Abstract
Nicotine may link maternal cigarette smoking with respiratory dysfunctions in sudden infant death syndrome (SIDS). Prenatal–perinatal nicotine exposure blunts ventilatory responses to hypercapnia and reduces central respiratory chemoreception in mouse neonates at Postnatal Days 0 (P0) to P3. This suggests that raphe neurons, which are altered in SIDS and contribute to central respiratory chemoreception, may be affected by nicotine. We therefore investigated whether prenatal–perinatal nicotine exposure affects the activity, electrical properties, and chemosensitivity of raphe obscurus (ROb) neurons in mouse neonates. Osmotic minipumps, implanted subcutaneously in 5- to 7-day-pregnant CF1 mice, delivered nicotine bitartrate (60 mg kg−1 d−1) or saline (control) for up to 28 days. In neonates, ventilation was recorded by head-out plethysmography, c-Fos (neuronal activity marker), or serotonin autoreceptors (5HT1AR) were immunodetected using light microscopy, and patch-clamp recordings were made from raphe neurons in brainstem slices under normocarbia and hypercarbia. Prenatal–perinatal nicotine exposure decreased the hypercarbia-induced ventilatory responses at P1–P5, reduced both the number of c-Fos–positive ROb neurons during eucapnic normoxia at P1–P3 and their hypercapnia-induced recruitment at P3, increased 5HT1AR immunolabeling of ROb neurons at P3–P5, and reduced the spontaneous firing frequency of ROb neurons at P3 without affecting their CO2 sensitivity or their passive and active electrical properties. These findings reveal that prenatal–perinatal nicotine reduces the activity of neonatal ROb neurons, likely as a consequence of increased expression of 5HT1ARs. This hypoactivity may change the functional state of the respiratory neural network leading to breathing vulnerability and chemosensory failure as seen in SIDS.
Keywords: perinatal nicotine exposure, sudden infant death syndrome, serotonin, serotonin autoreceptors, central chemoreception
Clinical Relevance
Prenatal nicotine may link maternal cigarette smoking with sudden infant death syndrome (SIDS). SIDS has been related to respiratory dysfunctions and abnormalities of raphe neurons, which contribute to respiratory central chemoreception. However, prenatal nicotine effects on raphe neuron properties have not been determined. This is the first report showing that prenatal nicotine exposure in mice reduces the firing discharge of serotonergic and nonserotonergic raphe neurons, preserving their electrical and chemosensitive properties and increasing their expression of 5-HT1A autoreceptors. Hypoactivity of raphe neurons can lead to breathing vulnerability and failure in responding to chemosensory demands, as seen in SIDS. However, these results also suggest that nicotine is not the unique and single factor responsible for anatomical findings found in SIDS.
Sudden infant death syndrome (SIDS) persists as a major cause of death in infants under 1 year of age in developed countries (1), whereas maternal cigarette smoking remains its main preventable risk factor (2). Certainly, infants born from smoking mothers and infants who have died from SIDS shared a higher incidence of respiratory-related deficits in arousability, chemoreflexes (3–5), and respiratory rhythm regularity (6). That respiratory dysfunction is involved in SIDS is reinforced by anatomical findings of abnormalities in respiratory-related brainstem nuclei (7).
Raphe neurons are chemosensitive (8–10), contribute to ventilatory drive (11–13), and are involved in promotion of waking (14). They are altered in SIDS (7, 15, 16), and suggestively, most deaths in SIDS occur during the transition between sleep and the awake state (17). In SIDS cases, medullary raphe neurons are more numerous and immature, and those that are serotonergic have lower serotonin transporter binding density than control subjects (7, 16). In addition, the raphe obscurus (ROb) and the arcuate nuclei show lower serotonin autoreceptor (5-HT1AR) binding densities (7, 16).
Nicotine may link maternal cigarette smoking with SIDS (18). In fact, prenatal–perinatal nicotine exposure causes hypoventilation (19, 20), increased apnea periods during normoxia in mice and rats (21), reduction of hypercarbia- and hypoxia-induced ventilatory chemoreflexes in mice (19), rats (20, 22), and sleeping lambs (23), reduction of autoresuscitation from primary apnea in rats (24), and delays in the hypoxia-induced awakening response in lambs (23). In the serotonergic system, prenatal nicotine increases serotonin 5-HT1AR binding in the ROb of baboon fetuses (25) as perinatal tobacco exposure does in the cerebral cortex of rhesus monkeys (26). Prenatal nicotine elevates both concentration and turnover of serotonin in the brainstem of rhesus monkey fetuses (27). In rats, prenatal nicotine reduces serotonin transporter binding in the cortex, whereas, in the midbrain and brainstem, it can increase it (28, 29).
Blunting of ventilatory chemoreflexes in nicotine-exposed mice is due, in part, to a failure in central chemoreception (19, 30). We hypothesized that, in mouse neonates, raphe neurons, which express functional nicotinic acetylcholine receptors (31, 32), could be targeted by prenatal–perinatal nicotine exposure, affecting their activities, their electrical properties, and their chemosensitivities. We studied CF1 neonates at Postnatal Days 1 (P1) to P8, because it is known that, within this period, prenatal cigarette smoke disrupts eupneic breathing and the ventilatory response to hypoxia in rats (33), whereas prenatal–perinatal nicotine exposure impairs ventilatory responses to hypercapnia and depresses central respiratory chemoreception in mice (19, 30). Therefore, we expect that the diminished hypercarbia-induced ventilatory responses that occur in nicotine-exposed mice at P1–P3 will be accompanied at the same time by changes in the electrical properties or CO2 responsiveness of raphe neurons, including both the serotonergic and nonserotonergic populations.
Materials and Methods
See the online supplement for additional information.
Animals and Prenatal–Perinatal Nicotine Administration
An osmotic minipump (2004; Alzet, Cupertino, CA), which was implanted subcutaneously between the scapulae into 5- to 7-day-pregnant adult mice (19), delivered saline (controls) or nicotine bitartrate (60 mg kg−1 d−1) for 28 days. A similar dose was used before (19, 34), and the reasons for choosing this dose are explained in previous work (34) and in the online supplement. This nicotine infusion is nontoxic, and does not affect the litter size, birth weight, or postnatal growth curve of mouse neonates (19, 21). After finishing the experiments, animals were killed with an anesthetic overdose.
Ventilatory Recordings
Tidal volume (Vt), instantaneous respiratory frequency (fR), and minute ventilation (e), calculated as Vt × fR, were measured in P1, P3, P5, and P8 control and nicotine-exposed neonates (n = 11–19 per group) using head-out, restricted-body, temperature-controlled plethysmography, as described previously (19, 21) and in the online supplement.
In Vivo Hypercapnic Stimulation
Neonates placed in the plethysmography chamber breathed humidified air spontaneously for 110 minutes (basal) or were stimulated, after 10 minutes of breathing air, with 10 minutes of inhalation of air enriched with 10% CO2 (21% O2, balance N2), and then maintained for 90 minutes breathing air before being killed for c-Fos immunohistochemistry.
