Abstract
Approximately one-half of the patients who develop clinical atherosclerosis have normal or only modest elevations in plasma lipids, indicating that additional mechanisms contribute to pathogenesis. In view of increasing evidence that inflammation contributes to atherogenesis, we studied the effect of human neutrophil α-defensins on low density lipoprotein (LDL) trafficking, metabolism, vascular deposition, and atherogenesis using transgenic mice expressing human α-defensins in their polymorphonuclear leukocytes (Def+/+). Accelerated Def+/+ mice developed α-defensin·LDL complexes that accelerate the clearance of LDL from the circulation accompanied by enhanced vascular deposition and retention of LDL, induction of endothelial cathepsins, increased endothelial permeability to LDL, and the development of lipid streaks in the aortic roots when fed a regular diet and at normal plasma levels of LDL. Transplantation of bone marrow from Def+/+ to WT mice increased LDL clearance, increased vascular permeability, and increased vascular deposition of LDL, whereas transplantation of WT bone marrow to Def+/+ mice prevented these outcomes. The same outcome was obtained by treating Def+/+ mice with colchicine to inhibit the release of α-defensins. These studies identify a potential new link between inflammation and the development of atherosclerosis.
Keywords: atherosclerosis, defensin, lipoprotein, low density lipoprotein (LDL), neutrophil, α-defensins, atherosclerosis
Introduction
Atherosclerosis and its thrombotic sequel, “atherothrombosis,” are likely to remain the predominant cause of death in “developed” countries for decades to come. The etiology of this syndrome is multifactorial, and current predictors provide an incomplete estimate of the risk and opportunity for intervention. In a pooled analysis of over 87,000 persons with diagnosed coronary heart disease, one in five lacked any of the conventional risk factors: hypertension, smoking, high cholesterol, or diabetes (1). Furthermore, half of all cardiovascular events occur in patients with normal lipid levels (2). These data reveal the need to identify and mitigate as yet undescribed, but clinically relevant, risk factors for cardiovascular disease beyond those targeted in current practice.
This problem is compounded by the lack of animal models that closely simulate human disease. Animal models used to study atherosclerosis, including its hyperlipidemic (3, 4) and inflammatory (5) components, are often characterized by striking elevations in plasma cholesterol, reaching plasma concentrations of 1500–2200 mg/dl in LDLR−/− and 400–450 mg/dl in ApoE−/− mice fed a high fat diet (3, 4), and most of the cholesterol is found in the VLDL fraction (3, 4). Neither these levels of lipids nor this distribution of lipoproteins is representative of findings in the vast majority of patients with atherosclerosis. Thus, there continues to be a need for new models to identify novel risk factors and novel approaches to intervention.
We have identified human α-defensins 1–4, also known as human neutrophil peptides (HNPs),2 as potentially having a role in the development of atherosclerosis. α-Defensins are antimicrobial proteins that constitute ∼5% of the total protein in polymorphonuclear leukocytes (PMNs). α-Defensins are released from a subset of azurophilic granules when the PMNs are activated by a variety of agonists (6, 7). α-Defensins are abundant in human atherosclerotic coronary and carotid arteries (8, 9), and there is a significant correlation between the deposition of α-defensins in skin tissue and the severity of coronary artery disease (10). α-Defensins inhibit the degradation of low density lipoprotein (LDL) and Lp(a) by vascular cells (11), increase their binding and retention in extracellular matrix (12), and inhibit tissue-type plasminogen activator (tPA)-mediated fibrinolysis (13, 14).
These observations have been confirmed and extended by others. Increased plasma levels of α-defensins are associated with acute myocardial infarction (15), cardiovascular mortality in patients with peripheral arterial disease (16), and chronic heart failure (17). α-Defensins stimulate foam cell formation (18), promote plaque instability (16), regulate aortic contractility, and activate leukocytes and platelets through low density lipoprotein receptor-related protein (LRP) (18, 19). However, the cause and effect relationships among these observations in vivo and their relevance to human disease remain to be established.
α-Defensins are not found in mouse PMNs (7). Therefore, existing mouse models of atherosclerosis do not reflect the complete contribution of inflammation, PMNs, or α-defensins to lesion development in humans. To help circumvent this problem, we recently characterized a transgenic mouse that expresses human α-defensins in its PMNs (Def+/+) (20). Def+/+ mice, which have a relatively low propensity to develop atherosclerosis even when fed a high fat diet (3, 21), were bred onto a C57Bl6 α-defensins background (20), enabling us to isolate the contribution of Def−/−.
Our data show that α-defensins induce a post-translational modification of LDL that alters its metabolism and disposition in the vasculature. α-Defensin·LDL complexes circulate in the plasma of Def+/+ mice and healthy human volunteers. Isolated or ex vivo reconstituted α-defensin·LDL complexes are cleared more rapidly from the circulation and show enhanced vascular deposition and retention compared with unmodified LDL. Accelerated LDL clearance in Def+/+ mice is associated with the development of lipid streaks in aortic roots even while they are consuming a regular diet; this effect was prevented in our study by bone marrow transplantation from wild-type mice or chronic ingestion of colchicine, which inhibits the release of α-defensins. These studies suggest that α-defensins released by activated PMNs may contribute to the development of atherosclerosis in patients with normal or moderately elevated levels of circulating LDL.
Experimental Procedures
Mice
C57BL/6 mice expressing HNP-1 and HNP-2 in their neutrophils (Def+/+) have been characterized previously (20, 22). Animal care and experimentation were conducted in accordance with protocols approved by the Animal Care Committee of the Hebrew University. Animals were fed a regular chow diet that contained 4.5% fat (PMI 5010, Harlan, Rehovot, Israel) or a high fat diet (HFD) that contained 15.8% fat and 1.25% cholesterol (TD.88051, Harlan). Colchicine was added to the drinking water at a concentration of 1 mg/liter, and the water was changed twice weekly.
Bone Marrow Transplantation
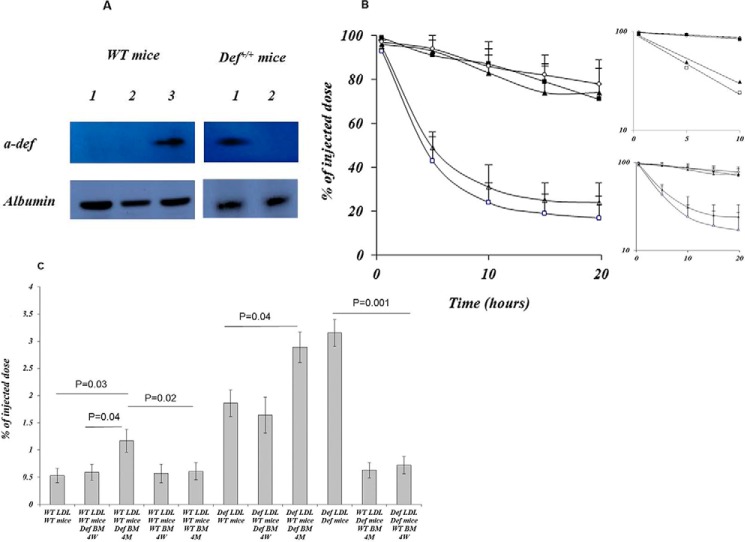
We transplanted bone marrow from male Def+/+ mice or wild-type (WT) mice into 6–8 week-old irradiated syngeneic male WT mice or bone marrow from WT mice into Def+/+ mice. All mice were on a C57BL/6 background. Whole bone marrow was collected from the femurs and tibias of donor WT and Def+/+ mice (23, 24). On the day of bone marrow transplantation, 6–8-week-old recipient WT and Def+/+ mice received 1000 centigrays total body irradiation in split doses (23, 24). Three hours post-irradiation, 2–5 × 106 bone marrow cells in a volume of 150–200 μl of PBS were injected via the tail vein (23, 24). White blood cell recovery and α-defensin levels were monitored by retro-orbital puncture. WT mice transplanted with Def+/+ bone marrow expressed α-defensins in their plasma (Fig. 2A), and plasma levels of α-defensins reached those found in Def+/+ mice by 4 weeks post-transplant (69 ± 9 μg/liter). In contrast, α-defensins were no longer detectable in the plasma and bone marrow of Def+/+ mice transplanted with WT bone marrow by 4 weeks.
