Abstract
Nonalcoholic fatty liver disease (NAFLD) associates with obesity and type 2 diabetes. Hypoactive AMP-activated protein kinase (AMPK), hyperactive mammalian target of rapamycin (mTOR) signaling, and macrophage-mediated inflammation are mechanistically linked to NAFLD. Studies investigating roles of arginase particularly the extrahepatic isoform arginase-II (Arg-II) in obesity-associated NAFLD showed contradictory results. Here we demonstrate that Arg-II−/− mice reveal decreased hepatic steatosis, macrophage infiltration, TNF-α and IL-6 as compared to the wild type (WT) littermates fed high fat diet (HFD). A higher AMPK activation (no difference in mTOR signaling), lower levels of lipogenic transcription factor SREBP-1c and activity/expression of lipogenic enzymes were observed in the Arg-II−/− mice liver. Moreover, release of TNF-α and IL-6 from bone marrow-derived macrophages (BMM) of Arg-II−/− mice is decreased as compared to WT-BMM. Conditioned medium from Arg-II−/−-BMM exhibits weaker activity to facilitate triglyceride synthesis paralleled with lower expression of SREBP-1c and SCD-1 and higher AMPK activation in hepatocytes as compared to that from WT-BMM. These effects of BMM conditioned medium can be neutralized by neutralizing antibodies against TNF-α and IL-6. Thus, Arg-II-expressing macrophages facilitate diet-induced NAFLD through TNF-α and IL-6 in obesity.
Nonalcoholic fatty liver disease (NAFLD) referred to as an intracellular accumulation of triglycerides in the liver (steatosis) with or without hepatitis can lead to liver fibrosis and cirrhosis and may contribute to development and progression of the most important chronic complications of diabetes, including cardiovascular disease and chronic kidney disease1,2. NAFLD is commonly associated with obesity, insulin resistance and type 2 diabetes3. Under the metabolic disorder conditions, hepatic de novo lipogenesis is paradoxically augmented despite insulin resistance4,5, which involves various mechanisms including activation of mammalian target of rapamycin complex 1 - small ribosomal protein S6 kinase 1 (mTORC1-S6K1) signaling and inhibition of 5′ adenosine monophosphate-activated protein kinase (AMPK) pathway, leading to up-regulation and activation of hepatic sterol regulatory element-binding protein-1c (SREBP-1c)4,5,6,7. SREBP-1c is a master transcription factor regulating expression of various enzymes in the lipogenesis pathway such as malic enzyme (ME), glucose-6-phosphate dehydrogenase (G6PDH), acetyl-CoA carboxylase-1 (ACC1), fatty acid synthase (FAS), and stearoyl-CoA desaturase-1 (SCD-1)8. While mTORC1 pathway has been shown to induce SREBP-1c and promote hepatic lipogenesis5,9, activation of AMPK pathway e.g., by the antidiabetic drug metformin and thiazolidinediones, accounts for its therapeutic effects on liver steatosis through inhibition of SREBP-1c7,10,11.
It is well recognized that obesity and inflammation are highly integrated processes in the pathogenesis of insulin resistance, type 2 diabetes and NAFLD12. Macrophages are heterogeneous and two extreme phenotypes, i.e., pro-inflammatory M1 and anti-inflammatory M2, are the most well investigated13. Macrophage infiltration accompanied by inflammation and metabolic alteration occurs in both adipose tissues and liver, contributing to insulin resistance, type 2 diabetes and NAFLD14,15,16,17. Recent studies provide convincing evidence that resident macrophages in the liver, i.e., Kupffer cells, are activated towards M1 phenotype in obesity18. Moreover, pro-inflammatory macrophages are also recruited to the liver under high fat diet (HFD) feeding19,20. These studies demonstrate that both activated M1 Kupffer cells and recruited macrophages in the liver cause hepatic inflammation and promote hepatic steatosis.
The regulatory mechanisms of macrophage phenotypes are complex and far from being characterized. Among other markers, iNOS and type-I arginase (Arg-I), the enzymes that are involved in L-arginine/NO metabolism, are associated with M1 and M2 macrophage phenotype, respectively13. A type-II arginase (Arg-II) also exists. In contrast to Arg-I which is constitutively and most abundantly expressed in hepatocytes21,22 and functions as a detoxifying enzyme in urea cycle to remove ammonia23, Arg-II is not expressed in hepatocytes but in many extrahepatic tissues/cells including kidney, brain, vasculature, and macrophages22,24. Moreover, Arg-II is up-regulated in macrophages by pro-inflammatory stimuli such as lipopolysaccharide (LPS) and associated with M1 phenotype25. Our recent study provides evidence demonstrating that Arg-II promotes macrophage pro-inflammatory responses, contributing to atherogenesis and obesity-associated insulin resistance26. Furthermore, we also showed that Arg-II forms a positive regulatory circuit with mTORC1-S6K1 and negatively regulates AMPK, resulting in functional changes of vascular cells, including eNOS-uncoupling, cell apoptosis/senescence, and impaired autophagy function27,28,29.
Despite the beneficial effects of Arg-II deficiency on the chronic inflammatory diseases, it is to note that pharmacological inhibition of arginase or genetic ablation of Arg-II in HFD-induced obesity mouse model yielded completely contradictory results on the pathology of liver steatosis as reported by the most recently published two studies30,31. Hence, it is important to further delineate the role of Arg-II in obesity-associated NAFLD and the underlying mechanisms. In this study, we demonstrate that Arg-II deficiency protects against obesity-associated NAFLD in mice through suppression of liver macrophage-mediated pro-inflammatory responses resulting in improved AMPK activation and decreased lipogenesis in hepatocytes.
Results
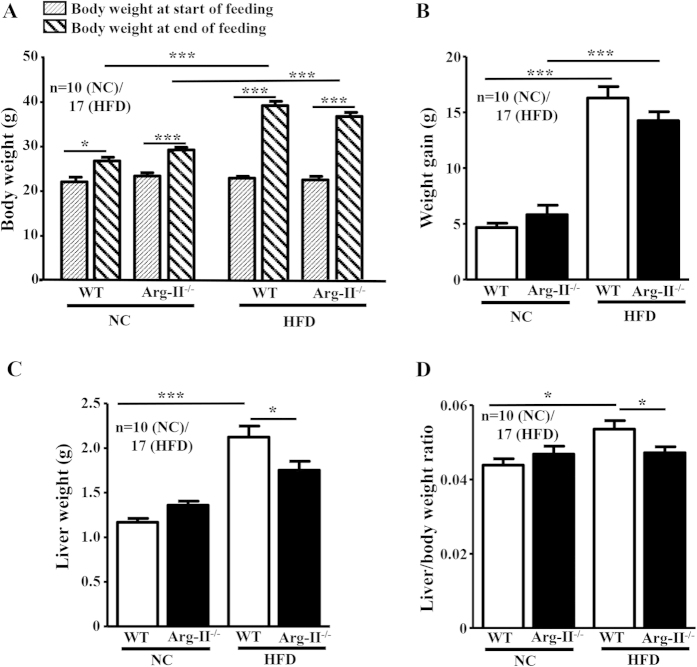
Reduced liver weight in obese Arg-II−/− mice fed HFD
After HFD feeding for 14 weeks, no significant difference in body weight and body weight gain between the WT and Arg-II−⁄− mice were observed (Fig. 1A,B). The liver weight and the liver weight/body weight ratio were, however, significantly lower in the Arg-II−⁄− mice as compared to the WT mice on HFD feeding (Fig. 1C,D).
Figure 1. Liver weight is reduced in obese Arg-II−/− mice.