Immunohistochemistry
Serotonergic neurons were recognized by detecting tryptophan hydroxylase (TpOH), the rate-limiting enzyme for the synthesis of serotonin, using polyclonal IgG sheep anti-TpOH antibody (Sigma, St. Louis, MO); as a marker of neuronal activity, c-Fos protein was detected with a monoclonal IgG rabbit anti–c-Fos antibody (Calbiochem, San Diego, CA), and 5-HT1A receptors were detected with polyclonal IgG rabbit anti-5HT1AR antibody (Santa Cruz Biotechnology, Santa Cruz, CA). For detailed methods, please see the online supplement.
Whole Cell Patch-Clamp Recording
Transverse brainstem slices from 3-day-old mice were superfused at 1–2 ml/min with artificial cerebrospinal fluid gassed with 95% O2–5% CO2, pH 7.4, at 22°C. Borosilicate glass electrodes (4–10 MΩ) were filled with solution containing 12 mM gluconic acid, 120 mM KOH, 10 mM KCl, 10 mM HEPES, 3 mM MgCl2, and 0.5 mM Na2-ATP, pH 7.4 (300 mOsm), and were positioned with near-infrared differential interference contrast optics (model E600FN; Nikon, Tokyo, Japan). Whole-cell voltage-clamp mode recordings were made with the Axoclamp 1D amplifier at a holding potential of −70 mV controlled by pClamp 5 software (Axon Instruments, Union City, CA). Recorded neurons were stained with Lucifer yellow for further measurement of their areas, as indicated in the online supplement.
Hypercapnic stimulation was done by increasing CO2 in the superfusion medium from 5 to 10% in the presence of bicuculline (20 μm) and kynurenic acid (1.5 mM) to block both ionotropic GABAergic and glutamatergic receptors.
Statistical Analysis
Values are expressed as mean (±SEM) unless otherwise stated. Differences for the results of immunohistochemistry and plethysmography were ascertained with two-way ANOVA followed by Newman-Keuls post hoc test. Electrophysiological data were analyzed using Mann-Whitney U test. Differences in distributions of somata size and firing rate of action potentials were assayed with the Kolgomorov-Smirnov two-sample test. The null hypothesis was rejected at P less than 0.05.
Results
Weight and Growth Curve of Neonates
We confirmed that prenatal nicotine administration did not affect the weight of newborns or the growth curve of neonates. P1, P3, P5, and P8 control neonates weighed 2.0 ± 0.3 g (mean ± SD; n = 92), 3.1 ± 0.4 g (n = 90), 4.9 ± 0.7 g (n = 59), and 7.9 ± 1.0 g (n = 49), respectively, whereas P1, P3, P5, and P8 nicotine-exposed neonates weighed 2.0 ± 0.2 g (n = 61), 2.9 ± 0.4 g (n = 57), 5.3 ± 0.6 g (n = 45), and 8.0 ± 0.7 g (n = 44), respectively. Two-way ANOVA showed no significant nicotine effect (df = 1, F ratio = 2.164, P = 0.1419), but a significant age effect (df = 3, F ratio = 6.01, P = 0.0005). Other variables, such as the number of neonates per litter, the weight of dams, and their food intake, were not affected (data not shown).
Morphological Effects of Prenatal–Perinatal Nicotine upon Raphe Neurons
We restricted our observations to the ROb, as this midline medullary nucleus plays a major role in modulating the respiratory rhythm (35, 36). In ROb, serotonergic (TpOH+) neurons are distributed around the mid-sagittal plane in medullary coronal sections, as illustrated in Figure 1A.
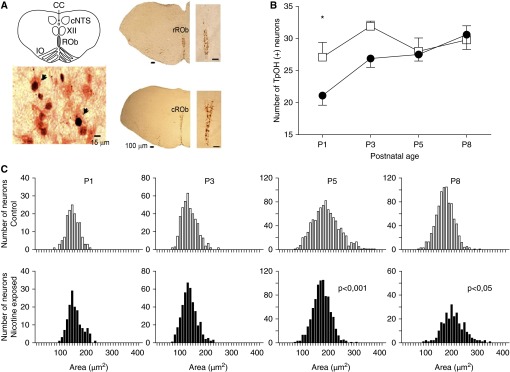
Figure 1.
Prenatal–perinatal nicotine reduces the number of serotonergic neurons at Postnatal Day 1 (P1) and alters distribution of cross-sectional areas of the somata of raphe obscurus (ROb) serotonergic neurons at P5 and P8. (A) Coronal sections of the brainstem represented in the schematic diagram containing the landmarks used to identify and standardize the level of the brainstem in which ROb neurons were studied. CC, central canal; cNTS, caudal part of the nucleus tractus solitarius; IO, inferior olive; XII, hypoglossal nucleus. Rostrally, ROb neurons are distributed equally on each side of the midsagittal plane (rROb), whereas, caudally, they are distributed in the shape of a “Y” (cROb); scale bars, 15 μm and 100 μm, as indicated. In the lower left, there is enlargement of the ROb showing c-Fos–positive (dark nucleus) and tryptophan hydroxylase (TpOH)–positive neurons (red cytoplasm). Arrowheads indicate examples of TpOH-positive and c-Fos–positive neurons. (B) Number of serotonergic ROb neurons in P1, P3, P5, and P8 control (open squares) and nicotine-exposed (solid circles) neonates (n = 10 for each group at any postnatal age). Prenatal–perinatal nicotine decreased the number of serotonergic neurons in ROb (df = 1, F ratio = 5.85, P = 0.0195); *P < 0.05 for difference between control and nicotine-exposed neonates (Newman-Keuls post hoc test). (C) Distribution of cross-sectional areas of cell bodies of serotonergic neurons in control (upper panels, open bars) and nicotine-exposed (lower panels, solid bars) P1–P8 neonates; comparison of distributions of somata areas at the same age for control and nicotine-exposed neonates was done using Kolmogorov-Smirnov test; P value is indicated when significant.
Prenatal–perinatal nicotine decreased the number of serotonergic neurons in ROb at P1 (Figure 1B; P < 0.05, post hoc Newman-Keuls test), and also modified the distribution of cross-sectional areas of the somata of ROb serotonergic neurons at P5 and P8 (Figure 1C). In P1–P3 control neonates, the cross-sectional areas ranged from 100 to 225 μm2, whereas, at P5, a subpopulation of neurons with bigger somata emerged (225–300 μm2). In control P8 neonates, the fraction of neurons having somata areas over 250 μm2 became very small. In P1–P3 nicotine-exposed neonates, the distributions of cross-sectional areas were similar to those in controls. Interestingly, in P5 nicotine-exposed pups, the subpopulation of serotonergic neurons with the largest somata was underrepresented compared with controls (Figure 1C; P < 0.001, Kolgomorov-Smirnov two-sample test). By contrast, at P8, many ROb neurons with the largest cross-sectional areas appeared in the nicotine-exposed mice (Figure 1C; P < 0.05, Kolgomorov-Smirnov two-sample test).