FIGURE 2.
Phenotypic conversion of LDL clearance following bone marrow transplantation. A, left, LDL·α-defensin complexes in the plasma of WT mice (lane 1) before and 1 month after transplantation with WT (lane 2) or Def+/+ (lane 3) bone marrow. Right, LDL·α-defensin complexes in the plasma of Def+/+ mice before (lane 1) and 1 month (lane 2) post-transplantation with WT bone marrow. The complexes were identified as described for Fig. 1C. B, LDL isolated from WT mice transplanted with Def+/+ bone marrow fed a HFD and injected into WT mice transplanted with Def+/+ bone marrow (open triangles) or injected into Def+/+ mice (open squares). LDL isolated from Def+/+ mice transplanted with WT bone marrow and injected into Def+/+ mice transplanted with WT bone marrow (solid triangles) or into WT mice (solid squares). Open circles show the clearance of LDL isolated from naive WT mice and injected into naive WT mice. Clearance was determined as described for Fig. 1A. The mean ± S.D. is shown. The inserts show the semi-log plot and the linear fits. C, LDL, isolated from WT mice, WT mice transplanted with Def+/+ bone marrow, Def+/+ mice, or Def+/+ mice transplanted with WT bone marrow, was radiolabeled and reinjected into WT mice or WT mice transplanted with WT bone marrow at 4 weeks (4W) or 4 months (4M) post-transplant. Radioactivity incorporated into the aortas was determined as described for Fig. 1E. The mean ± 2 S.D. and p values are shown.
Cell Lines
The mouse macrophage cell line RAW264.7 was purchased from American Type Culture Collection (Manassas, VA). Bovine endothelial aortic cells (BAEC) were the gift of I. Vlodavsky, Hadassah-Hebrew University.
Antibodies
Mouse monoclonal anti-HNP antibodies were purchased from Antibodies-Online (Aachen, Germany), affinity-purified polyclonal antibodies to mouse cathepsin B from R&D Systems (Minneapolis, MN), F4/80 and rat IgG2a monoclonal anti-mouse macrophage antibodies from Invitrogen, and rat monoclonal anti-mouse CD15 (anti-PMN) antibodies from Biotest (Bet Haemek, Israel).
Collection and Analysis of Blood Samples
Blood was taken by transcardiac puncture after 6 h of fasting. Total plasma cholesterol, high density lipoprotein cholesterol (HDL), and triacylglycerol were measured enzymatically using an autoanalyser (Cobas 6000, Roche), and LDL cholesterol was calculated. α-Defensins were measured by ELISA.
Isolation and Radiolabeling of LDL
Plasma was obtained from male Def+/+ and WT mice maintained on a HFD. LDL was isolated and pooled. Human plasma was collected from healthy volunteers with normal plasma levels of LDL. LDL was radiolabeled as described (11).
In Vivo Clearance of Plasma LDL
Def+/+ and WT mice were anesthetized with intraperitoneal zolazepam (25 mg/kg) and xylazine (50 mg/kg). 125I-LDL (human or murine) preincubated with buffer or α-defensins for 2 h (20 μg of protein, ∼ 2 × 106 cpm) in 150 μl of saline was injected through the tail vein. Radioactivity in the syringe and cannula was counted before and after injection to determine the amount of radioactivity administered. Blood samples were withdrawn at specified times and centrifuged at 10,000 × g for 5 min at 4 °C. Radioactivity in the trichloroacetic acid-precipitable fraction of plasma was measured, and LDL concentration-time curves were plotted. Clearance was assessed by determining half-life time (t½) values using curve fitting. When more than 95% of the radioactivity had been cleared from the blood, animals were sacrificed, major organs and aortas were harvested, washed, and homogenized, and radioactivity was measured.
Endothelial Cathepsin Activity
BAECs were incubated for 6 h with or without α-defensins alone or with a recombinant LRP inhibitor (recombinant receptor-associated protein (rRAP), 20 nm) or anti-LRP-1 antibody. Cathepsin B activity was measured by adding 100 μm Z-Arg-Arg-AMC (Calbiochem) to cell supernatants. Released free AMC was quantified at excitation/inhibition wavelengths of 360 and 460 nm, respectively, and the results were expressed in arbitrary fluorescence units (Packard Instrument Co.). Substrate specificity was determined by adding the selective cathepsin B inhibitor CA-074 (100 nm) or the competitive inhibitor GB111-NH2 (GB111) (2 μm) (both from Calbiochem). To image cathepsin activity, aortas were incubated with 0.2 μm GB123 for 1 h and washed with acetate buffer for 24 h, as described elsewhere (25). To determine nonspecific staining, samples were preincubated for 1 h with GB111. Images were obtained with an IVIS Kinetic system.
Endothelial Permeability
BAECs were plated onto culture inserts (3-μm pore size, BD Falcon, BD Biosciences) within 24-well plates, grown to confluence, and incubated for 4 h at 37 °C with 125I-LDL alone (saline) or with α-defensins with or without rRAP (20 nm), anti-LRP (20 nm), CA-074 (100 nm), or GB111-NH2 (2 μm). In other experiments, TNF-α (20 nm) was added instead of α-defensins with or without CA-074 or GB111-NH2. Permeability was assessed by measuring radioactivity in aliquots taken from the bottom chamber relative to saline-treated cells.
Tissue Staining/Immunofluorescence
Mice were sacrificed and the hearts and aortas removed and transected. Cryostat sections were stained with primary rabbit antibodies against mouse macrophages (F4/80), mouse cathepsin B, and human α-defensin. Cy2-anti-rabbit Ig was used as the secondary antibody. Nuclei were stained with DAPI. Lipid deposition was quantified using Oil Red O and Image-Pro Plus analysis software.
Statistical Analysis
Group comparisons were made using Student's t test or one-way analysis of variance with the Newman-Keuls post hoc test. For LDL clearance, “between group” comparisons were performed using the Mann-Whitney rank test and two-way analysis of variance with the Newman-Keuls post hoc test. Statistical significance was set at p < 0.05.
