Abstract
Idiopathic pulmonary fibrosis (IPF) is a disease with relentless course and limited therapeutic options. Nintedanib (BIBF-1120) is a multiple tyrosine kinase inhibitor recently approved by the U.S. Food and Drug Administration for the treatment of IPF. The precise antifibrotic mechanism(s) of action of nintedanib, however, is not known. Therefore, we studied the effects of nintedanib on fibroblasts isolated from the lungs of patients with IPF. Protein and gene expression of profibrotic markers were assessed by Western immunoblotting and real-time PCR. Autophagy markers and signaling events were monitored by biochemical assays, Western immunoblotting, microscopy, and immunofluorescence staining. Silencing of autophagy effector proteins was achieved with small interfering RNAs. Nintedanib down-regulated protein and mRNA expression of extracellular matrix (ECM) proteins, fibronectin, and collagen 1a1 while inhibiting transforming growth factor (TGF)-β1–induced myofibroblast differentiation. Nintedanib also induced beclin-1–dependent, ATG7-independent autophagy. Nintedanib’s ECM-suppressive actions were not mediated by canonical autophagy. Nintedanib inhibited early events in TGF-β signaling, specifically tyrosine phosphorylation of the type II TGF-β receptor, activation of SMAD3, and p38 mitogen-activated protein kinase. Nintedanib down-regulates ECM production and induces noncanonical autophagy in IPF fibroblasts while inhibiting TGF-β signaling. These mechanisms appear to be uncoupled and function independently to mediate its putative antifibrotic effects.
Keywords: fibrosis, nintedanib, fibroblasts, autophagy, transforming growth factor-β
Clinical Relevance
Nintedanib has been recently added to the armamentarium of therapeutics for the treatment of idiopathic pulmonary fibrosis. Nintedanib is a multiple tyrosine kinase inhibitor; the precise antifibrotic mechanism(s) of action of nintedanib, however, is not known. We have demonstrated that multiple mechanisms may contribute to the antifibrotic effects of nintedanib. Nintedanib inhibits transforming growth factor-β signaling, a potent effect that may explain its efficacy in clinical practice. This is the first study to show that nintedanib induces noncanonical autophagy in lung fibroblasts, which is uncoupled from its antifibrotic effects and might reflect a stress response.
Idiopathic pulmonary fibrosis (IPF) is a devastating disease with a natural history of inexorable progression, terminating in respiratory failure. Nintedanib (BIBF-1120; marketed under the trade name OFEV) has been recently approved by the U.S. Food and Drug Administration (FDA) based on clinical trials that demonstrated retardation of the disease progression in patients with IPF (1, 2). Remarkably, the precise mechanism of action of this drug in IPF is unclear, although it is known to inhibit the receptor tyrosine kinases of platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF). In preclinical studies using nintedanib, it was surmised that the mechanism of action for its antifibrotic effects is through the inhibition of one or more of these receptor tyrosine kinases. However, it was acknowledged that nonreceptor tyrosine kinases and as yet unidentified targets and/or cellular processes might play a role in exerting in vivo antifibrotic effects (3–5).
Elucidating mechanisms by which nintedanib mediates its antifibrotic effects will provide valuable insights into the future design of targeted therapies. The aim of this study was to determine the effects of nintedanib on lung fibroblasts, which are key effector cells in the pathogenesis of fibrosis (6). Some of the results of this study have been previously reported in the form of abstracts (7, 8).
Materials and Methods
Cell Culture and Reagents
We obtained primary human fibroblasts isolated from three failed-donor lungs (“Non-IPF”) and from explants of six patients with IPF undergoing lung transplant at University of Alabama at Birmingham (UAB), Birmingham, AL, as approved by the Institutional Review Board and normal human fetal diploid fibroblasts (IMR-90 cells) from Coriell Institute (Camden, NJ). Culture techniques are similar to those described earlier by our group (9). Briefly, the fibroblasts were cultured in DMEM (Life Technologies, Grand Island, NY) supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 μg/ml amphotericin-B and were incubated at 37°C in 5% CO2 and 95% air. Cells were allowed to grow to 70% confluence before the experiments. All experiments with IPF fibroblasts were performed on cells below the eighth passage. BIBF-1120 (or nintedanib, the active drug in OFEV) was obtained from Selleck Chemicals LLC (Houston, TX). Chloroquine was obtained from Sigma-Aldrich (St. Louis, MO). Transforming growth factor (TGF)-β1 was obtained from R&D Systems (Minneapolis, MN).
Western Immunoblotting, Immunoprecipitation, Immunofluorescence, and Confocal Microscopy
Immunoblotting, immunoprecipitation, and immunofluorescence studies using confocal laser microscopy were performed as described in earlier publications from our group (9, 10). Antibodies used are listed in Table E1 in the online supplement. Image densitometry was performed using ImageJ software (http://imagej.nih.gov/ij/).
RNA Interference
For ATG7 knockdown, we used Signalsilence ATG7 small interfering RNA (siRNA) I from Cell Signaling Technology, Danvers, MA. For beclin-1, light chain 3 (LC3) knockdown, and nontargeting control, we obtained siRNA from Dharmacon (Lafayette, CO) with sense sequences as in Table E2. We transfected fibroblasts with 100 nM targeting or nontargeting siRNA using Lipofectamine 2000 in OptiMEM medium (both from Life Technologies), according to the manufacturer’s protocol overnight, followed by recovery using DMEM with 10% FBS.