Ventilatory Activity of P1–P8 Neonates under Basal Conditions
Ventilatory activity during eucapnic normoxia showed periods of regular respiratory cycles interrupted by either variable-length apnea periods in P1–P3 neonates or movement artifacts in P1–P8 neonates (Figure 2A). Ventilatory variables under eucapnic normoxia are shown in Table 1. Two-way ANOVA revealed a significant age effect on Vt (df = 2, F ratio = 36.9, P < 0.0001), fR (df = 2, F ratio = 11.5, P < 0.0001), and e (df = 2, F ratio = 89.1, P < 0.0001). No nicotine effects were observed on Vt (df = 2, F ratio = 0.8, P = 0.35), fR (df = 2, F ratio = 0.1, P = 0.71), or e (df = 2, F ratio = 2.6, P = 0.10). Differences in neither the number or duration of apnea periods were found between control and nicotine-exposed mice at P1 and P3 (data not shown).
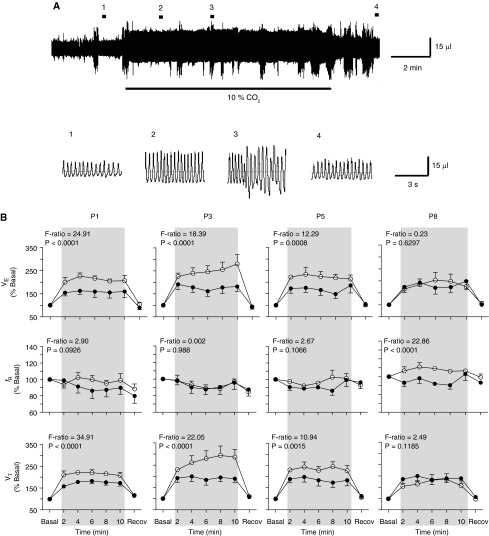
Figure 2.
Prenatal–perinatal nicotine reduces ventilatory responses to hypercapnia in neonates younger than P8. (A) Plethysmographic recording before (1), during (2 and 3), and after (4) inhalation of air enriched with 10% CO2 (horizontal black bars) by a P3 control neonate. Lower traces, recordings displayed at faster speed at the places indicated with numbers on the plethysmographic recording. Note that in 3, movement artifacts are overlapped on the ventilatory recording. (B) Time course of the changes in minute ventilation (e; upper panels), instantaneous respiratory frequency (fR; middle panels), and tidal volume (Vt; lower panels) during hypercapnia (inhalation of 10% CO2 for 10 min) in control (open circle) and nicotine-exposed (solid circles) P1, P3, P5, and P8 neonates. Ventilatory variables are expressed as percentage of respective basal value (% basal). Period of hypercapnia is indicated by gray shading. Basal and recovery values were measured 2 minutes before and 2 minutes after the hypercapnic stimulus, respectively. Data are expressed as mean (±SEM). Number of P1, P3, P5, and P8 neonates were 6, 6, 5, and 7 (controls) and 6, 7, 7, and 6 (nicotine-exposed neonates), respectively. Two-way ANOVA revealed significant nicotine effects upon Vt and e at P1, P3, and P5, but not at P8; F ratios and P values are indicated for each postnatal age.
Table 1.
Ventilatory Variables in Control and Nicotine-Exposed Postnatal Days 1–8 Mouse Neonates during Eucapnic Normoxia
| |
|
Vt |
fR |
e |
|---|---|---|---|---|
| n | (μl) | (Cycles min−1) | (ml/min) | |
| Control | ||||
| P1 | 16 | 14.1 ± 1.3 | 136.9 ± 7.6 | 1.8 ± 0.1* |
| P3 | 19 | 22.6 ± 1.7 | 148.4 ± 9.3 | 3.1 ± 0.1 |
| P8 | 13 | 42.7 ± 5.3 | 173.8 ± 11.5 | 6.8 ± 0.5 |
| Nicotine exposed | ||||
| P1 | 16 | 12.4 ± 1.7 | 113.7 ± 5.3 | 1.3 ± 0.1* |
| P3 | 12 | 20.3 ± 2.3 | 168.7 ± 10.6 | 3.2 ± 0.2 |
| P8 | 11 | 39.4 ± 7.0 | 168.0 ± 13.8 | 5.8 ± 0.7 |
Definition of abbreviations: fR, instantaneous respiratory frequency; P, Postnatal Day; e, minute volume; Vt, tidal volume.
Two-way ANOVA revealed significant aging effect on Vt, fR, and e (P < 0.0001). Mean ± SEM.
P = 0.041, ANOVA.
Prenatal–Perinatal Nicotine Exposure Reduces Ventilatory Responses to Hypercapnia
Control and nicotine-exposed P1–P8 neonates rapidly increased Vt and e as early as from the third and fourth cycles after starting to breath air enriched with 10% CO2 (Figures 2A and 2B). e and Vt remained high during the 10 minutes of hypercapnic stimulus (Figure 2A), and then decreased once the hypercapnia ended, eventually reaching baseline values within 5–10 minutes. The time course of the ventilatory changes induced by 10 minutes of hypercapnia, expressed as a percentage of baseline values, is illustrated in Figure 2B. The most pronounced increase in Vt and e relative to baseline values was observed in control P3 pups. By contrast, hypercapnia did not induce any significant change in fR at any age. This means that the time course profile for changes in e was followed closely only by Vt changes induced by hypercapnia. As reported previously (19), two-way ANOVA revealed a significant nicotine effect on the response of Vt and e to hypercapnia at P1, P3, and P5, but not at P8 (Figure 2B). By contrast, at P8, changes in e and Vt induced by hypercapnia became indistinguishable between nicotine-exposed and control pups.
Nicotine Reduced the Number of Serotonergic and Nonserotonergic c-Fos–Positive ROb Neurons in Normoxic Eucapnia
In nicotine-exposed P1–P3 pups, the level of activity of serotonergic and nonserotonergic ROb neurons, evaluated through c-Fos immunodetection, was decreased under normoxic eucapnia (Figure 3A). By contrast, the number of c-Fos–positive serotonergic and nonserotonergic neurons was not different in control and nicotine-exposed P5 and P8 neonates (data not shown).