Results
Endogenous α-Defensins Form Complexes with Plasma LDL That Promote Vascular Deposition
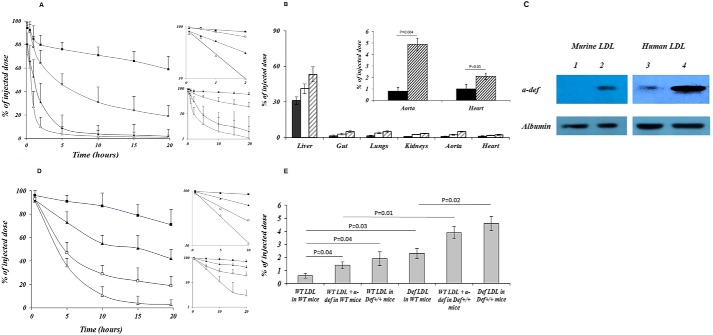
We reported previously that α-defensins bind to LDL and to Lp(a) and stimulate binding to subendothelial matrix of these lipoproteins to vascular cells in vitro (9, 11) and subendothelial matrix (12). To examine whether similar effects are observed in vivo, human 125I-LDL was preincubated with or without α-defensins and injected into WT mice, and radioactivity in the circulation and vasculature was measured. Preincubation with α-defensins stimulated the clearance of human 125I-LDL from the circulation, reducing t½ from more than 20 h to 1.16 h (p < 0.0001) (Fig. 1A) and significantly increasing its incorporation into major organs including the aorta (Fig. 1B).
FIGURE 1.
α-Defensins modulate LDL trafficking and tissue deposition. A, human 125I-LDL preincubated in the absence (solid squares) or presence (solid triangles) of α-defensin (∼2 × 106 cpm) was injected into the jugular vein of 4-month-old WT mice (n = 7–9 in each group). Serial 20-μl blood samples were withdrawn at the times indicated, and the radioactivity was measured. The experiment was repeated in Def+/+ mice (open symbols) with human 125I-LDL preincubated in the absence (open squares) or presence (open triangles) of α-defensin (n = 8–10 in each group). The mean and p values are shown. The inserts show the semi-log plot and the linear fits. B, human 125I-LDL preincubated with saline (solid bars) or α-defensin (open bars) was injected into anesthetized WT mice. In other experiments, human 125I-LDL preincubated with α-defensin was injected into Def+/+ mice (hatched bars). Twenty-four hours later, animals were sacrificed, major organs were harvested and washed, and radioactivity was measured. The insert shows incorporation of human 125I-LDL into the hearts and aortas of WT mice (solid bars) compared with human 125I-LDL preincubated with α-defensins given to Def+/+ mice (hatched bars). 6–9 animals/group were studied. The mean ± 2 S.D. and p values are shown. C, LDL was isolated from the plasma of WT mice (lane 1) as well as from Def+/+ mice (lane 2) fed a HFD for 1 month or from human plasma (lane 3). LDL was immunoprecipitated with anti-LDL antibodies and analyzed on 5–15% SDS-polyacrylamide gels (3). Purified human α-defensin was used control (lane 4). D, mice were fed a HFD for 1 month. LDL isolated from the plasma of WT mice (solid symbols) and Def+/+ mice (open symbols) was radiolabeled and injected (∼2 × 106 cpm) into the jugular vein of WT mice (squares) or Def+/+ mice (triangles) at 4 months of age as described in A. Serial 20-μl blood samples were withdrawn at the times indicated, and the radioactivity was measured. The inserts show the semi-log plot and the linear fits. E,125I-LDL (∼2 × 106 cpm) from WT mice preincubated with saline or α-defensins or 125I-LDL from Def+/+ mice was injected into WT or Def+/+ mice. When more than 95% of the radioactivity had been cleared from the blood, animals were sacrificed, the aortas were removed and washed, and radioactivity was measured. The mean ± S.D. and p values are shown.
To examine the role of endogenous α-defensins in the metabolism of LDL, we studied transgenic (Def+/+) mice that express fully processed human α-defensins in their PMNs like their human counterparts (20). Levels of α-defensins in the plasma of Def+/+ mice (74 ± 11 μg/liter) are comparable with plasma levels in healthy humans (15, 18). We first asked whether α-defensins bind to LDL in Def+/+ mice as we had observed in vitro (11). We found that α-defensins co-immunoprecipitated with LDL isolated from Def+/+ mice and from healthy humans with normal plasma levels of LDL (Fig. 1C).
To examine the effect of endogenous α-defensins on the clearance of endogenous LDL, LDL was purified from WT or Def+/+ mice, radiolabeled, and injected into their counterparts. 125I-LDL purified from Def+/+ mice (125I-LDL·α-defensin) injected into WT mice was cleared more rapidly than 125I-LDL purified from WT mice (125I-LDL·WT) with t½ > 20 h compared with 5.83 h (p < 0.0001) (Fig. 1D). Clearance of 125I-LDL·α-Def injected into Def+/+ mice was even more rapid than in WT mice (t½ 4.38 h, p = 0.026) (Fig. 1D). To examine the potential clinical relevance of these results, we repeated the experiments using human 125I-LDL. Human 125I-LDL and human 125I-LDL preincubated with α-defensins were both cleared more rapidly in Def+/+ mice than in WT mice, reducing the t½ of human LDL alone from >20 h to 4.29 h (p = 0.0071) and of LDL preincubated with α-defensins from 1.16 to 0.714 h (p = 0.019) (Fig. 1A).
The more rapid clearance of LDL seen in Def+/+ mice was accompanied by enhanced vascular deposition. There was minimal incorporation of 125I-LDL·WT into the aortas of naive WT mice (Fig. 1E). Greater retention was seen when 125I-LDL·α-defensin was injected into WT mice (Fig. 1E), and accumulation was augmented further when 125I-LDL·α-defensin was injected into other Def+/+ mice (Fig. 1E). These findings support the hypothesis that α-defensins modulate LDL metabolism in vivo through two interactive mechanisms, i.e. by increasing the clearance of the lipoprotein from the circulation and by increasing its retention in the vasculature.
Phenotypic Conversion of LDL Clearance following Bone Marrow Transplantation
We used bone marrow transplantation from Def+/+ mice into WT mice and from WT into Def+/+ and WT mice as an independent method to assess the effect of α-defensin on LDL. Plasma levels of α-defensin in WT mice transplanted with Def+/+ marrow reached the levels found in Def+/+ mice by 4 weeks post-transplant (69 ± 9 μg/liter); in contrast α-defensin became undetectable in the plasma of Def+/+ mice transplanted with WT bone marrow by this time. LDL·α-defensin complexes were detectable in the plasma of WT recipients of Def+/+ bone marrow by 1 month post-transplant (Fig. 2A); in contrast, at no time were such complexes detected in the plasma of Def+/+ mice transplanted with WT bone marrow (Fig. 2A). LDL purified 1 month post-transplant was radiolabeled and injected into WT and Def+/+ mice, and clearance was measured. The clearance of LDL isolated from WT mice transplanted with Def+/+ bone marrow and injected into WT mice transplanted with Def+/+ bone marrow or injected into Def+/+ mice was comparable and accelerated (t½ of about 4.5 h) compared with clearance in WT mice or Def+/+ mice transplanted with WT bone marrow (t½ > 20 h, p < 0.0001) (Fig. 2B). Furthermore, LDL purified from Def+/+ mice transplanted with WT bone marrow was cleared at the same rate in WT mice as LDL isolated from control WT mice (Fig. 2B).