Real-Time PCR
Techniques used for estimation of steady-state mRNA levels are similar to those described eleswhere (9). The primers sequences used are as in Table E3.
Statistical Analysis
We used GraphPad Prism v. 6.04 for graphical representation and statistical analysis. We performed paired or unpaired t tests where applicable. Statistical significance was defined at P < 0.05.
Results
Nintedanib Decreases Constitutive Expression of Fibronectin and Collagen in IPF Lung Fibroblasts
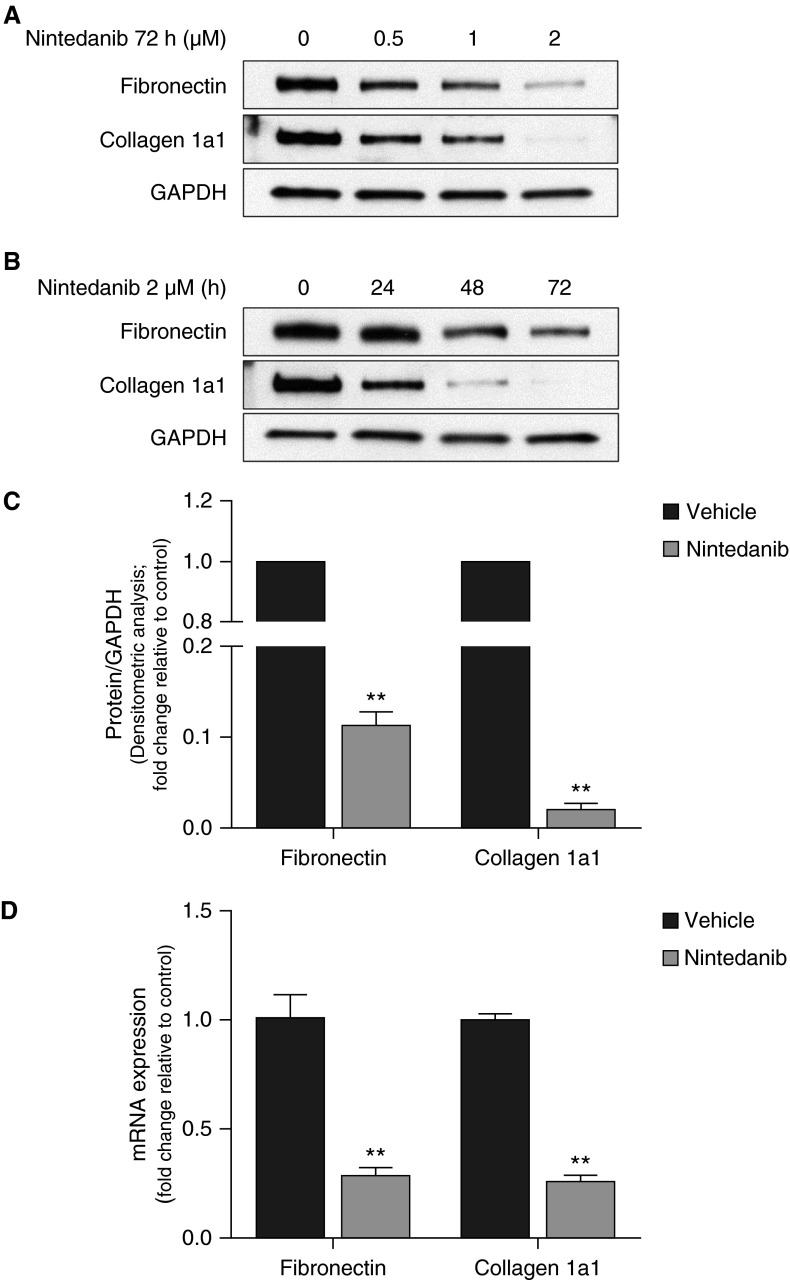
We first examined the effects of nintedanib on primary lung fibroblasts isolated from lung explants of patients with IPF undergoing lung transplantation. Cells were exposed to varying doses of nintedanib or vehicle control for up to 72 hours. Nintedanib decreased constitutive expression of the extracellular matrix (ECM) proteins, fibronectin, and collagen 1a1, as determined by Western immunoblotting in a dose- and time-dependent manner (Figures 1A–1C). These changes in proteins paralleled changes in mRNA levels as assessed by real-time PCR (Figure 1D). Treatment with nintedanib induced a change in morphology of the fibroblasts but did not decrease cell confluence or induce apoptosis (Figures E1A–E1C). These results suggest that nintedanib reduces the fibrogenic potential of fibroblasts by reducing steady-state levels of key ECM proteins.
Figure 1.