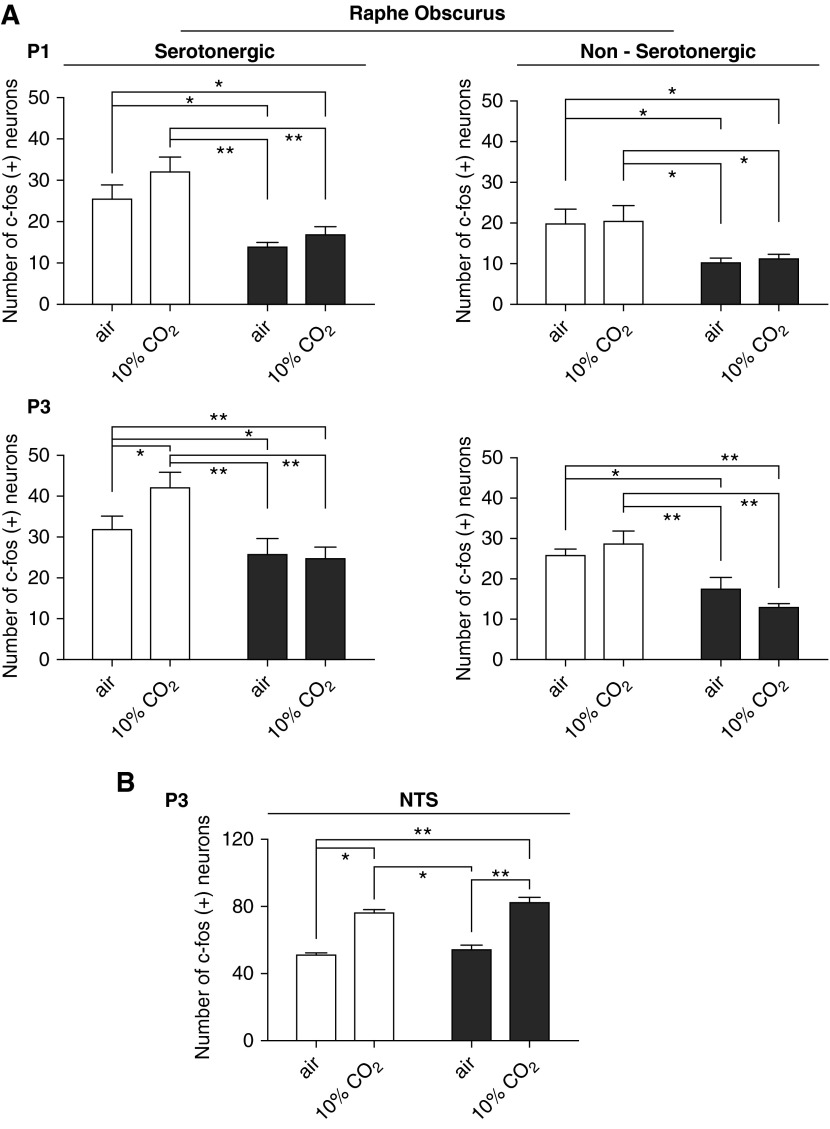
Figure 3.
Prenatal–perinatal nicotine decreases c-Fos activation of ROb neurons in vivo during eucapnia and hypercapnia. (A) Counting of active (c-Fos–positive) neurons in ROb 1.5 hours after inhalation of air alone (basal conditions) or air enriched with 10% CO2 (hypercapnic stimulation) for 10 minutes in control and nicotine exposed P1 and P3 neonates. Results were similar for serotonergic and nonserotonergic ROb neurons. Controls (open bars) and nicotine-exposed (solid bars) neonates inhaling air or 10% CO2. Two-way ANOVA revealed a significant nicotine effect on the counting of c-Fos–positive serotonergic (df = 1, F ratio = 29.630, P < 0.0001) and nonserotonergic (df = 1, F ratio = 14.263, P < 0.0017) neurons at P1. This nicotine effect was also observed at P3 (df = 1, F ratio = 33.275, P = 0.0001 for serotonergic and df = 1, F ratio = 13.664, P = 0.002 for nonserotonergic ROb neurons). At both ages, control neonates in eucapnic normoxia and during hypercapnia had a greater number of ROb serotonergic and nonserotonergic neurons coexpressing c-Fos than nicotine-exposed neonates (P < 0.05 or P < 0.01, Newman-Keuls post hoc test). In addition, significant hypercarbia-induced recruitment of serotonergic c-Fos–positive neurons at ROb was observed in P3 neonates (P < 0.05, Newman-Keuls post hoc test). Interestingly, this hypercapnia-induced recruitment of serotonergic ROb neurons was abolished in nicotine-exposed P3 neonates, and it was not observed in ROb nonserotonergic neurons. (B) Counting of active (c-Fos–positive) neurons in the nucleus tractus solitarius (NTS) of combined P1–P3 neonates. In contrast to ROb, no effect of nicotine exposure was detected in NTS neurons at normoxic eucapnia (df = 1, F ratio = 3.431, P = 0.078). Besides, the increase in the number of c-Fos–positive NTS neurons induced by hypercapnia (df = 1, F ratio = 114.853, P < 0.0001) was similar in both control and nicotine-exposed P1–P3 pups. Data are expressed as mean (±SEM); n = 5 for each group for ROb experiments and n = 6 for NTS experiments. Conditions showing significant differences are shown with connecting lines; *P < 0.05, **P < 0.01, Newman-Keuls post hoc test.
Nicotine Abolished the Activation of c-Fos by Hypercapnia in the ROb at P3
We found a significant hypercapnia-induced increase in the number of c-Fos–positive serotonergic ROb neurons in control P3 neonates. This hypercapnia-induced increase was abolished in nicotine-exposed P3 neonates (Figure 3A).
In contrast to ROb neurons, no effect of nicotine exposure was detected in nucleus tractus solitarius (NTS) neurons (Figure 3B). The baseline numbers of c-Fos–positive NTS neurons were similar in control and nicotine-exposed animals during normoxic eucapnia. Furthermore, the increase in the number of c-Fos–positive NTS neurons induced by hypercapnia was similar in control and nicotine-exposed P1–P3 pups (Figure 3B), suggesting that nicotine effects upon c-Fos expression were selective upon raphe neurons.
Prenatal–Perinatal Nicotine Exposure Decreased the Firing Rate of ROb Neurons in Absence of Synaptic Blockers
ROb neurons recorded from slices (Figure 4A) at P3 showed spontaneous, low-frequency (most <4 Hz), and irregular firing rates of action potentials (Figure 4B). Electrophysiological properties (Table 2) of nicotine-exposed neurons (n = 27) were similar to those of controls (n = 41), and these were concordant with electrophysiological characteristics of raphe neurons previously reported (37). Action potential (Figure 4B) and cell excitability (Figure 4D) were similar in controls and nicotine-exposed neurons. However, the average firing rates of nicotine-exposed raphe neurons were lower than those of controls (Figure 4C and Table 2; *P < 0.05 Mann-Whitney U Test).
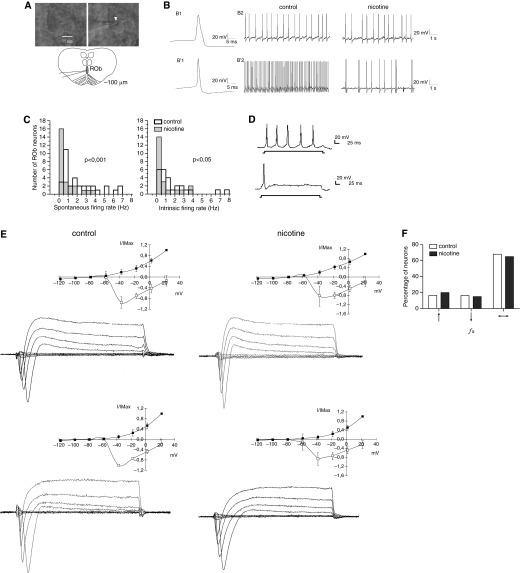
Figure 4.
