Abstract
Serpins regulate coagulation and inflammation, binding serine proteases in suicide-inhibitory complexes. Target proteases cleave the serpin reactive center loop scissile P1–P1′ bond, resulting in serpin-protease suicide-inhibitory complexes. This inhibition requires a near full-length serpin sequence. Myxomavirus Serp-1 inhibits thrombolytic and thrombotic proteases, whereas mammalian neuroserpin (NSP) inhibits only thrombolytic proteases. Both serpins markedly reduce arterial inflammation and plaque in rodent models after single dose infusion. In contrast, Serp-1 but not NSP improves survival in a lethal murine gammaherpesvirus68 (MHV68) infection in interferon γ-receptor-deficient mice (IFNγR−/−). Serp-1 has also been successfully tested in a Phase 2a clinical trial. We postulated that proteolytic cleavage of the reactive center loop produces active peptide derivatives with expanded function. Eight peptides encompassing predicted protease cleavage sites for Serp-1 and NSP were synthesized and tested for inhibitory function in vitro and in vivo. In engrafted aorta, selected peptides containing Arg or Arg-Asn, not Arg-Met, with a 0 or +1 charge, significantly reduced plaque. Conversely, S-6 a hydrophobic peptide of NSP, lacking Arg or Arg-Asn with −4 charge, induced early thrombosis and mortality. S-1 and S-6 also significantly reduced CD11b+ monocyte counts in mouse splenocytes. S-1 peptide had increased efficacy in plasminogen activator inhibitor-1 serpin-deficient transplants. Plaque reduction correlated with mononuclear cell activation. In a separate study, Serp-1 peptide S-7 improved survival in the MHV68 vasculitis model, whereas an inverse S-7 peptide was inactive. Reactive center peptides derived from Serp-1 and NSP with suitable charge and hydrophobicity have the potential to extend immunomodulatory functions of serpins.
Keywords: herpesvirus, inflammation, monocyte, peptides, serpin, artery, pox viruses, reactive center loop
Introduction
Serpins (serine protease inhibitors) are ubiquitous, complex, and highly active regulatory molecules that effectively control multiple coagulation, inflammatory, and neuroendocrine pathways (1–3). The amino acid sequence in the reactive center loop (RCL)3 of serpins acts as bait for target serine proteases, initiating structural changes in the serpin-protease complex and culminating in suicide inhibition (1–3). This same RCL can insert into the neighboring β-sheet A in other serpins in serpinopathies, causing serpin aggregates induced by genetic mutations and causing disease as for anti-thrombin III (SERPIN C1, ATIII), α-1 antitrypsin (SERPIN A1, AAT), and neuroserpin (SERPIN I1, NSP). Whereas the amino acid residues in the RCL provide target P1–P1′ sequences, referred to as a scissile bond, serpins also require the greater part of the protein structure to function with true serpin-protease inhibitory activity (4, 5). However, as for other proteins, peptides derived during protein metabolism may act to extend serpin activity beyond the initial suicide-inhibitory function, both increasing and decreasing responses (6–9). In prior work, significant and prolonged anti-inflammatory functions have been detected with myxomavirus-derived Serp-1 (10–17) and mammalian serpin NSP purified protein injections in animal models of vascular disease (18, 19). We have hypothesized that peptides produced by protease cleavage of the RCL sequence during natural proteolytic metabolism of Serp-1 or NSP may extend serpin activity, increasing anti-inflammatory activity after serpin-protease complex formation. Thus, these serpin RCL peptide metabolites have the theoretical potential to interfere with either protease activity by acting as a protease bait or inhibitors or to inhibit other serpins by inserting into the β-sheet.
Many proteins have active metabolites providing additional and/or expanded functions. Peptides derived from calreticulin (20) and apolipoprotein E (Ep1B) (21) have proven anti-atherogenic activity, reducing inflammation and plaque growth in animal models. Serpins also have reported active terminal peptide metabolites. Among the serpins, angiotensinogen is a protein with serpin structure but lacking serpin inhibitory activity (6). Angiotensinogen is cleaved first by renin and subsequently by the angiotensin-converting enzyme to form angiotensin I and angiotensin II, angiotensin II being a potent vasoconstrictor with a central role in regulation of blood pressure. A C-terminal peptide, C36, derived from AAT, induces a pro-inflammatory response in human monocytes (7), together with receptor interaction and the capacity to alter gene expression. One AAT C-terminal peptide is also reported to have antiviral functions, inhibiting HIV-1 (22). The N terminus of a second serpin, heparin cofactor II (SERPIN D1), is reported to block endotoxin activity (8). However, selective activity of peptides naturally processed from the RCL has not been examined for extended activity.
Among the most highly active mammalian serpins are those that regulate thrombolytic and thrombotic protease pathways, such as plasminogen activator inhibitor-1 (PAI-1; SERPIN E1) and ATIII as well as connective tissue-degrading enzymes, such as AAT. Serpins are found at all levels of evolution. Highly active serpins have evolved in poxviruses to provide a defense against host immune reactions to the virus (8, 10–17, 23). Serp-1 is a secreted myxomavirus-derived protein that binds and inhibits urokinase- and tissue-type plasminogen activators (uPA and tPA, respectively), plasmin, and factor X, with demonstrated inhibition of plaque growth and organ scarring in mouse, rat, and rabbit balloon angioplasty-induced neointimal plaque growth and in rodent transplant models (10–17, 23). NSP is a mammalian serpin that binds tPA and uPA with a greater predilection for tPA. NSP has been reported to reduce cerebral infarct size in mouse models (19). Transplant vasculopathy is an aggressive form of atherosclerosis and, together with chronic rejection, is a leading cause of transplanted organ loss after the first year post-transplant. When purified Serp-1 or NSP protein was given as a single bolus infusion immediately after aortic allograft transplant, both serpins significantly reduced plaque growth and inflammatory cell invasion at a 4-week follow-up (16). When Serp-1 is given for the first 10 days after cardiac or renal (10, 16, 23) allograft transplants, together with the immunosuppressant cyclosporine, there is a significant reduction in transplant vascular plaque growth and scarring in mice and rats at 3–5 months when compared with cyclosporine treatment alone. Serp-1 but not NSP improved outcomes and reduced inflammatory cell invasion in a lethal MHV68 mouse herpesviral infection in mice when the Serp-1 RCL scissile P1–P1′ bond sequence was Arg-Asn. When the Serp-1 RCL P1–P1′ Arg-Asn sequence was mutated to Ala-Ala, the anti-atherogenic function was lost, indicating that Serp-1 functions through a serpin-based mechanism. Additionally, Serp-1 has been tested in a recent Phase 2a clinical trial in the United States and Canada, in which a Serp-1 infusion was given for 3 days starting immediately after stent implantation in patients with acute unstable angina. In this small clinical trial, Serp-1 reduced early markers of myocardial damage (24). The short plasma half-life of Serp-1 (20 min to 1 h) indicates probable rapid metabolism; however, beneficial effects are detectable weeks to months later in animal models, suggesting extended function. These extended functions may be produced by active metabolites.
Natural protease cleavage sites for Serp-1 and NSP in the serpin RCL were predicted, and peptides were designed and synthesized based upon these predicted sequences. Inhibitory activity for RCL-derived Serp-1 and NSP peptides was assessed in vitro in human monocytes and in vivo in mouse ascites, aortic allograft transplant, and lethal mouse herpesviral (MHV68) vasculitis models.
Experimental Procedures
Peptide Expression and Purification
Neuroserpin was expressed in BL21 (DE3) pLysS cells (Invitrogen) as described previously (18). In brief, cells induced with 1 mm isopropyl β-d-1-thiogalactopyranoside for 3 h were pelleted, resuspended in elution buffer (20 mm Tris + 20 mm imidazole + 150 mm NaCl) containing EDTA-free complete protease inhibitors (Roche Applied Science) and 2 mm 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, and French-pressed. Cell debris was pelleted at 15,000 rpm, and supernatant was loaded onto 500 μl of cobalt nitrilotriacetic acid slurry (Sigma-Aldrich). The majority of the NSP was localized in inclusion bodies, but sufficient soluble active protein was expressed (39.5 μg/50 ml of culture).
Serp-1 was purified from the supernatant of recombinant Chinese hamster ovary (CHO) cell line as described previously (provided by Viron Therapeutics, Inc., London, Canada) (10–17, 23, 24). Serp-1 purity was over 95% as determined by overloaded Coomassie-stained SDS-polyacrylamide gels and reverse-phase HPLC.
Serpin peptide metabolite sequences were predicted by the PeptideCutter utility of the Expasy program (the Expasy Protein Analysis System, World Wide Web Server) and synthesized by the University of Florida Peptide Center. The physicochemical characteristics provided in Fig. 1 were calculated using PepDraw (Wimley laboratory, Tulane University, New Orleans LA).
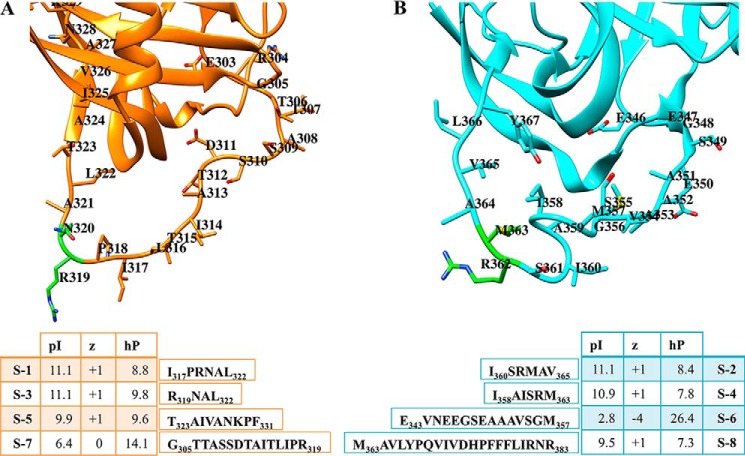
FIGURE 1.
