Abstract
Radiation-induced pulmonary fibrosis (RIF) is a severe complication of thoracic radiotherapy that limits its dose, intensity, and duration. The contribution of the endocannabinoid signaling system in pulmonary fibrogenesis is not known. Using a well-established mouse model of RIF, we assessed the involvement of cannabinoid receptor-1 (CB1) in the onset and progression of pulmonary fibrosis. Female C57BL/6 mice and CB1 knockout mice generated on C57BL/6 background received 20 Gy (2 Gy/min) single-dose thoracic irradiation that resulted in pulmonary fibrosis and animal death within 15 to 18 weeks. Some C57BL/6 animals received the CB1 peripherally restricted antagonist AM6545 at 1 mg/kg intraperitoneally three times per week. Animal survival and parameters of pulmonary inflammation and fibrosis were evaluated. Thoracic irradiation (20 Gy) was associated with marked pulmonary inflammation and fibrosis in mice and high mortality within 15 to 18 weeks after exposure. Genetic deletion or pharmacological inhibition of CB1 receptors with a peripheral CB1 antagonist AM6545 markedly attenuated or delayed the lung inflammation and fibrosis and increased animal survival. Our results show that CB1 signaling plays a key pathological role in the development of radiation-induced pulmonary inflammation and fibrosis, and peripherally restricted CB1 antagonists may represent a novel therapeutic approach against this devastating complication of radiotherapy/irradiation.
Keywords: radiation-induced pulmonary fibrosis, cannabinoid receptor 1, thoracic irradiation, peripherally restricted CB1 antagonist, AM6545
Clinical Relevance
We report for the first time the involvement of cannabinoid receptor 1 (CB1)-mediated signaling in the onset and progression of radiation-induced pulmonary fibrosis (RIF). We were able to delay the onset of RIF by genetic targeting of CB1 receptors as well as by its pharmacological inhibition. Thus, pharmacological targeting of CB1 receptors with peripherally restricted CB1 antagonists void of central nervous system complications may represent a novel strategy to prevent the development of RIF.
Radiation-induced pulmonary fibrosis (RIF) is a severe dose-limiting complication of thoracic radiotherapy. In most cases, RIF is preceded by radiation pneumonitis, but sometimes RIF can develop years after radiotherapy without symptomatic manifestations of early pulmonary inflammation. Regardless the extensive use of stereotactic radiotherapy, which limits the exposure of normal lung tissue to irradiation, as many as 35% of patients with lung cancer and breast cancer receiving thoracic radiotherapy develop radiation pneumonitis and are at strong risk of developing RIF months and years after initial radiotherapy (1–3). This risk of developing RIF limits the dose and intensity of irradiation, thus leaving a chance for a noncomplete removal of neoplasia by radiosurgery. Patients undergoing total body irradiation before bone marrow transplant are also at risk for developing radiation pneumonitis and RIF (3–4). When developed, pulmonary fibrosis is incurable and leads to partial loss of pulmonary functions or even to cor pulmonale (heart failure due to a long-term increase in pressure in pulmonary arteries) when significant lung areas are affected.
Unfortunately, pulmonary fibrosis, including RIF, is resistant to the available, mostly symptomatic, therapeutic approaches (5, 6). Lung transplantation is often the only option available to treat this devastating condition, and its benefits are limited. Therefore, new strategies leading to reversal or delay of the progression of pulmonary fibrosis are desperately needed. Within a complex family of pulmonary fibrotic diseases, RIF stands alone as the only nongenetic fibrotic disease when time and origin of the insult leading to the development of pulmonary fibrosis are known. Therefore, animal models of RIF provide a direct view of the events leading to RIF in patients undergoing radiotherapy and allow in-depth investigation of these devastating consequences of thoracic irradiation.
Cannabinoid receptor-mediated signaling emerges as a novel signaling pathway regulating fibrogenesis. Both cannabinoid receptor-1 (CB1) and cannabinoid receptor-2 (CB2) were recently implicated in pathogenesis of skin (7–10), liver (11–14), cardiac (15), and renal (16) fibrotic proliferative diseases. However, the role of the endocannabinoid system in pulmonary fibrogenesis remains enigmatic. Herein, using a well-established mouse model of RIF, we demonstrate that CB1 receptors are directly implicated in RIF and that RIF progression can be delayed by selective targeting of peripheral CB1 receptors using the brain-impenetrable novel CB1 antagonist AM6545.