Although LDL isolated from WT mice 1 month post-transplant with Def+/+ bone marrow was cleared from the circulation as rapidly as LDL isolated directly from Def+/+ mice (Fig. 2B), enhanced deposition of the lipoprotein in the vasculature of these transplanted mice was not evident at this time (Fig. 2C). To determine whether the enhanced deposition of LDL would proceed with more protracted follow-up, we repeated the experiments looking at the vasculature 6 months post-transplant. By 6 months post-transplant, the vasculature of WT mice transplanted with Def+/+ bone marrow displayed the accelerated clearance of WT LDL seen in Def+/+ mice (Fig. 2C).
Colchicine Prevents Release of α-Defensins by PMNs and Attenuates LDL Trafficking in Def+/+ Mice
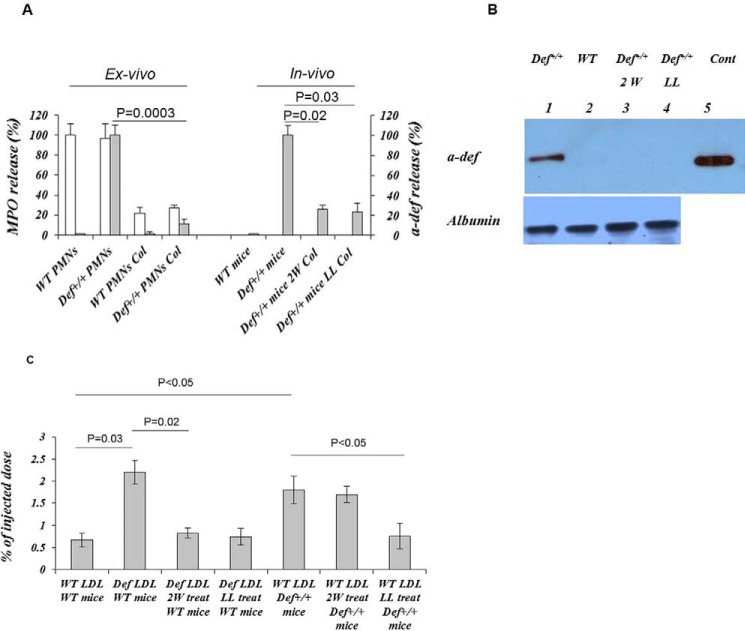
Colchicine inhibits the release of granular components from activated PMNs (26). Therefore, we next examined the effect of colchicine on release of α-defensins. Zymosan-activated serum stimulated the release of α-defensins and myeloperoxidase from isolated Def+/+ PMNs, which was inhibited by colchicine (Fig. 3A). Def+/+ mice given colchicine in their drinking water for as little as 2 weeks showed a 64% reduction in plasma α-defensins (p = 0.019) (Fig. 3A).
FIGURE 3.
Inhibition of PMN degranulation and release of α-defensins modulate LDL trafficking by colchicine. A, ex vivo, PMNs from Def+/+ and WT mice (n = 10) were activated with zymosan-activated serum in the presence or absence of colchicine (10 nm) (Col). α-Defensin (solid bars) and myeloperoxidase (MPO) (open bars) released into the supernatant fluids were measured by ELISA. In vivo, Def+/+ mice fed a regular chow diet or HFD and WT mice fed a HFD were given saline or colchicine (Col) for 2 weeks (2W) or 4 months (LL, lifelong). Blood samples were taken, and α-defensins were measured by ELISA. The mean ± S.D. and p values are shown. B, mice were fed a HFD for 4 months beginning at 4 weeks of age. Colchicine was introduced concurrent with the change in diet. LDL was isolated from the plasma of untreated Def+/+ mice (lane 1) and WT mice (lane 2) or from Def+/+ mice given colchicine for 2 weeks (lane 3) or 4 months (lane 4). LDL was immunoprecipitated with anti-LDL antibody, analyzed on 5–15% SDS-polyacrylamide gels (3), and developed with anti-α-defensin antibody as described for Figs. 1C and 2A. Purified human α-defensin was used as the control (Cont, lane 5). A gel representative of three independent experiments is shown. C, experiments were performed as described for Figs. 1E and 2C. LDL from WT mice (WT LDL), untreated Def+/+ mice (Def LDL), or Def+/+ mice given colchicine for 2 weeks or 4 months (LL) (n = 6–9/group) was radiolabeled with 125I. 125I-LDL was injected into WT mice, untreated Def+/+ mice, or Def+/+ mice given colchicine for 2 weeks (2W Def+/+ mice) or 4 months (LL Def+/+ mice). Clearance was measured as described for Figs. 1E and 2C. The mean ± S.D. is shown.
We next examined the effect of colchicine on the formation of LDL·α-defensin complexes in plasma and their deposition in the vasculature. LDL from mice given colchicine for as little as 2 weeks was almost completely devoid of α-defensins (Fig. 3B) and no longer showed enhanced deposition in the vasculature of WT mice (Fig. 3C). Persistent treatment with colchicine for 6 months also decreased the incorporation of 125I-LDL from WT mice into the vasculature of Def+/+ mice (p = 0.048) (Fig. 3C).
α-Defensin Stimulates Endothelial Cell Permeability to LDL
Increased endothelial permeability to LDL contributes to the formation of lipid streaks and more complex atherosclerotic lesions (27) and might contribute to vascular retention of LDL in Def+/+ mice (Fig. 1, B and E). Therefore, we examined the effect of α-defensins on the permeability of endothelial cells to LDL. The results shown in Fig. 4A demonstrate that α-defensins increased the permeability of cultured endothelial cells to LDL in a dose-dependent manner.
FIGURE 4.
α-Defensins stimulate expression of cathepsin activity by endothelial cells and macrophages and increase endothelial permeability. A, confluent monolayers of BAECs were incubated for 120 min at 37 °C with 125I-LDL alone (Saline) or with α-defensins (α-def, 50–150 nm), α-defensin and rRAP (20 nm), anti-LRP antibody (20 nm) ± GB111-NH2, or CA-074, and permeability was assessed. The results are expressed as the percent increase in radioactivity (cpm) in the bottom chamber relative to saline-treated cells. The mean ± S.D. and p values are shown. B, bovine aortic endothelial cells grown to confluence in 48-well culture plates (150,000 cells/well) were incubated with or without α-defensins (50 or 150 nm) and with or without rRAP or anti-LRP antibodies for 6 h. Z-Arg-Arg-AMC was added to the supernatants with or without GB111-NH2 or CA-074. The results are expressed in arbitrary fluorescence units (FU). The mean ± S.D. and p values are shown. C, RAW cells were incubated in saline buffer or saline supplemented with α-defensins (50 or 150 nm) with or without rRAP or anti-LRP antibodies for 6 h. Z-Arg-Arg-AMC was added to the supernatants alone or in the presence of GB111-NH2, and fluorescence was recorded over the ensuing 35 min as described in B. The mean ± S.D. and p values are shown. D, aortas from 4-month-old WT or Def+/+ mice fed a regular chow diet were incubated with the cathepsin catalytic site probe GB123, with or without the inhibitor GB111-NH2, to measure specific binding (25). Images were obtained with an IVIS Kinetic system using 640/695 excitation and emission filters, respectively (25). Representative sections (n = 5 animals) are shown. E, cathepsin expression was calculated as described in Methods. Vessels from 5–8 animals in each group were studied. The mean ± S.D. and p values are shown.