Serp-1 and NSP reactive center loops and their respective peptides. The RCL portions of Serp-1 and NSP are shown (A and B) along with the peptide sequences and their calculated pI, charge (z), and hydrophobicity (hP) values. RCL portions shown are from a homology model of Serp-1 (sequence UniProtKB/Swiss-Prot: P12393.2) generated by SWISS-MODEL (template, Protein Data Bank entry 4DTE) and crystal structure of NSP (Protein Data Bank entry 3F5N).
Cell Cultures
Human monocytic THP-1 cell line (TIB-202) and T-lymphocyte Jurkat cells (E6.1 clone, TIB-152) were procured from the American Type Culture Collection (Manassas, VA) and cultured in RPMI medium supplemented with 10% fetal bovine serum (Invitrogen Canada Inc., Burlington, Canada), penicillin (1 unit/ml), and streptomycin (1 mg/ml) (Invitrogen Canada), with 2% l-glutamine and 1% sodium pyruvate (Invitrogen Canada) added for Jurkat cells. Cells were maintained at a confluence of 0.5–1.0 × 106 cells/ml (12, 13, 23, 25).
In Silico Modeling of Potential for Protease and Serpin Inhibitory Functions
The RCL insertions were modeled into the β-sheet of a neighboring serpin. Complexes of two serpin-derived peptides (S-2 and S-7) with serpin ATIII and PAI-1 (Protein Data Bank entries 2B4X and 1DB2, respectively) were generated.
The central antiparallel β-strand of the β-sheet of either full-length serpin was deleted, and the S-2 and S-7 peptides were manually rigid body-docked into the vacated space. Steric clashes between the peptide and serpin were corrected by selection of alternate rotamers in the serpin and/or peptide. The following complexes were built: ATIII·S-2, ATIII·S-7, PAI-1·S-2, and PAI-1·S-7. The resulting complex coordinates were analyzed with PDBePISA (42) to measure the solvation energy upon ligation and to account for any favorable interactions between the peptide and serpin.
Protease and Serpin Inhibitory Activity Assay
Activity of purified NSP and Serp-1 was measured as inhibitory activity for uPA (Assaypro, St. Louis, MO) per the manufacturers' directions as described previously (10, 19, 23). Serpin inhibition activity of peptides was also assessed using assays for uPA, tPA, plasmin, PAI-1, and anti-thrombin III (ATIII). SensoLyte® fluorimetric assay kits were purchased from Anaspec (Fremont, CA) for tPA (AS-72160), plasmin (AS-72124), and thrombin (AS-72130), and assays were conducted per the manufacturer's specifications. Peptide inhibition of ATIII (Thermo Fisher Scientific) was measured as a reduction in its thrombin inhibition activity. uPA (Sigma, U0633) activity toward pefachrome uPA (Enzyme Research Laboratories (South Bend, IN), P082-33) was used for assaying peptide inhibition. Modulation of the inhibitory activity of PAI-1 (Peprotech (Rock Hill, NJ), 140-04) toward uPA by peptides was also assayed. Residual protease activity was calculated as compared with control samples without active serpin or peptides.
Animal Models
All research protocols and animal care conformed to the Guiding Principles for Animal Experimentation of the United States Council on Animal Care and were approved by the University of Florida laboratory animal research ethics committee (institutional animal care and use committee).
Mouse Peritoneal Cell Migration Assay
Mice were injected with monocyte chemoattractant protein-1 (MCP-1) and treated with Serp-1, NSP, or individual peptides (intravenously). Three mice were tested per serpin or peptide treatment. Cell counts were obtained by flow cytometry. Cells isolated from mouse ascites were stained with antibodies to surface or intracellular antigens and incubated for 30 min at room temperature (13, 25). Labeled cells were washed and resuspended with 150 μl of PBS and assessed by flow cytometry. For staining of intracellular antigens, cell pellets in 500 μl were incubated with fixation/permeabilization buffer (eBiosciences, San Diego, CA), incubated in the dark for 45 min, treated with permeabilization buffer (eBiosciences), and incubated with intracellular antibody mix, with further incubation for 30 min at 4 °C. All antibodies were purchased from eBiosciences and Biolegend (San Diego, CA). Flow cytometry was performed with a CyAn ADP analyzer (Dako, Ft. Collins, CO) (12, 13, 17, 18, 25). Data analysis was done using Gatelogic software (eBiosciences).
Mouse Aortic Transplant Models
93 mice had aortic allograft transplantation; 56 had PAI-1−/− (C57BL/6 background) donor to BALB/c (PAI-1+/+) recipient, and 37 had C57BL/6 wild type (PAI-1+/+) to BALB/c (PAI-1+/+) recipient. PAI-1-deficient mice (PAI-1−/−) have been variably reported to have increased and alternatively decreased plaque after vascular injury. Due to the large number of surgeries required, it was necessary to utilize both the PAI-1−/− and the WT mouse implants. The PAI-1−/− mice also allowed analysis of serpin response in a mouse transplant lacking a key thrombolysis regulating serpin, PAI-1. A 0.3-cm aortic segment isolated from the donor mouse was transplanted into the infrarenal aorta of recipient mice for aortic allograft studies, as described previously (10, 15, 25) (Table 1). The aortic transplant was anastomosed end-to-end using Sharpoint 11/0 nylon sutures (Surgical Specialties Corp., Reiding, MA) under general anesthetic (6.5 mg/100 g body weight Somnotrol (MTC Pharmaceuticals, Cambridge, Canada)) given by intramuscular injection, as described previously (10–17, 26). WT C57BL/6 mice were purchased directly from Jackson Laboratories (Bar Harbor, ME), and PAI-1−/− mice were bred in-house under an institutional animal care and use committee-approved breeding protocol using mice supplied by Jackson Laboratories prior to surgery. Mice were followed for 4 weeks after transplant. A single infusion of either individual proteins (Serp-1 or NSP) or individual serpin peptides (15 μg/mouse; 0.75 μg/g body weight in 0.2 ml). Once reduced plaque was detected at 15-μg doses, a dose titration curve was completed for two of the individual peptides that displayed apparent anti-inflammatory and transplant vasculopathy-inhibitory activity (4–10 mice/treatment; 1.5, 15, and 150 μg/mouse). Serpins or serpin-derived peptides were infused by intravenous tail vein injection immediately after transplant surgery, once aortic pulsation was again visible. At 4 weeks after aortic transplant, mice were euthanized with 0.05 ml of euthanyl (Bimeda MTC Animal Health Ltd., Cambridge, Canada) by intramuscular injection.
TABLE 1.
Mouse aortic allograft transplant surgeries
| Treatment | Dose/mouse | No. of mice transplanted (donor B6a/PAI-1−/−) | No. of mice surviving 4 weeks | Adverse events |
|---|---|---|---|---|
| μg | ||||
| Saline | 0 | 11 (6 B6/5 PAI-1−/−) | 10 | 1 thrombosis |
| NSP | 15 | 5 (0 B6/5 PAI-1−/−) | 4 | 1 thrombosis |
| NSP-PP RCL mutant | 15 | 5 (0 B6/5 PAI-1−/−) | 4 | 1 hematoma |
| S-1 | 1.5 | 6 (0 B6/6 PAI-1−/−) | 6 | 0 |
| S-1 | 15 | 10 (5 B6/5 PAI-1−/−) | 8 | 1 thrombosis, 1 fighting injury |
| S-1 | 150 | 4 (0 B6/4 PAI-1−/−) | 4 | 0 |
| S-2 | 15 | 5 (5 B6/0 PAI-1−/−) | 4 | 1 thrombosis |
| S-3 | 15 | 6 (6 B6/0 PAI-1−/−) | 6 | 0 |
| S-4 | 15 | 5 (5 B6/0 PAI-1−/−) | 5 | 0 |
| S-5 | 1.5 | 4 (0 B6/4 PAI-1−/−) | 4 | 0 |
| S-5 | 15 | 6 (0 B6/6 PAI-1−/−) | 6 | 0 |
| S-5 | 150 | 4 (4 B6/0 PAI-1−/−) | 4 | 0 |
| S-6 | 1.5 | 6 (6 B6/0 PAI-1−/−) | 6 | 1 thrombosis |
| S-6 | 15 | 6 (0 B6/6 PAI-1−/−) | 0 | 6 thromboses |
| S-7 | 15 | 5 (0 B6/5 PAI-1−/−) | 5 | 0 |
| S-8 | 15 | 5 (0 B6/5 PAI-1−/−) | 5 | 0 |
| Total no. of mice | 93 (37 B6/56 PAI-1−/−) | 81 | 12 |
a B6, C57BL/6.
MHV68 Infection in IFNγR−/− Mice
Viral MHV68 stocks were generated in NIH 3T12 fibroblasts (ATCC, Manassas, VA). Cells were cultured in DMEM + 10% FCS + 10 mm HEPES + 2 mm l-glutamine + 1% penicillin/streptomycin. At 50% confluence, cells were infected with a multiplicity of infection of 0.1. Seven days postinfection, cells underwent freeze-thaw, and lysate was transferred to Nalgene Oak Ridge PPCO tubes and centrifuged (15 min at 4300 × g). Cleared supernatant was centrifuged for 2 h at 12,000 × g. The pellet was rinsed with PBS, and resuspended in medium, vortexed, and stored at −80 °C in 250-μl aliquots. Virus was titered in duplicate.
Interferon γ receptor knockout (IFNγR−/−) mice (B6.129S7-Ifngr1tm1Agt/J) and wild type mice (BALB/c), 5–7 weeks of age, were purchased from Jackson Laboratories and bred under specific pathogen-free conditions. Littermate controls were used for all studies. 90 IFNγR−/− mice (6–10 mice/group) were infected by intraperitoneal injection of 12.5 × 106 pfu of MHV68 given in 0.1 ml of DMEM. Mice were treated with either saline control (100 μl) or individual serpin peptides (S-1, S-2, S-3, S-7, S-8, or the inverted sequence S-7) at 100 μg/kg/100 μl) given at the time of infection and then daily by intraperitoneal injection for a total of 10 days starting on the day of MHV68 infection. Mice were followed up for a maximum of 150 days. Mice were carefully monitored by the veterinary animal care staff to minimize potential suffering. Euthanasia was performed as described previously (14–21).