Nintedanib decreases constitutive expression of extracellular matrix proteins fibronectin and collagen 1a1 in idiopathic pulmonary fibrosis (IPF) fibroblasts. (A and B) IPF fibroblasts were treated with increasing doses of nintedanib (0.5, 1, or 2 μM) (A) or nintedanib (2 μM) for increasing durations (24, 48, or 72 h) (B). Expression of fibronectin and collagen 1a1 was evaluated by Western immunoblotting. (C) Densitometric analysis of independent experiments on different IPF fibroblast cell lines treated with nintedanib (2 μM) for 72 hours. Error bars represent mean ± SEM (n = 3). **P < 0.01. (D) IPF fibroblasts were treated with nintedanib (2 μM) for 72 hours, cells were lysed, and RNA was extracted. RT-PCR was performed to assess steady-state mRNA levels of fibronectin and collagen 1a1, compared with β-actin (control). Results are depicted graphically. Error bars represent mean ± SEM (n = 3). **P < 0.01. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Nintedanib Enhances Beclin-1–Dependent, ATG7-Independent Autophagic Flux in Fibroblasts
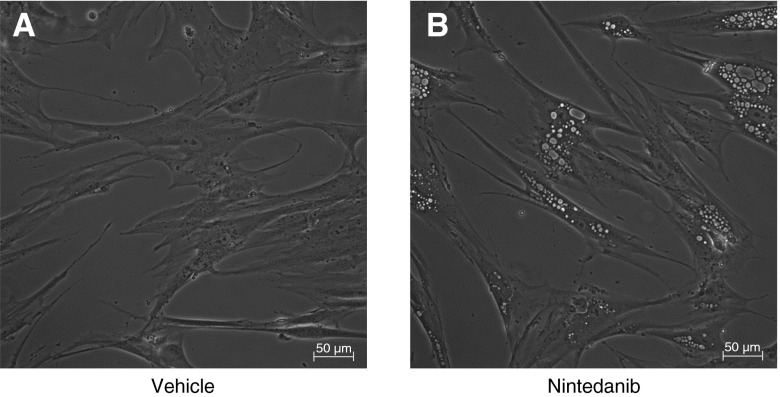
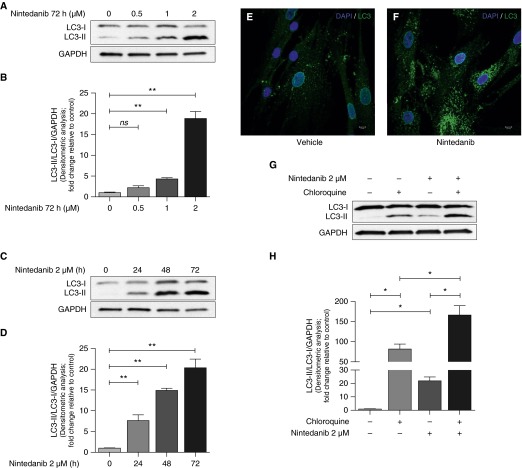
We observed a consistent effect of nintedanib that resulted in the formation of cytoplasmic vacuoles in fibroblasts within 6 hours of treatment (Figure 2), which suggested possible involvement of autophagy (which is associated with vacuole formation). Autophagy is an adaptive stress response that has been reported to be impaired in IPF (11–14). We assessed the autophagy markers microtubule-associated protein LC3B-I and 3B-II (LC3-I and LC3-II) in non-IPF and IPF primary lung fibroblasts by Western immunoblotting. We observed that IPF fibroblasts exhibited significantly lower autophagic flux than non-IPF fibroblasts at baseline as determined by the LC3-II to LC3-I ratio in Western immunoblotting (Figures E2A and E2B). Next, we analyzed nintedanib-treated fibroblasts for LC3-I and LC3-II. A dose- and time-dependent increase in LC3-II to LC3-I ratio was observed (Figures 3A–3D). We confirmed an increase in LC3-tagged vacuoles in nintedanib-treated fibroblasts by immunofluorescence microscopy (Figures 3E and 3F). To determine if this is related to a block in autophagy or an increase in autophagic flux, we tested the effect of adding chloroquine, an autophagy inhibitor that interferes with lysosomal degradation (15, 16). Enhanced LC3-II formation was noted in fibroblasts cotreated with nintedanib and chloroquine, suggesting an increase in autophagic flux induced by nintedanib (Figures 3G and 3H).
Figure 2.
Nintedanib induces vacuolar changes in fibroblasts. (A and B) IPF fibroblasts were treated with vehicle (A) or nintedanib (2 μM) (B) for 6 hours, and light microscopy was performed. Scale bars, 50 μm.
Figure 3.
Nintedanib enhances autophagic flux in fibroblasts. (A) IPF fibroblasts were treated with increasing doses of nintedanib for 72 hours. LC3 expression was evaluated by Western blotting. (B) Densitometric analysis of independent experiments performed as in A showing light chain 3B-II (LC3-II)/LC3-I/GAPDH. Error bars represent mean ± SEM (n = 3). **P < 0.01. (C) IPF fibroblasts were treated with nintedanib (2 μM) for increasing durations. (D) Densitometric analysis of independent experiments performed as in C showing LC3-II/LC3-I/GAPDH. Error bars represent mean ± SEM (n = 3). **P < 0.01. (E and F) IPF fibroblasts were treated with vehicle (E) or nintedanib (2 μM) (F) for 6 hours, and laser confocal microscopy was performed to detect LC3 by indirect immunofluorescence. Scale bars, 10 μm. (G) IPF fibroblasts were treated with nintedanib (2 μM) for 6 hours, and chloroquine (20 μM) was added 2 hours before harvest. LC3 expression was evaluated by Western blotting. (H) Densitometric analysis of independent experiments performed as in G showing LC3-II/LC3-I/GAPDH. Error bars represent mean ± SEM (n = 3). *P < 0.05. DAPI, 4′,6-diamidino-2-phenylindole; ns, not significant.
