Abstract
Aspergillus fumigatus (AF) infection and sensitization are common and promote Th2 disease in individuals with asthma. Innate immune responses of bronchial epithelial cells are now known to play a key role in determination of T cell responses upon encounter with inhaled pathogens. We have recently shown that extracts of AF suppress JAK-STAT signaling in epithelial cells and thus may promote Th2 bias. To elucidate the impact of AF on human bronchial epithelial cells, we tested the hypothesis that AF can modulate the response of airway epithelial cells to favor a Th2 response and explored the molecular mechanism of the effect. Primary normal human bronchial epithelial (NHBE) cells were treated with AF extract or fractionated AF extract before stimulation with poly I:C or infection with human rhinovirus serotype 16 (HRV16). Expression of CXCL10 mRNA (real-time RT-PCR) and protein (ELISA) were measured as markers of IFN-mediated epithelial Th1–biased responses. Western blot was performed to evaluate expression of IFN regulatory factor-3 (IRF-3), NF-κB, and tyrosine-protein phosphatase nonreceptor type 11 (PTPN11), which are other markers of Th1 skewing. Knockdown experiments for protease-activated receptor-2 (PAR-2) and PTPN11 were performed to analyze the role of PAR-2 in the mechanism of suppression by AF. AF and a high-molecular-weight fraction of AF extract (HMW-AF; > 50 kD) profoundly suppressed poly I:C– and HRV16-induced expression of both CXCL10 mRNA and protein from NHBE cells via a mechanism that relied upon PAR-2 activation. Both AF extract and a specific PAR-2 activator (AC-55541) suppressed the poly I:C activation of phospho–IRF-3 without affecting activation of NF-κB. Furthermore, HMW-AF extract enhanced the expression of PTPN11, a phosphatase known to inhibit IFN signaling, and concurrently suppressed poly I:C–induced expression of both CXCL10 mRNA and protein from NHBE cells. These results show that exposure of bronchial epithelial cells to AF extract suppressed poly I:C and HRV16 signaling via a mechanism shown to involve activation of PAR-2 and PTPN11. This action of AF may promote viral disease exacerbations and may skew epithelial cells to promote Th2 inflammation in allergic airway disorders mediated or exacerbated by AF, such as asthma and chronic rhinosinusitis.
Keywords: asthma, CRS, Aspergillus fumigatus, virus, bronchial epithelial cells
Clinical Relevance
This study demonstrates that Aspergillus fumigatus (AF) extract, especially high-molecular-weight AF extract, activated protease-activated receptor-2 and tyrosine-protein phosphatase nonreceptor type 11; these events in turn suppressed poly I:C– and human rhinovirus serotype 16–induced CXCL10 mRNA and protein expression in airway epithelial cells. These findings give new insight into the mechanism by which AF allergen modulates the antiviral/IFN response of airway epithelial cells. Suppression of this response, which is important in immunity to both viral and fungal pathogens, can increase susceptibility to such pathogens and may lead to an epithelial bias of subsequent adaptive immune responses promoting T-helper (Th) 2 or Th17 responses in favor of Th1 responses.
Th2-related chronic allergic diseases of both upper and lower airways, such as chronic rhinosinusitis (CRS) and bronchial asthma, are major health concerns in the modern world. The World Health Organization estimates that asthma accounts for 1 in every 250 deaths worldwide (1). The prevalence of asthma in developed countries is approximately 10% in adults and is even higher in children, whereas in developing countries the prevalence is lower but is increasing rapidly (2). Also, the prevalence of CRS with nasal polyps, a Th2-related upper respiratory inflammatory disorder, is around 3 to 5% of the United States population (3).