Materials and Methods
Animals and Reagents
All animal experiments were approved by the University of Illinois at Chicago Animal Care and Use Committee. Female C57BL/6 mice (8–10 wk old) were purchased from Jackson Laboratory (Bar Harbor, ME). Female CB1−/− mice (on C57BL6/J background) were provided by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (Intramural Research Program of NIH/NIAAA, Rockville, MD). CB1 antagonist AM6545 [5-(4-(4-cyanobut-1-yn-1-yl)phenyl)-1-(2,4-dichlorophenyl)-N-(1,1-dioxidothiomorpholino)-4-methyl-1H-pyrazole-3-carboxamide] was provided by Dr. A. Makriyannis (Center for Drug Discovery, Northeastern University, Boston, MA). Halt protease inhibitor cocktail and a BCA protein determination kit were purchased from Fisher Scientific (Pittsburgh, PA). The Sircol Soluble Collagen Assay kit was purchased from Biocolor (Carrickfergus, UK). All antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Animal Model of Radiation-Induced Pulmonary Fibrosis and AM6545 Administration
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (0.5 mg/kg) and administered radiation (20 Gy at the rate of 2 Gy/min) to the thorax as described previously (17). AM6545 (1 mg/kg intraperitoneally as a solution in 2% Tween-80 in saline) was administered three times per week starting 2 hours before irradiation. Control mice received just the solvent. For survival and biochemical/histological studies, mice were considered “dead” when loosing 25% body weight at the end of RIF phase. For biochemical and histological comparison of nontreated and treated C57BL/6 mice and CB1−/− mice, the same number of animals from each group was killed in a dependent fashion each time an animal in a control irradiated nontreated C57BL/6 group reached termination criterion (25% body weight loss). The “paired to control group” animals were selected based on the biggest decline in their body weight or at random if the body weight was not yet declining. Bronchoalveolar lavage (BAL) was collected by washing the lungs twice with 1 ml HBSS without calcium and magnesium (only the first BAL portion was used as “BAL” preparation; the second portion was needed to ensure maximum cell recovery) followed by blood collection via cardiac puncture, vascular system cleaning by perfusion with 10 ml HBSS without calcium and magnesium through the pulmonary artery, and lung tissue collection. The development of pulmonary fibrosis was assessed histologically by staining lung tissue slices with Sirius Red for collagen deposition and by microscopic evaluation, by measuring acid-soluble collagen content (per Biocolor instructions), and by determining the expression of α-smooth muscle actin (α-SMA) and collagen α2 type I (by Western blotting) in the lung. Before biochemical analyses, the harvested lung tissues were pulverized in liquid nitrogen to ensure sample homogeneity.
Immunoblotting
Lung tissue lysis and immunoblotting was performed as described elsewhere (18).
Statistical Analyses
Each animal group contained at least five animals. One-way ANOVA was used to determine statistical differences between groups. Post hoc Student t test was used where appropriate. A comparison of survival between different groups of animals was performed using Kaplan–Meier analysis. A GraphPad Prizm 5.02 package was used for statistical analyses. Differences between groups were considered statistically significant at P < 0.05. Results are expressed as means ± SEM. Cluster analysis of gene expression data was performed using the Bio-Rad CFX software package (Bio-Rad, Hercules, CA).
Details of RNA isolation, real-time RT-PCR analysis, and cytokine quantification by ELISA are provided in the online supplement.
Results
Genetic Deletion or Pharmacological Inhibition of CB1 Attenuates RIF-Associated Pulmonary Inflammation
To define the role of CB1 receptors in RIF, we compared the effect of thoracic irradiation (20 Gy, 2 Gy/min) on female C57BL/6 mice treated with the peripherally restricted selective CB1 antagonist AM6545 (19–21) (1 mg/kg, three times per week intraperitoneally) or solvent (2% Tween-80 in saline). Treatment was initiated 2 hours before irradiation and continued until animals were killed. Animals were killed and considered “dead for the purpose of the study” at the terminal stage of RIF development when mice lose 25% their body weight and developed massive pulmonary fibrosis and inflammation. In C57BL/6 mice this happens between 15 and 20 weeks after irradiation when a 20-Gy single irradiation dose is applied to the thorax at the rate of 2 Gy/min with the head, abdomen, and lower part of the body shielded (18).