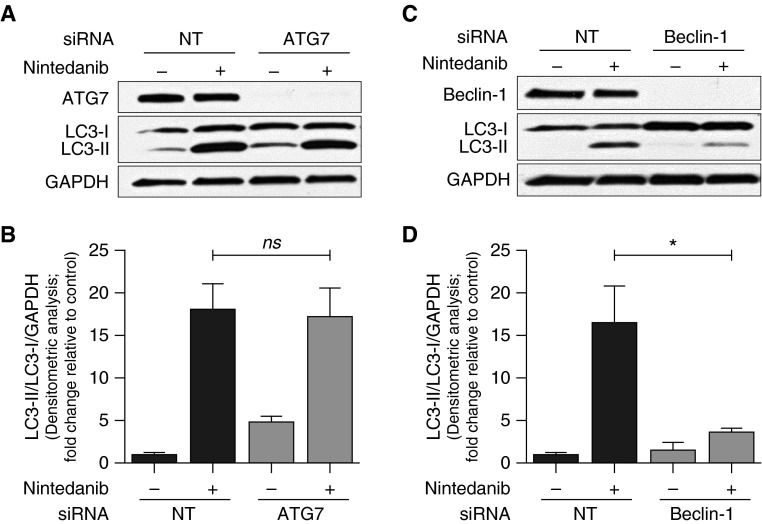
ATG7 and beclin-1 are key effector proteins in the canonical autophagy pathway (17). To determine if autophagy induced by nintedanib involves the canonical pathway, we performed siRNA-mediated knockdown of these proteins. Nintedanib-induced autophagy was preserved in ATG7-silenced cells, whereas it was markedly diminished in beclin-1–deficient cells (Figures 4A–4D). These results indicate that nintedanib induces beclin-1–dependent, ATG7-independent autophagy in fibroblasts.
Figure 4.
Autophagy induced by nintedanib is beclin-1 dependent and ATG7 independent. (A) IPF fibroblasts were subjected to small interfering RNA (siRNA)-mediated knockdown of ATG7 and treated with nintedanib (2 μM) for 48 hours. ATG7 and LC3 expression were evaluated by Western blotting. (B) Densitometric analysis of independent experiments performed as in A showing LC3-II/LC3-I/GAPDH. Error bars represent mean ± SEM (n = 3). (C) IPF fibroblasts were subjected to siRNA-mediated knockdown of beclin-1 and treated with nintedanib (2 μM) for 48 hours. Beclin-1 and LC3 expression were evaluated by Western blotting. (D) Densitometric analysis of independent experiments performed as in C showing LC3-II/LC3-I/GAPDH. Error bars represent mean ± SEM (n = 5). *P < 0.05. NT, nontargeting siRNA. ATG7, autophagy-related protein 7.
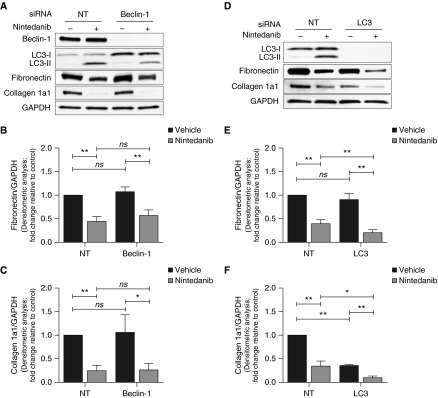
ECM-Suppressive Effect of Nintedanib Is Not Influenced by Beclin-1 Silencing
Next, we tested whether nintedanib-induced autophagy may, in part, account for the ECM-suppressive effects of the drug. Nintedanib’s effects on the down-regulation of fibronectin and collagen were not affected in beclin-1–silenced cells that demonstrated reduced autophagy (by decreased LC3-II:I ratio) (Figures 5A–5C). Interestingly, silencing of LC3 augmented the down-regulation of these ECM proteins by nintedanib (Figures 5D–5F). These results indicate that canonical autophagy does not account for the ECM-suppressive effect of nintedanib.
Figure 5.
Autophagy inhibition does not abrogate the effects of nintedanib on ECM proteins. (A) IPF fibroblasts were subjected to siRNA-mediated knockdown of beclin-1 and treated with nintedanib (2 μM) for 48 hours. Expression of ECM proteins (fibronectin and collagen 1a1) was evaluated by Western blotting. (B and C) Densitometric analysis of independent experiments performed as in A showing fibronectin (B) and collagen 1 (C) expression relative to GAPDH. Error bars represent mean ± SEM (n = 4). *P < 0.05; **P < 0.01. (D) IPF fibroblasts were subjected to siRNA-mediated knockdown of LC3 and treated with nintedanib (2 μM) for 48 hours. Expression of ECM proteins was evaluated by Western blotting. (E and F) Densitometric analysis of independent experiments performed as in D showing fibronectin (E) and collagen 1 (F) expression relative to GAPDH. Error bars represent mean ± SEM (n = 4). *P < 0.05; **P < 0.01.