Epithelial cells are known to play a pivotal role in innate responses against viral, bacterial, and fungal pathogens and to modulate the responses to inhaled allergens (4). Porter and colleagues recently reported that environmental fungi directly contribute to the pathogenesis of chronic Th2-related airway diseases (5). Recently, we have described the inhibition of IFN-β signaling through JAK-STAT1 by Aspergillus fumigatus (AF) in bronchial epithelium, resulting in reduced induction of CXCL10, also known as IFN-γ–induced protein 10, a chemokine that attracts Th1 cells (6). Inhibition of IFN signaling by AF in epithelium could bias the epithelial responses toward Th2 chemokines and cytokines and therefore may be important in the mechanism by which AF worsens allergic inflammatory diseases. Impairment of IFN-mediated antiviral responses could also undermine antiviral immunity in individuals colonized by AF. When viral infection occurs, viral recognition receptors, such as Toll-like receptor 3 (TLR3) expressed by epithelial cells, are activated to produce inflammatory cytokines and chemokines, including IFN-β, CCL20, CXCL8, and CXCL10 (7). IFN regulatory factor-3 (IRF-3) and NF-κB are thought to be important transcription factors downstream of TLR3 activation in airway epithelial cells (7, 8). An autocrine activation of IFN receptors occurs that also activates the JAK-STAT signaling pathway, which in turn promotes the antiviral state. We found that AF extracts are able to inhibit this JAK-STAT signaling and inhibit induction of antiviral marker genes, but we did not establish the mechanism of this effect (6). In the present report, we have explored the molecular mechanism of this potentially important property of extracts of AF and have mechanistically implicated protease-activated receptor-2 (PAR-2) and tyrosine–protein phosphatase nonreceptor type 11 (PTPN11; also known as Src homology 2–containing protein tyrosine phosphatase).
PAR-2 is a seven-transmembrane G-coupled protein receptor that is activated by proteases contained in aeroallergens, such as ragweed, cockroach, Aspergilllus species, Alternaria species, Candida species, and house dust mite (HDM) (9, 10). PAR-2 is also activated by mast cell–derived tryptase (11). In contrast, PAR-1, -3, and -4 are activated by thrombin. PAR-2 is expressed on airway epithelial cells (12), mast cells (13), alveolar macrophages, dendritic cells (14), and neutrophils (15). Levels of PAR-2 expression are increased in airway epithelial cells in subjects with asthma (16) and in subjects with CRS (17) when compared with control subjects.
PTPN11 is an evolutionarily conserved protein tyrosine phosphatase, containing two SH2 domains at the N terminus, a central catalytic domain, and a C-terminal tail (18, 19). PTPN11 positively regulates the signaling pathways of cytokines and growth factors, such as insulin, epidermal growth factor, fibroblast growth factor, and PDGF (20, 21); in contrast, PTPN11 negatively regulates the JAK-STAT signaling pathway activated by IFN-α and IFN-γ (22).
Defects of antiviral innate immune response have been described in airway epithelial cells derived from patients with asthma. Cultured airway epithelial cells from patients with asthma showed reduced production of type I and III IFNs when inoculated with human rhinovirus (HRV) (23, 24). Hussein and colleagues have shown that expression of the immune IFN, IFN-γ, was also decreased in a stable state of disease, suggesting that several types of IFNs may be defective within patients with asthma (25). Type 1 and type 2 responses are mutually suppressive at the level of T cell differentiation; in addition, it has been shown that IFN-γ suppresses IL-4–activated STAT6 signaling in airway epithelial cells (26, 27). These lines of evidence suggest that protective roles of IFNs may be deficient in patients with type 2 allergic disorders.
Due to the importance of IFN signaling in the initiation of Th1 responses, it is notable that fungi and fungal extracts can inhibit the IFN signaling pathway, and an understanding of the mechanism(s) becomes imperative. Alternaria alternata, a major inhaled fungal allergen, was found by Zhu and colleagues to inhibit virus-induced type I and III IFN secretion from epithelial cells via a mechanism not involving PAR-2 (28). On the other hand, Nhu and colleagues showed that the TLR3 pathway was inhibited via a mechanism involving PAR-2 in airway epithelial cells (29). As mentioned above, PTPN11 is a phosphatase and acts as an upstream inhibitor of several signaling phosphatase pathways, including JAK-STAT (20). Inhibition of TLR3 signaling by PTPN11 was previously shown, and PTPN11 was found to be activated by PAR-2 in two separate studies (18, 30). These lines of investigation indicate the potential importance of phosphatases in regulating innate immune response. Furthermore, protein phosphatase 2A, another signal regulating phosphatase, was inhibited by HDM, resulting in elevated induction of inflammatory cytokines by airway epithelial cells (31). As these studies have demonstrated, understanding both the positive and negative regulation of cytokine or chemokine expression in airway epithelial cells by products of organisms commonly found on mucosal surfaces may shed light on mechanisms of airway disorders triggered by allergic inflammation.