Histological and Morphometric Analysis
Donor aortic transplant implants and adjacent recipient aorta (0.5–0.6 cm long) were cut into two 0.25–0.3-cm pieces, fixed, paraffin-embedded, and cut into 5-μm sections (two per aortic section, providing four sections for analysis of each transplanted mouse aorta) and stained with hematoxylin and eosin for morphometric analysis as described previously. Areas of plaque, lumen, and internal elastic lamina were measured by means of the Olympus application program using a Sony Power HAD3CCD color video camera attached to the microscope and calibrated to the microscope objective. The mean total cross-sectional area of the intima, using sections with the largest detectable plaque area, was calculated for each aortic specimen (10, 15, 23, 24). Numbers of invading mononuclear cells were counted per high powered field area at three sites in the intimal, medial, and adventitial layers, and the mean count for each arterial layer and specimen was calculated.
Membrane Fluidity Cell Activation Studies
One million >95% viable THP-1, Jurkat T, or 5 × 105 human umbilical vein endothelial cells were labeled with 1,3-Bis-pyrenylpropane (BPP) (0.8 μm) to study core membrane fluidity 3 h prior to cell activation as described previously (12, 13, 25, 27, 28). Cells were activated with PMA (1 μg/ml) for 1 h, washed, resuspended in growth medium, and treated with either Serp-1 (500 ng/ml), NSP (500 ng/ml), or individual serpin peptides at 500, 1000, or 1500 ng/ml for 1 h. All assays were performed in triplicate. At the end of 1 h, cells were washed to remove excess fluorescent probe, and monomer and excimer fluorescence emission intensities were measured at 390 and 485 nm, respectively, during excitation at 320 nm using a fluorescent dual wavelength reader (Fluoroskan, Thermolab Systems). The ratio of excimer fluorescence to monomer fluorescence gives the measure of membrane fluidity.
RT-PCR Gene Expression Analyses
To further examine potential mechanisms of inhibitory activity for individual serpin peptides, THP-1 monocyte and Jurkat T cells in culture were treated with individual serpins or peptides with proven inhibitory activity for plaque growth in vivo (12, 13, 17, 25). RT-PCR signaling pathway gene arrays (Sigma-Aldrich). were analyzed in triplicate per cell type and predicted peptide or serpin treatment.
Statistics
Changes in plaque area, intimal/medial thickness ratios, inflammatory cell invasion, and membrane fluidity and cellular adhesion were assessed by analysis of variance and Student's t test (10–18, 23–25, 27, 28). Mean values were calculated for plaque area and intimal/medial thickness ratios for each mouse and used for subsequent statistical analysis. For PCR arrays, a two-tailed Student's t test was conducted between saline controls and the treatments. For the viral infection studies, Kaplan-Meier survival analyses were performed. p ≤ 0.05 was considered significant.
Results
Serpin Peptide Chemical Characteristics and Primary Structure
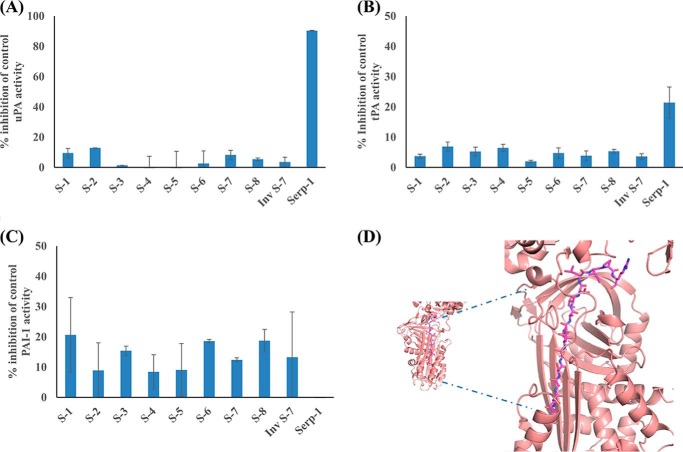
The eight peptides examined here represent predicted metabolites derived from natural proteolysis of either Serp-1 or NSP RCL sequences. The chemical characteristics of these peptide metabolites are provided in Fig. 1, together with the primary structure (Wimley Lab PepDraw, Tulane University, New Orleans, LA). The pI (isoelectric point), hydrophobicity, and net charge varied. Of note, peptides S-1 (IPRNAL), S-3 (RNAL), S-5 (TAIVANKPF), S-2 (ISRMAV), S-4 (IAISRM), and S-8 (MAVLYPQVIVDHPFFFLIRNR) all had a predicted net positive (+1) charge, whereas S-7 (GTTASSDTAITLIPR) had a predicted net 0 charge and S-6 (EVNEEGSEAAAVSGM) had a net negative (−4) charge. The pI for most peptides ranged from 6.42 to 11.11, but S-6 was an outlier with pI of 2.75. The hydrophobicity calculation was also similar for most peptides, with a range from +7.76 to +14.07; however, S-6 peptide was again an outlier with a calculated hydrophobicity of +26.40. The secondary structure was analyzed using the Online ExPasy site. The RNAL sequence is predicted to form a helix, whereas the other sequences are predicted to form either strands (AITLIPR) or coil (GTTASSDT). None of the RCL peptides had inhibitory activity for uPA (Fig. 2A) or tPA (Fig. 2B) activity by a protease activity assay; nor did any of the peptides inhibit the activity of plasmin or factor X (data not shown). In the chromogenic assay, the serpin-derived RCL peptides did demonstrate some inhibition of PAI-1. S-1, S-3, S-6, S-7, and S-8 demonstrated a trend toward greater inhibition of PAI-1 than S-2 and S-5 (Fig. 2C). ATIII was not inhibited significantly by the peptides (data not shown).
FIGURE 2.
Analysis of serpin and serpin-derived peptide inhibition of uPA, tPA, and PAI-1. Chromogenic assays for inhibition of serine proteases by 500 ng of Serp-1 or the RCL peptides are shown. A, inhibition of uPA activity; B, inhibition of tPA activity; C, inhibition of PAI-1 activity. D, molecular model showing S-7 docked in the β-sheet A of PAI-1. Error bars, S.E.
Selected Serpin RCL Peptides Reduce Plaque Growth
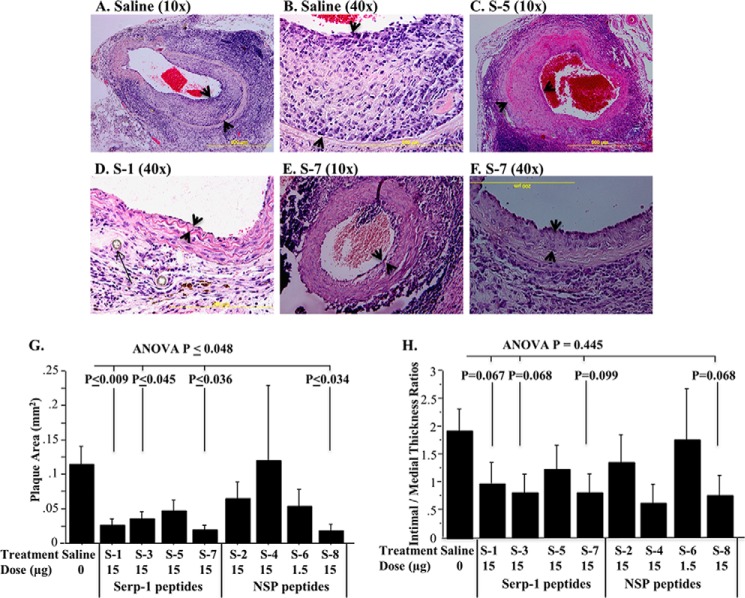
To assess potential anti-inflammatory actions of individual serpin peptides, peptide treatment was tested in mice after aortic allograft transplant at 4 weeks. In engrafted aorta, peptides containing Arg or Arg-Asn (specifically S-1, S-3, S-7, and S-8) significantly reduced plaque growth (Figs. 3 and 4). Conversely, the S-6 peptide lacking Arg or Arg-Asn with excess negative charge (−4) induced early thrombosis and mortality, and Arg-Met-containing peptide S-4 was inactive. Histological analysis detected increased plaque in cross-sections of engrafted aortic arteries with saline treatment (Fig. 3, A and B). Analysis of histologic cross-sections demonstrated significantly reduced inflammatory plaque area after treatment by either the Serp-1-derived RCL peptides S-1 (Figs. 3 (D, G, and H) and 4 (A and C)), S-3 (Fig. 3 (G and H); p ≤ 0.045), and S-7 (Fig. 3 (E–H); p ≤ 0.036) or NSP peptide S-8 (Fig. 3 (G and H); p ≤ 0.034) given at 15-μg doses. Serp-1 peptide S-5 (Figs. 3 (C, G, and H) and 4B) and NSP peptides S-2, S-4, and S-6 (Fig. 3, G and H) did not significantly alter plaque growth. Of interest, S-6 treatment at the same 15-μg dose as for other peptides produced universal early thrombosis at the site of aortic allograft transplant with 100% mortality (Table 1). When S-6 was given at 10-fold lower doses (1.5 μg) than other peptides given at 15-μg doses, there was no effect on plaque growth (Fig. 3, G and H).
FIGURE 3.