Endothelial cell permeability is increased by cathepsin B expressed by activated endothelium (28–30) and cathepsins are present in human atherosclerotic lesions (31, 32). Therefore, we examined the possibility that α-defensins stimulate endothelial permeability by inducing the expression of cathepsins. Incubation of BAECs with α-defensins increased cathepsin activity in the media in a dose-dependent manner (Fig. 4B). Cathepsin activity was inhibited by the cathepsin B inhibitor CA-074 (Fig. 4B) and by inhibiting the binding of α-defensins to endothelial LRP using anti-LRP antibody or the endogenous receptor antagonist rRAP (Fig. 4B). The effect of α-defensins on endothelial permeability to LDL (Fig. 4A) was likewise attenuated by the cathepsin B inhibitor CA-074, by anti-LRP antibody, and by rRAP (Fig. 4A).
Cathepsins B and S are secreted by activated macrophages found within atherosclerotic lesions (31–34). α-Defensins bind and activate monocytes (18, 35) and macrophages (18, 36). Therefore, we examined the possibility that α-defensins stimulate the expression of cathepsins in macrophages, which also contributes to increased endothelial permeability and enhanced retention of LDL in the vasculature of Def+/+ mice (Fig. 1B). Incubation of RAW cells, a mouse macrophage cell line (37), with α-defensins increased cathepsin activity in the media (Fig. 4C). Activity was blocked by GB111-NH2 (Fig. 4C), an inhibitor of cathepsins S and B. The effect of α-defensins on expression of cathepsin activity was dose-dependent and inhibited by anti-LRP antibody and by rRAP (Fig. 4C). Furthermore, cathepsin B and S activities were significantly higher (64 ± 9.7%) in the aortas of Def+/+ mice than in WT mice (p = 0.004) (Fig. 4, D and E).
Def+/+ Mice Develop Lipid Streaks on a Regular Diet
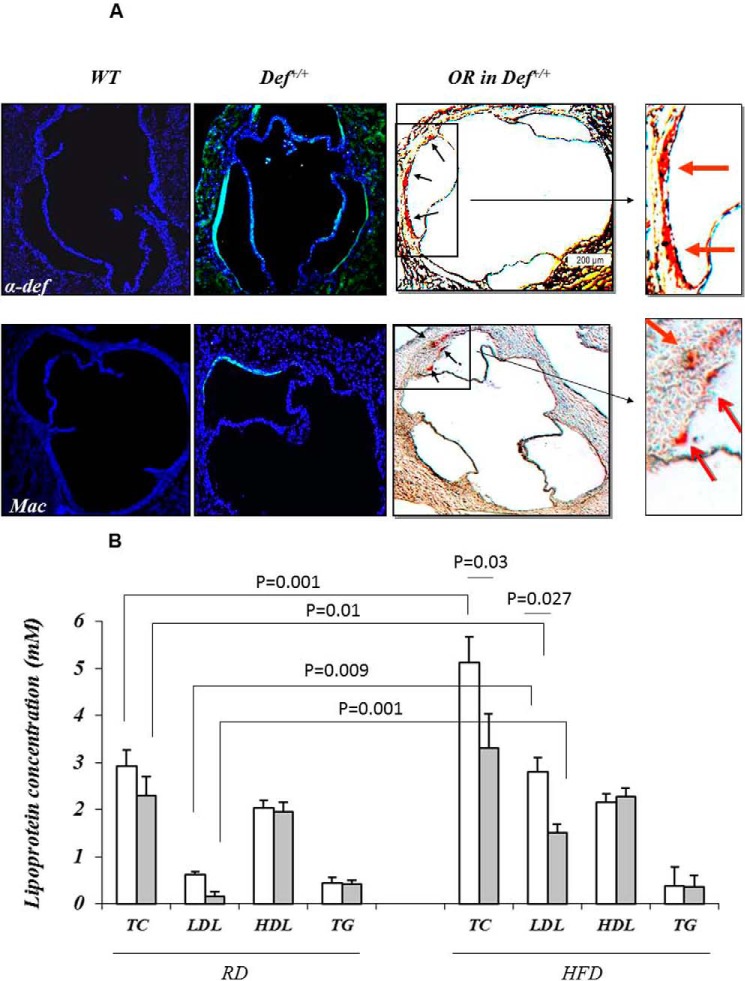
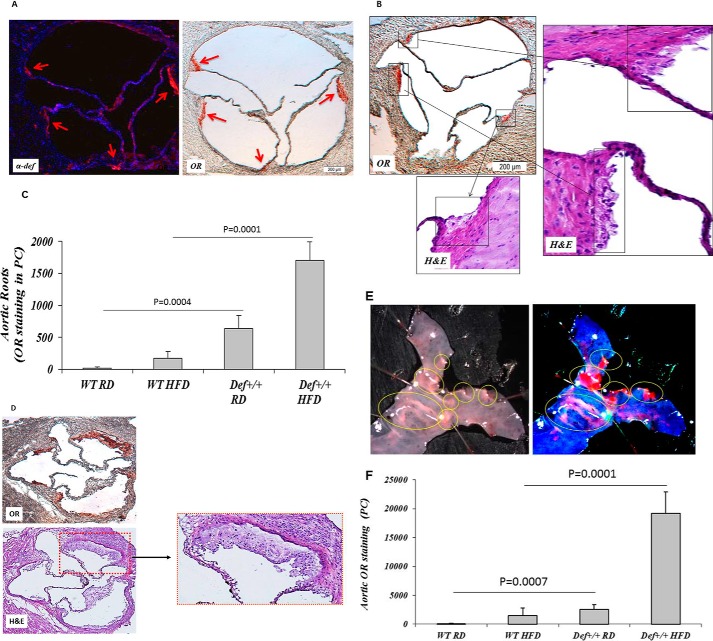
Increased cathepsin (33, 34), endothelial permeability (27), and retention of LDL in the blood vessel wall contribute to atherogenesis. Therefore, we next examined the effect of enhanced vascular deposition of α-defensin·LDL on the development of lipid streaks. Nine of eleven Def+/+ mice fed a regular diet for 16 weeks developed lipid streaks in the aortic roots (Fig. 5A) unlike WT mice, none of which developed lesions (not shown). Total plasma cholesterol, LDL, HDL, and triacylglycerol in these Def+/+ mice were all within the range measured in WT animals, with a trend toward lower total plasma cholesterol and LDL (Fig. 5B). This is consistent with the data presented in Fig. 1A showing increased plasma clearance of LDL in Def+/+ mice. α-Defensins were found in the aortic roots of Def+/+ mice but not in WT controls (Fig. 5A), consistent with prior findings in human lesions (8, 9) and with the increased incorporation of α-defensin·LDL complexes into the vasculature (Fig. 1, B and E). Macrophages, an essential component of atherosclerotic lesions, were found in sections of aortic roots from Def+/+ mice in apposition to regions staining for α-defensins but not in WT mice (Fig. 5A), consistent with the described chemotactic effect of α-defensins for monocytes (35) and the subsequent development of foam cells in lipid streaks (Fig. 6B).
FIGURE 5.
