Abstract
Endothelin-1 (ET-1) plays a critical role in endothelial dysfunction and contributes to the pathogenesis of pulmonary hypertension (PH). We hypothesized that peroxisome proliferator–activated receptor γ (PPARγ) stimulates microRNAs that inhibit ET-1 and pulmonary artery endothelial cell (PAEC) proliferation. The objective of this study was to clarify molecular mechanisms by which PPARγ regulates ET-1 expression in vitro and in vivo. In PAECs isolated from patients with pulmonary arterial hypertension, microRNA (miR)-98 expression was reduced, and ET-1 protein levels and proliferation were increased. Similarly, hypoxia reduced miR-98 and increased ET-1 levels and PAEC proliferation in vitro. In vivo, hypoxia reduced miR-98 expression and increased ET-1 and proliferating cell nuclear antigen (PCNA) levels in mouse lung, derangements that were aggravated by treatment with the vascular endothelial growth factor receptor antagonist Sugen5416. Reporter assays confirmed that miR-98 binds directly to the ET-1 3′-untranslated region. Compared with littermate control mice, miR-98 levels were reduced and ET-1 and PCNA expression were increased in lungs from endothelial-targeted PPARγ knockout mice, whereas miR-98 levels were increased and ET-1 and PCNA expression was reduced in lungs from endothelial-targeted PPARγ–overexpression mice. Gain or loss of PPARγ function in PAECs in vitro confirmed that alterations in PPARγ were sufficient to regulate miR-98, ET-1, and PCNA expression. Finally, PPARγ activation with rosiglitazone regimens that attenuated hypoxia-induced PH in vivo and human PAEC proliferation in vitro restored miR-98 levels. The results of this study show that PPARγ regulates miR-98 to modulate ET-1 expression and PAEC proliferation. These results further clarify molecular mechanisms by which PPARγ participates in PH pathogenesis and therapy.
Keywords: PPARγ, ET-1, pulmonary hypertension, hypoxia, miR-98
Clinical Relevance
These findings provide the first description that targeting peroxisome proliferator–activated receptor γ regulates critical mediators in pulmonary hypertension (PH) pathogenesis through modulation of microRNA (miRNA) levels. These results suggest that paradigms involving pharmacological manipulation of miRNA represent promising strategies to improve PH therapeutics.
Pulmonary hypertension (PH) is a complex disorder that involves the activation of pathways that stimulate endothelial dysfunction, increased production of vasoconstrictors, reduced production of vasodilators, and enhanced proliferation of cells in the pulmonary vascular wall (1). For example, endothelin-1 (ET-1) (2–4) is a potent vasoconstrictor whose concentrations in plasma are elevated in patients with pulmonary arterial hypertension (PAH) (5, 6), and ET-1 mRNA and protein expression are increased in endothelial cells isolated from patients with PAH and have been shown to correlate with increased pulmonary vascular resistance (5). Despite the use of pharmacological antagonists targeting ET-1 signaling, PH continues to cause significant morbidity and mortality (7, 8). These observations suggest that novel therapeutic strategies simultaneously targeting multiple dysregulated pathways in PH could provide additional benefit in this disease.
Recent studies have suggested that targeting the nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ) can favorably modulate several pathways widely believed to participate in PH pathogenesis (9, 10). PH has been associated with reductions in PPARγ (11–13), whereas activation of PPARγ with thiazolidinedione (TZD) ligands attenuates PH in experimental animal models (13–16). We recently demonstrated that TZD-mediated PPARγ targeting attenuated PH and ET-1 signaling pathway components in hypoxia-exposed mice (16, 17). Although TZD ligands, such as rosiglitazone and pioglitazone, are currently available for treatment of type 2 diabetes, their use has been associated with controversial adverse effects (18, 19), indicating that additional studies to define the therapeutic mechanisms of PPARγ action may facilitate development of new therapies for use in PH therapy.
Because the PPARγ ligand rosiglitazone attenuated PH and ET-1 signaling in experimental PH (17), the current study was designed to further define the molecular mechanisms underlying these beneficial effects. Based on recent observations that microRNA (miRNA or miR)-27a mediated hypoxic reductions in PPARγ (20), we sought to explore the ability of PPARγ to attenuate ET-1 expression through post-transcriptional mechanisms. miRNAs are endogenous, noncoding, single-stranded RNAs comprised of approximately 22 nucleotides that constitute a novel class of post-transcriptional gene regulators (21). miRNAs negatively regulate gene expression through translational repression or mRNA degradation (21). Currently, 2,588 unique mature human miRNAs have been identified that regulate approximately 30% of the genes in the human genome (miRBase version 20) (22, 23). Because miRNAs contribute to the regulation of fundamental cellular processes such as proliferation, differentiation, and apoptosis (24), they are widely believed to contribute to human physiology and disease (25). Altered miRNA expression after hypoxia (26) and in pulmonary vascular cells from patients with PAH (27) supports the roles of miRNAs in pulmonary vascular cell proliferation and PH pathogenesis (28).
Based on in silico analysis of the ET-1 3′ untranslated region (3′UTR), the current study focused on the role of miR-98 in the regulation of ET-1. Members of the ubiquitously expressed miR-98/let-7 family inhibit cell growth responses (29) and function as tumor suppressors in lung cancer (30, 31), in part through regulation of apoptosis (32, 33) and inflammation (34). Our study provides novel evidence that hypoxia, a common stimulus for pulmonary vascular cell proliferation and PH, decreases miR-98 levels to increase ET-1 expression and pulmonary artery endothelial cell proliferation. Further, PPARγ activation increases miR-98 levels to suppress ET-1 and pulmonary vascular cell proliferation and PH. These findings provide the first description that targeting PPARγ regulates critical mediators in PH pathogenesis through modulation of miRNA levels. These results indicate that paradigms involving pharmacological manipulation of miRNA represent promising strategies to improve PH therapeutics.
Materials and Methods
Primary Human Pulmonary Artery Endothelial Cells
Pulmonary artery endothelial cells (PAECs) isolated from the lungs of control subjects or patients with idiopathic pulmonary arterial hypertension (IPAH) (35) were generously provided by Dr. Suzy Comhair (Cleveland Clinic, Cleveland, OH).