Effects of serpin peptide infusions on plaque growth. Histological cross-sections demonstrate altered plaque growth when comparing serpin peptides (n = 93 mice, 15-μg dose immediately after transplant). Saline-treated (A, 10×; B, 40×) and S-5-treated (C, 10×) animals display larger plaque areas. S-1 significantly reduced plaque area (D, 40×), as did S-7 (E, 10×; F, 40×). The intimal hyperplasia is indicated by arrowheads. The long arrow in D shows the suture at the site of transplant. Bar graphs demonstrate significant reductions in plaque area by S-1, S-3, S-7, and S-8 peptides (G). Intimal to medial thickness ratios (H) for S-1, S-3, S-7, and S-8 also showed a trend toward reduction compared with saline controls. p ≤ 0.05 considered significant. Error bars, S.E.
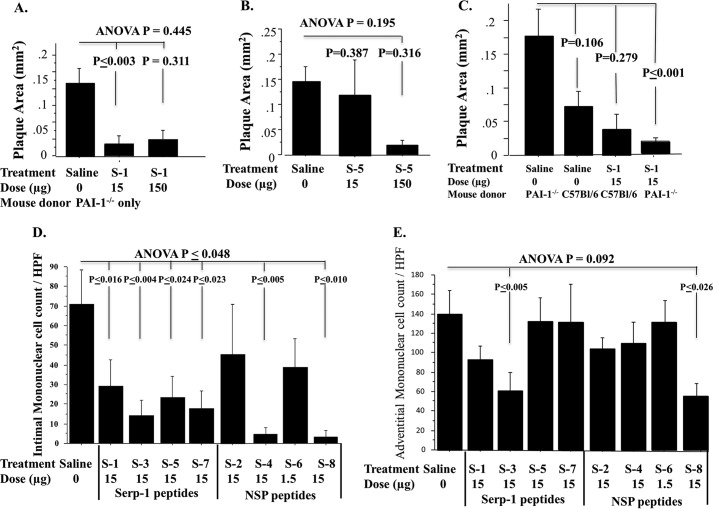
FIGURE 4.
Serpin peptide dose titration, effects on inflammatory mononuclear cell counts, and differential effects in PAI-1-deficient aorta. A and B, titration curves for Serp-1 peptide S-1 (A) and S-5 (B) treatments. S-1 significantly reduced plaque area at both doses (A), whereas S-5 displayed some inhibition of plaque growth but only at a 10-fold higher dose infusion (B). S-1 peptide significantly reduced plaque in PAI-1−/− aortic allografts but only displayed a trend toward reduced plaque in C57BL/6 WT aortic allografts (C). Counts of invading mononuclear cells (monocyte/macrophage and T cells) at 28 days in intimal (D) and adventitial (E) layers are shown. Invasion into intima was reduced by all of the Serp-1 peptides and by the S-4 and S-8 peptides from NSP. Adventitial cell counts were reduced significantly with S-3 and S-8. p ≤ 0.05 considered significant. Error bars, S.E.
PAI-1 is one of the main thrombolytic (clot-dissolving) pathway regulators functioning to inhibit tPA, uPA, and also the clotting factor thrombin in the presence of heparin. PAI-1 has been variably reported to act as an anti-atherosclerosis agent and a pro-atherogenic agent in differing animal models of vascular disease. In prior work, Serp-1 treatment reduced plaque growth and inflammation in PAI-1−/− aortic allograft implants. In this study, there was a trend toward increased plaque growth in engrafted PAI-1−/− aortas when compared with C57BL/6 WT aortic allograft transplants after implant in BALB/c mice; however, this increased plaque did not reach significance (p = 0.106). S-1 peptide treatment was compared for PAI-1−/− and for C57BL/6 donor allograft (Table 1 and Fig. 4C). When all data were combined for PAI-1−/− and C57BL/6 WT donor allografts, S-1 significantly inhibited plaque growth (Fig. 3, G and H; p ≤ 0.009). When data were separated for S-1 treatment after PAI-1−/− and C57BL/6 WT donor allograft transplant, S-1 inhibition was greatest in PAI-1-deficient aortic allografts with significant reductions in plaque area (Fig. 4C; p ≤ 0.001).
S-1 and S-5 peptides were assessed for dose-dependent reductions in plaque. S-1 had equivalent activity at both doses (Fig. 4, A and C), and S-5, although showing some improved efficacy at the higher dose, did not reach significance (Fig. 4B). S-3 was active as was S-1 in reducing plaque growth, with similar efficacy; however, S-3 was tested only in C57BL/6 (WT) aortic allograft implants. The majority of effective peptides were tested in PAI-1−/− donor allograft transplants, which might suggest that peptide efficacy was more dependent on the donor allograft implanted. S-6 was, however, tested at the lethal 15-μg dose in PAI-1−/− allografts, and an inactive NSP-PP with a mutated RCL P1-P1′ site was similarly tested in PAI-1−/− allografts, and neither reduced plaque growth (Figs. 3 and 4). The increased thrombosis in the S-6 15-μm-treated allograft transplants, as noted above, was detected in PAI-1−/− allograft implants. PAI-1−/− allografts might be predicted to have increased local thrombolysis, due to the lack of PAI-1-mediated blockade of tPA and uPA. The capacity of S-6 to markedly increase thrombosis, causing 100% mortality when infused at 15-μg doses, further suggests an extension of normal PAI-1 regulatory function as a thrombolysis inhibitor, causing clot formation (thrombosis).
In Silico Modeling of Potential for Protease and Serpin Inhibitory Functions
The potential for these peptides to bind to and inhibit other serpins, PAI-1 and ATIII, was also examined in silico and demonstrated potential for binding of S-7 to PAI-1 (Fig. 2D). The docking of the S-2 peptide into ATIII did not show any significant interactions between the peptide and serpin, with only a few potential hydrogen bonds observed, with a calculated ligation solvation energy of −27 kJ/mol (data not shown), whereas many more interactions were observed in the ATIII·S-7 complex. This longer peptide made significant contacts with neighboring side chains in ATIII (estimated ∼5-fold more than in the ATIII·S-2 complex). The majority of the interactions at the interface were hydrogen bonds, with a calculated ligation solvation energy of −24.7 kJ/mol.
The PAI-1·S-2 complex also shows little potential for hydrogen bonds, even fewer than in the ATIII·S-2 complex. This may be a consequence of the compact nature of the PAI-1 β-barrel, compared with ATIII. The S-2 peptide wedged into a narrow opening between two parallel β-strands. This most likely explains the lower ligation energy of −17 kJ/mol. In addition, unlike ATIII, which consists of five β-strands, PAI-1 consists of only four β-strands, which could also be of importance. The PAI-1·S-7 complex displayed the highest calculated ligation solvation energy among the four models, −38 kJ/mol. This observation is most likely due to the ability of the S-7 peptide to make additional interactions with a loop region distance from the β-sheet. In contrast, the shorter bulkier S-2 peptide was unable to make these interactions. In the case of both ATIII and PAI-1, it appears that both serpins favor interactions with the longer S-7 peptide due to its ability to form interactions in the neighboring side chains of amino acids within the β-sheet.
Analysis of Inflammatory Cell Invasion
Inflammatory mononuclear cell infiltrates into the arterial wall were measured in the intimal layer. Increased cell invasion was seen at 28 days follow-up in aortic allograft sections. There were reduced counts for invading MC for all but S-4 and S-6, demonstrating some correlation with plaque reductions seen for S-1, S-3, S-7 and S-8, but not correlating with S-2 and S-5 treatment, where no significant reduction in plaque was detected (Fig. 4D). For the adventitial layer, only S-3 and S-8 significantly reduced mononuclear cell counts (Fig. 4E), and S-1 and S-7 did not. Thus, a consistent association was not detected between peptide treatments, plaque inhibition, and inflammatory cell invasion into the arterial wall. This may be due to the fact that these sections were taken at late follow-up times after aortic grafting and treatment. Thus, effects on early inflammatory cell invasion were assessed in a mouse ascites model, as described below.
Selected Peptides Modify Spleen Cell Responses
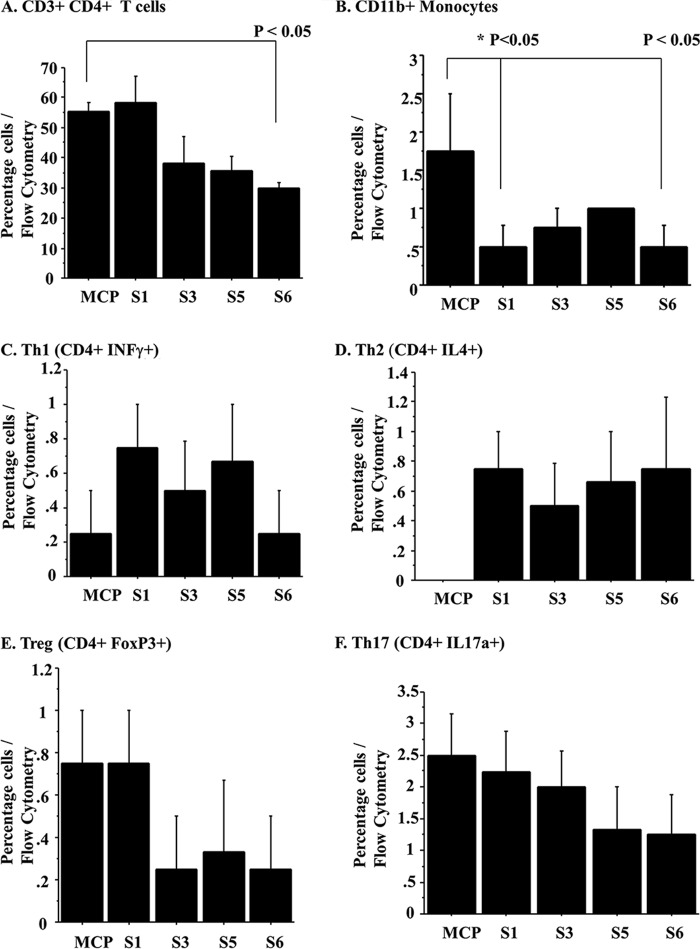
In order to investigate whether peptides would also actively modify cell invasion for selected cell types, we examined cellular migration responses after chemokine injection into the peritoneum (intraperitoneal injection). Prior work demonstrated that Serp-1 and NSP modify Th1 and Th2 responses. Intraperitoneal chemokine (MCP-1) injection was used to induce mononuclear cell migration into the peritoneal cavity of mice. None of the peptides altered cell migration into ascites fluid at 18 h (data not shown). However, when examining these same mice for systemic effects on spleen cell populations, cells isolated from spleens in mice after MCP-1 injection demonstrated altered cell responses with S-1 and S-6 treatment. S-1 and S-6 significantly reduced CD11b (Fig. 5B; p < 0.05), whereas S-3 and S-5 did not. Although S-3 showed a trend toward reduced CD11b percentage, this trend did not achieve significance. Of the peptides, only S-6 significantly altered CD3+CD4+ T cell counts (p < 0.05; Fig. 5A). Th1 (CD4+IFNγ+; Fig. 5C), CD4+Th2 (IL4+, Fig. 5D), Treg (FoxP3; Fig. 5E), and Th17 (IL17a+; Fig. 5F) were not altered with peptide treatment.