When developed, RIF in mice is characterized by significant collagen deposition in pulmonary interstitium (18, 22, 23). To define the role of CB1 receptors in RIF progression, we analyzed lung tissues and BAL fluids collected from AM6545-treated C57BL/6 mice and from CB1−/− mice for collagen deposition, expression of fibrotic markers, and cellular and humoral markers of inflammation. Because irradiated animals attained RIF stage within a relatively wide time window (mostly between 15 and 19 wk), animals from the C57BL/6 control, AM6545-treated, or CB1−/− groups were killed, and lung tissue and BAL were collected each time an animal from the irradiated C57BL/6 group attained 25% body weight loss. This collection pattern allowed minimizing data spread and creating coherent sets of data.
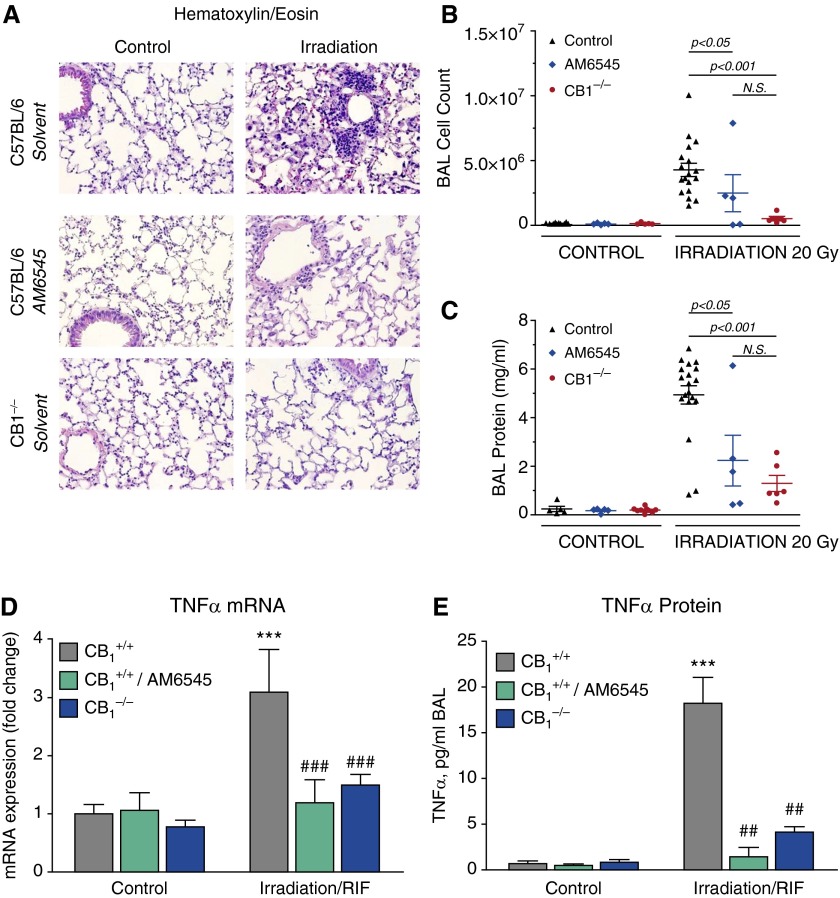
RIF development is accompanied by escalating pulmonary inflammation characterized by increased vascular leakiness, immune cell infiltration into the lung, and local and systemic increase in the expression of multiple inflammatory cytokines (18, 22, 23). We found that the inhibition of CB1-mediated signaling by AM6545 or the lack of functioning CB1 receptors markedly reduced immune cell infiltration into the lung tissue (Figure 1A), the amount of cells recovered from alveolar lumens into BAL (Figure 1B), and the protein level in the BAL (Figure 1C). This was accompanied by attenuation of the tissue mRNA level of proinflammatory cytokine TNF-α (Figure 1D) and by a marked decline of TNF-α level in BAL (Figure 1E). Lymphocyte infiltration was significant at RIF stage in nontreated CB1+/+ mice, but the lack of functioning CB1 receptors in CB1−/− mice did not affect lymphocyte percentage within the population of infiltrating cells (see Figure E1 in the online supplement). Interestingly, the long-term (5 mo) application of AM6545 markedly decreased lymphocyte percentage in BAL from irradiated animals (Figure E1).
Figure 1.
Pharmacological or genetic targeting of cannabinoid receptor-1 (CB1) decreases radiation-induced pulmonary inflammation in mice administered a single thoracic dose (20 Gy) of irradiation. Here and thereafter, lung tissues from control, irradiated (20 Gy, thoracic, 2 Gy/min), AM6545-treated, and CB1−/− mice were taken when solvent-treated irradiated C57BL/6 mice reached radiation-induced pulmonary fibrosis (RIF) stage. (A) Lung tissue immune cell infiltration is decreased in animals that received the peripheral CB1 antagonist AM6545 (1 mg/kg intraperitoneally three times per week) and in CB1−/− mice. Hematoxylin and eosin staining of lung sections (representative images; original magnification: ×200). (B and C) BAL fluid was collected and assessed for total protein and cell counts. Compared with irradiated control animals, CB1 receptor inhibition with AM6545 or the lack of functional CB1 receptors in knockout animals substantially decreased bronchoalveolar lavage (BAL) cell count (B) and protein concentration (C). (D and E) Lung tissue TNF-α messenger RNA (mRNA) level (D) and TNF-α BAL level (E) are up-regulated at RIF stage and normalized by CB1 inhibition with AM6545 or in CB1−/− animals. ***P < 0.001 versus nonirradiated control animals. ##P < 0.01 and ###P < 0.001 versus irradiated control animals. N.S., not significant.
Genetic Deletion or Pharmacological Inhibition of CB1 Attenuates RIF-Associated Pulmonary Oxidative and Nitrative Stress
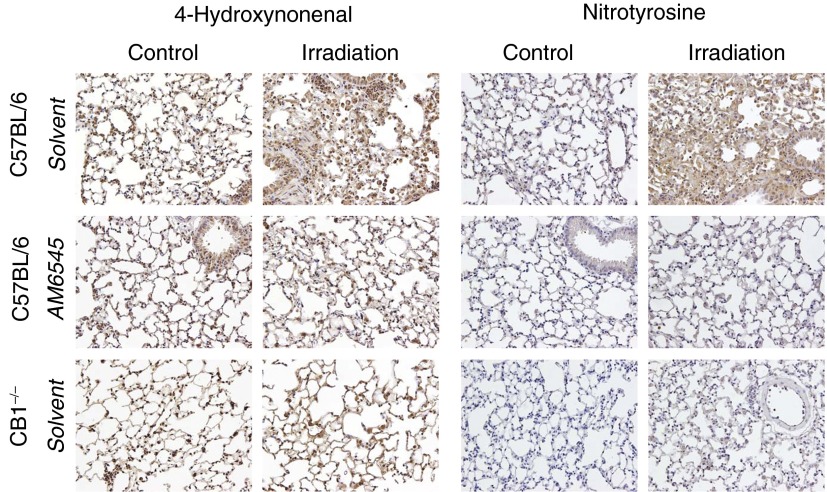
The inflammatory response is associated with increased reactive oxygen species and reactive nitrogen species production by immune cells (24, 25). Lung tissue staining for 4-hydroxynonenal and nitrotyrosine, markers of tissue oxidative and nitrative stress, were substantially increased in control irradiated C57BL/6 mice at RIF stage (Figure 2). RIF-associated up-regulation of both markers of oxidative/nitrative stress was markedly attenuated by pharmacological inhibition or by genetic deletion of CB1 (Figure 2).
Figure 2.