Mouse Model of PH
To examine PH in vivo, two established mouse models were used (16, 36). Male C57BL/6 mice (8–12 wk old), purchased from the Jackson Laboratory (Bar Harbor, ME), were exposed to hypoxia (HYP, 10% O2) or normoxia (NOR, room air, 21% O2) for 3 weeks, and animals were gavaged daily with rosiglitazone (RSG, 10 mg/kg/d) or with an equivalent volume of vehicle for the final 10 days of exposure as we have reported (16). To induce more severe PH, as recently reported (37, 38), C57BL/6 mice were also exposed to hypoxia for 3 weeks with or without treatment with the vascular endothelial growth factor receptor (VEGFR) antagonist SU5416 (20 mg/kg by subcutaneous injection once per week for 3 wk) or with vehicle (0.5% wt/vol carboxymethylcellulose, 0.9% wt/vol sodium chloride, 0.4% vol/vol polysorbate 80, and 0.9% vol/vol benzyl alcohol in deionized water) (37) alone. PH was assessed as reported (16). Additional information is provided in the online supplement.
Endothelial-Targeted PPARγ Knockout and PPARγ Overexpression Mice
To examine the role of PPARγ in regulating miR-98, mice with endothelial-targeted loss (39) or gain of PPARγ function were used. For endothelial-targeted PPARγ knockout (ePPARγ KO) mice, Tie2-Cre transgenic mice expressing Cre recombinase driven by the Tie2 promoter were crossed with mice carrying a floxed PPARγ gene to produce ePPARγ KO mice as we reported (39). The ePPARγ KO mice have been previously reported to develop spontaneous PH under normoxic conditions (12) but display no other gross phenotypic changes. Littermate control and ePPARγ KO mice on a C57BL/6 background were expanded from breeding pairs. For endothelial-targeted PPARγ overexpression (ePPARγ OE) mice, a constitutively active PPARγ mutant (VP16-PPARγ) was generated by fusing the transcriptional activation domain of HSV VP16 to the N terminus of mouse PPARγ1 as previously reported (40). DNA isolated from tail snips of offspring from these crosses were genotyped, and littermate control (floxVP16-PPARγ−; Tie2Cre×) and ePPARγ OE mice (floxVP16-PPARγ+; Tie2Cre+) were selected for additional study (see Figure E1 in the online supplement). The ePPARγ OE mice have not been previously published, and our manuscript characterizing this model is currently under revision. The ePPARγ OE mice are normal in appearance and behavior. All mice were fully backcrossed into a C57BL/6 background. All animal studies were approved by the Institutional Animal Care and Use Committee of the Atlanta Veterans Affairs Medical Center.
Laser-Capture Microdissection
To further confirm up-regulation of ET-1 in the pulmonary vasculature of hypoxia-exposed mice, laser-capture microdissection was used to select the profiles of small pulmonary arteries (50–100 μm in diameter) from lung sections of control and hypoxia-exposed mice. We used the same mouse lungs that we have previous published (16). Additional information is provided in the online supplement.
Immunohistochemistry
To conform the impact of Hypoxia-Sugen5416 in the mouse on the remodeling and muscularization of small pulmonary vessels, the lungs were isolated, pressure perfused, fixed, sectioned, and stained with antibodies to α smooth muscle actin (α-SMA). The number of α-SMA–positive vessels that were 50 to 100 μm in diameter was determined per mm2 as previously described (16). Additional information is provided in the online supplement.
PPARγ Knockdown or Overexpression in PAECs In Vitro
Once 40 to 50% confluent, selected PAECs were transfected with PPARγ small interfering RNA (siRNA) (100 nM) using the GeneSilencer (Genlantis, San Diego, CA) transfection reagent according to the manufacturer’s instructions as we have reported (20). Control cells were exposed to transfectant containing scrambled siRNA. To induce PPARγ overexpression, human PAECs (HPAECs) were transfected with adenovirus containing a PPARγ plasmid (25–50 multiplicity of infection) or control green fluorescent protein (GFP) plasmid. Six hours after transfection, adenovirus-containing medium was replaced by fresh 10% FBS endothelial growth medium, and alterations in miR-98, ET-1, proliferating cell nuclear antigen (PCNA), and PPARγ levels were confirmed by quantitative RT-PCR (qRT-PCR).
ET-1 3′UTR Luciferase Reporter Studies
The pLightSwitch empty-vector (pLS) and pLS-ET-1–3′UTR construct containing the 3′UTR of human ET-1 (NM_001955), the putative binding site for miR-98, were purchased from SwitchGear Genomics (Carlsbad, CA). PAECs (1 × 104) were seeded into 24-well plates, incubated for 24 hours, and washed with PBS, and then fresh growth medium was added before the addition of transfection complexes. For luciferase assays, 200 ng of pLS or pLS-ET-1–3′UTR construct with 10 nM miR-98 mimic or scrambled siRNA were transiently cotransfected into PAECs using lipofectamine RNAiMax (Invitrogen, Grand Island, NY). PAEC lysates were harvested and analyzed for luciferase activity using the Luciferase Reporter Assay System (SwitchGear Genomics), and luciferase activity was measured using a Luminometer (PerkinElmer). Relative light units were normalized to control group luciferase activity.
miR-98 Down-Regulation and Overexpression
To confirm the role of miR-98 in alterations in ET-1 expression, PAECs (passages 2–7) were transfected with lipofectamine RNAiMax containing anti–miR-98 (5–50 nM), anti-miR negative control (50 nM), mimic miR-98 (1–10 nM), or scrambled mimic (10 nM) according to the manufacturer’s (Qiagen) instructions as we have previously reported (20). Six hours after transfection, serum-free medium was replaced with 10% FBS endothelial growth medium. HPAECs were exposed to control conditions (21% O2) in a standard incubator or to hypoxia (1% O2) in a Biospherix exposure chamber for 72 hours as we have previously reported (17), and then alterations in miR-98, ET-1, and PCNA levels were confirmed by qRT-PCR. The relative abundance of these targets was reflected by the mean number of cycles of qRT-PCR amplification required for their detection (mean Ct values: miR-98 = 21.3 and RNU6B = 14.4 in HPAECs; miR-98 = 19.3 and RNU6B = 12.1 in mouse lungs).
Statistical Analysis
Data from studies with more than two groups were analyzed using ANOVA. Post hoc analysis using the Student-Neuman-Keuls test was used to detect differences between specific groups. In studies comparing only two experimental groups, data were analyzed with Student’s t test to determine the significance of treatment effects. The level of statistical significance was taken as P < 0.05. All results are reported as mean ± SE.
Results
miR-98 Is Down-Regulated in Hypoxia-Exposed Mice and PAECs
Increased levels of ET-1 contribute to PH pathogenesis. To explore the role of post-transcriptional regulation in increased ET-1 levels, ET-1–targeted miRNAs were identified with a bioinformatic approach using multiple prediction algorithms (miRBase, PicTar, and TargetScan v6.1). These analyses confirmed that the ET-1 3′UTR contains binding sites for the seed sequences of miR-1/206 and miR-98/let-7 families (41), which are highly conserved in human and mouse. We then screened for hypoxia-induced alterations in the levels of these miRNAs. Of all the miRNAs examined, only miR-98 was significantly down-regulated by hypoxia in PAECs and in mouse lung (Figure E2). Therefore, we focused on miR-98 to examine its potential role in regulating ET-1 in PH pathogenesis and therapy.