Starting from 7 weeks of age, male wild-type (WT) and Arg-II−/− mice were fed a HFD for 14 weeks to induce obesity or maintained on normal chow diet (NC) as controls. (A) Body weight was measured at the start and at the end of HFD or NC feeding. (B) Weight gains during HFD feeding period. (C) Liver weight at the end of HFD. (D) Ratio of the liver weight and body weight at the end of HFD. *p < 0.05 and ***p < 0.001 between the indicated groups.
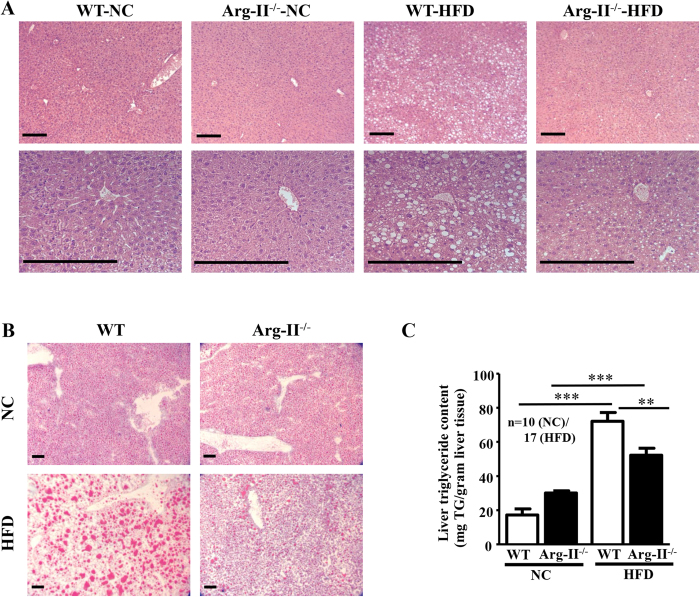
Arg-II deficiency reduces HFD–induced liver steatosis
In line with above observations, Arg-II−/− mice on HFD feeding displayed reduced hepatic steatosis as revealed by histomorphometric analysis of hematoxylin and eosin (HE) staining (Fig. 2A) and Oil Red O staining (Fig. 2B) in the liver sections, and significantly reduced hepatic triglyceride content when compared to the WT mice on HFD (Fig. 2C).
Figure 2. HFD-induced hepatic steatosis is reduced in Arg-II−/− mice.
Presented are images of (A) HE staining taken at two different magnifications. (B) Oil Red O staining in liver of WT and Arg-II−/− mice on either normal chow diet (NC) or high fat diet (HFD). Scale bar = 0.2 mm. (C) Liver triglyceride content. **p < 0.01, ***p < 0.001 between the indicated groups.
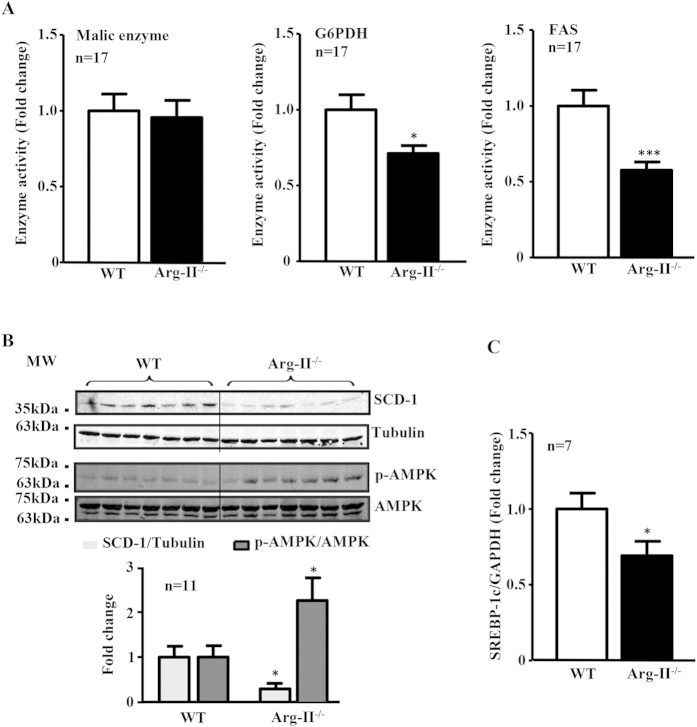
Arg-II deficiency suppresses hepatic lipogenic enzymes and signaling
Next, we examined the hepatic lipogenic signaling and enzymes. Arg-II−/− mice on HFD feeding revealed decreased activities of lipogenic enzymes in the liver such as G6PDH and FAS but not ME (Fig. 3A). Also the hepatic SCD-1 levels were significantly lower in the Arg-II−/− mice as compared to the WT littermates on HFD feeding (Fig. 3B). The reduced SCD-1 levels in the Arg-II−/− mice were associated with increased AMPK activation as measured by enhanced p-AMPK levels (Fig. 3B). Furthermore, expression of SREBP-1c was significantly reduced in the liver of Arg-II−/− mice on HFD (Fig. 3C). No difference in S6K1 activity as monitored by S6-S235/236 level was observed between the WT and Arg-II−/− mice fed HFD (data not shown). The results demonstrate a role of Arg-II in hepatic lipogenesis linking to the development of liver steatosis induced by HFD feeding. Taking into account that inhibition of SCD-1 in the context of the overflow of free fatty acids coming to the liver on HFD feeding has been shown to cause the buildup of toxic lipids resulting in increased hepatocyte cell death and liver injury despite the decreased TG deposition32,33, we assessed the liver injury by monitoring plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST). As shown in Fig. S1, plasma ALT and AST are significantly increased upon HFD feeding in both WT and Arg-II−/− mice. However, the increase in plasma ALT and AST tends to be smaller in obese Arg-II−/− mice as compared to obese WT mice.
Figure 3. Reduced lipogenic enzyme activity/expression and SREBP-1c expression, and enhanced AMPK signaling in obese Arg-II−/− mice.
(A) Activity of malic enzyme (ME), glucose-6-phosphate-dehydrogenase (G6PDH) and FAS in liver from obese WT and Arg-II−/− mice. (B) Shown is immunoblotting analysis of SCD-1 and phosphorylated AMPK-T172 (p-AMPK) and total AMPK levels from obese WT and Arg-II−/− liver. Tubulin served as a loading control. Quantification of the signals is presented in the graphics below. (C) qRT-PCR analysis of SREBP-1c mRNA expression in liver from obese WT and Arg-II−/− mice. *p < 0.05 and ***p < 0.001 vs WT-HFD.
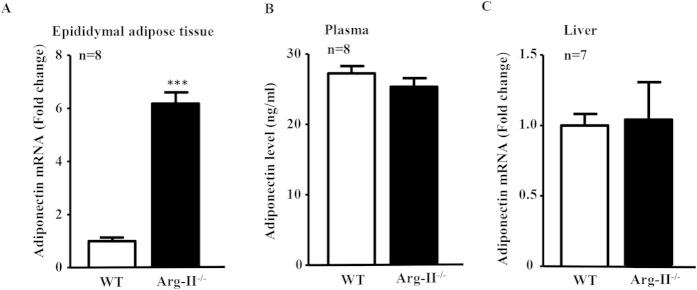
Arg-II-deficiency enhances adiponectin levels in adipose tissue, but not in plasma and in liver
Taking into account that Arg-II is an extrahepatic isoform of arginase and was not detectable in WT mouse liver and that Arg-I expression was not affected by Arg-II-deficiency (Fig. S2), the protective effect of Arg-II-deficiency on HFD-induced liver steatosis must be secondary to its effect on Arg-II-expressing cells/tissue. Given that adiponectin exerts its protective effects against liver steatosis through activating AMPK signaling pathway34 which is enhanced in Arg-II−/− mouse liver (Fig. 3B), we examined the adiponectin levels in adipose tissues, plasma and liver. Though the adiponectin mRNA level in epididymal adipose tissue as monitored by qRT-PCR is indeed significantly increased in Arg-II−/− mice (Fig. 4A), no difference in adiponectin levels was found in plasma or liver between WT and Arg-II−/− mice on HFD (Fig. 4B,C). No difference in weight of epididymal adipose tissue was found between WT and Arg-II−/− mice on HFD either (Fig. S3). These results rule out the endocrine and paracrine effects of adiponectin as a mechanism for enhanced AMPK and reduced hepatic lipogenesis in the Arg-II−/− mice.