Nicotine reduces the spontaneous firing rate of raphe neurons without modifying other electrophysiological properties or chemosensitivity. (A) Upper left, infrared differential interference contrast visualization of a ROb neuron in a slice from a P3 control neonate; upper right, same neuron is touched by a patch pipette; lower, drawing of slice indicating patch-clamp electrode position. Arrowhead indicates the tip of the patch electrode touching a soma of a raphe neuron. Scale bar, 15 μm as indicated. (B) Current-clamp recording from ROb neurons in slices from P3 mice. In control and nicotine-exposed slices, 34 out of 41 and 21 out of 27 neurons, respectively, showed an inflection or “hump” on the repolarization phase (upper recording). (C) Distribution of firing rate of ROb neurons in controls (open bars, bold sides) and nicotine-exposed slices (shaded bars). Left panel, distribution of spontaneous firing in absence of glutamatergic and GABAergic blockers. Right panel, distribution of intrinsic firing in presence of 1.5 mM kynurenic acid and 20 μM bicuculline; P level of significance of difference in distributions, assayed with Kolmogorov-Smirnov test, is indicated for each panel. (D) Depolarizing current pulses (300 ms, 20 pA) evoked a tonic discharge of action potentials in 19 out of 25 control and in 13 out of 21 nicotine-exposed neurons. The rest of the neurons responded with a phasic discharge with spike frequency adaptation. (E) Voltage-clamp recordings and current-voltage (I-V) curves from ROb neurons in control and nicotine-exposed slices. I-V curves were obtained using 50 ms voltage pulses from the holding potential of −70 mV to imposed membrane potentials ranging from −120 to +20 mV in steps of 20 mV. Control and nicotine-exposed neurons having action potentials with a hump in the repolarization phase showed an inward current followed by outward currents compatible either with one outward current with adaptation or the activation of two different outward currents, one with a rapid peak and decay, and a second one that was sustained during the 50-ms step (upper I-V curves). By contrast, neurons without a hump on the repolarization phase showed an inward current followed by a sustained outward current in response to a voltage pulse (lower I-V curves). Data are expressed as mean (±SEM). (F) ROb neurons were stimulated with hypercapnia (switching the artificial cerebrospinal fluid equilibration from 5 to 10% CO2) for 5 minutes while they were current-clamp recorded in the presence of glutamatergic and GABAergic receptor blockade. The percentage of ROb neurons increasing (↑), decreasing (↓), or not modifying (↔) their firing rate in response to hypercarbic stimulation is indicated, and no difference was found between controls (open bars) and nicotine-exposed (solid bars) neurons. fx, firing rate of ROb neurons.
Table 2.
Electrophysiological Properties of Raphe Obscurus Neurons in Slices from Control and Nicotine-Exposed Postnatal Day 3 Mouse Neonates
| Control |
Nicotine Exposed |
|||
|---|---|---|---|---|
| Shape of Repolarization | Inflection | Noninflection | Inflection | Noninflection |
| Area, μm2 | 222 ± 59 | 176 ± 44 | 208 ± 56 | 238 ± 94 |
| Vm, mV | −54.2 ± 4.1 | −56.3 ± 2.6 | −56.6 ± 6.7 | −51.3 ± 2.5 |
| Cm, μF | 17.3 ± 3.7 | 15.0 ± 3.6 | 16.3 ± 4.5 | 22.3 ± 15.7 |
| Ri, MΩ | 1,544 ± 998 | 2,099 ± 780 | 1,475 ± 762 | 1,138 ± 896 |
| AHP, mV | 15.7 ± 5.1 | 10.3 ± 4.5 | 15.5 ± 3.9 | 13.3 ± 10.7 |
| fX1, Hz | 2.1 ± 2.0 | 1.9 ± 1.7 | * 0.9 ± 1.1 | * 0.4 ± 0.4 |
| n1 | 34 | 7 | 21 | 6 |
| fX2, Hz | 1.4 ± 2.0 | 0.8 ± 1.1 | ||
| n2 | 22 | 17 | ||
Definition of abbreviations: AHP, after hyperpolarization; Area, area of somata of ROb neurons determined with fluorescence microscopy after Lucifer yellow injection; Cm, membrane capacitance; fx1, firing rate of ROb neurons in absence of synaptic blockers in intact slices; fx2, firing rate of ROb neurons in presence of synaptic blockers; n1 and n2, number of ROb neurons recorded in absence or presence of synaptic blockers, respectively; ROb, raphe obscurus; Ri, input resistance; Vm, resting membrane potential.
Values are expressed as mean ± SEM. Shape of repolarization refers to presence of inflection in the repolarization phase of the action potential.
P < 0.05, Mann-Whitney U test to assess statistical difference between control and nicotine-exposed preparations.
Prenatal–Perinatal Nicotine Exposure Decreased the Firing Rate of ROb Neurons in Presence of Synaptic Blockers
Spontaneous firing rate of raphe neurons was estimated during glutamatergic (1.5 mM kynurenic acid) and GABAergic (20 μM bicuculline) synaptic blockade. This blockade silenced totally the recording of fast excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) in raphe neurons (data not shown) and decreased the firing rate of control neurons from 2.1 (±2.0) to 1.4 (±2.0) Hz. Such a decrease in firing rate was not observed in nicotine-exposed neurons, in which the firing rate in the absence of blockers was 0.9 (±1.1) and in their presence was 0.8 (±1.1) (Table 2). Notably, the firing rate of nicotine-exposed neurons in the presence of synaptic blockers was still lower than that of controls (Table 2). The lower firing rate was related to the displacement to the left of the distribution of firing rates found in raphe neurons recorded from slices exposed to nicotine, resulting in a significant difference in respect to that found in controls (Figure 4C; P < 0.05 Kolgomorov-Smirnov two-sample test). No differences in current-voltage I-V curves were observed between control and nicotine-exposed neonates (Figure 4E).
Responses of Raphe Neurons to Hypercapnic Acidosis
The percentage of raphe neurons that increased, decreased, or did not respond to the hypercapnic stimulus were similar in control and nicotine-exposed slices (Figure 4F). Notably, most of the ROb neurons in control (72%) and nicotine-exposed (67%) slices did not modify their firing rate during hypercapnic acidosis. In 3 out of 25 control neurons that increased their firing rate in response to hypercapnia, the magnitude of change ranged from 165 to 435%, whereas, in 4 out of 21 nicotine-exposed neurons, this ranged from 113 to 405% (P = 0.052, Mann-Whitney U test). Similar analysis applied to the group of neurons that decreased their firing rate in response to hypercapnia indicated that the magnitude of reduction was not significantly different between control (n = 4) and nicotine-exposed slices (n = 3; P = 0.172, Mann-Whitney U test).