Nintedanib Inhibits Early Events in TGF-β1 Signaling
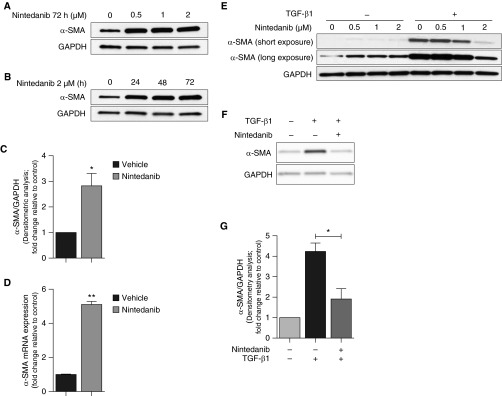
In contrast to ECM proteins, nintedanib induced expression of α-smooth muscle actin (α-SMA), a marker for myofibroblast transformation in IPF fibroblasts (Figures 6A–6D, E3A, and E3B). As with ECM proteins, canonical autophagy did not account for this effect of nintedanib (Figures E4A–E4D). Interestingly, previous studies have reported that nintedanib inhibits TGF-β1–induced myofibroblast differentiation (3, 5), although mechanisms are not well defined. Consistent with these reports, nintedanib at doses of ≥2 μM inhibited TGF-β1–induced production of α-SMA in IPF as well as IMR-90 fibroblasts (Figures 6E–6G). These findings suggest that distinct mechanisms of nintedanib action are involved in the (increased) constitutive and (decreased) TGF-β1–stimulated α-SMA expression.
Figure 6.
Nintedanib has divergent effects on constitutive and transforming growth factor (TGF)-β1–induced α-smooth muscle actin (α-SMA) expression. (A and B) Expression of α-SMA by Western blotting was assessed in IPF fibroblasts treated with increasing doses of nintedanib (0.5, 1, or 2 μM) (A) or nintedanib (2 μM) for increasing duration (24, 48, or 72 h) (B). (C) Densitometric analysis of independent experiments on different IPF fibroblast cell lines treated with nintedanib (2 μM) for 72 hours showing α-SMA expression relative to GAPDH. Error bars represent mean ± SEM (n = 3). *P < 0.05. (D) IPF fibroblasts were treated with nintedanib (2 μM) for 72 hours, cells were lysed, and RNA was extracted. RT-PCR was performed to assess steady-state mRNA levels of α-SMA compared with β-actin (control). Results are depicted graphically. Error bars represent mean ± SEM (n = 3). **P < 0.01. (E) IPF fibroblasts serum starved overnight were cotreated with or without TGF-β1 (2.5 ng/ml) and increasing doses of nintedanib (0.5, 1, and 2 μM) for 48 hours. α-SMA expression was evaluated by Western blotting. (F) Expression of α-SMA by Western blotting was assessed in IPF fibroblasts serum starved overnight and cotreated with or without TGF-β1 (2.5 ng/ml) and nintedanib (2 μM) for 48 hours. (G) Densitometric analysis of independent experiments performed as in F showing α-SMA expression relative to GAPDH. Error bars represent mean ± SEM (n = 3). *P < 0.05.
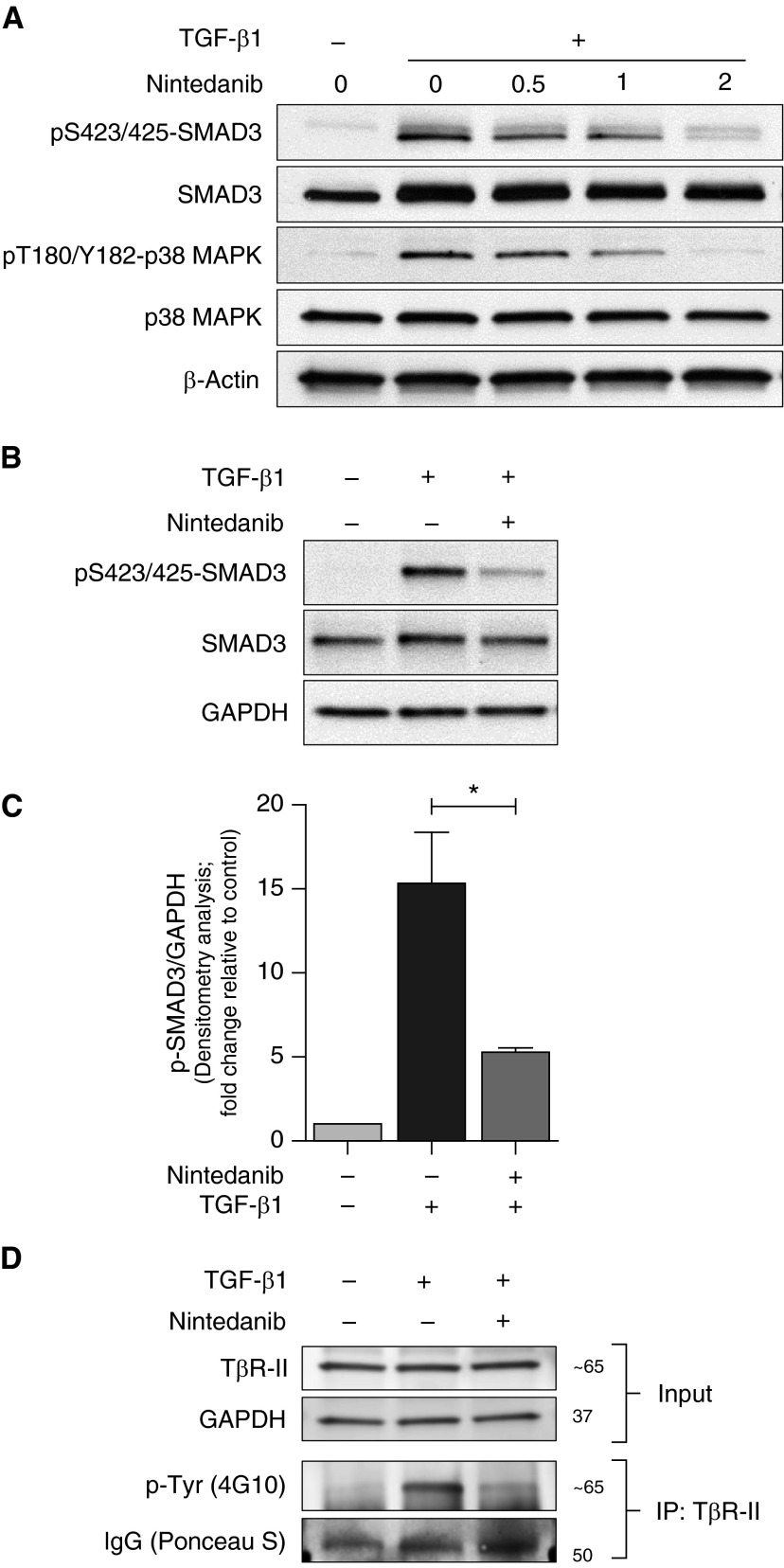
To further explore the mechanisms of nintedanib-mediated inhibition of TGF-β1–induced myofibroblast differentiation, human lung fibroblasts were treated with TGF-β1 (2.5 ng/ml) in the presence or absence of increasing doses of nintedanib for 60 minutes. We observed that nintedanib dose-dependently inhibited phosphorylation of S423/425-SMAD3 as well as T180/Y182-p38 mitogen-activated protein kinase (MAPK) (Figure 7A), suggesting inhibitory effects of this tyrosine kinase inhibitor on early signaling events via the TGF-β receptor(s). Nintedanib effectively inhibited SMAD3 phosphorylation at time points as early as 