Figure 4. Enhanced adiponectin in fat tissue but not in plasma or liver in Arg-II−/− mice.
(A) qRT-PCR analysis of adiponectin mRNA expression in epididymal fat tissue from obese WT and Arg-II−/− mice. ***p < 0.001 vs WT-HFD group. (B) Adiponectin level in plasma measured by ELISA. (C) qRT-PCR analysis of mRNA expression of adiponectin in liver tissue from obese WT and Arg-II−/− mice.
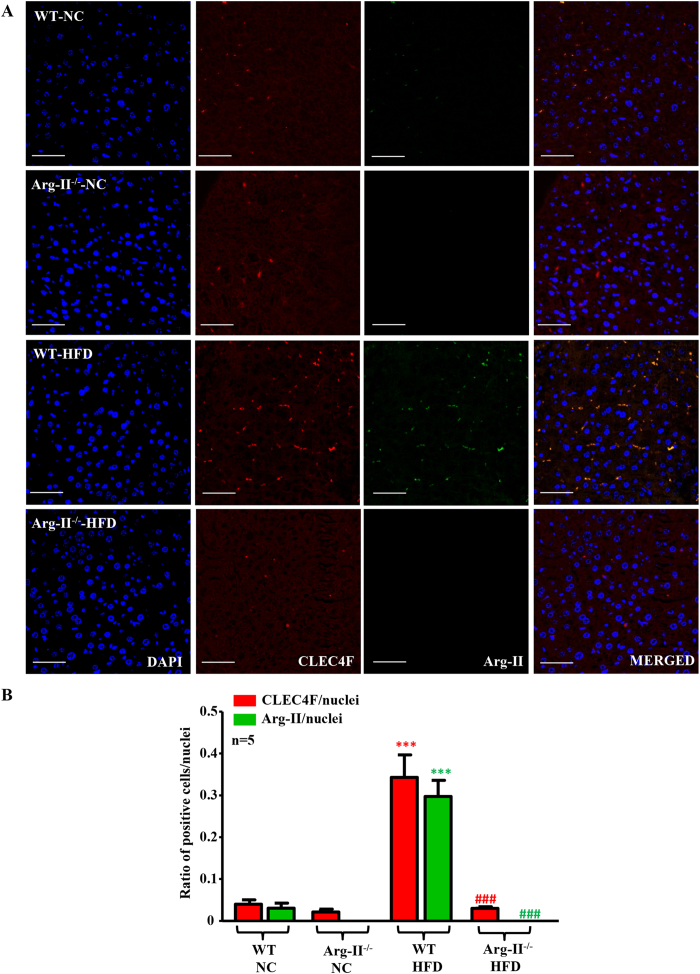
Arg-II-positive macrophages are increased in fatty liver
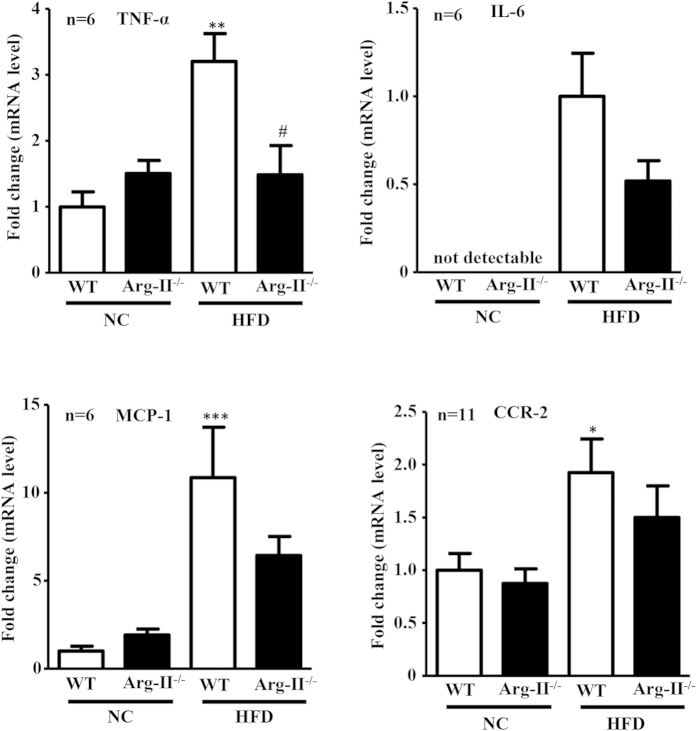
Macrophage-mediated inflammation has been mechanistically linked to the augmented hepatic lipogenesis in obesity17. Our previous studies demonstrate that Arg-II-deficiency suppresses obesity-associated systemic macrophage-mediated inflammation26. We thus examined the hepatic macrophage inflammation. Under HFD feeding, the number of CLEC4F positive and F4/80 positive macrophages in the WT-liver was increased, which was prevented in Arg-II−/− mice (Fig. 5 and Fig. S4). Moreover, Arg-II was exclusively expressed in these cells and was enhanced on HFD feeding as demonstrated by immunofluorescence staining (Fig. 5). In line with these observations, the pro-inflammatory cytokine TNF-α was significantly lower in obese Arg-II−/− mice liver (Fig. 6). We also observed a lower IL-6, MCP-1 and CCR-2 levels in Arg-II−/− mice liver, which statistically did not reach significance (Fig. 6). These results indicate that suppression of liver steatosis in obese Arg-II−/− mice might be attributable to reduced hepatic macrophage-mediated inflammation.
Figure 5. Enhanced hepatic inflammation upon HFD in WT is associated with increased Arg-II expression in macrophages in liver, which is abrogated by Arg-II-deficiency.
(A) Shown are representative images of immunostaining of the liver sections with macrophage marker CLEC4F (red) and Arg-II (green) followed by nuclei counterstaining with DAPI (blue). Scale bar = 50 μm. (B) Quantification of the signal is expressed as a ratio of CLEC4F- or Arg-II-positive cells to tissue nuclei number. ***P < 0.001 vs WT-NC. ###P < 0.001 vs WT-HFD.
Figure 6. Decreased cytokine production in liver of Arg-II−/− mice.
qRT-PCR analysis of mRNA expression of cytokines in liver tissue from WT and Arg-II−/− mice either on NC or HFD. Except for IL-6, all data are presented as fold change from WT-NC group, since IL-6 is not detectable in WT-NC; the data for IL-6 are presented as fold change from WT-HFD group. *p < 0.05, **p < 0.01, ***p < 0.001 vs WT-NC group; #p < 0.05 vs WT-HFD group.