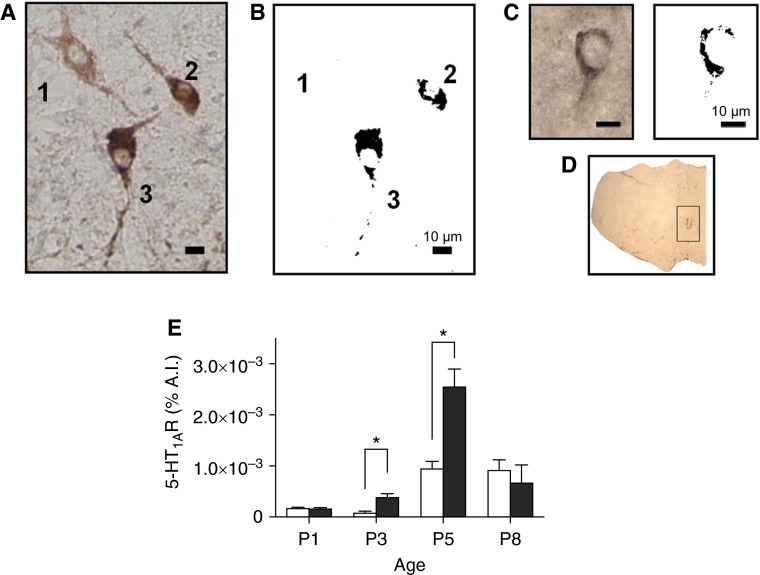
Changes in 5-HT1A Receptor Expression
Firing rate of raphe neurons is under 5-HT1A autoreceptor control (38). Most serotonergic (TpOH+) raphe neurons coexpressed 5-HT1ARs (Figures 5A and 5B). The 5-HT1AR labeling was located on somata and proximal processes within the ROb (Figure 5C). Two-way ANOVA revealed an age effect on 5-HT1AR labeling in ROb area (df = 3, F ratio = 25.780, P < 0.001), increasing from P3 to P5 in both control and nicotine-exposed pups (Figure 5E). This increment was more pronounced in nicotine-exposed pups at P5. In fact, two-way ANOVA also revealed a nicotine effect on 5-HT1AR labeling (df = 1, F ratio = 8.087, P < 0.05) being the 5-HT1AR labeling in P3 and P5 nicotine-exposed mouse pups higher than that observed in controls (Figure 5E).
Figure 5.
5-HT1AR labeling in neonatal ROb is increased by nicotine. (A) Photomicrograph taken at 40× magnification with brightfield illumination showing three TpOH-positive neurons in the ROb of control P3 neonate, two of them (neurons 2 and 3) coexpressing 5-HT1AR. (B) Images containing the darkest grains obtained through filtering gray-tone pictures of neurons in A. Note that filtering eliminates anti-TpOH labeling, leaving the label ascribed to 5-HT1AR label (darkest pixels generated by nickel intensification). (C) ROb neuron immunostained for 5-HT1AR. Note that the distribution of 5-HT1AR labeling on the soma is similar to that obtained after filtering gray-tone pictures in neurons 2 and 3 in B. (D) Coronal section showing area of interest, which contains ROb, used to evaluate 5-HT1AR labeling. (E) Average % area of interest (A.I.) with 5-HT1AR immunolabel expressed as percentage of total pixels in area of interest in ROb. Data are expressed as mean (±SEM). *P < 0.05 for difference between control (open bars) and nicotine-exposed (solid bars) neonates, respectively (n = 3 for each age and experimental condition; two-way ANOVA, Newman-Keuls post hoc test).
Discussion
In this work, we show that the number of ROb serotonergic neurons at P1, the distribution of the areas of their somata at P5–P8, and their activity at P1–P3 can be affected by prenatal–perinatal nicotine exposure. In particular, this is the first report of a reduction of the firing rate of ROb neurons induced by this challenge. Such hypoactivity was not only observed in vitro in the absence of peripheral input, as evidenced by current-clamp recordings in whole-cell mode at P3 in slices, but also in vivo at P1–P3 by detecting the expression of c-Fos protein, a neuronal activity marker in both serotonergic and nonserotonergic neurons. Interestingly, at P0–P5, the ventilatory responses to hypercapnia are depressed by prenatal–perinatal nicotine exposure, reaching a peak of depression at P3 (19). Reduction of the firing rate was not dependent on glutamatergic or GABAergic synaptic inputs, because it was also found in slices after glutamatergic and GABAergic blockade. Neither was it secondary to a concomitant modification of any active or passive electrophysiological properties. We also did not detect any change in intrinsic chemosensitivity of raphe neurons recorded in slices from P3 neonates exposed to prenatal–perinatal nicotine. We found an increase in 5-HT1AR immunostaining in the raphe nuclei. This is consistent with hypoactivity of raphe neurons due to activation of autoreceptors, a well established inhibitory feedback of firing rate of serotonergic neurons (39). Comparison of the time courses of prenatal nicotine-induced upregulation of 5-HT1ARs and the reduction in number of serotonergic c-Fos–positive neurons in the ROb is consistent with the possibility that there is a decrease in ROb neuron activity due to the increase in 5-HT1AR levels, although the evidence is indirect. Notably, both variables did not differ between control and nicotine-exposed pups on P8, the same postnatal day in which ventilatory responses normalize in nicotine-exposed pups.
The absence of modification of intrinsic neuron chemosensitivity in nicotine-exposed raphe neurons, at first glance, does not seem compatible with our findings showing a reduction in hypercapnia-induced recruitment of serotonergic c-Fos–positive neurons in ROb in 3-day-old neonates and a decrease in hypercapnia-induced ventilatory responses in 1- to 5-day-old neonates. To reconcile our results, we can consider two explanations that are not necessarily mutually exclusive. First, intrinsic chemosensitivity of serotonergic raphe neurons increases with age in brain slices and in culture (40). Thus, raphe neuron chemosensitivity is not well developed during the first week of postnatal life, being expressed only by a small fraction of the neuronal population, which can make it difficult to detect subtle changes. Second, any mechanism that reduces serotonergic neuron activity will reduce the ventilatory response to hypercapnia. In fact, in adult serotonin transporter knockout mice, the reduction of serotonergic neuron activity (41) is accompanied by reduction in the ventilatory response to hypercapnia (42), although this knockout mouse exhibits decreased 5-HT1AR binding (41). Reduction of serotonergic neuron activity can be achieved by 5-HT1AR overexpression or 5-HT1AR activation. Thus, excessive serotonin autoinhibition by overexpression of 5-HT1ARs reduces the firing rate responses to hypercapnia of serotonergic neurons in the dorsal raphe in slices (43). Furthermore, activation of 5-HT1ARs reduces the respiratory response to hypercapnic challenge in the in situ rat brainstem preparation (44). Therefore, reduction of the firing rate by increased expression of 5-HT1ARs could explain reduction of the respiratory responses to hypercapnia. It remains unknown if upregulation of 5-HT1ARs is secondary to a direct effect of nicotine on raphe neurons, which express functional nicotinic receptors (31). Likewise, it is an open question whether an effect of nicotine on the serotonin system during development can interfere with the developmental role of serotonin (45), and produce permanent changes in the respiratory neural network.