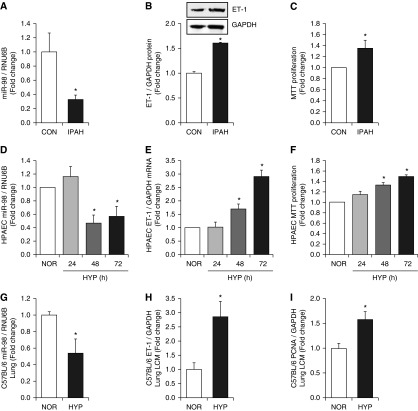
To establish that miR-98 dysregulation occurs in PH, we analyzed miR-98 levels in PAECs isolated from control subjects or patients with IPAH. miR-98 expression was reduced in PAECs isolated from patients with IPAH compared with control subjects (Figure 1A) and was associated with enhanced PAEC ET-1 protein levels (Figure 1B) and PAEC proliferation (Figure 1C). Similarly, hypoxia, a common stimulus causing PH, reduced miR-98 levels by 60% after 48 hours of hypoxia exposure (Figure 1D) and caused concomitant increases in ET-1 levels (Figure 1E) and PAEC proliferation (Figure 1F). Exposing mice to hypoxic regimens in vivo that induce significant PH, right ventricular hypertrophy (RVH), and pulmonary vascular remodeling (16) had similar effects. Laser-capture microdissection was used to isolate tissue from small pulmonary arteries in the mouse lung (Figure E3). Hypoxia reduced miR-98 expression (Figure 1G) and increased ET-1 (Figure 1H) and PCNA levels (Figure 1I) in small pulmonary arteries in hypoxia-exposed mouse lung. As illustrated in Figure E4, correlation analysis showed that hypoxia-induced reductions in mouse lung miR-98 levels were associated with previously reported (16) increases in right ventricular systolic pressure (RVSP) (r = −0848; P < 0.002).
Figure 1.
Reductions in microRNA (miR)-98 are associated with increased endothelin-1 (ET-1) levels and proliferation in pulmonary artery endothelial cells (PAECs) in vitro and in lung in vivo. (A–C) PAECs were isolated from the lungs of control (CON) subjects or patients with idiopathic pulmonary arterial hypertension (IPAH) as reported (35). (A) Quantitative RT-PCR was performed for miR-98, and the results are expressed relative to RNU6B as fold-change versus control. PAECs were derived from five CON subjects and four patients with IPAH. (B) ET-1 levels were measured with Western blotting and expressed relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein as fold-change versus control. Representative immunoblots are presented above the bar graph (n = 3). (C) Cell proliferation was determined using MTT assays, and results are expressed as fold-change versus control (n = 4–5). *P < 0.05 versus CON. (D–F) PAECs were exposed to normoxia (NOR, 21% O2) or hypoxia (HYP, 1% O2) for 24, 48, or 72 hours as reported (17). (D) miR-98 levels are expressed relative to RNU6B as fold change versus NOR (n = 3). (E) ET-1 mRNA levels are expressed relative to GAPDH mRNA as fold-change versus NOR (n = 3–6). (F) Cell proliferation was determined using MTT assays, and results are expressed as fold-change versus NOR (n = 3). (G–I) Whole lung homogenates were collected from mice exposed to normoxia (NOR, 21% O2) or hypoxia (HYP, 10% O2) for 3 weeks. (G) Lung miR-98 levels are expressed relative to RNU6B as fold change versus NOR (n = 5). Small pulmonary arteries were isolated from sections of mouse lung with laser capture microscopy (LCM), and mRNA levels of ET-1 (H) and proliferating cell nuclear antigen (PCNA) (I) are expressed relative to GAPDH as fold change versus NOR (n = 6–8). *P < 0.05 versus NOR.
miR-98 Is Down-Regulated in a Mouse Model of Severe PH
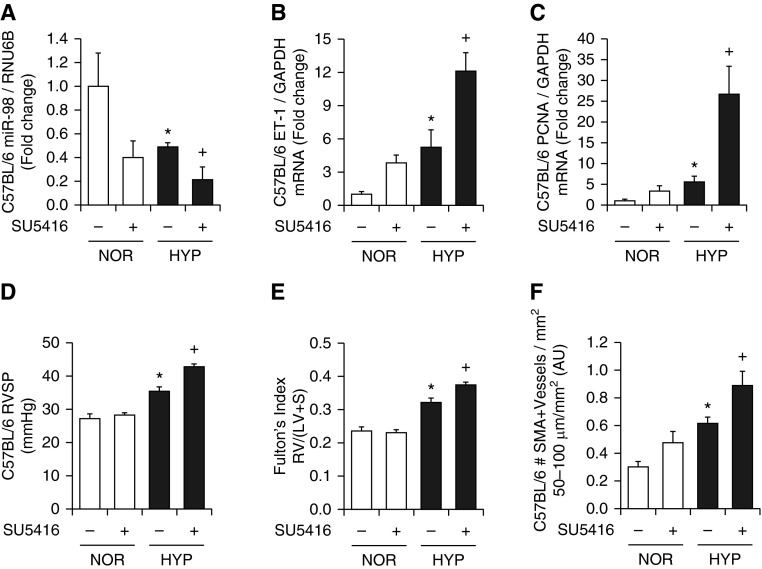
We previously demonstrated that chronic hypoxia increased ET-1 expression and PH in mice in vivo and in PAECs in vitro (17). Because treatment with chronic hypoxia and the VEGFR antagonist SU5416 produces more severe PH in the mouse than hypoxia alone (37, 38), we also examined miR-98 and ET-1 levels in this model. Treatment with either hypoxia or SU5416 alone reduced lung miR-98 levels, and the addition of SU5416 exacerbated hypoxic reductions in miR-98 (Figure 2A). Treatment with SU5416 also exacerbated hypoxic increases in ET-1 (Figure 2B) and PCNA (Figure 2C) mRNA levels, RVSP (Figure 2D) and RVH (Figure 2E), and α-SMA (Figure 2F).
Figure 2.