20 minutes after exposure to TGF-β1 (Figures 7B and 7C). Next, we cotreated the fibroblasts with or without TGF-β1 in the presence or absence of nintedanib (2 μM for 20 min) and performed immunoprecipitation studies of the type II TGF-β receptor (TβR-II) to assess its tyrosine phosphorylation. We observed an increase in tyrosine phosphorylation of TβR-II with treatment of TGF-β1 alone, which was inhibited by nintedanib (Figure 7D). These results suggest that nintedanib mediates an inhibitory effect on TGF-β receptor(s) signaling.
Figure 7.
Nintedanib inhibits early events in TGF-β1 signaling. (A) IMR-90 fibroblasts were serum starved overnight and cotreated with or without TGF-β1 (2.5 ng/ml) and increasing doses of nintedanib (0.5, 1, and 2 μM) for 1 hour. Expression of pS423/425-SMAD3, SMAD3, pT180/Y182-p38 mitogen-activated protein kinase (MAPK), and p38 MAPK was evaluated by Western blotting. (B) Expression of pS423/425-SMAD3, and SMAD3 by Western blotting was assessed in IMR-90 fibroblasts that were serum starved overnight and cotreated with or without TGF-β1 (2.5 ng/ml) and nintedanib (2 μM) for 20 minutes. (C) Densitometric analysis of independent experiments performed as in B showing pS423/425-SMAD3 relative to GAPDH. Error bars represent mean ± SEM (n = 3). *P < 0.05. (D) Levels of phospho-tyrosine residues were assessed by Western blotting in IMR-90 fibroblasts under experimental conditions similar to those in B and subjected to immunoprecipitation (IP) to enrich TβR-II. Input levels of TβR-II, along with GAPDH and IgG staining with Ponceau S in the immunoprecipitated samples, represent loading controls. p-Tyr, phospho-tyrosine; TβR, TGF β receptor, type II.
Discussion
Nintedanib has recently been approved by the FDA for the treatment of IPF based on phase III clinical trials that demonstrated retardation in disease progression (based on slower rates of FVC decline) and increased time to first acute exacerbation (2). Nintedanib is known to be a multiple tyrosine kinase inhibitor (TKI) that targets the receptor kinase(s) of PDGF, VEGF, and FGF. However, the putative antifibrotic effects of nintedanib have not been clearly defined. For example, although the PDGF receptor (PDGFR) pathway has been implicated in animal models of fibrosis (18), our group has shown that the strategy of inhibition of fibroblast proliferation (by a TKI) is insufficient to mediate antifibrotic effects in late stages of tissue injury repair (19). Indeed, a clinical trial of imatinib (which inhibits PDGFR) for IPF failed to show clinical benefit (20). In this study, we show that multiple coordinated mechanisms that modulate the profibrotic actions of fibroblasts, key effector cells in fibrosis, may account for clinical benefits of this drug in IPF; these actions of nintedanib include (1) inhibition of TGF-β receptor(s) signaling, (2) inhibition of fibronectin and collagen 1a1 mRNA expression independent of TGF-β signaling, and (3) induction of noncanonical autophagy.