Serotonergic abnormalities described in children who died from SIDS (7, 15, 16) were not completely reproduced by prenatal–perinatal nicotine in mice. Upregulation of 5-HT1ARs, similar to that reported by us, has been observed in rats (46) and in fetal nonhuman primates after chronic prenatal nicotine administration (25). The same changes have also been observed in offspring from pregnant Rhesus monkeys exposed to environmental tobacco smoke during gestation and for up to 3 months postnatally (26). Infants who were victims of SIDS show an increased number of serotonergic neurons and decreased binding for both 5-HT1ARs and serotonin transporters in the brainstem (7). These discrepancies between human cases and mice exposed prenatally to nicotine could be due to a unique way that the human serotonergic system responds to a nicotinic challenge, or the timing of nicotine exposure, or the association to different risk factors. Nicotine administered during the first 2 weeks of postnatal life in piglets can reduce the expression of 5-HT1ARs in the dorsal motor nucleus of the vagus, inferior olivary nucleus, and NTS (47). Thus, such effects may be dependent on the timing of nicotine exposure and the nuclei analyzed. Discrepancies between findings in SIDS and the mouse cannot be ascribed to the occurrence of fetal hypoxia events. The intensity of fetal hypoxia induced by cigarette smoking or nicotine injections is higher than that due to delivery by an osmotic minipump, because the steady-state plasma levels of nicotine attained with osmotic minipumps are lower than those that produce uterine flow reduction (48). Furthermore, hypoxia and prenatal nicotine reduce the number of raphe serotonergic neurons (49), which is the opposite of what is observed in SIDS. Fetal hypoxia and nicotine can reproduce other features of SIDS, such as the reduction of serotonin transporter expression and raphe neural projections, which might be responsible for the reduced serotonin content in rodent forebrain (7, 49). It is likely that any explanation of a disparity in results from SIDS cases and prenatal mice treated with nicotine will require consideration of other concomitant contributors that could modify the response of the serotonergic system in humans. For example, potential contributors may include multiple neurotoxins also found in cigarette smoke. A concomitant risk factor could also be prenatal exposure to alcohol, which can reduce serotonin receptor binding in the arcuate nucleus, including SIDS cases (50). One can speculate that reduction of serotonergic receptors by alcohol could predominate over upregulation induced by nicotine exposure.
In summary, prenatal–perinatal nicotine exposure transiently reduces the number and modifies the firing rate of ROb neurons in the medulla. Notably, it reduces the activity of raphe neurons in 3-day-old mouse neonates, which is associated with an increase in expression of 5-HT1A autoreceptors. Reduction of serotonergic drive is sufficient cause for explaining depression of the ventilatory response to hypercapnia observed in 3-day-old mouse neonates. As a corollary, alteration of the serotonergic system by nicotine exposure can impair respiratory responses required to maintain organism homeostasis. Our results also suggest that nicotine alone may not account for all the serotonergic abnormalities found in SIDS. Likely, multiple factors acting concomitantly, in addition to nicotine, are required to determine all the features of this syndrome.
Footnotes
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico grants 1090375 and 1130874 (J.L.E.), Comisión Nacional de Ciencia y Tecnología grant AT-4040216 (V.J.C.), Iniciativa Cientifica Milenio grant P01-007-F (M.d.l.L.O.A.), and National Institutes of Health grants P01HD36379 and R01HD052772 (G.B.R.).
Part of this work was submitted by V.J.C. to the P. Universidad Católica de Chile in partial fulfillment of the requirements for the Ph.D. in Biological Sciences (Physiology); E.U.B. and S.B.-C. are graduate students of Universidad de Santiago de Chile in the Biotechnology and in the Neuroscience Ph.D. programs, respectively.
Author Contributions: V.J.C.—design, acquisition, analysis, and drafting of the morphological and electrophysiological work; M.d.l.L.O.A.—supervision, acquisition, and drafting of the electrophysiological work; S.B.-C.—acquisition, analysis, interpretation, and drafting of the morphological work; E.U.B.—acquisition, analysis, and drafting of the morphological work; I.R.L.—supervision of morphological studies, analysis, and drafting of the article; G.B.R.—acquisition of funding, supervision of morphological work, analysis, and critical review of the article; J.L.E.—conception, design, supervision, funding, and acquisition of morphofunctional studies, and writing of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0329OC on February 19, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Matthews TJ, MacDorman MF. Infant mortality statistics from the 2010 period linked birth/infant death data set. Natl Vital Stat Rep. 2013;62:1–26. [PubMed] [Google Scholar]
- 2.Trachtenberg FL, Haas EA, Kinney HC, Stanley C, Krous HF. Risk factor changes for sudden infant death syndrome after initiation of Back-to-Sleep campaign. Pediatrics. 2012;129:630–638. doi: 10.1542/peds.2011-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato I, Franco P, Groswasser J, Scaillet S, Kelmanson I, Togari H, Kahn A. Incomplete arousal processes in infants who were victims of sudden death. Am J Respir Crit Care Med. 2003;168:1298–1303. doi: 10.1164/rccm.200301-134OC. [DOI] [PubMed] [Google Scholar]
- 4.Ueda Y, Stick SM, Hall G, Sly PD. Control of breathing in infants born to smoking mothers. J Pediatr. 1999;135:226–232. doi: 10.1016/s0022-3476(99)70026-0. [DOI] [PubMed] [Google Scholar]
- 5.Shannon DC, Kelly DH, O’Connell K. Abnormal regulation of ventilation in infants at risk for sudden-infant-death syndrome. N Engl J Med. 1977;297:747–750. doi: 10.1056/NEJM197710062971403. [DOI] [PubMed] [Google Scholar]
- 6.Kato I, Groswasser J, Franco P, Scaillet S, Kelmanson I, Togari H, Kahn A. Developmental characteristics of apnea in infants who succumb to sudden infant death syndrome. Am J Respir Crit Care Med. 2001;164:1464–1469. doi: 10.1164/ajrccm.164.8.2009001. [DOI] [PubMed] [Google Scholar]
- 7.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol. 1998;511:433–450. doi: 10.1111/j.1469-7793.1998.433bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- 11.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nattie EE, Li A, Richerson GB, Lappi DA.Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo J Physiol 2004556235–253.[Published erratum appears in J Physiol 587:4377.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphé. J Appl Physiol (1985) 1996;80:108–115. doi: 10.1152/jappl.1996.80.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Monti JM. The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep Med Rev. 2010;14:307–317. doi: 10.1016/j.smrv.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, et al. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 17.Daltveit AK, Irgens LM, Oyen N, Skjaerven R, Markestad T, Wennergren G. Circadian variations in sudden infant death syndrome: associations with maternal smoking, sleeping position and infections: the Nordic Epidemiological SIDS Study. Acta Paediatr. 2003;92:1007–1013. [PubMed] [Google Scholar]