The vascular endothelial growth factor receptor antagonist SU5614 exacerbates hypoxia-induced reductions in miR-98, increases in lung ET-1 and PCNA expression, and increases in right ventricular systolic pressure (RVSP) and right ventricular hypertrophy. Mice were exposed to NOR or HYP with or without SU5416 (20 mg/kg) weekly for 3 weeks. Whole lung homogenates were prepared, and quantitative RT-PCR was performed. (A) miR-98 levels are expressed relative to RNU6B as fold change versus NOR. ET-1 (B) and PCNA (C) mRNA levels are expressed relative to GAPDH as fold-change versus NOR (n = 3–5). (D) RVSP is presented in mm Hg (n = 3–5). (E) The right ventricular to left ventricular plus septum weight ratio [RV:(LV + S)] is presented as an index of right ventricular hypertrophy (n = 3–5). *P < 0.05 versus NOR; +P < 0.05 versus HYP. (F) The number of α smooth muscle actin (α-SMA)-positive vessels (50–100 μm in luminal diameter) per mm2 was determined. Each bar represents the mean ± SE α-SMA–positive vessels per mm2 (n = 8). *P < 0.05 versus NOR; +P < 0.05 versus HYP. AU, arbitrary units.
ET-1 Is a miR-98 Target Gene
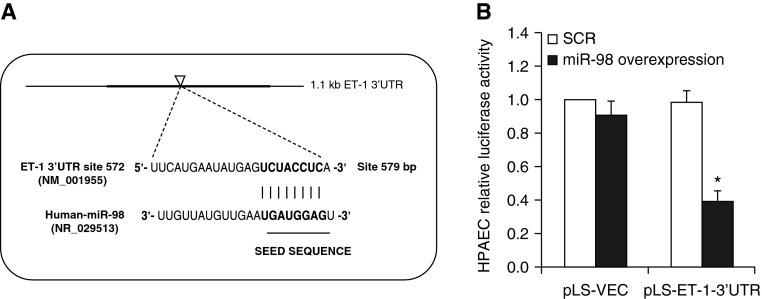
To determine if miR-98 binds to the ET-1 3′UTR, the human 1,199-bp ET-1 mRNA 3′-UTR containing a putative miR-98 binding site (Figure 3A) was cloned into a luciferase reporter vector. PAECs were cotransfected with miR-98 mimic and pLS-ET-1–3′UTR luciferase reporter constructs. Compared with scrambled miRNA, miR-98 mimic caused 60% repression of ET-1 3′UTR luciferase activity (Figure 3B).
Figure 3.
ET-1 is a miR-98 target gene. (A) Schematic illustration of the human ET-1 3′UTR, which contains a putative binding site (arrowhead) for miR-98. The miR-98 seed sequence is shown in bold font. (B) PAECs were transfected with pLightSwitch empty-vector (pLS-ET-1)-3′UTR (ET-1–3′UTR) luciferase reporter constructs or empty luciferase vector (VEC) + miR-98 mimic or scrambled miRNA (SCR) as described in Materials and Methods. After 72 hours, PAEC lysates were harvested and analyzed for luciferase activities. Each bar represents luciferase activity in relative light units as fold control versus PAECs transfected with VEC and treated with SCR construct (n = 3). *P < 0.05 versus SCR.
PAEC ET-1 Expression and Proliferation Are Reduced by miR-98 Overexpression and Increased by a miR-98 Antagonist
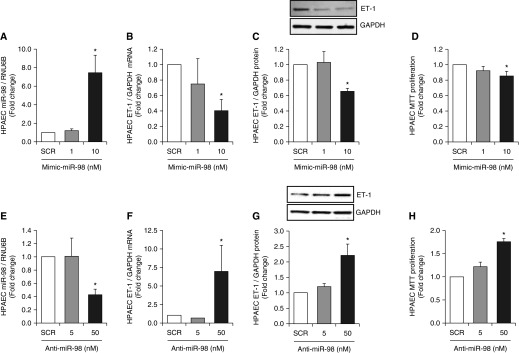
To examine the effect of miR-98 overexpression on ET-1 and PAEC proliferation, miR-98 gain-of-function was accomplished by transfecting PAECs with a miR-98 mimic. As shown in Figure 4A, 10 nM, but not 1 nM, miR-98 mimic significantly increased miR-98 concentrations. Overexpression of miR-98 also reduced ET-1 mRNA and protein expression (Figures 4B and 4C) and reduced PAEC proliferation (Figure 4D). In contrast, miR-98 loss-of-function was accomplished by transfecting PAECs with a miR-98 inhibitor. As shown in Figure 4E, 50 nM, but not 5 nM, anti–miR-98 reduced PAEC miR-98 expression and increased PAEC ET-1 mRNA and protein expression (Figures 4F and 4G) and PAEC proliferation (Figure 4H). Taken together, the findings in Figures 3 and 4 provide additional evidence that post-transcriptional mechanisms involving miR-98 regulate PAEC ET-1 expression and proliferation.
Figure 4.
miR-98 gain and loss of function causes divergent effects on PAEC ET-1 expression and proliferation. (A–D) PAECs were transfected with either scrambled (SCR, 10 nM) or 1 to 10 nM mimic–miR-98 (n = 4–6). (E–H) PAECs were transfected with either scrambled (SCR, 50 nM) or 5 to 50 nM anti–miR-98 (n = 3–6). In A and E, quantitative RT-PCR was performed to measure miR-98 levels that are expressed relative to RNU6B as fold change versus SCR. In B and F, quantitative RT-PCR was performed to measure ET-1 mRNA levels expressed relative to GAPDH as fold change versus SCR. In C and G, Western blotting was used to measure PAEC ET-1 protein levels that are expressed relative to GAPDH as fold-change versus SCR. Representative immunoblots are presented below their corresponding bar graphs. In D and H, PAEC proliferation was determined using MTT assays and is expressed as fold-change versus SCR. *P < 0.05 versus SCR.