Pharmacological or genetic targeting of CB1 receptors decreases radiation-induced oxidative and nitrative stress in the lung. Lung tissues were stained for markers of oxidative (4-hydroxynonenal [4-HNE]) and nitrative (S-nitrotyrosine) stress. RIF results in a marked increase in lipid peroxidation (4-HNE staining) and protein nitrosylation (nitrotyrosin staining) (original magnification: ×200). Animal treatment with AM6545 or the lack of CB1 receptors substantially decreases lung tissue reactive oxygen species and reactive nitrogen species formation, as evidenced by normalization of tissue staining for 4-HNE and nitrotyrosine (representative images are shown).
Genetic Deletion or Pharmacological Inhibition of CB1 Attenuates Radiation-Induced Pulmonary Fibrosis
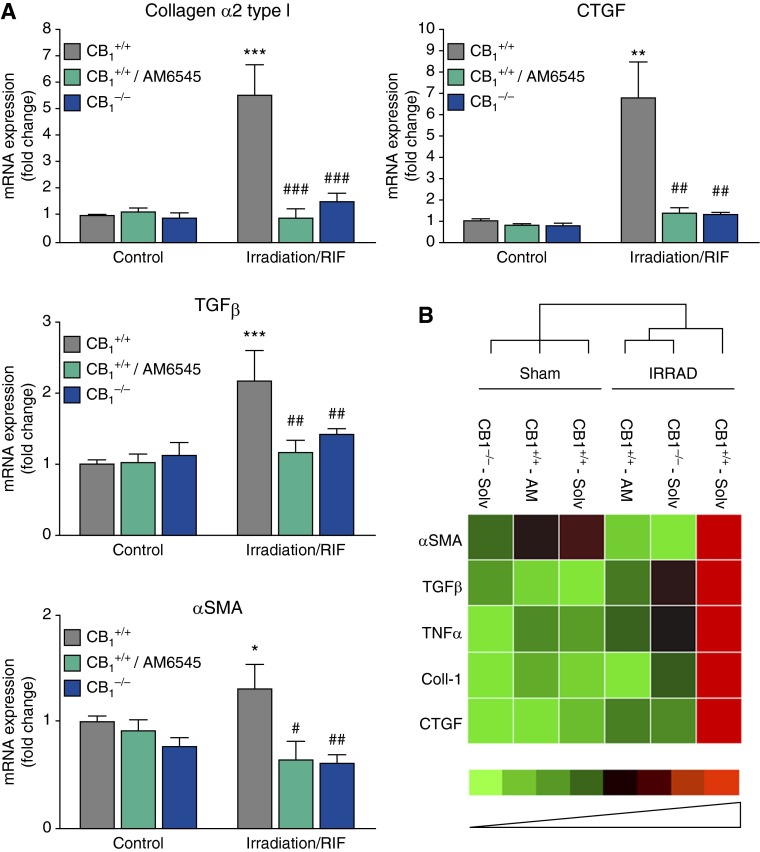
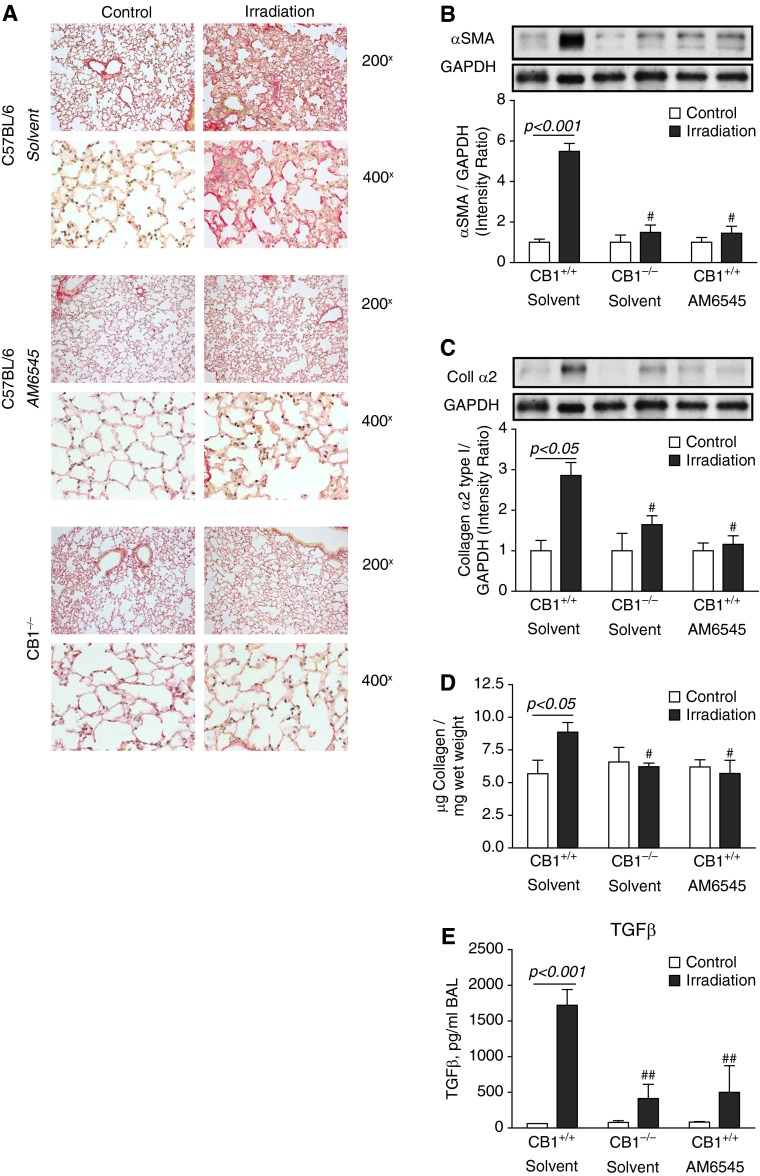
The analysis of the lung tissues for mRNA expression of several major markers of fibrosis, such as transforming growth factor-β, the connective tissue growth factor, α-SMA, and collagen α2 type I, showed substantial increase in mRNA expression of these fibrosis-associated proteins at RIF stage (Figure 3A). Cluster analysis of dysregulation in gene expression associated with active fibrogenesis and inflammation at RIF stage clearly demonstrated attenuation of profibrotic changes by inhibition of CB1 receptors with the peripherally restricted CB1 antagonist AM6545 or by the lack of CB1 receptors in CB1−/− animals (Figure 3B). In parallel to up-regulated gene expression, RIF was characterized by the increased lung tissue collagen deposition (Figure 4A, Sirius red staining), acid-soluble collagen content (Figure 4D), and protein expression of α-SMA and collagen α2 type I (Figures 4B–4D). Collectively, the peripherally restricted CB1 antagonist AM6545 or the deletion of CB1 receptors markedly attenuated the expression of these profibrotic markers at both mRNA and protein levels (Figures 3 and 4).