FIGURE 5.
Serpin peptides modify splenocyte responses after intraperitoneal MCP-1 chemokine injection. Treatment with S-6 peptide significantly reduced splenocyte CD3+CD4+ T cell isolates at 18 h (A), whereas S-1 and S-6 reduced CD11b+ mononuclear splenocyte cell isolates (B). Th1 (C), Th2 (D), Treg (E), and Th17 (F) were not significantly altered by peptide treatment, although non-significant trends were detected. p ≤ 0.05 is considered significant. Error bars, S.E.
Peptides Modify Cell Activation Measured by Membrane Fluidity
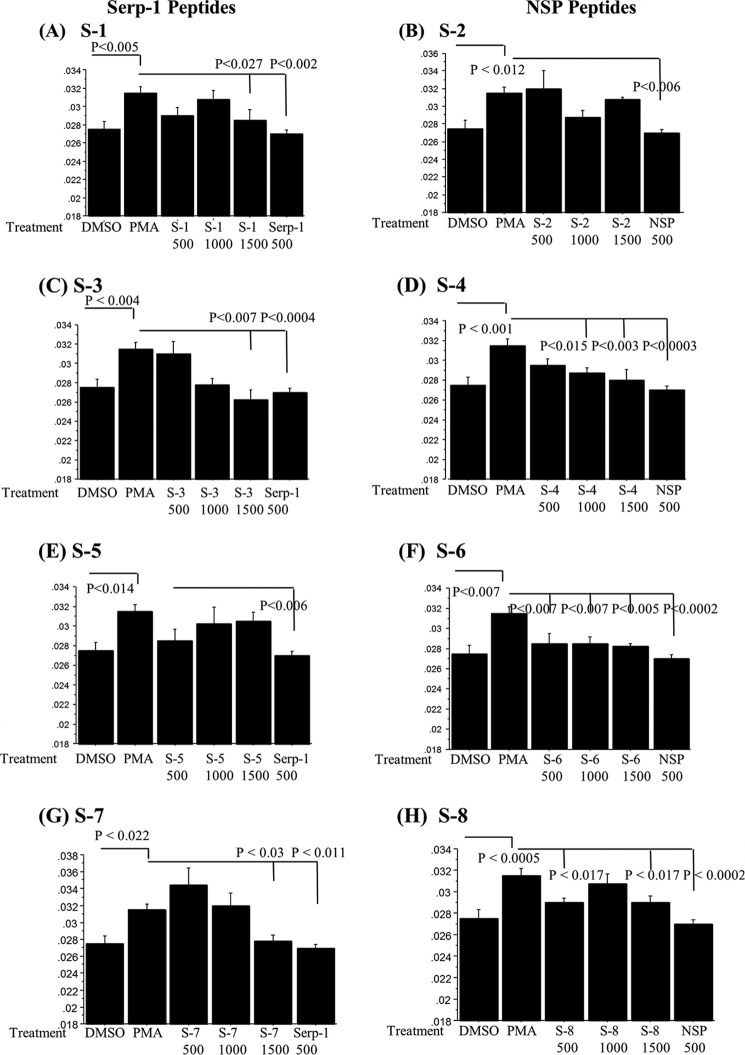
Cellular membrane fluidity is measured by pyrene dimer formation in the lipid bilayer and is used as a nonspecific measure of cell activation (28). Increases in THP-1 human monocyte membrane fluidity after PMA activation correlate with changes in cell activation, adhesion, and motility. BPP (pyrene)-labeled cells treated with Serp-1 or NSP demonstrated significant changes in the excimer (dimer) to monomer fluorescence emission intensity (Iex/Imon) ratios (Fig. 6) in PMA-activated human THP-1 cells in culture. All peptides were tested at three doses: 500, 1000, and 1500 ng/ml. Serp-1 peptides S-1 and S-3 and NSP peptides S-4, S-6, and S-8 significantly reduced PMA-mediated increases in the Iex/Imon ratio, a measure for cell activation, with varying concentration-dependent activity.
FIGURE 6.
Serpin peptides modify cell activation as measured by membrane fluidity in vitro. Membrane fluidity assays measured as Iex/Imon fluorescence of BPP-labeled THP-1 monocytes demonstrated increased Iex/Imon ratios with PMA activation. Peptide treatments paralleled in vivo activity with the one exception being the S-4 peptide. The S-6 peptide, which caused excess thrombosis, displayed inhibitory activity for cells. Serp-1 peptides S-1 (A), S-3 (C), and S-7 (G) and NSP peptides S-4 (D), S-6 (F), and S-8 (H) significantly reduced membrane fluidity in PMA-activated THP-1 monocytes. Serp-1 peptide S-5 (E) and NSP peptide S-2 did not reduce membrane fluidity in PMA-activated THP-1 monocytes. Serp-1 and NSP consistently reduced membrane fluidity significantly. Error bars, S.E.
S-6 reduced Iex/Imon ratios at all doses (p ≤ 0.007), whereas S-4 was active at the two higher concentrations (p ≤ 0.015). S-1, S-3, S-7, and S-8 were inhibitory at higher concentrations, 1500 ng (S-1, p < 0.027; S-3, p < 0.007; S-7, p < 0.03; S-8, p < 0.017). S-2 and S-5 did not significantly alter Iex/Imon (S-2, p = 0.07; S-5, p = 0.057). Thus, analysis of membrane fluidity detected concentration-dependent variations in cell responses to serpin peptide treatments, with the greatest sensitivity to treatment seen with S-6 followed by S-3 and S-4.
The data from this cell activation assay suggest that peptides S-2 and S-5, which lack anti-atherogenic activity in vivo after allograft transplant, did not modify THP-1 cell activity. The other peptides that displayed activity in the in vivo allograft transplant model, both protective and anti-atherogenic (S-1, S-3, S-7, and S-8) or pro-thrombotic (S-6), modified cell activation in the membrane fluidity assay. The capacity to produce beneficial inhibition of vascular inflammation and plaque growth was inversely proportional to the capacity to modify THP-1 human monocyte activation, with S-6 having the greatest activity at lower doses than S-1, S-3, and S-7. The lack of activity for S-2 and S-5 did correspond to a lack of inhibitory activity for plaque growth. S-4 was an exception, having effects on membrane fluidity but no efficacy in the allograft model.
Peptide-mediated Changes in Gene Expression
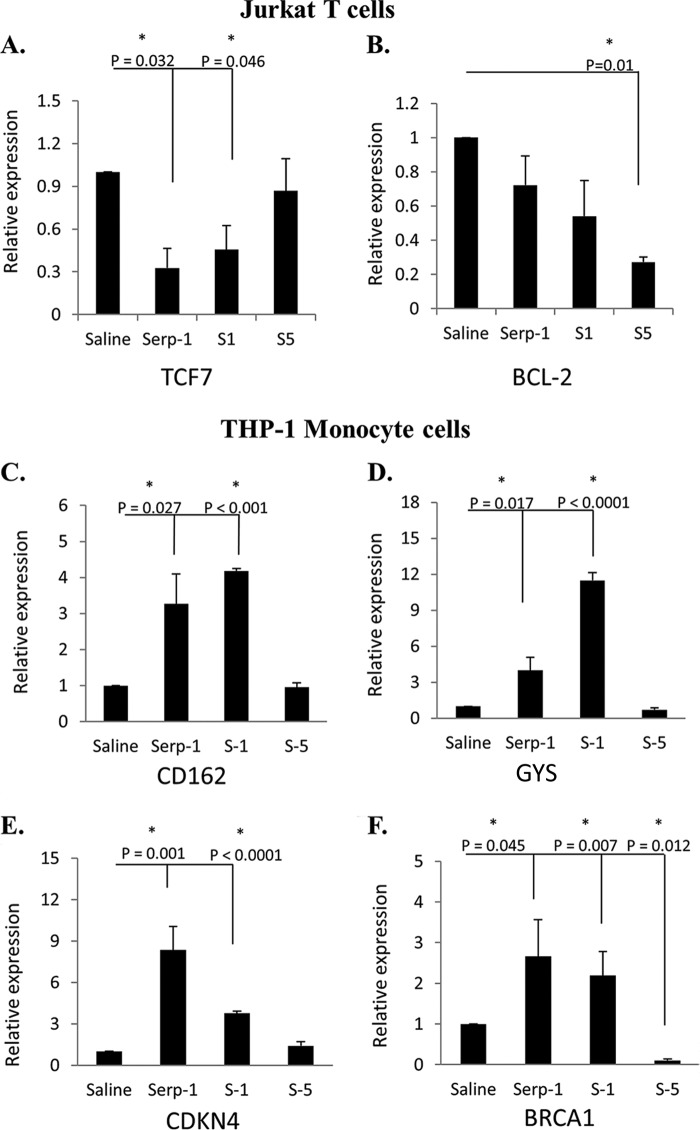
Shared effects of Serp-1 and S-1, one of the active Serp-1 RCL peptide inhibitors for plaque growth, on transcription of representative genes in signaling pathways were studied in human Jurkat T cells (Fig. 7, A and B) and THP-1 monocytes (Fig. 7, C–F). The inactive S-5 peptide, which did not reduce plaque, was similarly tested in parallel for effects on gene expression in the same cell lines. Serp-1 and the Serp-1 peptides S-1 and S-5 were compared with saline-treated controls. Genes with 2-fold change in expression with p <0.05 by Student's t test were considered significant.