PPARγ Regulates miR-98 and ET-1 Expression
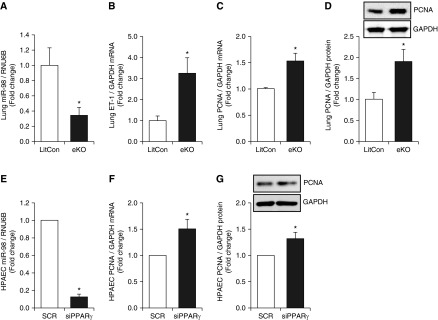
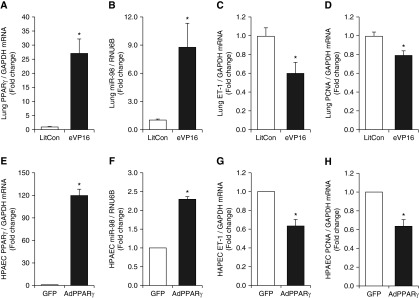
We have previously reported that hypoxic regimens that stimulate PAEC ET-1 expression and proliferation in vitro and ET-1 expression and PH in vivo are sufficient to significantly reduce PPARγ and that activating PPARγ attenuates these hypoxic alterations (17). To determine if alterations in PPARγ expression are sufficient to regulate miR-98 expression, mice with endothelial-targeted loss- or gain-of-function were used. In lungs from mice with endothelial-targeted PPARγ loss-of-function, miR-98 levels were reduced (Figure 5A), whereas levels of ET-1 (Figure 5B) and PCNA (Figures 5C and 5D) were significantly increased. We previously reported that PPARγ knockdown increased PAEC ET-1 expression in vitro (20). siRNA-mediated PPARγ knockdown reduced miR-98 expression (Figure 5E) and increased PCNA mRNA (Figure 5F) and protein (Figure 5G) expression. Compared with lungs from littermate control mice, lungs from ePPARγ OE mice had increased PPARγ mRNA (Figure 6A) and miR-98 (Figure 6B) levels and decreased ET-1 (Figure 6C) and PCNA (Figure 6D) mRNA levels. These findings indicate that PPARγ positively regulates miR-98 to attenuate ET-1 and cellular proliferation. Similarly, adenoviral-mediated PAEC PPARγ overexpression increased PPARγ mRNA (Figure 6E) and miR-98 (Figure 6F) levels and decreased ET-1 (Figure 6G) and PCNA (Figure 6H) mRNA levels. Collectively, the findings in Figures 5 and 6 demonstrate that endothelial PPARγ regulates miR-98, ET-1, and PAEC proliferation.
Figure 5.
Peroxisome proliferator–activated receptor γ (PPARγ) loss of function reduces miR-98 in vivo and in vitro and stimulates ET-1 expression and proliferation. (A–D) Whole lung homogenates were collected from littermate control (LitCon) or ePPARγ knockout mice (eKO) as previously reported (39) (n = 4–8). (A) Lung miR-98 levels are expressed relative to lung RNU6B as fold-change versus LitCon. Lung ET-1 (B) and PCNA (C) mRNA levels are expressed relative to GAPDH as fold-change versus LitCon. (D) Lung PCNA protein levels were measured with Western blotting and are expressed relative to GAPDH protein as fold-change versus LitCon. *P < 0.05 versus LitCon. (E–G) PAECs were transfected with SCR or PPARγ siRNA (siPPARγ) for 72 hours. (E) miR-98 levels are expressed relative to RNU6B as fold-change versus SCR (n = 3). (F) PCNA mRNA levels are expressed relative to GAPDH as fold change versus SCR (n = 10). (G) PAEC PCNA protein levels were determined with Western blotting and are expressed relative to GAPDH protein as fold-change versus SCR. A representative Western blot is presented above the bar graph. *P < 0.05 versus SCR.
Figure 6.
PPARγ gain-of-function increases miR-98 in vivo and in vitro and attenuates ET-1 expression and proliferation. (A–D) Whole lung homogenates were collected from LitCon or ePPARγ-overexpression mouse (eVP16) lungs (n = 5). (A) Lung PPARγ mRNA levels are expressed relative to GAPDH in the same sample as fold-change versus LitCon. (B) Lung miR-98 levels are expressed relative to lung RNU6B as fold-change versus LitCon. (C) ET-1 mRNA levels are expressed relative to GAPDH as fold-change versus LitCon. (D) PCNA mRNA levels are expressed relative to GAPDH as fold-change versus LitCon. *P < 0.05 versus LitCon. (E–H) PAECs were transfected with adenovirus containing a PPARγ plasmid (AdPPARγ) or green fluorescent protein (GFP) constructs for 72 hours, and miRNA or mRNA were isolated and subjected to quantitative RT-PCR analysis (n = 3). (E) PAEC PPARγ mRNA levels are expressed relative to GAPDH in the same sample as fold-change versus GFP. (F) PAEC miR-98 levels are expressed relative to RNU6B as fold-change versus GFP. (G) PAEC ET-1 mRNA levels are expressed relative to GAPDH as fold-change versus GFP. (H) PAEC PCNA mRNA levels are expressed relative to GAPDH as fold-change versus GFP. *P < 0.05 versus GFP.
PPARγ Activation Restores miR-98 Levels in Hypoxia-Exposed Mouse Lung or PAECs
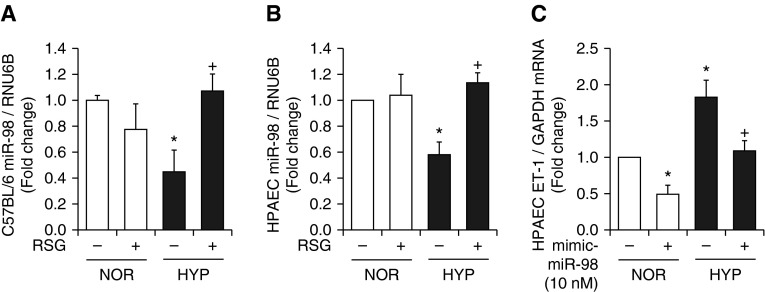
Based on prior evidence that hypoxic regimens that stimulated PAEC ET-1 expression and proliferation in vitro and ET-1 expression and PH in vivo also reduced PPARγ (16, 17) and that activating PPARγ attenuates ET-1 and PH (16, 17), we examined the impact of PPARγ ligands on hypoxic alterations in miR-98 levels in vitro and in vivo. For in vivo studies, these analyses were performed on archived mouse lung samples where the hemodynamic outcomes were previously published (16). Consistent with the findings in Figure 1G, hypoxia exposure significantly reduced miR-98 levels in mouse lung (Figure 7A). Treating mice with the PPARγ ligand rosiglitazone using a regimen that attenuated hypoxia-mediated PH, RVH, vascular remodeling, and ET-1 expression (17) restored miR-98 levels in the lung. Similarly, in PAECs exposed to a hypoxia regimen that reduced PAEC PPARγ and increased ET-1 and proliferation (17), hypoxia reduced miR-98 (Figure 7B) and increased ET-1 levels (Figure 7C), and these derangements were attenuated by treatment with RSG or miR-98 mimic, respectively. These findings demonstrate that hypoxic alterations in miR-98 are attenuated by PPARγ activation.
Figure 7.