Figure 3.
Pharmacological or genetic targeting of CB1 normalizes radiation-induced dysregulation in the expression of genes associated with active fibrogenesis and inflammation at RIF stage. (A) Lung tissue mRNA levels for genes linked to active fibrogenesis were quantified by quantitative RT-PCR (qRT-PCR), and their expression was normalized to that of 18S. The normalized expression of each gene was further normalized to the gene expression in solvent-treated control C57BL/6 mice. *P < 0.05, **P < 0.01, and ***P < 0.001 versus nonirradiated control animals. #P < 0.05, ##P < 0.01, and ###P < 0.001 versus irradiated control animals. (B) Cluster analysis of the effect of pharmacological or genetic targeting of CB1 receptors on dysregulation in the expression of genes associated with active fibrogenesis and inflammation at RIF stage. Cluster analysis was performed using BioRad software for processing qRT-PCR data. α-SMA, α-smooth muscle actin; AM, AM6545; Coll-1, collagen α2 type I; CTGF, connective tissue growth factor; IRRAD, irradiation; Solv, solvent; TGF-β, transforming growth factor β.
Figure 4.
Pharmacological or genetic targeting of CB1 decreases radiation-induced pulmonary fibrosis in mice administered a single thoracic dose (20 Gy) of irradiation. (A) Collagen deposition is decreased in animals that received the peripheral CB1 antagonist AM6545 (1 mg/kg intraperitoneally three times per week) and in CB1−/− mice. Sirius red staining of lung sections. (B–D) The lung tissue levels of the markers of fibrogenesis α-SMA (B), Coll α2 (C), acid-soluble collagen (D), and BAL level of TGFβ (E) are decreased or completely normalized in C57Bl/6 mice treated with AM6545 and in CB1−/− mice. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. #P < 0.05, ##P < 0.01 versus irradiated control animals.
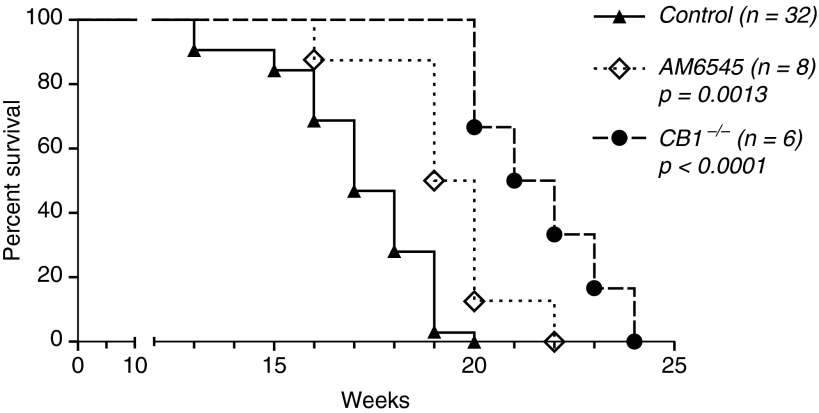
The Peripherally Restricted CB1 Antagonist AM6545 or Genetic Deletion of CB1 Receptors Improves Survival of Mice Subjected to Thoracic Irradiation
Control female C57BL/6 mice subjected to 20 Gy (2 Gy/min) thoracic irradiation die predominantly between 15 and 18 weeks after irradiation. The treatment of animals with AM6545 statistically significantly prolonged animal survival by 2 to 3 weeks in comparison to solvent-treated irradiated control mice (Figure 5). To further confirm the role of CB1 receptors in pulmonary RIF, we subjected female global knockout CB1−/− mice generated on the same C57BL/6 background (Intramural Research Program of NIH/NIAAA) to the same dose and intensity of thoracic irradiation (20 Gy, 2 Gy/min). Animals received solvent treatment in the same way as it was done to C57BL/6 mice. As shown in Figure 5, the lack of functioning CB1 receptor provided significant extension in animal survival that superseded that provided by AM6545.
Figure 5.
Pharmacological or genetic targeting of CB1 improves survival of mice subjected to thoracic irradiation. Female C57BL/6 (CB1+/+) or CB1−/− mice weighing 18 to 22 g were subjected to a single 20 Gy (2 Gy/min) dose of thoracic irradiation. Some animals received AM6545 (1 mg/kg intraperitoneally three times per week) as a solution in 2% Tween-80 in saline; control mice received just the solvent. Inhibition of CB1 receptors with AM6545 or the lack of CB1 receptors extended animal survival. Statistical difference was calculated using a log-rank (Mantel-Cox) test.