Expression of α-defensin and presence of macrophages and lipid streaks in Def+/+ and WT mice on a regular chow diet. A, Def+/+ and WT mice fed regular chow diets were sacrificed at 4 months of age. Paired cyrostat sections through the coronary ostia, coronary sinuses, and aortic leaflets were stained with rabbit antibodies against mouse macrophages (F4/80) (Mac) or human α-defensins followed by Cy2-labeled anti-rabbit Ig. The blue fluorescence represents nuclear staining with DAPI; green fluorescence represents binding to macrophages or α-defensins. Lipid deposition (arrows) was assessed by Oil Red O (OR) staining (5). Representative sections (n = 9 animals) are shown. B, mice (n = 9) were placed on a regular diet (RD) or a HFD beginning at 4 weeks of age. At 4 months of age, blood samples were taken after a 6-h fast. Plasma α-defensins, total cholesterol (TC), HDL, and triacylglycerol (TG) were measured, and LDL levels were calculated. The mean ± 2 S.D. and p values are shown.
FIGURE 6.
Effect of a HFD on progression of lipid streaks in Def+/+ and WT mice. A, Def+/+ and WT mice (n = 10) fed a regular chow diet (RD) or HFD for 6 months were sacrificed. Sections from the aortic roots were stained for human α-defensin and lipid deposition (arrows) as described for Fig. 5A. Representative sections are shown. B, H&E staining of the lesions depicted in A show lipid-engorged macrophage foam cells characterized by pale, bubbly cytoplasm and single central or slightly eccentric nuclei. Representative sections are shown. C, lipid deposition, expressed in pixels (PC), was quantified in aortic roots (n = 10 mice/group) using Image-Pro Plus analysis software. The mean ± 2 S.D. and p values are shown. D, aortic roots from Def+/+ mice (n = 8) fed a HFD for 12 months were stained with Oil Red O (OR) or H&E. Representative sections are shown. E, aortas from Def+/+ mice (n = 12) fed a HFD for 6 months were stained with Oil Red O. Representative sections are shown. F, quantification of Oil Red O staining and statistical analyses were performed as described in B. The mean ± 2 S.D. and p values are shown.
Formation of Lipid Streaks in Def+/+ Mice Is Exacerbated by a High Fat Diet
A cohort of Def+/+ mice was converted to a HFD at 4 weeks of age. By 6 months, all converted mice developed lipid streaks in their aortic roots (Fig. 6, A–C). In contrast, only 3 of 11 WT mice developed small lesions on the same diet (Fig. 6C). Lesion size in Def+/+ mice fed a HFD increased significantly over baseline (p = 0.0001) (Fig. 6C). The lipid streaks were much more extensive by 12 months of age (Fig. 6D). α-Defensins co-localized with lipids in the aortic roots of Def+/+ mice following diet conversion (Fig. 6A) in the absence of intact PMNs or PMN antigens. These lesions had the appearance typical of early fatty streaks on H&E staining (Fig. 6, B and D), containing lipid-engorged macrophage foam cells characterized by pale, “bubbly” cytoplasm and a single central or slightly eccentric nucleus. Def+/+ mice fed a HFD also developed extensive lipid streaks in their thoracic aortas (Fig. 6E), whereas WT mice did not show any extension of the lesions (Fig. 6F). Seemingly paradoxically, plasma LDL and total plasma cholesterol increased to a greater extent in WT than in Def+/+ mice (Fig. 5B).
Colchicine Attenuates Lipid Streak Formation in Def+/+ Mice
Colchicine inhibits the release of α-defensins from PMNs in vitro and in vivo (Fig. 3A), formation of LDL/α-defensin complexes (Fig. 3B), accelerated clearance LDL from the circulation (Fig. 3C), and increased endothelial cell permeability (Fig. 4A). Therefore, we examined the effect of colchicine on lesion development in Def+/+ mice.
Def+/+ mice were fed a regular diet or HFD for 8 months beginning at 4 weeks of age. Colchicine or saline was introduced concurrent with the change in diet. Animals fed a regular diet and given colchicine for 8 months showed a significant decrease in the size of lipid streaks in their aortic roots (p = 0.043) (Fig. 7). Def+/+ mice fed a HFD and placed on colchicine for 8 months also showed a decrease in the size of lipid streaks in aortic roots by ∼55% (p = 0.036) (Fig. 7).
FIGURE 7.

Colchicine inhibits formation of lipid streaks in aortic roots of Def+/+ mice. Def+/+ mice were fed a regular chow diet (RD) or HFD for 8 months beginning at 4 weeks of age (n = 10–12 mice/group). Colchicine (Col) was introduced concurrent with the change in diet. Lipid streaks were quantified in aortic roots as described for Fig. 6C. The mean ± S.D. is shown. OR, Oil Red O; PC, pixels.
Discussion
Efforts to prevent atherosclerosis by lowering LDL and raising HDL have left a substantial proportion of patients unprotected. To help identify other approaches that prevent disease progression, increased attention has been focused on the role of chronic inflammation (reviewed in Ref. 38). A correlation between circulating neutrophil counts and clinical disease has been identified repeatedly over the past 4 decades (reviewed in Ref. 38). Yet, the involvement of neutrophils in the inception, progression, and terminal thrombotic events that characterize human atherosclerosis remains incompletely defined.
Previously we identified an abundance of α-defensins in human coronary and cerebral arterial atherosclerotic vessels (8, 9). α-Defensins are stored almost exclusively in a subset of the azurophilic granules of neutrophils (40, 41). In vitro, α-defensins have been implicated in the activation of macrophages (18, 36), the production of TNFα (36), increased expression of scavenger receptors, and the activation of endothelial cells with release of reactive oxygen species (18, 42, 43). However, the absence of α-defensins in murine PMNs has precluded an assessment of their effects on lipoprotein metabolism and the development of early atherosclerotic lesions in this species, a gap in knowledge we sought to address in this study.
Our bipartite hypothesis was that any inflammatory stimulus that leads to activation of neutrophils could release α-defensins into the circulation, altering the composition and behavior of circulating lipoproteins and the response of the vasculature, effects that act in concert to perturb LDL trafficking and enhance its vascular retention (Fig. 7). Our data support this concept of two independent but interdependent processes that alter LDL metabolism and thereby enhance lesion formation and progression in mice expressing human α-defensins. First, Def+/+ mice developed novel complexes in their plasma composed of α-defensins and LDL not found in WT mice (Fig. 2A). The relevance of this new finding is supported by the presence of comparable α-defensin·LDL complexes in plasma from healthy human donors with normal total plasma LDL. α-Defensin·LDL complexes injected into WT mice were cleared more rapidly from the circulation and exhibited more extensive vascular deposition than did LDL similarly isolated from WT mice (Fig. 1D). With time, and as the animals age, the vasculature of Def+/+ mice showed a greater capacity to retain LDL and α-defensin·LDL than was seen in WT mice (Fig. 1E). These findings are consistent with prior in vitro observations that α-defensins bind LDL, forming stable α-defensin·LDL complexes that increase lipoprotein binding to endothelial cells (11) and extracellular matrix (12). Deposition of α-defensins in the vasculature of Def+/+ mice was associated with increased expression of cathepsin by endothelial cells and macrophages, which in turn increased endothelial permeability to LDL (Fig. 7B). α-Defensins may thereby create a positive feed-forward amplification process that accelerates the deposition and retention of LDL within the vasculature.