Treatment with rosiglitazone restores miR-98 levels in hypoxia-exposed mouse lung or PAECs. (A) Whole lung homogenates were collected from mice exposed to normoxia (NOR, 21% O2) or hypoxia (HYP, 10% O2) for 3 weeks. During the last 10 days of this exposure, selected animals were treated with or without rosiglitazone (RSG; 10 mg/kg/d by gavage) as reported (16). Lung miR-98 levels are expressed relative to RNU6B as fold-change versus NOR/RSG−. *P < 0.05 versus NOR/RSG−; +P < 0.05 versus HYP/RSG− (n = 3–6). (B) PAECs were exposed to NOR (21% O2) or HYP (1% O2) for 72 hours. Selected cells were treated during the final 24 hours of exposure with RSG (10 μM). PAEC miR-98 levels are expressed relative to RNU6B as fold-change versus NOR/RSG. *P < 0.05 versus NOR/RSG; +P < 0.05 versus HYP/RSG (n = 3–6). (C) PAECs were transfected with or without miR-98 mimic and then exposed to NOR or HYP for 72 hours. PAEC ET-1 mRNA levels are expressed relative to GAPDH as fold-change versus NOR/miR-98 mimic. *P < 0.05 versus NOR/miR-98 mimic; +P < 0.05 versus HYP/miR-98 mimic (n = 3–6).
Discussion
The results presented herein provide several new observations pertinent to PH pathogenesis and therapy. These studies demonstrate for the first time that miR-98 negatively regulates ET-1 in hypoxia-induced PAEC proliferation in vitro and PH in vivo. In addition, endothelial-targeted PPARγ gain or loss of function models provide novel evidence that PPARγ regulates miR-98 expression. Consistent with these findings, stimulating PPARγ with pharmacological ligands increases miR-98 levels, attenuates ET-1 expression, and reduces pulmonary vascular cell proliferation. Collectively these findings indicate that strategies targeting PPARγ can regulate not only transcriptional but also post-transcriptional regulation of vasoactive mediators to favorably modulate pulmonary vascular cell proliferation and PH pathogenesis. Coupled with previous reports, these findings further support a role for impaired PPARγ function in PH and indicate that targeting PPARγ provides a novel strategy to therapeutically modulate programs of dysregulated gene expression that contribute to endothelial proliferation, pulmonary vascular remodeling, and PH pathogenesis.
ET-1 plays a critical role in the pathophysiology of PH (3–6) by mediating the hyperproliferative, glycolytic pulmonary vascular phenotype (42). Nonselective (43) as well as endothelin A receptor–selective antagonists (44) have been used in the treatment of PH (45, 46). Although treatment with endothelin receptor antagonists improves functional class and walk distance in patients with PAH, clinical trials have failed to detect a mortality benefit (34, 47). This limited efficacy of endothelin receptor antagonism likely relates in part to the complex pathobiology of PAH and to the multiple complex pathways involved in its pathogenesis. Thus, new strategies that regulate not only ET-1 signaling but also other pathways that participate in PH pathogenesis may provide more effective therapeutic approaches for PH therapy. The current study therefore further explored PPARγ as a novel strategy that regulates not only ET-1 (17) but also additional pathogenic mechanisms in PH, including Nox4 (16), platelet-derived growth factor (16), and thrombospondin-1 (48).
Rapidly evolving evidence demonstrates that miRNAs are essential for pulmonary vascular development and function and that miRNA dysfunction participates in the pathogenesis of PH due to chronic hypoxia (20, 27, 49–54). For example, we reported that miR-27a reduced PPARγ expression, leading to increased ET-1 expression and cellular proliferation in hypoxia-induced PH and that PPARγ activation reduced miR-27a expression and function through suppression of transcription factors such as EGR1 and SP1 (20). However, to our knowledge, the regulation of ET-1 by specific miRNAs in PH has not been investigated. Therefore, based on in silico analysis of the ET-1 3′-UTR, we postulated that miR-98 negatively regulates ET-1 expression and that PPARγ increases miR-98 to reduce ET-1 expression and PAEC proliferation. To address this question, we used gain- or loss-of-function approaches targeting miR-98 or PPARγ using in vitro and in vivo models. The potential biological relevance of these pathways to human PAH was supported by evidence that PAECs isolated from patients with PAH had lower miR-98 levels, higher ET-1 levels, and higher proliferation rates compared with PAECs isolated from control patients. Similarly, in vitro hypoxia exposure for 48 hours reduced human PAEC miR-98, and this reduction was associated with increased levels of ET-1 and PAEC proliferation. Similar reductions in lung miR-98 levels were observed in mice after exposure to hypoxia for 3 weeks and were associated with increased ET-1 levels and markers of cellular proliferation. Further, adding treatment with the VEGFR antagonist Sugen to hypoxia, which causes a more severe degree of PH in mice (55), aggravated reductions in miR-98, caused increases in ET-1 and proliferative markers, and caused more severe increases in RVSP and RVH. As predicted, miR-98 directly regulated the ET-1 3′-UTR, as illustrated by studies where miR-98 mimic significantly down-regulated the activity of an ET-1 3′-UTR luciferase reporter. The causal role of miR-98 in ET-1 regulation was demonstrated further by studies in which miR-98 mimic increased PAEC miR-98 levels and reduced ET-1 levels and proliferation, whereas anti–miR-98 reduced PAEC miR-98 levels and increased ET-1 levels and proliferation. Collectively, these findings provide compelling evidence that miR-98 regulates ET-1 expression and that dysregulation of miR-98 levels in pulmonary vascular wall cells can contribute to a proliferative phenotype.
To our knowledge, factors contributing to alterations in levels of miR-98 during PH pathogenesis have not been previously examined. Our lab and others have previously reported that PPARγ expression is reduced in pulmonary vascular wall cells during PH (12) and that stimulating PPARγ attenuates PH (16). To further determine if alterations in PPARγ regulate miR-98, mice with endothelial-targeted gain or loss of PPARγ function were used. These findings demonstrated that loss of endothelial-targeted PPARγ reduced miR-98 levels and increased ET-1 and proliferative markers, whereas gain of endothelial-targeted PPARγ increased miR-98 levels and reduced ET-1 and proliferative markers. These relationships were confirmed in vitro using human PAECs treated with either PPARγ siRNA or adenovirus containing a PPARγ plasmid. Taken together, these data suggest that stimulating PPARγ provides a novel approach whereby miR-98 levels could be pharmacologically increased to reduce ET-1 and pulmonary vascular wall cell proliferation and thereby attenuate PH pathogenesis (Figure 8). This concept was supported by studies in which hypoxia-induced reductions in miR-98 levels in mice were reversed by in vivo treatment with the PPARγ ligand rosiglitazone. Similarly, hypoxia-induced reductions in PAEC miR-98 levels in vitro were restored by treatment with rosiglitazone. These findings demonstrate that activation of PPARγ promotes miR-98 expression to reduce ET-1 expression, pulmonary vascular cell proliferation, and PH.