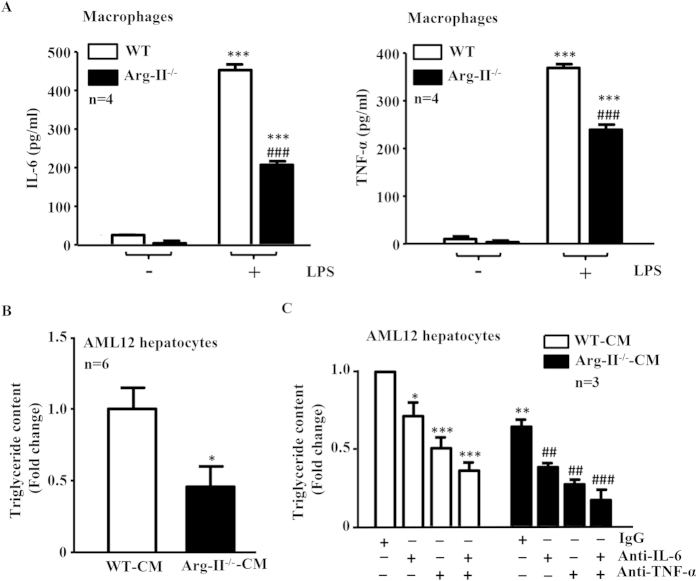
Suppressed production of IL-6 and TNF-α in Arg-II−/− macrophages accounts for reduced hepatic lipogenesis
To further confirm the above-mentioned findings, we prepared macrophages derived from bone marrow cells (BMM) that were isolated from WT or Arg-II−/− mice. The levels of secreted TNF-α and IL-6 in the BMM-conditioned medium (CM) from BMM upon LPS stimulation (100 ng/ml, 8 h) were determined. As shown in Fig. 7A, production of TNF-α and IL-6 upon LPS stimulation was significantly reduced in BMM from Arg-II−/− mice as compared to those from WT control animals. We then evaluated the effects of the CM from LPS-treated BMM on triglyceride synthesis in a murine hepatocyte cell line (AML12) in the presence of oleic acid (OA) that enhances hepatic triglyceride synthesis35. Remarkably, triglyceride synthesis in the hepatocytes was significantly lower in the presence of Arg-II−/−-BMM-CM as compared to WT-BMM-CM (Fig. 7B), suggesting that the lower IL-6 and TNF-α levels released from Arg-II−/− BMM accounts for the reduced triglyceride synthesis induced by Arg-II−/−-BMM-CM.
Figure 7. Suppressed production of IL-6 and TNF-α in Arg-II−/− macrophages accounts for reduced lipogenesis in hepatocyte AML12 cells.
The serum-starved (overnight) WT- or Arg-II−/−-BMM were incubated in the absence or presence of 100 ng/ml LPS for 8 hours, the conditioned medium (CM) was then collected. (A) IL-6 and TNF-α production in CM collected from WT- or Arg-II−/−-BMMs in the absence or presence of LPS was evaluated by ELISA. ***p < 0.001 vs controls (-LPS), ###p < 0.001 vs WT+LPS. (B) AML12 hepatocytes were serum-starved for 6 hours in 0.2% bovine serum albumin (BSA)-ITS-DMEM-F-12 Ham medium and then incubated with CM of LPS-treated BMM from WT or Arg-II−/− mice as indicated in the presence of oleic acid (OA, 20 mmol/L) for 24 hours. The lipid was then extracted for measurement of the triglyceride content in the cells. *p < 0.05 vs WT-CM. (C) Experiment procedure is the same as in panel B, except that BMM-CM was pre-treated for 2 hours with control rat IgG (10 μg/ml) or neutralizing antibodies anti-IL-6 (18 μg/ml) or anti-TNF-α (0.4 μg/ml) or anti-IL-6 (18 μg/ml) plus anti-TNF-α prior to the addition to the cells. *p < 0.05, **p < 0.01, ***p < 0.001 vs (WT-BMM-CM + IgG); ##p < 0.01, ###p < 0.001 vs (Arg-II−/− -BMM-CM + IgG).
To further reinforce the above conclusion, BMM-CM was first incubated with control IgG or neutralizing antibodies against IL-6, TNF-α or both and then used to treat the AML12 cells in the presence of OA. Fig. 7C shows that anti-TNF-α or anti-IL-6 antibody alone reduced triglyceride synthesis stimulated by BMM-CM of WT or Arg-II−/−. The strongest suppressing effects were achieved with the combination of both antibodies, supporting our conclusion drawn above.
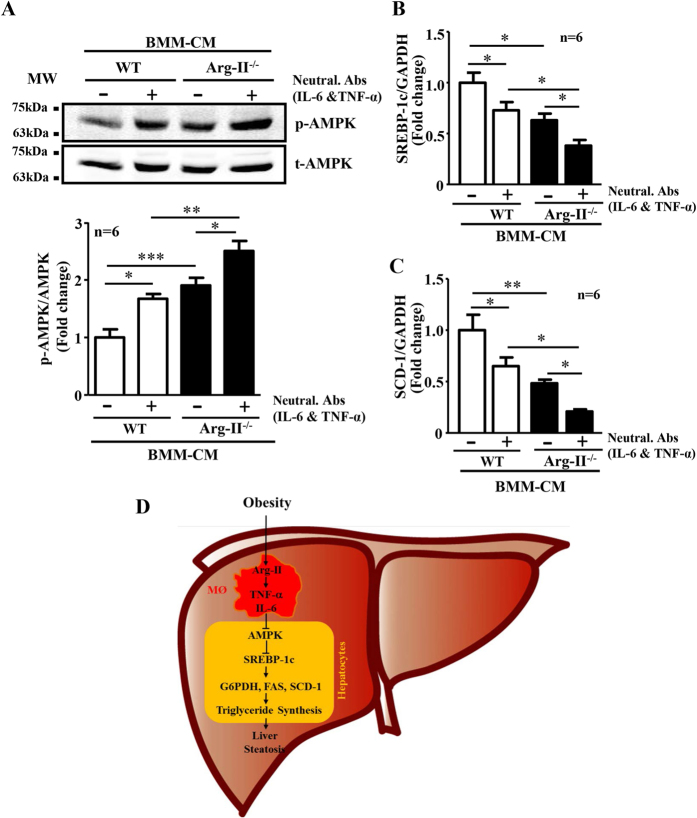
Arg-II−/−-BMM-CM elicits enhanced AMPK activation and reduced expression of SREBP-1c and SCD-1 in hepatocytes
Next, we determined the effect of BMM-CM on AMPK activation, SREBP-1c and SCD-1 expression in AML12 hepatocytes. In line with their effects on triglyceride synthesis, AMPK activation as monitored by phospho-AMPK-T172 in the hepatocytes was significantly higher when the cells were treated with Arg-II−/−-BMM-CM as compared to the cells treated with control WT-BMM-CM (Fig. 8A). BMM-CM pretreated with neutralizing antibodies against IL-6 and TNF-α elicited enhanced AMPK activation (Fig. 8A). In accordance, the expression levels of SREBP-1c and SCD-1 in the hepatocytes were significantly lower when the cells were treated with Arg-II−/−-BMM-CM as compared to the cells treated with control WT-BMM-CM (Fig. 8B,C). Pretreatment of BMM-CM with neutralizing antibodies against both cytokines further reduced the expression levels of SREBP-1c and SCD-1 (Fig. 8B,C).
Figure 8. CM from Arg-II−/−-BMM elicits increased AMPK signaling and decreased SREPBP-1c and SCD-1 in hepatocytes.
Experiment procedure is the same as in Fig. 7B, except that (A) AML12 hepatocyte cell lysates were prepared for immunoblotting analysis of AMPK activation. Quantification of the signals is presented below. (B,C) RNA was extracted for qRT-PCR analysis of mRNA expression of SREBP-1c and SCD-1. For all the panels *p < 0.05, **p < 0.01, ***p < 0.001 between the indicated groups. Neutral. Abs means neutralizing antibodies. IgG was used as control (-) for neutralizing antibodies. (D) Schematic illustration of the major findings of this study.