FIGURE 7.
Serpin peptides display differential effects on gene expression in signaling pathways in human T cells and monocyte/macrophage cell lines. The activation or repression of signaling genes by Serp-1, S-1, and S-3 treatments in Jurkat T cells (A and B) and THP-1 (C–F) monocyte cells was compared with saline treatment. In Jurkat T cells, the transcription factor 7 gene (TCF7) (A) was repressed by Serp-1 and S-1 but not by S-5, and the BCL-2 gene (B) was repressed by S-5 treatment, whereas S-1 and Serp-1 did not alter gene expression. In THP-1 monocytes, CD162 (C), GYS (D), and CDKN4 (E) gene expression was significantly increased by both Serp-1 and S-1 but not by S-5. BRCA1 gene (F) expression was significantly increased by Serp-1 and S-1 treatment and repressed by S-5 when compared with saline treatment. Error bars, S.E.
Treatment of Jurkat cells did not effect a 2-fold change in any of the genes tested, but both Serp-1 and S-1 inhibited transcription factor 7 (TCF7; p < 0.046), whereas S-5 treatment did not (Fig. 7A). Other genes were significantly reduced by S-5 peptide in T cells but were not modified by Serp-1 or S-1. Of these genes, S-5 reduced BCL-2 (Fig. 7B) and also chemokine CCL20 (p < 0.03), CCND-1 (p < 0.01), interleukin-8 (IL-8; p < 0.009), and matrix metalloproteinase 7 (MMP7; p < 0.004) (data not shown), whereas S-1 and Serp-1 had no effect, suggesting that reductions in the expression of these genes in T cells may provide no protection in arterial disease.
In THP-1 cells, both Serp-1 and S-1 increased expression for four genes by ≥2-fold. Selectin P ligand (CD162; p < 0.027), glycogen synthase (GYS; p < 0.017), and cyclin-dependent kinase inhibitor 1B (p27; p < 0.001) were induced by Serp-1 and S-1 peptide treatment, whereas S-5 peptide treatment did not increase expression (Fig. 7, C–E). Both Serp-1 and S-1 induced the expression of breast cancer 1 gene (BRCA1; p < 0.045) in THP-1 cells, whereas S-5 inhibited BRCA1 significantly (p < 0.012) (Fig. 7F). BRCA1 has demonstrated protective activity in mice against endothelial cell apoptosis, dysfunction, and inflammation. GYS (glycogen synthase), which is associated with glycogen storage disease, and CDKN1 (cyclin-dependent kinase inhibitor 1B) do not have reported direct effects on atherogenesis or arterial inflammation to date. These studies suggest that the serpin Serp-1 and the Serp-1 RCL peptide S-1 share the capacity to significantly alter gene expression in human monocytes and Jurkat T cells and, more specifically, inflammatory responses, such as inflammatory monocyte and T cell differentiation in atherogenesis. Changes in gene expression for Serp-1 and S-5 differed from the RCL peptide S-5, potentially representing differing pathways modified by the serpins and peptides that reduce plaque growth and those that do not.
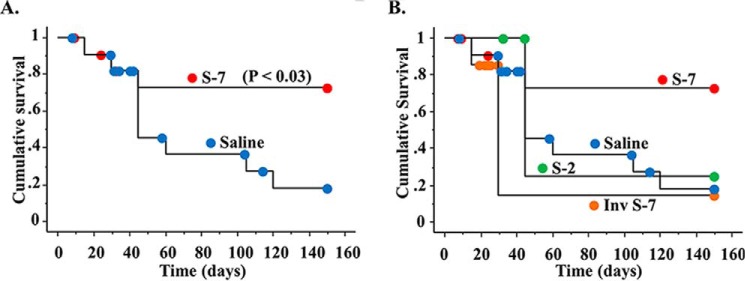
Serpin RCL S-7 Peptide Improves Survival in Lethal MHV68 Infections in IFNγR−/− Mice
To further determine whether serpin RCL peptides with anti-inflammatory activity in the aortic transplant model had beneficial functions in other in vivo models, peptides were assessed in a lethal MHV68 infection in IFNγR-deficient mice, a model for virus-induced vasculitis. Infected mice were treated with either S-1, S-2, S-3, S-7, an inverted sequence S-7, or S-8 peptides. S-7 was again active, with a significant improvement in survival (Fig. 8A). As for the aortic transplants, S-2 showed no activity (Fig. 8B). Differing from the aortic transplant model, no other RCL peptides examined improved survival in this model (data not shown). S-7 was tested in two independent studies with two differing MHV68 stocks. The S-7 inverted peptide did not improve survival, demonstrating that the peptide sequence was important to this activity.
FIGURE 8.
Serpin peptide S-7 improves survival in a lethal MHV68 vasculitis model. A, Kaplan-Meier survival curves demonstrate a significantly prolonged survival with S-7 treatment (p ≤ 0.03). B, in contrast, the inverse S-7 peptide sequence and the S-2 peptide did not improve survival.
Discussion
With these studies, we identify potent anti-inflammatory activity in peptides generated from the RCL of two serpins, the mammalian serpin NSP and the myxomaviral serpin Serp-1. Eight RCL peptides were designed based upon predicted natural protease cleavage sites of the RCL of either NSP or Serp-1. Treatment of mouse aortic allograft transplants with peptides containing either Arg alone or Arg-Asn possessed anti-atherogenic activity at long term follow-up after single-dose infusion, similar to Serp-1 and NSP treatment in this model. One peptide, S-7, also improved survival in a lethal mouse MHV68 lethal vasculitis model. An inverse sequence S-7 peptide was not active in the MHV68 model, demonstrating sequence specificity for peptide function.
Arg-Asn is the scissile P1-P1′ sequence in Serp-1, the target sequence that is cleaved by thrombolytic and thrombotic serine proteases, and Arg-Met is the P1-P1′ scissile bond for NSP that is cleaved by thrombolytic serine proteases. The presence or Arg or Arg-Asn in the serpin-derived peptides was associated with anti-inflammatory and anti-atherogenic activity. There was a high proportion of active peptides, particularly those containing Arg-Asn or Arg alone in the sequence. These active peptides may act to extend the natural life span of serpin inhibitory functions. In contrast, one peptide lacking Arg or Arg-Asn, with markedly negative charge and hydrophobicity (S-6), had highly non-beneficial activity. It caused early local allograft thrombosis and had no anti-inflammatory or anti-atherogenic activity. This peptide was derived from NSP and may thus be postulated to extend the anti-thrombolytic (e.g. pro-thrombotic) functions of NSP. Serpins are suicide inhibitors forming 1:1 stoichiometric complexes, wherein the protease cleaves the serpin RCL P1-P1′ scissile bond, forming a complex with the serpin such that both the serpin and protease lose function after binding (1–5, 29). These active peptides may represent a class of anti-inflammatory peptides similar to the highly active expanding classes of defensin peptides.
The serpins Serp-1 and NSP have been previously demonstrated to possess potent inhibitory activity, preventing inflammatory cell activation and late vascular plaque growth after single-dose infusions at the time of arterial injury or transplant (10–15, 18) and also in mouse models of lethal viral sepsis (17). The prolonged efficacy of single doses of Serp-1 and NSP would suggest a potential for additional functions. This prolonged activity for a serpin, with a presumed limited life span, would suggest a mechanism that would extend functions such as inhibition of pivotal central regulatory steps, modification of cell activation and gene expression, or perhaps though active proteolytic metabolites such as the serpin peptides. The serpin reactive center and specifically the P1-P1′ scissile bond, which is cleaved by serine proteases, represents a natural peptide processing site for serpins.
Upon review of the defensin and cathelicidin active peptide sequences, as published (26, 30–34), no overlapping sequences were found except for one cow defensin where an LANR sequence was reported. One study has reported that the β-defensins are more dependent upon the secondary and tertiary structure for anti-microbial activity than the primary amino acid sequence. When the secondary peptide structure was assessed, the active peptides S-1 and S-3 with Arg-Asn or RNL had potential for helical sections (Expasy). Other studies have suggested that some peptides function by binding and then acquiring a conformation complementary to the target proteins. Use of a pharmacophore model to understand the characteristics required for such binding (viz. the charge, hydrophobicity, and hydrogen bonding properties of immunosuppressive peptides) has been reported recently (35). Altering the peptide hydrophobicity or charge in the T cell receptor nonapeptide (core peptide) derived from the T cell receptor transmembrane region by removing hydrophobic or positively charged residues modified binding and inhibition of IL-2 (35). Analysis to identify potential binding partners for the active serpin peptides will require further investigation.
NSP blocks uPA and tPA. One negatively charged, hydrophobic RCL peptide, S-6, derived from NSP that lacks Arg or Arg-Asn caused significant thrombosis at sites of aortic allograft implant with early mortality at doses equivalent to those used for other peptides. This increased clotting was not seen at a lower dose infusion, but although less toxic, there was no significant reduction in inflammation or plaque growth at the lower dose. As noted, the S-6 peptide may extend the pro-thrombotic, anti-thrombolytic functions of NSP. The Serp-1 peptides S-1, S-3, and S-7 as well as NSP peptide S-8 displayed anti-atherogenic and anti-inflammatory actions (Figs. 3 and 4). Serp-1 anti-inflammatory activity has been linked to binding to uPA and the uPA receptor (12, 13, 15). However, none of these active peptides had the capacity to bind to or to inhibit uPA or tPA in vitro (Fig. 2). Thus, the peptide activity detected in these assays is probably unrelated to direct uPA or tPA binding and/or inhibition.