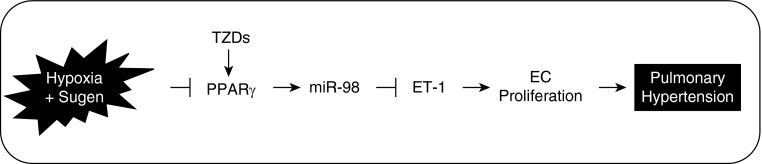
Figure 8.
PPARγ regulates ET-1 expression through miR-98 in hypoxia-induced pulmonary hypertension (PH). Hypoxia decreases PPARγ and miR-98 expression to increase levels of ET-1 that stimulate PAEC proliferation and contribute to pulmonary vascular remodeling and PH. Rosiglitazone activates PPARγ and restores miR-98 expression and attenuates hypoxic up-regulation of ET-1, which can attenuate derangements such as pulmonary vasoconstriction and vascular remodeling. TZD, thiazolidinedione. EC, endothelial cells.
Our study has several important limitations that merit additional consideration. As recently reviewed (56), the mouse models of PH used in the current study fail to replicate the proliferative, plexogenic arteriopathy seen in patients with advanced PAH. The hypoxia + SU5416 model used in the current study caused more severe PH than hypoxia alone and was reported to induce proliferative vascular lesions in the mouse (57). Reduced miR-98 levels, increased ET-1, and PAEC proliferation were common features observed in PAH PAECs and in hypoxia or hypoxia + Sugen mouse lung. Further, although we focus on PPARγ-mediated regulation of miR-98 and miR-98–mediated regulation of ET-1, this reductionist approach fails to address additional PPARγ or miR-98 targets that may also participate in PH pathogenesis. Nonetheless, the present study confirms the role of PPARγ in regulating miR-98, ET-1, and pulmonary vascular cell proliferation. The precise molecular mechanisms underlying PPARγ-mediated increases in miR-98 remain an area of active investigation in our lab. In silico analysis of the miR-98 promoter using PuTmiR software (58) indicated that the upstream promoter region of miR-98 (chromosome X: 53,587,546–53,587,567 bp) contains a putative PPARγ binding site, suggesting that PPARγ may directly regulate transcriptional expression of miR-98. However, PPARγ-mediated stimulation or repression of other gene targets may well contribute to the integrated effects of activation of this receptor in PH pathogenesis and therapy.
In summary, these findings provide novel evidence that PPARγ regulates miR-98 to modulate ET-1 and pulmonary vascular cell proliferation in PH pathogenesis. These results demonstrate that targeting PPARγ activation with pharmacological ligands can provide a novel approach to restore PPARγ activity under pathophysiological conditions where PPARγ expression is reduced. Increasing PPARγ activity increased miR-98 levels to reduce transcript and protein levels of ET-1, an important mediator of PH pathobiology. These findings suggest that modulating miR-98 levels through PPARγ may provide a new therapeutic approach to regulate ET-1 and other pathobiological mediators of PH.
Footnotes
This work was supported by Veterans Affairs Merit Review Award 1I01BX001910 (C.M.H.), by the National Institutes of Health grant HL102167 (R.L.S. and C.M.H.), by the Emory/Children’s Healthcare of Atlanta grant CEB F16788–00 (B.-Y.K.), and by the American Heart Association National Scientist Development grant 13SDG14150004 (B.-Y.K.). The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government.
Author Contributions: Conception, hypothesis delineation, and design: B.-Y.K. and C.M.H. Acquisition of data, analysis, and interpretation: B.-Y.K., K.K.P., J.M.K., T.C.M., and C.M.H. Writing of the article, B.-Y.K., D.E.G., K.M.B., S.M.Y., K.A.C., C.D.S., R.L.S., and C.M.H.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0337OC on June 22, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122:4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cacoub P, Dorent R, Nataf P, Carayon A. Endothelin-1 in pulmonary hypertension. N Engl J Med. 1993;329:1967–1968. doi: 10.1056/NEJM199312233292618. [DOI] [PubMed] [Google Scholar]
- 3.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 5.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 6.Yoshibayashi M, Nishioka K, Nakao K, Saito Y, Matsumura M, Ueda T, Temma S, Shirakami G, Imura H, Mikawa H. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects: evidence for increased production of endothelin in pulmonary circulation. Circulation. 1991;84:2280–2285. doi: 10.1161/01.cir.84.6.2280. [DOI] [PubMed] [Google Scholar]
- 7.Lilienfeld DE, Rubin LJ. Mortality from primary pulmonary hypertension in the United States, 1979–1996. Chest. 2000;117:796–800. doi: 10.1378/chest.117.3.796. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Chen J, Gao Y, Deng B, Liu K. Endothelin receptor antagonists for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2013;2:CD004434. doi: 10.1002/14651858.CD004434.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabinovitch M. PPARgamma and the pathobiology of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:447–458. doi: 10.1007/978-1-60761-500-2_29. [DOI] [PubMed] [Google Scholar]
- 10.Sutliff RL, Kang BY, Hart CM. PPARgamma as a potential therapeutic target in pulmonary hypertension. Ther Adv Respir Dis. 2010;4:143–160. doi: 10.1177/1753465809369619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 12.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes PDGF receptor-beta-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1082–L1090. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, et al. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L885–L897. doi: 10.1152/ajplung.00258.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology. 2010;15:659–668. doi: 10.1111/j.1440-1843.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 16.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol. 2010;42:482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang BY, Kleinhenz JM, Murphy TC, Hart CM. The PPARgamma ligand rosiglitazone attenuates hypoxia-induced endothelin signaling in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2011;301:L881–L891. doi: 10.1152/ajplung.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Awasaki Y, Kandori H, Tanakamaru ZY, Nagai H, Baron D, Yamamoto M. Suppressive effects of acid-forming diet against the tumorigenic potential of pioglitazone hydrochloride in the urinary bladder of male rats. Toxicol Appl Pharmacol. 2011;251:234–244. doi: 10.1016/j.taap.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Kang BY, Park KK, Green DE, Bijli KM, Searles CD, Sutliff RL, Hart CM. Hypoxia mediates mutual repression between microRNA-27a and PPARgamma in the pulmonary vasculature. PLoS One. 2013;8:e79503. doi: 10.1371/journal.pone.0079503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 22.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 23.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 24.Chhabra R, Dubey R, Saini N. Cooperative and individualistic functions of the microRNAs in the miR-23a∼27a∼24–2 cluster and its implication in human diseases. Mol Cancer. 2010;9:232. doi: 10.1186/1476-4598-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Marin-Muller C, Bharadwaj U, Chow KH, Yao Q, Chen C. MicroRNAs: control and loss of control in human physiology and disease. World J Surg. 2009;33:667–684. doi: 10.1007/s00268-008-9836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guimbellot JS, Erickson SW, Mehta T, Wen H, Page GP, Sorscher EJ, Hong JS. Correlation of microRNA levels during hypoxia with predicted target mRNAs through genome-wide microarray analysis. BMC Med Genomics. 2009;2:15. doi: 10.1186/1755-8794-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan K, Orcholski M, Tian X, Liao X, de Jesus Perez VA. MicroRNAs: promising therapeutic targets for the treatment of pulmonary arterial hypertension. Expert Opin Ther Targets. 2013;17:557–564. doi: 10.1517/14728222.2013.765863. [DOI] [PubMed] [Google Scholar]