The centrality of α-defensins in lesion progression is supported by the salutary effects of bone marrow transplantation from WT mice and by the provision of colchicine to Def+/+ mice (Fig. 7C). The bidirectional phenotypic transfer using bone marrow transplantation between Def2+ and WT mice excludes a contribution of α-defensins from non-hematopoetic cells, and it also excludes the possibility that colchicine acts by inhibiting the release of another mediator.
The differential effects of short-term and more protracted courses of colchicine together with the outcomes in transplanted mice help dissociate the pathways by which α-defensins promote lesion progression. α-Defensin·LDL complexes were detected by the 4th week after transplantation of bone marrow from Def+/+ mice into WT mice, but accelerated retention of WT LDL required 4 months before it was detected. Conversely, a 2-week course of colchicine decreased the levels of α-defensin·LDL complexes in the plasma of Def+/+ mice by more than 65% but did not affect vascular retention of LDL, suggesting that the vascular damage induced by this novel lipoprotein complex is only slowly reversible. In support of this conclusion, protracting the administration of colchicine to 8 months lead to a reduction in the size of newly formed lipid streaks (Fig. 7). Together, these findings are consistent with the concept that biologically active molecules released from activated neutrophils in plasma or tissue retain proatherogenic effects that endure long after other evidence of neutrophil involvement has passed, with the finding of greater benefit when therapy with colchicine is extended in experimental (39) and clinical settings (5).
In summary, the findings described in this study suggest that α-defensins modify the composition and trafficking of LDL and promote their vascular deposition even at normal to low plasma levels, as occurs in a large subset of affected patients. α-Defensins may therefore represent a trackable link between inflammation and the development of atherosclerosis.
Author Contributions
A. A. H. conceived the study, A. A. H. and R. A. designed the study, and A. A. H., R. A., and D. B. C. wrote the paper. R. A., A. H., S. A., N. H., and E. M. performed and analyzed the experiments shown in Figs. 1–6, and I. A. and G. B. designed, performed, and analyzed the experiments shown in Fig. 4, D and E. All authors analyzed the results and approved the final version of the manuscript.
This work was supported by National Institutes of Health Grants HL077760, HL805429, HL82545, and HL123912 and by Grant 930/04 from the Israeli Science Foundation. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- HNP
- human neutrophil peptide
- PMN
- polymorphonuclear leukocyte
- LRP
- lipoprotein receptor-related protein
- HFD
- high fat diet
- BAEC
- bovine endothelial aortic cell
- Z
- benzyloxycarbonyl
- Lp(a)
- lipoprotein(a)
- ELISA
- enzyme-linked immunosorbent assay
- AMC
- 7-amino-4-methylcoumarin
- rRAP
- recombinant receptor-associated protein.
References
- 1.Khot U. N., Khot M. B., Bajzer C. T., Sapp S. K., Ohman E. M., Brener S. J., Ellis S. G., Lincoff A. M., and Topol E. J. (2003) Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 290, 898–904 [DOI] [PubMed] [Google Scholar]
- 2.Ross R. (1999) Atherosclerosis: an inflammatory disease. N. Engl. J. Med. 340, 115–126 [DOI] [PubMed] [Google Scholar]
- 3.Ishibashi S., Goldstein J. L., Brown M. S., Herz J., and Burns D. K. (1994) Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Invest. 93, 1885–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S. H., Reddick R. L., Piedrahita J. A., and Maeda N. (1992) Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258, 468–471 [DOI] [PubMed] [Google Scholar]
- 5.Drechsler M., Megens R. T., van Zandvoort M., Weber C., and Soehnlein O. (2010) Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122, 1837–1845 [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. (2003) Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunology 3, 710–720 [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer P. B., and Lehrer R. I. (1992) Mouse neutrophils lack defensins. Infect. Immun. 60, 3446–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higazi A. A., Lavi E., Bdeir K., Ulrich A. M., Jamieson D. G., Rader D. J., Usher D. C., Kane W., Ganz T., and Cines D. B. (1997) Defensin stimulates the binding of lipoprotein (a) to human vascular endothelial and smooth muscle cells. Blood 89, 4290–4298 [PubMed] [Google Scholar]
- 9.Barnathan E. S., Raghunath P. N., Tomaszewski J. E., Ganz T., Cines D. B., and Higazi A. al-R. (1997) Immunohistochemical localization of defensin in human coronary vessels. Am. J. Pathol. 150, 1009–1020 [PMC free article] [PubMed] [Google Scholar]
- 10.Nassar H., Lavi E., Akkawi S., Bdeir K., Heyman S. N., Raghunath P. N., Tomaszewski J., and Higazi A. (2007) α-Defensin: link between inflammation and atherosclerosis. Atherosclerosis 194, 452–457 [DOI] [PubMed] [Google Scholar]
- 11.Higazi A. A., Nassar T., Ganz T., Rader D. J., Udassin R., Bdeir K., Hiss E., Sachais B. S., Williams K. J., Leitersdorf E., and Cines D. B. (2000) The α-defensins stimulate proteoglycan-dependent catabolism of low-density lipoprotein by vascular cells: a new class of inflammatory apolipoprotein and a possible contributor to atherogenesis. Blood 96, 1393–1398 [PubMed] [Google Scholar]
- 12.Bdeir K., Cane W., Canziani G., Chaiken I., Weisel J., Koschinsky M. L., Lawn R. M., Bannerman P. G., Sachais B. S., Kuo A., Hancock M. A., Tomaszewski J., Raghunath P. N., Ganz T., Higazi A. A., and Cines D. B. (1999) Defensin promotes the binding of lipoprotein(a) to vascular matrix. Blood 94, 2007–2019 [PubMed] [Google Scholar]
- 13.Higazi A. A., Barghouti I. I., and Abu-Much R. (1995) Identification of an inhibitor of tissue-type plasminogen activator-mediated fibrinolysis in human neutrophils. A role for defensin. J. Biol. Chem. 270, 9472–9477 [DOI] [PubMed] [Google Scholar]
- 14.Higazi A. A., Ganz T., Kariko K., and Cines D. B. (1996) Defensin modulates tissue-type plasminogen activator and plasminogen binding to fibrin and endothelial cells. J. Biol. Chem. 271, 17650–17655 [DOI] [PubMed] [Google Scholar]
- 15.Zhao H., Yan H., Yamashita S., Li W., Liu C., Chen Y., Zhou P., Chi Y., Wang S., Zhao B., and Song L. (2012) Acute ST-segment elevation myocardial infarction is associated with decreased human antimicrobial peptide LL-37 and increased human neutrophil peptide-1 to 3 in plasma. J. Atheroscler. Thromb. 19, 357–368 [DOI] [PubMed] [Google Scholar]
- 16.Urbonaviciene G., Frystyk J., Flyvbjerg A., Urbonavicius S., Henneberg E. W., and Lindholt J. S. (2012) Markers of inflammation in relation to long-term cardiovascular mortality in patients with lower-extremity peripheral arterial disease. Int. J. Cardiol. 160, 89–94 [DOI] [PubMed] [Google Scholar]