- 18.Nattie E, Kinney H. Nicotine, serotonin, and sudden infant death syndrome. Am J Respir Crit Care Med. 2002;166:1530–1531. doi: 10.1164/rccm.2210001. [DOI] [PubMed] [Google Scholar]
- 19.Eugenín J, Otárola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von Bernhardi R. Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J Neurosci. 2008;28:13907–13917. doi: 10.1523/JNEUROSCI.4441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St-John WM, Leiter JC. Maternal nicotine depresses eupneic ventilation of neonatal rats. Neurosci Lett. 1999;267:206–208. doi: 10.1016/s0304-3940(99)00364-x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol. 2002;538:957–973. doi: 10.1113/jphysiol.2001.012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simakajornboon N, Vlasic V, Li H, Sawnani H. Effect of prenatal nicotine exposure on biphasic hypoxic ventilatory response and protein kinase C expression in caudal brain stem of developing rats. J Appl Physiol (1985) 2004;96:2213–2219. doi: 10.1152/japplphysiol.00935.2003. [DOI] [PubMed] [Google Scholar]
- 23.Hafström O, Milerad J, Sundell HW. Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. Am J Respir Crit Care Med. 2002;166:1544–1549. doi: 10.1164/rccm.200204-289OC. [DOI] [PubMed] [Google Scholar]
- 24.Fewell JE, Smith FG, Ng VK. Prenatal exposure to nicotine impairs protective responses of rat pups to hypoxia in an age-dependent manner. Respir Physiol. 2001;127:61–73. doi: 10.1016/s0034-5687(01)00232-8. [DOI] [PubMed] [Google Scholar]
- 25.Duncan JR, Garland M, Myers MM, Fifer WP, Yang M, Kinney HC, Stark RI. Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: implications for sudden infant death syndrome. J Appl Physiol (1985) 2009;107:1579–1590. doi: 10.1152/japplphysiol.91629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slotkin TA, Pinkerton KE, Tate CA, Seidler FJ. Alterations of serotonin synaptic proteins in brain regions of neonatal Rhesus monkeys exposed to perinatal environmental tobacco smoke. Brain Res. 2006;1111:30–35. doi: 10.1016/j.brainres.2006.06.094. [DOI] [PubMed] [Google Scholar]
- 27.Slotkin TA, Seidler FJ, Spindel ER. Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome: amelioration by vitamin C. Neurotoxicol Teratol. 2011;33:431–434. doi: 10.1016/j.ntt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- 29.Muneoka K, Ogawa T, Kamei K, Mimura Y, Kato H, Takigawa M. Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur J Pharmacol. 2001;411:279–282. doi: 10.1016/s0014-2999(00)00925-0. [DOI] [PubMed] [Google Scholar]
- 30.Coddou C, Bravo E, Eugenín J. Alterations in cholinergic sensitivity of respiratory neurons induced by pre-natal nicotine: a mechanism for respiratory dysfunction in neonatal mice. Philos Trans R Soc Lond B Biol Sci. 2009;364:2527–2535. doi: 10.1098/rstb.2009.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduno J, Frias-Dominguez C, Drucker-Colin R, Mihailescu S. Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse. 2008;62:601–615. doi: 10.1002/syn.20526. [DOI] [PubMed] [Google Scholar]
- 32.Commons KG Alpha4 containing nicotinic receptors are positioned to mediate postsynaptic effects on 5-HT neurons in the rat dorsal raphe nucleus Neuroscience 2008153:851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pendlebury JD, Wilson RJ, Bano S, Lumb KJ, Schneider JM, Hasan SU. Respiratory control in neonatal rats exposed to prenatal cigarette smoke. Am J Respir Crit Care Med. 2008;177:1255–1261. doi: 10.1164/rccm.200711-1739OC. [DOI] [PubMed] [Google Scholar]
- 34.Campos M, Bravo E, Eugenín J. Respiratory dysfunctions induced by prenatal nicotine exposure. Clin Exp Pharmacol Physiol. 2009;36:1205–1217. doi: 10.1111/j.1440-1681.2009.05214.x. [DOI] [PubMed] [Google Scholar]
- 35.Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J Neurophysiol. 1997;77:1349–1361. doi: 10.1152/jn.1997.77.3.1349. [DOI] [PubMed] [Google Scholar]
- 38.Evrard A, Laporte AM, Chastanet M, Hen R, Hamon M, Adrien J. 5-HT1A and 5-HT1B receptors control the firing of serotoninergic neurons in the dorsal raphe nucleus of the mouse: studies in 5-HT1B knock-out mice. Eur J Neurosci. 1999;11:3823–3831. doi: 10.1046/j.1460-9568.1999.00800.x. [DOI] [PubMed] [Google Scholar]
- 39.McCall RB, Clement ME. Identification of serotonergic and sympathetic neurons in medullary raphe nuclei. Brain Res. 1989;477:172–182. doi: 10.1016/0006-8993(89)91405-4. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience. 1999;90:1001–1011. doi: 10.1016/s0306-4522(98)00505-3. [DOI] [PubMed] [Google Scholar]
- 41.Gobbi G, Murphy DL, Lesch K, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–995. [PubMed] [Google Scholar]
- 42.Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol. 2008;586:2321–2329. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baccini G, Mlinar B, Audero E, Gross CT, Corradetti R. Impaired chemosensitivity of mouse dorsal raphe serotonergic neurons overexpressing serotonin 1A (Htr1a) receptors. PLoS One. 2012;7:e45072. doi: 10.1371/journal.pone.0045072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corcoran AE, Richerson GB, Harris MB. Serotonergic mechanisms are necessary for central respiratory chemoresponsiveness in situ. Respir Physiol Neurobiol. 2013;186:214–220. doi: 10.1016/j.resp.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 46.Slotkin TA, Ryde IT, Tate CA, Seidler FJ. Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: separate or sequential exposure during fetal development and adulthood. Brain Res Bull. 2007;73:259–272. doi: 10.1016/j.brainresbull.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Say M, Machaalani R, Waters KA. Changes in serotoninergic receptors 1A and 2A in the piglet brainstem after intermittent hypercapnic hypoxia (IHH) and nicotine. Brain Res. 2007;1152:17–26. doi: 10.1016/j.brainres.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 48.Monheit AG, VanVunakis H, Key TC, Resnik R. Maternal and fetal cardiovascular effects of nicotine infusion in pregnant sheep. Am J Obstet Gynecol. 1983;145:290–296. doi: 10.1016/0002-9378(83)90713-5. [DOI] [PubMed] [Google Scholar]
- 49.Buller KM, Wixey JA, Reinebrant HE. Disruption of the serotonergic system after neonatal hypoxia-ischemia in a rodent model. Neurol Res Int. 2012;2012:650382. doi: 10.1155/2012/650382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, et al. Serotonergic brainstem abnormalities in Northern Plains Indians with the sudden infant death syndrome. J Neuropathol Exp Neurol. 2003;62:1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]