Abstract
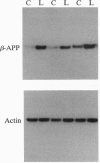
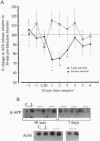
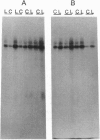
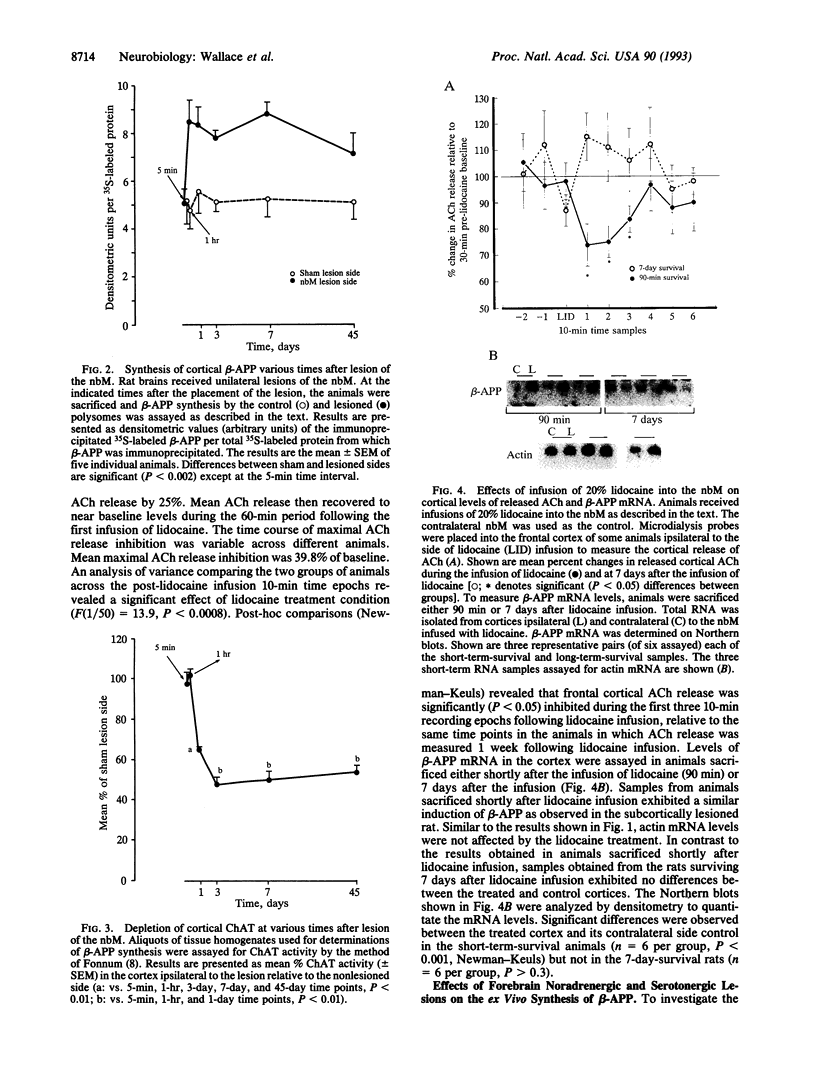
Lesions of the cholinergic nucleus basalis of Meynert elevate the ex vivo synthesis of beta amyloid precursor protein (beta-APP) in the cerebral cortex, a major projection region. We have found that this elevation is reflected by increased levels of beta-APP mRNA. The induction is rapid (occurring 60 min after placement of the lesion) and persistent (remaining for at least 45 days after lesioning). Two other subcortical lesions, which result in reductions of cortical adrenergic and serotonergic innervation, similarly induced cortical beta-APP. The beta-APP induction is reversible and does not require loss of the subcortical neurons. Infusion of lidocaine, a calcium antagonist that disrupts neurotransmitter release, into the nucleus basalis of Meynert leads to the temporary reduction of released acetylcholine in the cortex. In this model, beta-APP mRNA levels are elevated shortly after the infusion of lidocaine (90 min) but return to preinfusion levels 7 days after the lidocaine treatment. However, metabolic stresses of the brain, including chronic physostigmine, glucocorticoid, and diabetogenic treatments, fail to induce the beta-APP response. These results suggest that the induction of beta-APP is a specific response to the loss of functional innervation in the cortex. Importantly, these studies show that cortical beta-APP is induced by lesions that mimic the neurochemical deficits most frequently observed in Alzheimer disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. P., Refolo L. M., Wallace W., Mehta P., Krishnamurthi M., Gotlib J., Bierer L., Haroutunian V., Perl D., Robakis N. K. Differential brain expression of the Alzheimer's amyloid precursor protein. EMBO J. 1989 Dec 1;8(12):3627–3632. doi: 10.1002/j.1460-2075.1989.tb08536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coirini H., Magariños A. M., De Nicola A. F., Rainbow T. C., McEwen B. S. Further studies of brain aldosterone binding sites employing new mineralocorticoid and glucocorticoid receptor markers in vitro. Brain Res. 1985 Dec 30;361(1-2):212–216. doi: 10.1016/0006-8993(85)91291-0. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Haroutunian V., Kanof P. D., Tsuboyama G., Davis K. L. Restoration of cholinomimetic activity by clonidine in cholinergic plus noradrenergic lesioned rats. Brain Res. 1990 Jan 22;507(2):261–266. doi: 10.1016/0006-8993(90)90280-o. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Brain glucose and energy metabolism during normal aging. Aging (Milano) 1990 Sep;2(3):245–258. doi: 10.1007/BF03323925. [DOI] [PubMed] [Google Scholar]
- Johnston M. V., McKinney M., Coyle J. T. Neocortical cholinergic innervation: a description of extrinsic and intrinsic components in the rat. Exp Brain Res. 1981;43(2):159–172. doi: 10.1007/BF00237760. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Wallace W. C., Bragin V., Robakis N. K., Sambamurti K., VanderPutten D., Merril C. R., Davis K. L., Santucci A. C., Haroutunian V. Increased biosynthesis of Alzheimer amyloid precursor protein in the cerebral cortex of rats with lesions of the nucleus basalis of Meynert. Brain Res Mol Brain Res. 1991 May;10(2):173–178. doi: 10.1016/0169-328x(91)90108-a. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., Delon M. R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982 Mar 5;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Woodruff G. N., Foster A. C., Gill R., Kemp J. A., Wong E. H., Iversen L. L. The interaction between MK-801 and receptors for N-methyl-D-aspartate: functional consequences. Neuropharmacology. 1987 Jul;26(7B):903–909. doi: 10.1016/0028-3908(87)90068-2. [DOI] [PubMed] [Google Scholar]
- el-Defrawy S. R., Boegman R. J., Jhamandas K., Beninger R. J., Shipton L. Lack of recovery of cortical cholinergic function following quinolinic or ibotenic acid injections into the nucleus basalis magnocellularis in rats. Exp Neurol. 1986 Mar;91(3):628–633. doi: 10.1016/0014-4886(86)90058-0. [DOI] [PubMed] [Google Scholar]