- 17.Christensen H. M., Frystyk J., Faber J., Schou M., Flyvbjerg A., Hildebrandt P., Raymond I., Klausen T. W., and Kistorp C. (2012) α-Defensins and outcome in patients with chronic heart failure. Eur. J. Heart Fail. 14, 387–394 [DOI] [PubMed] [Google Scholar]
- 18.Quinn K. L., Henriques M., Tabuchi A., Han B., Yang H., Cheng W. E., Tole S., Yu H., Luo A., Charbonney E., Tullis E., Lazarus A., Robinson L. A., Ni H., Peterson B. R., Kuebler W. M., Slutsky A. S., and Zhang H. (2011) Human neutrophil peptides mediate endothelial-monocyte interaction, foam cell formation, and platelet activation. Arterioscler. Thromb. Vasc. Biol. 31, 2070–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nassar T., Akkawi S., Bar-Shavit R., Haj-Yehia A., Bdeir K., Al-Mehdi A. B., Tarshis M., and Higazi A. (2002) Human α-defensin regulates smooth muscle cell contraction: a role for low-density lipoprotein receptor-related protein/α2-macroglobulin receptor. Blood 100, 4026–4032 [DOI] [PubMed] [Google Scholar]
- 20.Bdeir K., Higazi A. A., Kulikovskaya I., Christofidou-Solomidou M., Vinogradov S. A., Allen T. C., Idell S., Linzmeier R., Ganz T., and Cines D. B. (2010) Neutrophil α-defensins cause lung injury by disrupting the capillary-epithelial barrier. Am. J. Respir. Crit. Care Med. 181, 935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishina P. M., Verstuyft J., and Paigen B. (1990) Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J. Lipid Res. 31, 859–869 [PubMed] [Google Scholar]
- 22.Glenthøj A., Cowland J. B., Heegaard N. H., Larsen M. T., and Borregaard N. (2011) Serglycin participates in retention of α-defensin in granules during myelopoiesis. Blood 118, 4440–4448 [DOI] [PubMed] [Google Scholar]
- 23.Greene T. K., Wang C., Hirsch J. D., Zhai L., Gewirtz J., Thornton M. A., Miao H. Z., Pipe S. W., Kaufman R. J., Camire R. M., Arruda V. R., Kowalska M. A., and Poncz M. (2010) In vivo efficacy of platelet-delivered, high specific activity factor VIII variants. Blood 116, 6114–6122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene T. K., Lyde R. B., Bailey S. C., Lambert M. P., Zhai L., Sabatino D. E., Camire R. M., Arruda V. R., and Poncz M. (2014) Apoptotic effects of platelet factor VIII on megakaryopoiesis: implications for a modified human FVIII for platelet-based gene therapy. J. Thromb. Haemost. 12, 2102–2112 [DOI] [PubMed] [Google Scholar]
- 25.Blum G., Weimer R. M., Edgington L. E., Adams W., and Bogyo M. (2009) Comparative assessment of substrates and activity-based probes as tools for non-invasive optical imaging of cysteine protease activity. PLoS ONE 4, e6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuki G. (2008) Colchicine: its mechanism of action and efficacy in crystal-induced inflammation. Curr. Rheumatol. Rep. 10, 218–227 [DOI] [PubMed] [Google Scholar]
- 27.Wong B. W., Wong D., Luo H., and McManus B. M. (2011) Vascular endothelial growth factor-D is overexpressed in human cardiac allograft vasculopathy and diabetic atherosclerosis and induces endothelial permeability to low-density lipoproteins in vitro. J. Heart Lung Transplant. 30, 955–962 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y., Li X., Boini K. M., Pitzer A. L., Gulbins E., Zhang Y., and Li P. L. (2015) Endothelial Nlrp3 inflammasome activation associated with lysosomal destabilization during coronary arteritis. Biochim. Biophys. Acta 1853, 396–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schraufstatter I. U., Trieu K., Zhao M., Rose D. M., Terkeltaub R. A., and Burger M. (2003) IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J. Immunol. 171, 6714–6722 [DOI] [PubMed] [Google Scholar]
- 30.Peterson M. W., Gruenhaupt D., and Shasby D. M. (1989) Neutrophil cathepsin G increases calcium flux and inositol polyphosphate production in cultured endothelial cells. J. Immunol. 143, 609–616 [PubMed] [Google Scholar]
- 31.Kim D., Kim J., Schellingerhout D., Shon S., Jeong S., Kim E., and Kim W. (2009) Molecular imaging of cathepsin B proteolytic enzyme activity reflects the inflammatory component of atherosclerotic pathology and can quantitatively demonstrate the antiatherosclerotic therapeutic effects of atorvastatin and glucosamine. Mol. Imaging 5, 291–301 [PubMed] [Google Scholar]
- 32.Sukhova G. K., Zhang Y., Pan J. H., Wada Y., Yamamoto T., Naito M., Kodama T., Tsimikas S., Witztum J. L., Lu M. L., Sakara Y., Chin M. T., Libby P., and Shi G. P. (2003) Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 111, 897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitamoto S., Sukhova G. K., Sun J., Yang M., Libby P., Love V., Duramad P., Sun C., Zhang Y., Yang X., Peters C., and Shi G. P. (2007) Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation 115, 2065–2075 [DOI] [PubMed] [Google Scholar]
- 34.Sasaki T., Kuzuya M., Nakamura K., Cheng X. W., Hayashi T., Song H., Hu L., Okumura K., Murohara T., Iguchi A., and Sato K. (2010) AT1 blockade attenuates atherosclerotic plaque destabilization accompanied by the suppression of cathepsin S activity in apoE-deficient mice. Atherosclerosis 210, 430–437 [DOI] [PubMed] [Google Scholar]
- 35.Territo M. C., Ganz T., Selsted M. E., and Lehrer R. (1989) Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Invest. 84, 2017–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soehnlein O., Kai-Larsen Y., Frithiof R., Sorensen O. E., Kenne E., Scharffetter-Kochanek K., Eriksson E. E., Herwald H., Agerberth B., and Lindbom L. (2008) Neutrophil primary granule proteins HBP and HNP1–3 boost bacterial phagocytosis by human and murine macrophages. J. Clin. Invest. 118, 3491–3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Q., Mei Y., Shoji T., Han X., Kaminski K., Oh G. T., Ongusaha P. P., Zhang K., Schmitt H., Moser M., Bode C., and Liao J. K. (2012) Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation 126, 2236–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soehnlein O. (2012) Multiple roles for neutrophils in atherosclerosis. Circ. Res. 110, 875–888 [DOI] [PubMed] [Google Scholar]
- 39.Nidorf S. M., Eikelboom J. W., Budgeon C. A., and Thompson P. L. (2013) Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. College Cardiol. 61, 404–410 [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez-García M., Oliva H., Climent N., García F., Gatell J. M., and Gallart T. (2007) Human immature monocyte-derived dendritic cells produce and secrete α-defensins 1–3. J. Leukocyte. Biol. 82, 1143–1146 [DOI] [PubMed] [Google Scholar]
- 41.Agerberth B., Charo J., Werr J., Olsson B., Idali F., Lindbom L., Kiessling R., Jörnvall H., Wigzell H., and Gudmundsson G. H. (2000) The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood 96, 3086–3093 [PubMed] [Google Scholar]
- 42.Zughaier S. M., Shafer W. M., and Stephens D. S. (2005) Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages. Cell. Microbiol. 7, 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kougias P., Chai H., Lin P. H., Yao Q., Lumsden A. B., and Chen C. (2006) Neutrophil antimicrobial peptide α-defensin causes endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 43, 357–363 [DOI] [PubMed] [Google Scholar]