TGF-β signaling has been shown to be central to fibrogenesis involving multiple organ systems, including the lung (6, 21, 22); additionally, TGF-β1 is up-regulated in the lungs of patients with IPF (23, 24). Our studies are the first, to our knowledge, to show that nintedanib inhibits early TGF-β receptor(s)-activated SMAD3 and p38 MAPK phosphorylation. Our data also confirm previous findings that nintedanib inhibits TGF-β1–mediated fibroblast-to-myofibroblast differentiation (3, 5). The TGF-β receptor is a heterodimer of type I (TβRI) and type II (TβRII) receptors; the ligand binds to the TβRII which activates the TβRI serine-threonine kinase, which in turn phosphorylates serine residues in receptor-activated SMADs, primarily SMAD2 and SMAD3 (25). Recent studies have shown that phosphorylation of tyrosine residues in the cytoplasmic tail of the TβRII subunit regulates activation of the receptor and its downstream effects (26, 27). In particular, the nonreceptor tyrosine kinase Src is known to phosphorylate one of these residues (Y284) that is necessary for downstream p38 MAPK signaling (28). It is noteworthy that nintedanib is known to inhibit nonreceptor tyrosine kinases belonging to the Src family, the significance of which is unknown in the context of its antifibrotic effects (5). It is not known if the effect of nintedanib on inhibiting SMAD3 and p38 MAPK phosphorylation (in our studies) is due to a direct effect on the TβRII or includes an indirect effect via inhibition of nonreceptor Src kinases (Figures E5A and E5B). Our group recently published that saracatinib (AZD0530), a potent inhibitor of Src family kinases, abrogates TGF-β1–mediated myofibroblast differentiation in vitro and bleomycin-induced fibrosis in mice (29). These studies and our current data indicate that nintedanib has the potential to mediate important antifibrotic effects in vivo either via its direct effect on TβRII and/or nonreceptor tyrosine kinases.
In the current study, we observed an effect of nintedanib on suppressing gene and protein expression of the ECM proteins, fibronectin, and collagen 1a1. Interestingly, this effect appears to be independent of TGF-β signaling based on the ability of nintedanib to down-regulate the constitutive expression of these ECM proteins despite a divergent effect on α-SMA. We speculate that nintedanib inhibits protein kinase pathways that converge on the regulation of fibronectin and collagen 1a1 gene expression, in particular the extracellular signal–regulated kinase-1/2 (ERK1/2) MAPK pathway (30, 31). The finding of a divergent effect on α-SMA was unexpected and suggests that the inhibitory effect of nintedanib on TGF-β/SMAD signaling could be counterbalanced by an independent effect(s) on the transcriptional regulation of α-SMA. This is plausible based on our own findings of reciprocal signaling between TGF-β1/SMAD and PDGFR/ERK1/2-MAPK that regulates myofibroblast differentiation via downstream effects on the transcription factor MyoD (32). Further studies are required to determine the relative roles of these differentiation-inducing versus proliferative signaling events in the pathogenesis of IPF.
Our studies have uncovered a novel effect of nintedanib in promoting autophagy in fibroblasts. Autophagy is an adaptive stress response that has been reported to be impaired in IPF (11–14). However, it is still unclear whether deficient autophagy is a causative factor for IPF or is an epiphenomenon that accompanies aging (33). We found that nintedanib-induced autophagy is ATG7 independent and beclin-1 dependent and thus represents a noncanonical pathway (17, 34). Several TKIs, most of which are used in cancer therapies, have been shown to induce autophagy; the mechanisms for autophagy induction by these TKIs include blockade of signaling by growth factors and direct actions on autophagy pathways (35, 36). A decrease in autophagic clearance of ECM proteins may lead to their accumulation, contributing to fibrosis; conversely, autophagy enhancers might be predicted to help resolve fibrosis. However, our data show that canonical autophagy is not the primary mechanism for a reduction in ECM proteins in nintedanib-treated fibroblasts. Autophagy might represent an adaptive cell survival response to blockade in growth factor signaling by nintedanib. If this is the case, combination therapy of nintedanib with an autophagy inhibitor (such as chloroquine) may offer a therapeutic advantage by switching the autophagic fate from cell survival to cell death. This paradigm for blocking the autophagic cellular stress response to targeted therapy has been proposed as a novel therapeutic approach in cancer (37, 38) and lymphangioleiomyomatosis (39). Whether such an approach would be beneficial in IPF deserves further study.
In conclusion, we report that nintedanib, a TKI that has been recently approved by the FDA for use in IPF, inhibits TGF-β signaling, down-regulates ECM gene/protein expression, and promotes noncanonical autophagy. Importantly, we found that the autophagy-promoting effects of nintedanib did not account for its putative antifibrotic effects on IPF lung fibroblasts. On the contrary, this study suggests that a therapeutic approach combining an antifibrotic TKI (such as nintedanib) with autophagy inhibition might enhance the therapeutic effect of the TKI. It is also of interest that both nintedanib and another newly approved drug, pirfenidone, target multiple pathways and processes, suggesting that such multitargeted approaches will be necessary and could be further optimized for the treatment of IPF.
Acknowledgments
Acknowledgments
The authors thank Naomi Logsdon for technical assistance.
Footnotes
This work was supported by National Institutes of Health grants P01 HL114470, R01 AG046210, and T32 HL105346 (V.J.T.).