Thus, our data clearly demonstrate the involvement of CB1 receptors in the process of radiation-induced pulmonary fibrogenesis and support the notion that pharmacological or genetic targeting of CB1 receptor–mediated signaling represents a novel strategy to alleviate long-term deleterious consequences of high-dose irradiation to the lung.
Discussion
RIF represents the most deleterious side-effect of thoracic radiotherapy and is capable of leading to cor pulmonale within a few years after patient treatment. Being part of the process called “radiation-induced lung injury,” RIF is preceded in most cases with radiation pneumonitis but can develop without prior manifestations of pulmonary inflammation. Noncontrolled “healing” of normal lung tissue in response to radiation-induced damage leads to excessive matrix deposition in the alveolar space that excludes the affected area of the lung from air exchange. RIF represents a special concern when significant lung areas are subjected to radiotherapy and when concomitant chemotherapy is applied that increases the risk of RIF development (26, 27). RIF is incurable, and all efforts are required to minimize the risk of its development and/or to delay its onset. Although the use of animal models of RIF identified several promising targets, like kinases linked to transforming growth factor-β and PDGF receptor–mediated signaling taking part in RIF progression (28–30), so far such approaches have failed to translate into targeted therapies for the treatment of radiation-induced fibrosis in humans. This failure is due in part to the complexity of pulmonary fibrogenesis, which is an intricate process involving multiple cell types and signaling pathways (5, 31). Therefore, the identification of novel therapeutic targets involved in pulmonary fibrogenesis is absolutely needed.
Based on the use of global (brain-penetrable) CB1 antagonists, which were withdrawn from the clinical development because of introduction of increased anxiety in human subjects, cannabinoid-mediated signaling emerges as a promising therapeutic target in liver (11–14, 32, 33), skin (7–10), and cardiac (15, 34) fibrogenesis. Accumulating evidence also suggests that activation of CB1 may exert proinflammatory or prooxidant effects (34–39). CB1 receptors are known to be expressed in the lung tissue (40), bronchial epithelial cells (41), and alveolar type II cells (42), yet no information is available on the role of CB1 in pulmonary fibrotic diseases. In this study we provide evidence on the key role of CB1 receptor signaling in the development of radiation-induced pulmonary inflammation and fibrosis and show that it can be targeted for therapeutic gain by novel peripherally restricted CB1 antagonists.
Irradiation resulted in marked inflammation, oxidative and nitrative stress, and fibrosis in lungs of control mice; these results were associated with high mortality within 15 and 18 weeks after exposure. Genetic deletion or pharmacological inhibition of CB1 with AM6545 not only significantly attenuated the radiation-induced lung inflammation, oxidative and nitrative stress, and fibrosis; it also prolonged the survival of animals, which is extremely important from a clinical point of view. Of note, the above-mentioned radiation-induced pathological pulmonary alterations were affected by a relatively moderate dose of peripherally restricted CB1 antagonist (1 mg/kg three times per week), in contrast to the usually used high daily dose of antagonist (5–10 mg/kg) in most published studies (19, 21, 43). Therefore, it is expected that more intensive regiments of AM6545 application will approach maximal protection seen in CB1−/− mice in terms of survival benefits (Figure 5). Although not fully blocking RIF development (animals still die from RIF; selected analysis of the lung tissues from AM6545-treated and CB1−/−–irradiated animals reaching the end-point criterion showed the development of fibrosis and inflammation similar to that in CB1+/+ mice [data not shown]), the observed delay in RIF progression through CB1 inhibition is significant and may provide important benefits when combined with other antiinflammatory and antifibrotic therapies.
When targeting CB1 receptors for therapeutic gain, it is important to remember the consequences of prolonged inhibition of CB1 receptors in the central nervous system (CNS) (in particular, neuropsychiatric complications in patients receiving the CB1 antagonist SR141716A [rimonabant] as an antiobesity drug [44]). Therefore, the development of a novel generation of peripherally restricted CB1 antagonists provides an important tool to selectively affect CB1-mediated signaling in peripheral organs without affecting neurotransmission in CNS. AM6545 represents one of the first new-generation CB1 antagonists largely excluded from CNS (∼0.03 as brain/plasma ratio) (21, 45); hence, the long-term use of AM6545 or similar CB1 antagonists required to affect RIF development should be void of significant neurological complications. Conversely, CB1 agonists (Δ9-tetrahydrocannabinol, marinol) should be used with caution in the treatment of cachexia in cancer patients receiving chemotherapy together with thoracic radiosurgery due to the unveiled novel role of CB1-mediated signaling in pulmonary fibrogenesis.
In summary, we provide the first evidence on the key pathological role of CB1 signaling in radiation-induced pulmonary fibrogenesis and show that peripherally restricted CB1 antagonists may represent a novel therapeutic approach against this devastating and untreatable complication of radiotherapy/irradiation. Our results also suggest that targeting CB1 may provide benefits in other lung diseases associated with inflammation and fibrosis.
Footnotes
This work was supported by National Institutes of Health grants 5P01 HL098050–04 (E.V.B.) and R01DA007215, R37DA023142, R37DA003801, and P01DA009158 (A.M.) and by the National Institute of Alcohol Abuse and Alcoholism Intramural Research Program (P.P.).