Discussion
In the present study, we provide both in vitro and in vivo evidences that Arg-II plays a role in HFD-induced liver steatosis through promoting macrophage inflammation in liver. Here we show that Arg-II−/− mice exhibit significantly less hepatic steatosis with decreased triglyceride content, lower hepatic weight, reduced inflammatory cytokines and macrophage infiltration, which is accompanied by enhanced AMPK signaling and reduced expression/activity of hepatic lipogenic regulator and enzymes, including SREBP-1c, G6PDH, FAS and SCD-1, leading to mitigated de novo lipogenesis in the liver in vivo. The reduced liver steatosis in Arg-II−/− mice on HFD is in line with the results of our previous study showing an improved glucose tolerance and insulin sensitivity in these mice26. The decreased hepatic steatosis in the Arg-II−/− mice does not seem to be the result of decreased lipid delivery to the liver or increased lipid secretion from liver into the blood stream in form of lipoprotein, since there is no difference in plasma triglyceride levels between WT and Arg-II−/− mice fed HFD as shown in our previous study26. Our data suggest that the reduced hepatic de novo lipogenesis in the obese Arg-II−/− mice ends up in decreased intrahepatic triglyceride accumulation versus lipid secretion. A dissociation of plasma triglyceride levels from liver steatosis has been reported by numerous studies36,37. Moreover, we show that in vitro, the CM from Arg-II−/− macrophages elicited lower triglyceride synthesis in cultured hepatocytes in the presence of oleic acid, which is accompanied by enhanced AMPK signaling and suppressed lipogenic regulator/enzyme SREBP-1c and SCD-1 and is attributable to the reduced production of the pro-inflammatory cytokines TNF-α and IL-6 from Arg-II−/− macrophages. Our findings thus suggest that targeting Arg-II is beneficial for obesity-associated NAFLD.
In agreement with the previous studies on Arg-I and -II expression in various tissues22,38, we could not detect Arg-II expression in liver from mice by immunoblotting analysis, nor Arg-II was detectable in hepatocytes by immunostaining. Given that Arg-II-deficiency protects mice from obesity-associated fatty liver despite undetectable Arg-II in hepatocytes, we postulate that the protective effects are secondary to an effect on other tissues or cells. Studies have provided convincing evidence showing that liver macrophages including resident Kupffer cells and recruited myeloid cells from circulating blood, when activated, promote hepatic inflammation and accelerate hepatic lipogenesis leading to lipid accumulation in the hepatocytes through release of pro-inflammatory cytokines17,19. A recent study by Morinaga et al. elegantly demonstrated that, in HFD-induced obesity mouse model, Kupffer cells become activated to express MCP-1 (although the number of Kupffer cells does not increase), which then recruit macrophages from blood to liver, enhancing hepatic inflammation and hepatic insulin resistance20. In line with this study, we observed increased macrophages in the liver of obese WT mice, which was reduced in obese Arg-II−/− mice. Importantly, Arg-II is mainly expressed and markedly enhanced in hepatic macrophages with concomitant enhanced accumulation of macrophages in obese WT mice as demonstrated by immunostaining, since the immunostaining signals of Arg-II in macrophages as well as the number of CLEC4F/Arg-II double positive or F4/80/Arg-II double positive cells are increased in the obese WT mouse liver. These observations are in accordance with our previous study26. Consistent with these observations, we also found significantly reduced TNF-α levels as well as reduced IL-6, MCP-1 and CCR-2 levels (although statistically not significant) in the liver of obese Arg-II−/− mice as compared to the obese WT control animals. These findings demonstrate that Arg-II is associated with macrophage-mediated pro-inflammatory responses, which is in agreement with our previous findings that Arg-II is upregulated in macrophages of mice fed HFD and plays a causative role in promoting macrophage pro-inflammatory responses in obesity and insulin resistance26. Moreover, vascular inflammation and endothelial dysfunction associated with aging are protected in the Arg-II−/− mice27. In support of our notions, the pro-inflammatory effects of Arg-II in endotoxin-induced uveitis and in diabetic nephropathy have been reported39,40. All the findings demonstrate a pro-inflammatory role of Arg-II in several inflammation disease models. In the present study, we further demonstrate that the Arg-II−/−-BMM-CM elicits a reduced triglyceride synthesis in the presence of oleic acid in cultured hepatocytes as compared to that of WT-BMM-CM, which is attributable to mitigated production of TNF-α and IL-6 from Arg-II−/−-BMM, since neutralizing antibody against TNF-α or IL-6 and combination of the two antibodies exhibit inhibition of triglyceride accumulation in the hepatocytes. These in vitro studies using AML12 hepatocytes were aimed to reinforce our findings in vivo. Although it is not optimal to use this cell line, which is established from a mouse hepatocyte transgenic for human TGF-α, the results on triglyceride accumulation and intracellular signaling in response to treatment with conditioned medium from WT and Arg-II−/−-BMM are in line with the in vivo experiments and support our conclusion. The fact that silencing Arg-II in monocytes decreased their adhesion activity to endothelial cells26 suggest that this may at least in part accounts for suppressed macrophage-infiltration in liver of Arg-II−/− mice fed HFD. It is of great interest to further evaluate the precise mechanisms of Arg-II in regulating adhesion of monocytes to endothelial cells. Regarding the molecular mechanism regulating Arg-II expression in macrophages in obesity, evidence has been presented that hyperactive S6K1 upregulates Arg-II in cardiovascular system27,28. Taking into account that HFD has been reported to activate S6K1 in various tissues41,42, it is attempting to speculate that S6K1 may also mediate HFD-induced increase in Arg-II expression in macrophage. Further experiments will be required to verify this hypothesis. Taken together, our in vivo and in vitro experiments demonstrate that Arg-II deficiency protects mice from obesity-linked liver steatosis through suppression of macrophage-mediated hepatic inflammation.
Both mTORC1-S6K1 and AMPK pathways have been implicated in insulin resistance and lipogenesis in the liver in obesity at least in part through regulation of SREBP-1c gene expression and activation4,5,6,7,43. In the vascular cells, we showed a positive crosstalk between Arg-II and mTORC1-S6K1 as well as a negative crosstalk between Arg-II and AMPK pathway27,28,29. In the present study, we observed a higher hepatic AMPK signaling in obese Arg-II−/− mice as compared to the obese WT mice, whereas no difference in hepatic mTORC1-S6K1 signaling between the two genotypes of obese mice was detected. Taking into account that AMPK inhibits mTORC1-S6K1 signaling pathway44, the fact that elevated AMPK signaling in Arg-II−/− liver is not accompanied by reduced mTORC1-S6K1 signaling suggests that an AMPK-independent mechanism in activating mTORC1-S6K1 is dominant. Moreover, these results also suggest that elevated AMPK suppresses hepatic lipogenesis and ultimately liver steatosis through mTORC1-S6K1-independent mechanism(s) in obese Arg-II−/− mice. Indeed, AMPK has been shown to suppress SREBP-1c expression and activity by directly phosphorylating SREBP-1c-S3726,7,45. Since Arg-II is not detectable in the liver of WT mice fed either NC or HFD, the difference in hepatic AMPK signaling between WT and Arg-II−/− does not result from the crosstalk between Arg-II and AMPK as observed in vascular cells29. Adiponectin, an important adipocyte-derived factor that has inhibitory effects on insulin resistance, hepatic steatosis and inflammation46, is a well-known endogenous AMPK activator47. However, a role of adiponectin in activation of AMPK in the liver of obese Arg-II−/− mice can be excluded, since no differences in circulating or hepatic levels of adiponectin were evident between obese WT and Arg-II−/− mice, although adiponectin level was significantly higher in adipose tissue of Arg-II−/− mice than the WT controls. Since there is no difference in epididymal fat weight between the obese Arg-II−/− and WT mice, this suggests that the discrepancy of the difference in adiponectin levels in adipose tissue and plasma between the two genotypes is not a consequence of a decrease in fat mass in Arg-II−/− mice. It rather indicates an increased local paracrine/autocrine but not endocrine secretion of adiponectin from adipose tissue into the circulation in Arg-II−/− mice. The fact that suppressed macrophage-mediated hepatic inflammation accounts for the reduced lipogenesis and liver steatosis in Arg-II−/− mice prompted us to hypothesize that the enhanced AMPK in hepatocytes is attributable to the reduced hepatic inflammation. The hypothesis is confirmed by our in vitro experiments showing that hepatocytes treated with the Arg-II−/−-BMM-CM exhibits higher AMPK activation, lower mRNA levels of SREBP-1c and SCD-1 as compared to the cells treated WT-BMM-CM, which could be further improved by neutralizing antibodies against IL-6 and TNF-α.