Of great interest, when a serpin RCL is cleaved at the P1-P1′ scissile bond, one peptide arm inserts into the β-sheet A, forming the serpin-protease suicide-inhibitory complex. In serpin genetic disorders, termed serpinopathies, as for AAT and NSP, the RCL of one serpin inserts into a neighboring serpin β-sheet, forming a dysfunctional serpin aggregate. We postulated that the serpin peptide derived here can inhibit the function of other serpins by insertion into the β-sheet of unrelated serpins. Upon modeling, we detected a capacity for the S-7 peptide to bind PAI-1 and ATIII. There was a correlative ability for peptides to inhibit PAI-1 in vitro. Thus, serpin-derived RCL peptides have the potential to function as serpin inhibitors. More extensive analyses in future work will be required to identify potential serpin targets.
The effects on monocyte activation were varied, but some correlation between inhibition of vascular plaque growth after aortic engrafting for peptides and inhibition of monocyte activation in vivo was demonstrated. S-1, S-3, S-6, S-7, and S-8 reduced membrane fluidity, whereas S-2 and S-5 were inactive (Fig. 6). S-4 was an outlier, demonstrating reduced membrane fluidity and marked variation in plaque growth from very small to very large plaque. This variability led to a non-significant change in plaque growth with S-4 treatment. In a mouse ascites assay, S-1, S-3, and S-6 altered splenocyte CD3+CD4+ and Cd11b responses to varying extents (Fig. 6). These data support systemic immune modulatory actions for selected peptides. In the model used here, an inflammatory response was induced by injection of a chemokine into the peritoneal cavity, followed by intravenous administration of the serpin peptides. Whereas no consistent effect was detected on mouse peritoneal mononuclear cell migration into the peritoneal cavity, a systemic effect on spleen cell population subsets was detected. Nonspecific changes in CD3+CD4+ were detected for S-6 alone and on CD11b for S-1 and S-6 with a trend for S-3. None of the peptides produced a significant change in T helper (Th) subsets Th1, Th2, and Th17 or Treg. These findings may be due to the small effect of intraperitoneal injection on systemic inflammation, because serpin activity, in our prior work, has often been more pronounced in animal models with marked increases in inflammation (e.g. angioplasty, stent implant of allograft transplants).
Analysis of the exact function and mechanism of action of these peptides will require further work. Certainly, analysis of the secondary and tertiary structures, as for the defensins, would be of interest. Prior studies have also reported activity for polyarginine peptides (34), suggesting some relevance for the Arg and Arg-Asn sequences associated with activity for the Serp-1 peptides. The fibrinogen RGD peptide sequence has known effects on thrombosis, modifying integrin fibrinogen interactions and platelet activation (36). This sequence is the basis for a class of anti-platelet inhibitors used for clinical prevention of thrombosis after stent implant and in unstable coronary syndromes to prevent thrombosis and heart attacks. Aside from S-6 induction of thrombosis with associated early mortality, no increased thrombosis was detected for the other peptides tested. There is thus no indication that these peptides are acting through modification of platelet activation and clot formation.
The differences in the expression of signaling genes between Serp-1 and S-1 treatments on one hand and S-5 treatment on the other offer potential mechanisms of action by which peptides such as S-1 may exert anti-atherogenic actions (Fig. 7). The breast cancer 1 gene, BRCA1, has recently been shown to be an essential regulator of heart function (37) and anti-atherogenic (38). Transcription factor 7 is involved in the differentiation of thymocytes and is indispensable for their development (39). The modulation of these genes may be linked to the protective effects of Serp-1 and S-1. Also, the relevance, if any, for selectin P ligand gene induction in the observed anti-atherogenic activity of Serp-1 and S-1 requires further investigation because the inhibition of this gene has been shown to be anti-atherogenic in mice (40, 41). Further work is needed to parse out the exact mechanism of action of these serpin-derived RCL peptides and to connect the modulation of these genes and their pathways to the effects of the anti-atherogenic peptides.
In conclusion, we report herein a series of serpin reactive center-derived peptides synthesized based upon natural protease cleavage sites. These RCL peptides display additional anti-inflammatory, anti-atherogenic, and (for one peptide, S-6) pro-thrombotic functions. We have further demonstrated that one serpin peptide, S-7, has the capacity to improve survival in a lethal mouse herpes infection in IFNγR-deficient mice. Preliminary in vitro assays detected inhibition of an unrelated mammalian serpin, PAI-1. Serpin RCL peptide metabolites have the potential to extend serpin regulatory activity. We have postulated that peptides derived from the serpin RCL function as immune modulators through insertion into the β-sheet A of unrelated serpins.
Author Contributions
A. L., S. A., G. M.-R., R. M., and S. T. designed the study. A. L., S. A., and G. M.-R. wrote the paper. S. A., G. M.-R., D. Z., C. S., B. M., and S. M. conducted experimental work. E. D. performed surgeries. E. F. cultured the virus. M. P. conducted in silico modeling studies.
This work was funded by National Institutes of Health Grants 1RC1HL100202-01 and 1 R01 AI100987-01A1 and American Heart Association Grant 0855421 E. Dr. Lucas consults for and holds minor shares in a small biotechnology company, Viron Therapeutics, Inc. (London, Canada). Viron did not provide research funding for these studies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- RCL
- reactive center loop
- ATIII
- anti-thrombin III
- AAT
- α-1 antitrypsin
- NSP
- neuroserpin
- PAI-1
- plasminogen activator inhibitor-1
- uPA
- urokinase-type plasminogen activator
- tPA
- tissue-type plasminogen activator
- MCP-1
- monocyte chemoattractant protein-1
- PMA
- phorbol 12-myristate 13-acetate
- BPP
- 1,3-Bis-pyrenylpropane.
References
- 1.Silverman G. A., Bird P. I., Carrell R. W., Church F. C., Coughlin P. B., Gettins P. G., Irving J. A., Lomas D. A., Luke C. J., Moyer R. W., Pemberton P. A., Remold-O'Donnell E., Salvesen G. S., Travis J., and Whisstock J. C. (2001) The serpins are an expanding superfamily of structurally similar but functionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 276, 33293–33296 [DOI] [PubMed] [Google Scholar]
- 2.Law R. H., Zhang Q., McGowan S., Buckle A. M., Silverman G. A., Silverman G. A., Wong W., Rosado C. J., Langendorf C. G., Pike R. N., Bird P. I., and Whisstock J. C. (2006) An overview of the serpin superfamily. Genome Biol. 7, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooptu B., and Lomas D. A. (2009) Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu. Rev. Biochem. 78, 147–176 [DOI] [PubMed] [Google Scholar]
- 4.Lucas A., Liu L., Dai E., Bot I., Viswanathan K., Munuswamy-Ramunujam G., Davids J. A., Bartee M. Y., Richardson J., Christov A., Wang H., Macaulay C., Poznansky M., Zhong R., Miller L., Biessen E., Richardson M., Sullivan C., Moyer R., Hatton M., Lomas D. A., and McFadden G. (2009) The serpin saga; development of a new class of virus derived anti-inflammatory protein immunotherapeutics. Adv. Exp. Med. Biol. 666, 132–156 [DOI] [PubMed] [Google Scholar]
- 5.Sutherland J. S., Bhakta V., and Sheffield W. P. (2007) The appended tail region of heparin cofactor II and additional reactive centre loop mutations combine to increase the reactivity and specificity of α1-proteinase inhibitor M358R for thrombin. Thromb. Haemost. 98, 1014–1023 [PubMed] [Google Scholar]
- 6.Zhou A., Carrell R. W., Murphy M. P., Wei Z., Yan Y., Stanley P. L., Stein P. E., Broughton Pipkin F., and Read R. J. (2010) A redox switch in angiotensinogen modulates angiotensin release. Nature 468, 108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Q., Jiang X., Yu F., Qiu J., Kang X., Cai L., Li L., Shi W., Liu S., Jiang S., and Liu K. (2012) Short cyclic peptides derived from the C-terminal sequence of α1-antitrypsin exhibit significant anti-HIV-1 activity. Bioorg. Med. Chem. Lett. 22, 2393–2395 [DOI] [PubMed] [Google Scholar]
- 8.Kalle M., Papareddy P., Kasetty G., Tollefsen D. M., Malmsten M., Mörgelin M., and Schmidtchen A. (2013) Proteolytic activation transforms heparin cofactor II into a host defense molecule. J. Immunol. 190, 6303–6310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shmueli R. B., Ohnaka M., Miki A., Pandey N. B., Lima e Silva R., Koskimaki J. E., Kim J., Popel A. S., Campochiaro P. A., and Green J. J. (2013) Long-term suppression of ocular neovascularization by intraocular injection of biodegradable polymeric particles containing a serpin-derived peptide. Biomaterials 34, 7544–7551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai E., Guan H., Liu L., Little S., McFadden G., Vaziri S., Cao H., Ivanova I. A., Bocksch L., and Lucas A. (2003) Serp-1, a viral anti-inflammatory serpin, regulates cellular serine proteinase and serpin responses to vascular injury. J. Biol. Chem. 278, 18563–18572 [DOI] [PubMed] [Google Scholar]