Author Contributions: E.V.B. designed the study, gathered animal and biochemical data, and wrote the manuscript. I.B. performed immunoblotting, biochemical, and histological analyses. B.S. and B.A. facilitated animal thoracic irradiation. R.R.W. provided general discussion regarding long-term consequences of thoracic irradiation. K.V. and A.M. designed AM6545 and helped with manuscript writing. K.E. performed immunohistochemical analysis of lung tissues. P.P. provided cannabinoid receptor 1−/− animals for the study and wrote the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2014-0331OC on March 10, 2015
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001;13:242–248. doi: 10.1097/00001622-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Yu TK, Whitman GJ, Thames HD, Buzdar AU, Strom EA, Perkins GH, Schechter NR, McNeese MD, Kau SW, Thomas ES, et al. Clinically relevant pneumonitis after sequential paclitaxel-based chemotherapy and radiotherapy in breast cancer patients. J Natl Cancer Inst. 2004;96:1676–1681. doi: 10.1093/jnci/djh315. [DOI] [PubMed] [Google Scholar]
- 3.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ ASCO Cancer Survivorship Expert Panel. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 4.Marnitz S, Garcia-Gonzalez E, Selvi E, Akhmetshina A, Palumbo K, Lorenzini S, Maggio R, Lucattelli M, Galeazzi M, Distler JW. Long-term results of total body irradiation in adults with acute lymphoblastic leukemia. Strahlenther Onkol. 2014;190:453–458. doi: 10.1007/s00066-014-0607-3. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, Bar-Joseph Z, Bitterman P, Blackburn MR, Bradford W, et al. Future directions in idiopathic pulmonary fibrosis research: an NHLBI workshop report. Am J Respir Crit Care Med. 2014;189:214–222. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gogali A, Wells AU. New pharmacological strategies for the treatment of pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:353–366. doi: 10.1177/1753465810379454. [DOI] [PubMed] [Google Scholar]
- 7.Marquart S, Zerr P, Akhmetshina A, Palumbo K, Reich N, Tomcik M, Horn A, Dees C, Engel M, Zwerina J, et al. Inactivation of the cannabinoid receptor CB1 prevents leukocyte infiltration and experimental fibrosis. Arthritis Rheum. 2010;62:3467–3476. doi: 10.1002/art.27642. [DOI] [PubMed] [Google Scholar]
- 8.Balistreri E, Garcia-Gonzalez E, Selvi E, Akhmetshina A, Palumbo K, Lorenzini S, Maggio R, Lucattelli M, Galeazzi M, Distler JW. The cannabinoid WIN55, 212–2 abrogates dermal fibrosis in scleroderma bleomycinmodel. Ann Rheum Dis. 2011;70:695–699. doi: 10.1136/ard.2010.137539. [DOI] [PubMed] [Google Scholar]
- 9.Servettaz A, Kavian N, Nicco C, Deveaux V, Chéreau C, Wang A, Zimmer A, Lotersztajn S, Weill B, Batteux F. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am J Pathol. 2010;177:187–196. doi: 10.2353/ajpath.2010.090763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazzerini PE, Natale M, Gianchecchi E, Capecchi PL, Montilli C, Zimbone S, Castrichini M, Balistreri E, Ricci G, Selvi E, et al. Adenosine A2A receptor activation stimulates collagen production in sclerodermic dermal fibroblasts either directly and through a cross-talk with the cannabinoid system. J Mol Med (Berl) 2012;90:331–342. doi: 10.1007/s00109-011-0824-5. [DOI] [PubMed] [Google Scholar]
- 11.Patsenker E, Stoll M, Millonig G, Agaimy A, Wissniowski T, Schneider V, Mueller S, Brenneisen R, Seitz HK, Ocker M, et al. Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Mol Med. 2011;17:1285–1294. doi: 10.2119/molmed.2011.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 13.Trebicka J, Racz I, Siegmund SV, Cara E, Granzow M, Schierwagen R, Klein S, Wojtalla A, Hennenberg M, Huss S, et al. Role of cannabinoid receptors in alcoholic hepatic injury: steatosis and fibrogenesis are increased in CB2 receptor-deficient mice and decreased in CB1 receptor knockouts. Liver Int. 2011;31:860–870. doi: 10.1111/j.1478-3231.2011.02496.x. [DOI] [PubMed] [Google Scholar]
- 14.Reichenbach V, Ros J, Fernández-Varo G, Casals G, Melgar-Lesmes P, Campos T, Makriyannis A, Morales-Ruiz M, Jiménez W. Prevention of fibrosis progression in CCl4-treated rats: role of the hepatic endocannabinoid and apelinsystems. J Pharmacol Exp Ther. 2012;340:629–637. doi: 10.1124/jpet.111.188078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slavic S, Lauer D, Sommerfeld M, Kemnitz UR, Grzesiak A, Trappiel M, Thöne-Reineke C, Baulmann J, Paulis L, Kappert K, et al. Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J Mol Med (Berl) 2013;91:811–823. doi: 10.1007/s00109-013-1034-0. [DOI] [PubMed] [Google Scholar]
- 16.Lin CL, Hsu YC, Lee PH, Lei CC, Wang JY, Huang YT, Wang SY, Wang FS. Cannabinoid receptor 1 disturbance of PPARγ2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. J Mol Med (Berl) 2014;92:779–792. doi: 10.1007/s00109-014-1125-6. [DOI] [PubMed] [Google Scholar]