There is an increase in serum liver enzymes ALT and AST in Arg-II−/− mice fed HFD, but this increase is actually smaller than those from WT-HFD group, although it does not reach statistical significance. Together with the results of hepatic steatosis and inflammation, these results demonstrate that Arg-II deficiency reduces but does not abolish liver injury in HFD-induced obesity. Extensive studies have shown that inhibition of SCD-1 in the context of the overflow of free fatty acids coming to the liver on HFD feeding causes the build-up of toxic lipids resulting in increased hepatocyte cell death and liver injury even there is decreased triglyceride deposition32,33. However, we show here that significantly reduced SCD-1 in the liver of obese Arg-II−/− mice as compared to that of obese WT mice does not lead to an accelerated liver injury while suppressing hepatic triglyceride synthesis, suggesting that the decreased hepatic SCD-1 is not necessarily accompanied by an exacerbation of liver damage. It remains elusive what is the mechanism that prevents hepatocyte damage from a decreased hepatic SCD-1 level under certain conditions such as in Arg-II−/− mice.
During preparation of this manuscript, two studies on the role of arginase in obesity-associated liver steatosis with opposite conclusions were reported30,31. In the study of Moon et al.30, using arginase inhibitor nor-NOHA that is an isoform non-specific inhibitor, the authors showed some similar findings to the ones we obtained with Arg-II−/− mice including reduced liver steatosis and liver triglyceride content, reduced expression of SCD-1 as well as elevated AMPK signaling. By demonstrating the direct inhibitory effect of nor-NOHA on oleic acid-induced triglyceride synthesis with concurrent increase in NO production and AMPK signaling in HepG2 hepatocytes in vitro, they conclude that arginase inhibition reduces obesity-induced hepatic lipid abnormality most likely attributable to the inhibition of Arg-I. However, this mechanism unlikely accounts for the beneficial effect of Arg-II deficiency in obesity-induced liver steatosis in our study, since Arg-II is not expressed in hepatocytes and no change in Arg-I level or total arginase activity in liver of Arg-II−/− mice was observed. In contrast to this study and also to our present study, Navarro et al. using Arg-II−/− mice, reported an increased macrophage inflammation and liver steatosis accompanied by an increase in de novo hepatic lipogenesis in Arg-II−/− mice on HFD feeding. Although reasons of the discrepancy between the studies are not clear, yet, the following points could be taken into consideration. In the study by Navarro et al. all the control C57BL/6J mice were purchased from Jackson laboratory, whereas the Arg-II−/− mice were obtained from another laboratory. In our study, we used offspring of WT/WT and Arg-II−/−/Arg-II−/− derived from heterozygous breeding. Evidence has been presented that multiple substrains will be created within an inbred strain that became increasingly more genetically divergent through generations of independent inbreeding48. Although Arg-II−/− mice have been backcrossed to C57BL/6J mice over 10 generations, which is considered as congenic to C57BL/6J, the independent breeding of the mice may lead to creation of a substrain that became genetically divergent from C57BL/6J mice purchased from Jackson Laboratory. It could thus not be excluded that the difference in macrophage inflammation associated with liver steatosis phenotype observed in their study is consequence of other genetic differences. Moreover, the numbers of animals used were not indicated throughout their report. If the animal number used in the study is too small, the results could be biased. In addition, Arg-II is highly expressed in the liver of WT type mice in their report, which is in contrast to studies published by other groups including our present study22,38. It is puzzling that Arg-II is even detectable in the Arg-II−/− liver, although it is at lower levels as compared to the WT mice. This raises concerns about the genetic modified mouse model used in their study. Nevertheless, despite very few reported studies on the role of Arg-II in macrophage inflammatory responses, our work showing the pro-inflammatory role of Arg-II in macrophages is in accordance with several other studies25,39,40. Finally, our previous studies also provide convincing evidence showing a protective and healthier metabolic and aging phenotype of Arg-II−/− mice on HFD or high cholesterol diet26,27,28,29.
In summary, our present study demonstrates that targeting Arg-II protects mice from HFD-induced liver steatosis through suppression of macrophage inflammation and suppression of the release of TNF-α and IL-6, which leads to improved AMPK activation, resulting in inhibition of SREBP-1c and ultimately the suppression of lipogenic enzymes and inhibition of hepatic lipogenesis (Fig. 8D). Our findings implicate that targeting Arg-II is beneficial for obesity-associated NAFLD.
Methods
Materials
All chemicals including those used for immunoblotting and anti-tubulin (T5168) were obtained from Sigma (Buchs, Switzerland); anti-phospho-AMPK (Thr-172) (2535S), anti-AMPKα (2793S), and anti-SCD-1 (2438S) were from Cell Signaling (Allschwil, Switzerland); anti-Arg-II (sc-20151) and anti-CLEC4F (SC-242467) were from Santa Cruz (Nunningen, Switzerland). Anti-F4/80 (MCA497G) was from AbD Serotec (Dusseldorf, Germany). Bio-Rad DCTM protein assay kit was from Bio-Rad (Basel, Switzerland); Alexa Fluor680-conjugated anti-mouse IgG (A21057) was from Molecular Probes ⁄ Invitrogen (Lucerne, Switzerland); IRDye800-conjugated anti-rabbit IgG (926-32211) were from LI-COR Biosciences (Bad Homburg, Germany). Neutralizing rat anti-mouse-IL-6 (BMS178) and control rat IgG1 (16-4301-85) were from eBioscience (Vienna, Austria). Neutralizing anti-TNF-α (AB-410-NA) was from R&D systems (Minneapolis, USA). All reagents for the activity assay of lipogenic enzymes including ME, G6PDH and FAS were kindly provided by Jean-Francois Cajot (University of Fribourg, Switzerland).
Animals
The breeding and genotyping of wild type (WT) and Arg-II-deficient mice (Arg-II−⁄−) were as previously described26,49. Starting at the age of 7 weeks, the male WT and Arg-II−/− mice were given free access for 14 weeks to a HFD (energy content: 55% fat, 21% protein, and 24% carbohydrate, Harlan Teklad TD 93075) and maintained on a 12-h light-dark cycle and tap water. After 14 weeks of HFD, the animals were sacrificed after intraperitoneal anesthesia with xylazine (10 mg kg−1body weight) and ketamine (100 mg kg−1 body weight). Blood and liver tissues were then collected for histological analysis or snap-frozen in liquid nitrogen and kept at −80 °C until processed. Animal work was approved by the Ethical Committee of Veterinary Office of Fribourg (number 174/07), Switzerland and was performed in compliance with guidelines on animal experimentation at our institution.
