002. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res Hum Retroviruses 18:567–576. doi: 10.1089/088922202753747914. [DOI] [PubMed] [Google Scholar]
- 42.Rainwater SM, Wu X, Nduati R, Nedellec R, Mosier D, John-Stewart G, Mbori-Ngacha D, Overbaugh J. 2007. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr HIV Res 5:189–197. doi: 10.2174/157016207780076986. [DOI] [PubMed] [Google Scholar]
- 43.Becquart P, Chomont N, Roques P, Ayouba A, Kazatchkine MD, Bélec L, Hocini H. 2002. Compartmentalization of HIV-1 between breast milk and blood of HIV-infected mothers. Virology 300:109–117. doi: 10.1006/viro.2002.1537. [DOI] [PubMed] [Google Scholar]