- 29.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 31.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Tang Y, Cui H, Zhao X, Luo X, Pan W, Huang X, Shen N. Let-7/miR-98 regulate Fas and Fas-mediated apoptosis. Genes Immun. 2011;12:149–154. doi: 10.1038/gene.2010.53. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Zhang C, Li Y, Wang P, Yue Z, Xie S. miR-98 regulates cisplatin-induced A549 cell death by inhibiting TP53 pathway. Biomed Pharmacother. 2011;65:436–442. doi: 10.1016/j.biopha.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Chen Q, Song Y, Lai L, Wang J, Yu H, Cao X, Wang Q. MicroRNA-98 negatively regulates IL-10 production and endotoxin tolerance in macrophages after LPS stimulation. FEBS Lett. 2011;585:1963–1968. doi: 10.1016/j.febslet.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 36.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 37.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, et al. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:1171–1182. doi: 10.1164/rccm.201103-0412OC. [DOI] [PubMed] [Google Scholar]
- 38.Vitali SH, Hansmann G, Rose C, Fernandez-Gonzalez A, Scheid A, Mitsialis SA, Kourembanas S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: long-term follow-up. Pulm Circ. 2014;4:619–629. doi: 10.1086/678508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleinhenz JM, Kleinhenz DJ, You S, Ritzenthaler JD, Hansen JM, Archer DR, Sutliff RL, Hart CM. Disruption of endothelial peroxisome proliferator-activated receptor-gamma reduces vascular nitric oxide production. Am J Physiol Heart Circ Physiol. 2009;297:H1647–H1654. doi: 10.1152/ajpheart.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saez E, Rosenfeld J, Livolsi A, Olson P, Lombardo E, Nelson M, Banayo E, Cardiff RD, Izpisua-Belmonte JC, Evans RM. PPAR gamma signaling exacerbates mammary gland tumor development. Genes Dev. 2004;18:528–540. doi: 10.1101/gad.1167804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs ME, Wingo CS, Cain BD. An emerging role for microRNA in the regulation of endothelin-1. Front Physiol. 2013;4:22. doi: 10.3389/fphys.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X, Kumar S, Sharma S, Aggarwal S, Lu Q, Gross C, Rafikova O, Lee SG, Dasarathy S, Hou Y, et al. Endothelin-1 induces a glycolytic switch in pulmonary arterial endothelial cells via the mitochondrial translocation of endothelial nitric oxide synthase. Am J Respir Cell Mol Biol. 2014;50:1084–1095. doi: 10.1165/rcmb.2013-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui B, Cheng YS, Dai DZ, Li N, Zhang TT, Dai Y. CPU0213, a non-selective ETA/ETB receptor antagonist, improves pulmonary arteriolar remodeling of monocrotaline-induced pulmonary hypertension in rats. Clin Exp Pharmacol Physiol. 2009;36:169–175. doi: 10.1111/j.1440-1681.2008.05044.x. [DOI] [PubMed] [Google Scholar]
- 44.DiCarlo VS, Chen SJ, Meng QC, Durand J, Yano M, Chen YF, Oparil S. ETA-receptor antagonist prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol. 1995;269:L690–L697. doi: 10.1152/ajplung.1995.269.5.L690. [DOI] [PubMed] [Google Scholar]
- 45.Jasmin JF, Lucas M, Cernacek P, Dupuis J. Effectiveness of a nonselective ET(A/B) and a selective ET(A) antagonist in rats with monocrotaline-induced pulmonary hypertension. Circulation. 2001;103:314–318. doi: 10.1161/01.cir.103.2.314. [DOI] [PubMed] [Google Scholar]
- 46.Price LC, Howard LS. Endothelin receptor antagonists for pulmonary arterial hypertension: rationale and place in therapy. Am J Cardiovasc Drugs. 2008;8:171–185. doi: 10.2165/00129784-200808030-00004. [DOI] [PubMed] [Google Scholar]
- 47.Ryerson CJ, Nayar S, Swiston JR, Sin DD. Pharmacotherapy in pulmonary arterial hypertension: a systematic review and meta-analysis. Respir Res. 2010;11:12. doi: 10.1186/1465-9921-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green DE, Kang BY, Murphy TC, Hart CM. Peroxisome proliferator-activated receptor gamma (PPARγ) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm Circ. 2012;2:483–491. doi: 10.4103/2045-8932.105037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2012;35:3203–3211. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 50.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 51.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 52.Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, Liu G. MiR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012;302:L521–L529. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bockmeyer CL, Maegel L, Janciauskiene S, Rische J, Lehmann U, Maus UA, Nickel N, Haverich A, Hoeper MM, Golpon HA, et al. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transplant. 2012;31:764–772. doi: 10.1016/j.healun.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 54.Drake KM, Zygmunt D, Mavrakis L, Harbor P, Wang L, Comhair SA, Erzurum SC, Aldred MA. Altered microRNA processing in heritable pulmonary arterial hypertension: an important role for Smad-8. Am J Respir Crit Care Med. 2011;184:1400–1408. doi: 10.1164/rccm.201106-1130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West J, Hemnes A. Experimental and transgenic models of pulmonary hypertension. Compr Physiol. 2011;1:769–782. doi: 10.1002/cphy.c100003. [DOI] [PubMed] [Google Scholar]
- 56.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 57.Sakao S, Tatsumi K. The effects of antiangiogenic compound su5416 in a rat model of pulmonary arterial hypertension. Respiration. 2011;81:253–261. doi: 10.1159/000322011. [DOI] [PubMed] [Google Scholar]
- 58.Bandyopadhyay S, Bhattacharyya M. PuTmiR: a database for extracting neighboring transcription factors of human microRNAs. BMC Bioinformatics. 2010;11:190. doi: 10.1186/1471-2105-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]