Cell Culture
The mouse AML12 hepatocytes cell line from American Type Culture Collection were maintained in DMEM/F-12 Ham medium supplemented with 10% fetal bovine serum (FBS) and human insulin- transferrin- sodium selenite (ITS) media supplement. Treatments were carried out after 6 hours starvation in 0.2% bovine serum albumin (BSA)-ITS-DMEM-F-12 Ham.
Bone marrow-derived macrophages (BMM) were prepared from bone marrow-derived cells by incubation with 10% HIFBS-RPMI, supplemented with 20% of L929-conditioned medium containing macrophage colony–stimulating factor, for 6 to 7 days to induce differentiation as described previously26. After 6 hours starvation in 0.2% BSA–RPMI, cells were either untreated or treated with LPS (100 ng/ml, 8 h). The conditional medium (CM) was then collected for further study.
Enzyme-Linked Immunosorbent Assay (ELISA)
The plasma adiponectin levels was evaluated according to the manufacturer’s instruction with the Adiponectin ELISA Kit (Assaypro LLC, Saint Charles, Missouri, USA), the IL-6 and TNF-α level in BMM-CM were with the ELISA MAXTM Deluxe kit from BioLegend (Lucerna Chem AG, Luzern, Switzerland).
Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction Analysis (qRT-PCR)
Total RNA was extracted from tissues with Trizol Reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) following the supplier’s protocol. The mRNA expression was evaluated by two-step qRT-PCR analysis as described previously26. The mRNA expression levels of all genes were normalized to the reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primer sequences are as follows:
mIL-6F: GACAACCACGGCCTTCCCTA ;
mIL-6R: GCCTCCGACTTGTGAAGTGGT.
mTNF-αF: GGCAGGTCTACTTTGGAGTCATTGC;
mTNF-αR: ACATTCGAGGCTCCAGTGAATTCGG.
mMCP-1F: AGCACCAGCCAACTCTCAC;
mMCP-1R: TCTGGACCCATTCCTTCTTG.
mAdiponectinF: TGTTCCTCTTAATCCTGCCCA;
mAdiponectinR: CCAACCTGCACAAGTTCCCTT.
mGAPDHF: ACCCAGAAGACTGTGGATGG;
mGAPDHR: ACACATTGGGGGTAGGAACA.
mSREBP-1cF: AAGCAAATCACTGAAGGACCTGG
mSREBP-1cR: AAAGACAAGGGGCTACTCTGGGAG
mSCD-1F: TTCTTACACGACCACCACCA
mSCD-1R: GCAGGAGGGAACCAGTATGA
Interaction between BMM and Hepatocytes in vitro
After 6 h starvation, AML12 hepatocytes were changed to BMM-CM and incubated in presence of oleic acid (OA, 20 mmol/L) for 24 h till the extraction.
For antibody blocking studies, the BMM-CM was incubated with control IgG or neutralizing anti-IL-6 (18 μg/ml), anti-TNF-α (0.4 μg/ml) or combination of anti-IL-6 and anti-TNF-α for 2h prior to the addition to the AML12 cells as described above.
Measurement of Triglyceride Levels in Cultured Hepatocytes and in Liver Tissues
The lipid extraction and triglyceride measurement in cultured hepatocytes were carried out with the triglyceride quantification kit (BioVision, Mountain View, CA) according to the manufacturer’s instructions. Measurement of liver triglyceride was performed as previously described50. Briefly, Livers were homogenized and the hepatic triglyceride was extracted by digesting 100–300 mg liver tissues overnight at 55 °C in 350 μl ethanolic potassium hydroxide (2:1 v/v of 100% ethanol and 30% potassium hydroxide). Samples were neutralized with water:ethanol (1:1 v/v; total volume of 1 ml). After centrifugation (8000 g, 5 min), 1 ml of supernatant was collected and brought to 1.2 ml volume with water:ethanol (1:1). Then, 200 μl was removed and proceeded to saponification with 215 μl of 1 mol/L MgCl2 by vortexing and incubating on ice for 10 min. Saponified liver extracts (the upper phase) were separated by centrifugation and kept frozen at −80 °C. After thawing the samples, triglyceride quantity was determined by an enzymatic colorimetric assay (TRIGL:ACN 781) on Roche/Hitachi cobas c system (Roche, Switzerland). The color intensity of the red dye stuff formed was proportional to the triglyceride concentration (mmol/L) and was determined at 505 nm.
Lipogenic Enzyme Activity Assay
To assess the activities of the lipogenic enzymes including ME, G6PDH and FAS in liver, liver tissues were homogenized in extraction buffer consisting of 250 mmol/L sucrose, 50 mmol/L Tris/HCl pH 7.3 and 30 mmol/L mercaptoethanol, and centrifuged at 13000 rpm for 10 min at 4 °C. Protein concentrations of the supernatants were determined by Bradford assay solution from Applichem (A6932, 0500).
The activity of ME and G6PDH was determined spectrophotometrically by measuring the increase in absorbance at 340 nm resulted from the reduction of NADP as previously described51,52, whereas FAS activity was determined spectrophotometrically by measuring the decrease in absorbance at 340 nm resulted from the oxidation of NADPH as described previously53. All the enzyme activity assays were run at 37 °C in duplicate in a prewarmed Bio-Rad (Model 680) microplate Spectrophotometer. Absorbance at 340 nm (37 °C) was measured using software programmed for a kinetic read sequence of 1 min intervals over 10 minutes for ME and G6PDH, every 2 min over 20 minutes for FAS. The enzyme activity was calculated by obtaining the ΔA340 nm/minute with the maximum and the minimum value using 5 data points in the linear range with respect to time and to sample concentration. Data are presented as fold change in the enzyme activity.
Immunoblotting Analysis
Cell lysate preparation, SDS-PAGE, immunoblotting, antibody incubation and signal detection were performed as described previously26. Quantification of the signals was performed using NIH Image 1.62 software.
Hematoxylin and eosin (HE) and Oil Red O Staining
Liver tissues embedded in paraffin or frozen in OCT compounds were cut at 7 μm, then stained with HE or oil red O, respectively, as described previously26,54.
Tissue Immunofluorescence Staining and Confocal Microscopy
Preparation of paraffin-embedded sections (7 μm) and immunostaining were performed as described previously26. The immunofluorescence signals were visualized under LEICA’s DIM6000 Confocal microscope. The fluorescence signals were quantified with NIH IMAGE J and results are presented as the ratio of F4/80, Arg-II, CLEC4F to DAPI-positive nucleus.
Measurement of blood parameters
Measurement of plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were performed by Laboratory HFR, Hospital Fribourgeois. Plasma adiponectin was determined by ELISA.
Statistics
Data are given as mean±SEM. In all experiments, n represents the number of animals. Statistical analysis was performed with unpaired t test or ANOVA with Dunnett or Bonferroni post-test. Differences in mean values were considered significant at p ≤ 0.05.
Additional Information
How to cite this article: Liu, C. et al. Targeting arginase-II protects mice from high-fat-diet-induced hepatic steatosis through suppression of macrophage inflammation. Sci. Rep. 6, 20405; doi: 10.1038/srep20405 (2016).
Supplementary Material
Acknowledgments
The work was supported by the Swiss National Science Foundation (310030_141070/1 and 31003A_159582/1), Swiss Heart Foundation, and National Centre of Competence in Research Program (NCCR-Kidney.CH). Chang Liu was supported by the Chinese Scholarship Council.