- 17.Mathew B, Jacobson JR, Berdyshev E, Huang Y, Sun X, Zhao Y, Gerhold LM, Siegler J, Evenoski C, Wang T, et al. Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. FASEB J. 2011;25:3388–3400. doi: 10.1096/fj.11-183970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorshkova I, Zhou T, Mathew B, Jacobson JR, Takekoshi D, Bhattacharya P, Smith B, Aydogan B, Weichselbaum RR, Natarajan V, et al. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J Lipid Res. 2012;53:1553–1568. doi: 10.1194/jlr.M026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limebeer CL, Vemuri VK, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A, Parker LA. Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–349. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ, Santerre JL, Makriyannis A, Salamone JD. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol Biochem Behav. 2010;97:179–184. doi: 10.1016/j.pbb.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Down JD, Yanch JC. Identifying the high radiosensitivity of the lungs of C57L mice in a model of total-body irradiation and bone marrow transplantation. Radiat Res. 2010;174:258–263. doi: 10.1667/RR2149.1. [DOI] [PubMed] [Google Scholar]
- 23.Jackson IL, Xu P, Hadley C, Katz BP, McGurk R, Down JD, Vujaskovic Z. Preclinical rodent model of radiation-induced lung injury for medical countermeasure screening in accordance with the FDA animal rule. Health Phys. 2012;103:463–473. doi: 10.1097/HP.0b013e31826386ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178:505–523. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gostner JM, Becker K, Fuchs D, Sucher R. Redox regulation of the immune response. Redox Rep. 2013;18:88–94. doi: 10.1179/1351000213Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girinsky T, Cosset JM. Pulmonary and cardiac late effects of ionizing radiations alone or combined with chemotherapy. Cancer Radiother. 1997;1:735–743. doi: 10.1016/s1278-3218(97)82950-2. [DOI] [PubMed] [Google Scholar]
- 27.Tsuboi M, Le Chevalier T. Interstitial lung disease in patients with non-small-cell lung cancer treated with epidermal growth factor receptor inhibitors. Med Oncol. 2006;23:161–170. doi: 10.1385/MO:23:2:161. [DOI] [PubMed] [Google Scholar]
- 28.Li M, Ping G, Plathow C, Trinh T, Lipson KE, Hauser K, Krempien R, Debus J, Abdollahi A, Huber PE. Small molecule receptor tyrosine kinase inhibitor of platelet-derived growth factor signaling (SU9518) modifies radiation response in fibroblasts and endothelial cells. BMC Cancer. 2006;6:79. doi: 10.1186/1471-2407-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Abdollahi A, Gröne HJ, Lipson KE, Belka C, Huber PE. Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model. Radiat Oncol. 2009;4:66. doi: 10.1186/1748-717X-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monceau V, Pasinetti N, Schupp C, Pouzoulet F, Opolon P, Vozenin MC. Modulation of the Rho/ROCK pathway in heart and lung after thorax irradiation reveals targets to improve normal tissue toxicity. Curr Drug Targets. 2010;11:1395–1404. doi: 10.2174/1389450111009011395. [DOI] [PubMed] [Google Scholar]
- 31.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen SW, Wu BY, Xu SP, Fan KX, Yan L, Gong Y, Wen JB, Wu DH. Suppression of CB1 cannabinoid receptor by lentivirus mediated small interfering RNA ameliorates hepatic fibrosis in rats. PLoS One. 2012;7:e50850. doi: 10.1371/journal.pone.0050850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Y, Kang XL, Wang X. The peripheral cannabinoid receptor 1 antagonist VD60 efficiently inhibits carbon tetrachloride-intoxicated hepatic fibrosis progression. Exp Biol Med (Maywood) 2014;239:183–192. doi: 10.1177/1535370213514922. [DOI] [PubMed] [Google Scholar]
- 34.Rajesh M, Bátkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horváth B, Holovac E, Cinar R, Liaudet L, Mackie K, et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–727. doi: 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Remessy AB, Rajesh M, Mukhopadhyay P, Horváth B, Patel V, Al-Gayyar MM, Pillai BA, Pacher P. Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia. 2011;54:1567–1578. doi: 10.1007/s00125-011-2061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han KH, Lim S, Ryu J, Lee CW, Kim Y, Kang JH, Kang SS, Ahn YK, Park CS, Kim JJ. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc Res. 2009;84:378–386. doi: 10.1093/cvr/cvp240. [DOI] [PubMed] [Google Scholar]
- 37.Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. 2009;119:28–36. doi: 10.1161/CIRCULATIONAHA.108.811992. [DOI] [PubMed] [Google Scholar]
- 38.Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukhopadhyay P, Rajesh M, Bátkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Haskó G, Pacher P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773–784. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 41.Gkoumassi E, Dekkers BG, Dröge MJ, Elzinga CR, Schmidt M, Meurs H, Zaagsma J, Nelemans SA. Virodhamine and CP55,940 modulate cAMP production and IL-8 release in human bronchial epithelial cells. Br J Pharmacol. 2007;151:1041–1048. doi: 10.1038/sj.bjp.0707320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice W, Shannon JM, Burton F, Fiedeldey D. Expression of a brain-type cannabinoid receptor (CB1) in alveolar type II cells in the lung: regulation by hydrocortisone. Eur J Pharmacol. 1997;327:227–232. doi: 10.1016/s0014-2999(97)89665-3. [DOI] [PubMed] [Google Scholar]
- 43.Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathan PJ, O'Neill BV, Napolitano A, Bullmore ET. Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci Ther. 2011;17:490–505. doi: 10.1111/j.1755-5949.2010.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chorvat RJ. Peripherally restricted CB1 receptor blockers. Bioorg Med Chem Lett. 2013;23:4751–4760. doi: 10.1016/j.bmcl.2013.06.066. [DOI] [PubMed] [Google Scholar]