- 11.Lucas A., Liu L., Macen J., Nash P., Dai E., Stewart M., Graham K., Etches W., Boshkov L., Nation P. N., Humen D., Hobman M. L., and McFadden G. (1996) Virus-encoded serine proteinase inhibitor SERP-1 inhibits atherosclerotic plaque development after balloon angioplasty. Circulation 94, 2890–2900 [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan K., Richardson J., Togonu-Bickersteth B., Dai E., Liu L., Vatsya P., Sun Y. M., Yu J., Munuswamy-Ramanujam G., Baker H., and Lucas A. R. (2009) Myxoma viral serpin, Serp-1, inhibits human monocyte adhesion through regulation of actin-binding protein filamin B. J. Leukoc. Biol. 85, 418–426 [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan K., Liu L., Vaziri S., Dai E., Richardson J., Togonu-Bickersteth B., Vatsya P., Christov A., and Lucas A. R. (2006) Myxoma viral serpin, Serp-1, a unique interceptor of coagulation and innate immune pathways. Thromb. Haemost. 95, 499–510 [DOI] [PubMed] [Google Scholar]
- 14.Bédard E. L., Jiang J., Arp J., Qian H., Wang H., Guan H., Liu L., Parry N., Kim P., Garcia B., Li X., Macaulay C., McFadden G., Lucas A., and Zhong R. (2006) Prevention of chronic renal allograft rejection by SERP-1 protein. Transplantation 81, 908–914 [DOI] [PubMed] [Google Scholar]
- 15.Dai E., Viswanathan K., Sun Y. M., Li X., Liu L. Y., Togonu-Bickersteth B., Richardson J., Macaulay C., Nash P., Turner P., Nazarian S. H., Moyer R., McFadden G., and Lucas A. R. (2006) Identification of myxomaviral serpin reactive site loop sequences that regulate innate immune responses. J. Biol. Chem. 281, 8041–8050 [DOI] [PubMed] [Google Scholar]
- 16.Jiang J., Arp J., Kubelik D., Zassoko R., Liu W., Wise Y., Macaulay C., Garcia B., McFadden G., Lucas A. R., and Wang H. (2007) Induction of indefinite cardiac allograft survival correlates with toll-like receptor 2 and 4 downregulation after serine protease inhibitor-1 (Serp-1) treatment. Transplantation 84, 1158–1167 [DOI] [PubMed] [Google Scholar]
- 17.Chen H., Zheng D., Abbott J., Liu L., Bartee M. Y., Long M., Davids J., Williams J., Feldmann H., Strong J., Grau K. R., Tibbetts S., Macaulay C., McFadden G., Thoburn R., Lomas D. A., Spinale F. G., Virgin H. W., and Lucas A. (2013) Myxomavirus-derived serpin prolongs survival and reduces inflammation and hemorrhage in an unrelated lethal mouse viral infection. Antimicrob. Agents Chemother. 57, 4114–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munuswamy-Ramanujam G., Dai E., Liu L., Shnabel M., Sun Y. M., Bartee M., Lomas D. A., and Lucas A. R. (2010) Neuroserpin, a thrombolytic serine protease inhibitor (serpin), blocks transplant vasculopathy with associated modification of T-helper cell subsets. Thromb. Haemost. 103, 545–555 [DOI] [PubMed] [Google Scholar]
- 19.Liang W., Chuan-Zhen L., Qiang D., Jian Q., Hui-Min R., and Bao-Guo X. (2004) Reductions in mRNA of the neuroprotective agent, neuroserpin, after cerebral ischemia/reperfusion in diabetic rats. Brain Res. 1015, 175–180 [DOI] [PubMed] [Google Scholar]
- 20.Dai E., Stewart M., Ritchie B., Mesaeli N., Raha S., Kolodziejczyk D., Hobman M. L., Liu L. Y., Etches W., Nation N., Michalak M., and Lucas A. (1997) Calreticulin, a potential vascular regulatory protein, reduces intimal hyperplasia after arterial injury. Arterioscler. Thromb. Vasc. Biol. 17, 2359–2368 [DOI] [PubMed] [Google Scholar]
- 21.Bocksch L., Rider B. J., Stephens T., Dai E., Liu L., Diao H., Viswanathan K., Munuswamy-Ramanujam G., Singh B., and Lucas A. (2007) C-terminal apolipoprotein E-derived peptide, Ep1.B, displays anti-atherogenic activity. Atherosclerosis 194, 116–124 [DOI] [PubMed] [Google Scholar]
- 22.Congote L. F. (2006) The C-terminal 26-residue peptide of serpin A1 is an inhibitor of HIV-1. Biochem. Biophys. Res. Commun. 343, 617–622 [DOI] [PubMed] [Google Scholar]
- 23.Chen H., Zheng D., Davids J., Bartee M. Y., Dai E., Liu L., Petrov L., Macaulay C., Thoburn R., Sobel E., Moyer R., McFadden G., and Lucas A. (2011) Viral serpin therapeutics from concept to clinic. Methods Enzymol. 499, 301–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tardif J. C., L'Allier P. L., Grégoire J., Ibrahim R., McFadden G., Kostuk W., Knudtson M., Labinaz M., Waksman R., Pepine C. J., Macaulay C., Guertin M. C., and Lucas A. (2010) A randomized controlled, phase 2 trial of the viral serpin Serp-1 in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 3, 543–548 [DOI] [PubMed] [Google Scholar]
- 25.Viswanathan K., Bot I., Liu L., Dai E., Turner P. C., Togonu-Bickersteth B., Richardson J., Davids J. A., Williams J. M., Bartee M. Y., Chen H., van Berkel T. J., Biessen E. A., Moyer R. W., and Lucas A. R. (2012) Viral cross-class serpin inhibits vascular inflammation and T lymphocyte fratricide; a study in rodent models in vivo and human cell lines in vitro. PLoS One 7, e44694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagaoka I., Hirota S., Niyonsaba F., Hirata M., Adachi Y., Tamura H., Tanaka S., and Heumann D. (2002) Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin. Diagn. Lab. Immunol. 9, 972–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christov A., Kostuk W. J., Jablonsky G., and Lucas A. (2001) Fluorescence spectroscopic analysis of circulating platelet activation during coronary angioplasty. Lasers Surg. Med. 28, 414–426 [DOI] [PubMed] [Google Scholar]
- 28.Zalai C. V., Kolodziejczyk M. D., Pilarski L., Christov A., Nation P. N., Lundstrom-Hobman M., Tymchak W., Dzavik V., Humen D. P., Kostuk W. J., Jablonsky G., Pflugfelder P. W., Brown J. E., and Lucas A. (2001) Increased circulating monocyte activation in patients with unstable coronary syndromes. J. Am. Coll. Cardiol. 38, 1340–1347 [DOI] [PubMed] [Google Scholar]
- 29.van Gent D., Sharp P., Morgan K., and Kalsheker N. (2003) Serpins: structure, function and molecular evolution. Int. J. Biochem. Cell Biol. 35, 1536–1547 [DOI] [PubMed] [Google Scholar]
- 30.Lee E., Kim J. K., Shin S., Jeong K. W., Lee J., Lee D. G., Hwang J. S., and Kim Y. (2011) Enantiomeric 9-mer peptide analogs of protaetiamycine with bacterial cell selectivities and anti-inflammatory activities. J. Pept. Sci. 17, 675–682 [DOI] [PubMed] [Google Scholar]
- 31.Doss M., Ruchala P., Tecle T., Gantz D., Verma A., Hartshorn A., Crouch E. C., Luong H., Micewicz E. D., Lehrer R. I., and Hartshorn K. L. (2012) Hapivirins and diprovirins: novel θ-defensin analogs with potent activity against influenza A virus. J. Immunol. 188, 2759–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadler K., Eom K. D., Yang J. L., Dimitrova Y., and Tam J. P. (2002) Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7. Biochemistry 41, 14150–14157 [DOI] [PubMed] [Google Scholar]
- 33.Barata T. S., Teo I., Brocchini S., Zloh M., and Shaunak S. (2011) Partially glycosylated dendrimers block MD-2 and prevent TLR4-MD-2-LPS complex mediated cytokine responses. PLoS Comput. Biol. 7, e1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anbanandam A., Albarado D. C., Tirziu D. C., Simons M., and Veeraraghavan S. (2008) Molecular basis for proline- and arginine-rich peptide inhibition of proteasome. J. Mol. Biol. 384, 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raguine L., Ali M., Bender V., Diefenbach E., Doddareddy M. R., Hibbs D., and Manolios N. (2013) Alanine scan of an immunosuppressive peptide (CP): analysis of structure-function relationships. Chem. Biol. Drug Des. 81, 167–174 [DOI] [PubMed] [Google Scholar]
- 36.Bennett J. S. (2001) Platelet-fibrinogen interactions. Ann. N.Y. Acad. Sci. 936, 340–354 [DOI] [PubMed] [Google Scholar]
- 37.Shukla P. C., Singh K. K., Quan A., Al-Omran M., Teoh H., Lovren F., Cao L., Rovira I. I., Pan Y., Brezden-Masley C., Yanagawa B., Gupta A., Deng C. X., Coles J. G., Leong-Poi H., Stanford W. L., Parker T. G., Schneider M. D., Finkel T., and Verma S. (2011) BRCA1 is an essential regulator of heart function and survival following myocardial infarction. Nat. Commun. 2, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh K. K., Shukla P. C., Quan A., Al-Omran M., Lovren F., Pan Y., Brezden-Masley C., Ingram A. J., Stanford W. L., Teoh H., and Verma S. (2013) BRCA1 is a novel target to improve endothelial dysfunction and retard atherosclerosis. J. Thorac. Cardiovasc. Surg. 146, 949–960.e4 [DOI] [PubMed] [Google Scholar]
- 39.Ma J., Wang R., Fang X., and Sun Z. (2012) β-Catenin/TCF-1 pathway in T cell development and differentiation. J. Neuroimmune Pharmacol. 7, 750–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.An G., Wang H., Tang R., Yago T., McDaniel J. M., McGee S., Huo Y., and Xia L. (2008) P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation 117, 3227–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips J. W., Barringhaus K. G., Sanders J. M., Hesselbacher S. E., Czarnik A. C., Manka D., Vestweber D., Ley K., and Sarembock I. J. (2003) Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation 107, 2244–2249 [DOI] [PubMed] [Google Scholar]
- 42.Krissinel E., and Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]