Author Contributions: Conception and design: S.R., A.K., D.K., K.B., Y.Y.S., Q.D., J. Zhang, and V.J.T. Analysis and interpretation: S.R., A.K., D.K., K.B., Y.Y.S., Q.D., J. Zhang, J. Zmijewski, and V.J.T. Drafting/revising the manuscript: S.R., V.B.A., J. Zhang, J. Zmijewski, and V.J.T.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0445OC on June 13, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 2.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhary NI, Roth GJ, Hilberg F, Muller-Quernheim J, Prasse A, Zissel G, Schnapp A, Park JE. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29:976–985. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- 4.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 5.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 6.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 7.Rangarajan S, Dodson M, Zhang J, Thannickal V. Enhanced autophagy as a potential mechanism for the anti-fibrotic effects of BIBF-1120 in IPF [abstract] Am J Respir Crit Care Med. 2013;187:A5162. [Google Scholar]
- 8.Rangarajan S, Kurundkar A, Zhang J, Thannickal V. Non-canonical autophagy and the anti-fibrotic effects of BIBF-1120 in IPF [abstract] Am J Respir Crit Care Med. 2014;189:A5395. [Google Scholar]
- 9.Desai LP, Zhou Y, Estrada AV, Ding Q, Cheng G, Collawn JF, Thannickal VJ. Negative regulation of NADPH oxidase 4 by hydrogen peroxide-inducible clone 5 (Hic-5) protein. J Biol Chem. 2014;289:18270–18278. doi: 10.1074/jbc.M114.562249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 12.Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, Morse D. Autophagy in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e41394. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L56–L69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- 14.Ricci A, Cherubini E, Scozzi D, Pietrangeli V, Tabbi L, Raffa S, Leone L, Visco V, Torrisi MR, Bruno P, et al. Decreased expression of autophagic beclin 1 protein in idiopathic pulmonary fibrosis fibroblasts. J Cell Physiol. 2013;228:1516–1524. doi: 10.1002/jcp.24307. [DOI] [PubMed] [Google Scholar]
- 15.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 16.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Codogno P, Mehrpour M, Proikas-Cezanne T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol. 2012;13:7–12. doi: 10.1038/nrm3249. [DOI] [PubMed] [Google Scholar]
- 18.Aono Y, Nishioka Y, Inayama M, Ugai M, Kishi J, Uehara H, Izumi K, Sone S. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2005;171:1279–1285. doi: 10.1164/rccm.200404-531OC. [DOI] [PubMed] [Google Scholar]
- 19.Vittal R, Zhang H, Han MK, Moore BB, Horowitz JC, Thannickal VJ. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J Pharmacol Exp Ther. 2007;321:35–44. doi: 10.1124/jpet.106.113407. [DOI] [PubMed] [Google Scholar]
- 20.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR Imatinib IPF Study Investigators. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 21.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 22.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis: aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190:867–878. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil N, O'Connor RN, Unruh HW, Warren PW, Flanders KC, Kemp A, Bereznay OH, Greenberg AH. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 24.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Wang H, Liao HJ, Hu W, Gewin L, Mernaugh G, Zhang S, Zhang ZY, Vega-Montoto L, Vanacore RM, et al. Integrin-mediated type II TGF-beta receptor tyrosine dephosphorylation controls SMAD-dependent profibrotic signaling. J Clin Invest. 2014;124:3295–3310. doi: 10.1172/JCI71668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allison SJ. Fibrosis: regulation of fibrotic signalling by TGF-beta receptor tyrosine phosphorylation. Nat Rev Nephrol. 2014;10:484. doi: 10.1038/nrneph.2014.127. [DOI] [PubMed] [Google Scholar]
- 28.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 29.Hu M, Che P, Han X, Cai GQ, Liu G, Antony V, Luckhardt T, Siegal GP, Zhou Y, Liu RM, et al. Therapeutic targeting of Src kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther. 2014;351:87–95. doi: 10.1124/jpet.114.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaelson JE, Ritzenthaler JD, Roman J. Regulation of serum-induced fibronectin expression by protein kinases, cytoskeletal integrity, and CREB. Am J Physiol Lung Cell Mol Physiol. 2002;282:L291–L301. doi: 10.1152/ajplung.00445.2000. [DOI] [PubMed] [Google Scholar]
- 31.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 32.Hecker L, Jagirdar R, Jin T, Thannickal VJ. Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res. 2011;317:1914–1921. doi: 10.1016/j.yexcr.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 34.Juenemann K, Reits EA. Alternative macroautophagic pathways. Int J Cell Biol. 2012;2012:189794. doi: 10.1155/2012/189794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han W, Pan H, Chen Y, Sun J, Wang Y, Li J, Ge W, Feng L, Lin X, Wang X, et al. EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PLoS One. 2011;6:e18691. doi: 10.1371/journal.pone.0018691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rikiishi H. Autophagic action of new targeting agents in head and neck oncology. Cancer Biol Ther. 2012;13:978–991. doi: 10.4161/cbt.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabretta B, Salomoni P. Inhibition of autophagy: a new strategy to enhance sensitivity of chronic myeloid leukemia stem cells to tyrosine kinase inhibitors. Leuk Lymphoma. 2011;52:54–59. doi: 10.3109/10428194.2010.546913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sui X, Kong N, Zhu M, Wang X, Lou F, Han W, Pan H. Cotargeting EGFR and autophagy signaling: a novel therapeutic strategy for non-small-cell lung cancer. Mol Clin Oncol. 2014;2:8–12. doi: 10.3892/mco.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Parkhitko A, Henske EP. Autophagy: an 'Achilles' heel of tumorigenesis in TSC and LAM. Autophagy. 2011;7:1400–1401. doi: 10.4161/auto.7.11.17652. [DOI] [PMC free article] [PubMed] [Google Scholar]