Footnotes
Author Contributions C.L. and A.G.R. performed experiments, analyzed data, and prepared the figures. E.R. and F.B. analyzed plasma and liver triglyceride as well as plasma ALT and AST. M.-C.B. reviewed HE staining of liver section. J.-P.M., Z.Y. and X.-F.M. did manuscript editing. Z.Y. and X.-F.M. carried out the project design, wrote the manuscript. C.L., Z.Y. and X.-F.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed the manuscript.
References
- Anderson N. & Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev 60, 311–357 (2008). [DOI] [PubMed] [Google Scholar]
- Targher G. & Byrne C. D. Clinical Review: Nonalcoholic fatty liver disease: a novel cardiometabolic risk factor for type 2 diabetes and its complications. J Clin Endocrinol Metab 98, 483–495 (2013). [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 346, 1221–1231 (2002). [DOI] [PubMed] [Google Scholar]
- Brown M. S. & Goldstein J. L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7, 95–96 (2008). [DOI] [PubMed] [Google Scholar]
- Li S., Brown M. S. & Goldstein J. L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA 107, 3441–3446 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap F., Craddock L. & Yang J. Mechanism of AMPK suppression of LXR-dependent Srebp-1c transcription. Int J Biol Sci 7, 645–650 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13, 376–388 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strable M. S. & Ntambi J. M. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol 45, 199–214 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstmann T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8, 224–236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang M. et al. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem 279, 47898–47905 (2004). [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Alquier T., Carling D. & Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1, 15–25 (2005). [DOI] [PubMed] [Google Scholar]
- Hijona E., Hijona L., Arenas J. I. & Bujanda L. Inflammatory mediators of hepatic steatosis. Mediators Inflamm 2010, 837419 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. & Ming X. F. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front Immunol 5, 533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–1808 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsouris D. et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8, 301–309 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton M. C. et al. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J Inflamm (Lond) 8, 8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 59, 347–357 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard J. I. et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab 7, 496–507 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obstfeld A. E. et al. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59, 916–925 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga H. et al. Characterization of Distinct Subpopulations of Hepatic Macrophages in HFD/Obese Mice. Diabetes 64, 1120–1130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizikes G. J., Grody W. W., Kern R. M. & Cederbaum S. D. Isolation of human liver arginase cDNA and demonstration of nonhomology between the two human arginase genes. Biochem Biophys Res Commun 141, 53–59 (1986). [DOI] [PubMed] [Google Scholar]
- Choi S., Park C., Ahn M., Lee J. H. & Shin T. Immunohistochemical study of arginase 1 and 2 in various tissues of rats. Acta Histochem 114, 487–494 (2012). [DOI] [PubMed] [Google Scholar]
- Crombez E. A. & Cederbaum S. D. Hyperargininemia due to liver arginase deficiency. Mol Genet Metab 84, 243–251 (2005). [DOI] [PubMed] [Google Scholar]
- Gotoh T. et al. Molecular cloning of cDNA for nonhepatic mitochondrial arginase (arginase II) and comparison of its induction with nitric oxide synthase in a murine macrophage-like cell line. FEBS Lett 395, 119–122 (1996). [DOI] [PubMed] [Google Scholar]
- Khallou-Laschet J. et al. Macrophage plasticity in experimental atherosclerosis. PLoS One 5, e8852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X. F. et al. Arginase II Promotes Macrophage Inflammatory Responses Through Mitochondrial Reactive Oxygen Species, Contributing to Insulin Resistance and Atherogenesis. J Am Heart Assoc 1, e000992 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepuri G. et al. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell 11, 1005–1016 (2012). [DOI] [PubMed] [Google Scholar]
- Xiong Y., Yu Y., Montani J. P., Yang Z. & Ming X. F. Arginase-II induces vascular smooth muscle cell senescence and apoptosis through p66Shc and p53 independently of its l-arginine ureahydrolase activity: implications for atherosclerotic plaque vulnerability. J Am Heart Assoc 2, e000096 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y. et al. ARG2 impairs endothelial autophagy through regulation of MTOR and PRKAA/AMPK signaling in advanced atherosclerosis. Autophagy 10, 2223–2238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J., Do H. J., Cho Y. & Shin M. J. Arginase inhibition ameliorates hepatic metabolic abnormalities in obese mice. PLoS One 9, e103048 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L. A. et al. Arginase 2 Deficiency Results in Spontaneous Steatohepatitis: A Novel Link Between Innate Immune Activation and Hepatic De Novo Lipogenesis. J Hepatol 62, 412–420 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K. et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45, 1366–1374 (2007). [DOI] [PubMed] [Google Scholar]
- Li Z. Z., Berk M., McIntyre T. M. & Feldstein A. E. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem 284, 5637–5644 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M. & Rogers C. Q. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 234, 850–859 (2009). [DOI] [PubMed] [Google Scholar]
- Chang J. J. et al. Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J Agric Food Chem 61, 6069–6076 (2013). [DOI] [PubMed] [Google Scholar]
- Horton J. D., Goldstein J. L. & Brown M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 109, 1125–1131 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P. Obesity Notches up fatty liver. Nat Med 19, 969–971 (2013). [DOI] [PubMed] [Google Scholar]
- Yu H. et al. Widespread expression of arginase I in mouse tissues. Biochemical and physiological implications. J Histochem Cytochem 51, 1151–1160 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am J Pathol 175, 891–902 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H., Gao T., Cooper T. K., Morris S. M. Jr. & Awad A. S. Arginase inhibition mediates renal tissue protection in diabetic nephropathy by a nitric oxide synthase 3-dependent mechanism. Kidney Int 84, 1189–1197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um S. H. et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 (2004). [DOI] [PubMed] [Google Scholar]
- Khamzina L., Veilleux A., Bergeron S. & Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146, 1473–1481 (2005). [DOI] [PubMed] [Google Scholar]
- Pende M. et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408, 994–997 (2000). [DOI] [PubMed] [Google Scholar]
- Shaw R. J. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 196, 65–80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108, 1167–1174 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T. et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116, 1784–1792 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8, 1288–1295 (2002). [DOI] [PubMed] [Google Scholar]
- Beck J. A. et al. Genealogies of mouse inbred strains. Nat Genet 24, 23–25 (2000). [DOI] [PubMed] [Google Scholar]
- Shi O., Morris S. M. Jr., Zoghbi H., Porter C. W. & O’Brien W. E. Generation of a mouse model for arginase II deficiency by targeted disruption of the arginase II gene. Mol Cell Biol 21, 811–813 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. D., Jones M. E., Prelle K., Simpson E. R. & Boon W. C. A selective estrogen receptor alpha agonist ameliorates hepatic steatosis in the male aromatase knockout mouse. J Endocrinol 210, 323–334 (2011). [DOI] [PubMed] [Google Scholar]
- Ceddia R. B. et al. Leptin stimulates uncoupling protein-2 mRNA expression and Krebs cycle activity and inhibits lipid synthesis in isolated rat white adipocytes. Eur J Biochem 267, 5952–5958 (2000). [DOI] [PubMed] [Google Scholar]
- Lama J. L., Bell R. A. & Storey K. B. Glucose-6-phosphate dehydrogenase regulation in the hepatopancreas of the anoxia-tolerant marine mollusc, Littorina littorea. PeerJ 1, e21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P. & Denton R. M. Insulin and the regulation of adipose tissue acetyl-coenzyme A carboxylase. Biochem J 132, 509–517 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Zacarias J. L., Castro-Munozledo F. & Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 97, 493–497 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.