The studies described above, including our own study showing suppression of JAK-STAT signaling by AF (6, 20), suggest that components of AF bias epithelial responses toward Th2 inflammation. In the present study, we have investigated the mechanism by which AF suppresses IFN signaling because this may be important not only in immunity to fungi and viruses but also in skewing immune responses toward Th2 bias. Our studies suggest that AF inhibits JAK-STAT signaling by activating the protease receptor PAR-2, which in turn induces the phosphatase PTPN11 (SHP2) and dephosphorylates STAT protein. These findings have relevance to fungal activation of allergic responses as well as the propensity of fungi to undermine Th1 responses in vivo.
Materials and Methods
Reagents
The AF, HDM, and cockroach extracts (Hollister Stier Laboratories, Spokane, WA) used were commercial allergen products used to conduct skin prick testing of allergy patients in the clinical setting and contain, in addition to the extract allergens, 50% (wt/vol) glycerol as preservative, 0.5% sodium chloride, and 0.275% sodium bicarbonate. AF extract was fractionated into a high-molecular-weight fraction (HMW-AF, >50 kD) and a low-molecular-weight fraction (LMW-AF, <50 kD) using Amicon ultra 4 centrifugal force filters (EMD Millipore, Billerica, MA). Poly I:C (InvivoGen, San Diego, CA), IL-13, IL-17A, and AC-55541 (a specific PAR-2 activator; R&D Systems, Minneapolis, MN), DMSO (Sigma-Aldrich, St. Louis, MO), and NSC-87877 (a specific PTPN11 inhibitor; EMD Millipore) were purchased from listed suppliers. Small interfering RNA (siRNA) against PAR-2, PTPN11, and the no-effect control (Santa Cruz Biotechnology, Santa Cruz, CA) and HiPerFect transfection reagent (Qiagen, Valencia, CA) were purchased from listed suppliers.
Cell Culture, Treatments, Transfection, and Human Rhinovirus Infection
Primary normal human bronchial epithelial (NHBE) cells (Lonza, Walkersville, MD) from at least three donors were plated in 12-well culture plates and grown in submerged and air–liquid interface (ALI) conditions as described (32). NHBE cells were deprived of hydrocortisone from the medium for 24 hours before treatment with AF extract or cytokine. NHBE cells were treated with 1:320 wt/vol AF, HMW-AF, LMW-AF extract, or AC-55541 (1 nM) for 1 hour before stimulation with poly I:C (5 μg/ml), IL-13 (100 ng/ml), or IL-17A (100 ng/ml) for the indicated time periods. For knockdown studies, NHBE cells were transfected with siRNA (10 nM) against nonspecific control, PAR-2, or PTPN11 at 50% confluency using HiPerFect transfection reagent (Qiagen). For inhibition studies, cells were treated with NSC-87877 (100 nM) for 3 hours before stimulation. HRV16 stocks were amplified and purified based on a previously published protocol (33). NHBE cells grown in an ALI culture system were infected with HRV16 at a multiplicity of infection of 1. HRV16-infected NHBE cells were then cultured for 48 hours at 33°C in the presence or absence of 1:320 wt/vol HMW-AF extract. Each experiment was performed at least three times.
Real-Time RT-PCR
Total RNA was isolated, cDNA was synthesized from cells, and real-time RT-PCR was performed as described previously (6, 32). The levels of expression of mRNA were normalized to the housekeeping gene β-actin. The sequences of primers and probe sets for detection of β-actin, CXCL8, CXCL10, and CCL26 are listed in Table E1 in the online supplement. Primer and probe sets for PTPN2, -6, -10, and -11 and PAR-2 were purchased from Life Technologies (Grand Island, NY).
ELISA
Levels of CXCL10 in the supernatants of cultured cells were determined with a commercially available ELISA kit following the manufacturer’s instructions (R&D Systems).
Western Blot
Stimulated cells lysates were collected for Western blot analysis as described previously (6). Primary antibody against actin was purchased from MP Biomedicals (Solon, OH). Phospho–IRF-3 Ser396 (S396), IRF-3, phospho–NF-κB, NF-κB, phospho–STAT-1 Tyr701 (Y701), STAT1, phospho–PTPN11 Tyr542 (Y542), and PTPN11 were from Cell Signaling Technology (Danvers, MA).
Statistical Analysis
All data are presented as the mean ± SEM unless otherwise mentioned. Data were normally distributed, and differences between groups were analyzed using the paired Student’s t test, with P < 0.05 considered to be statistically significant. All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA).
Results
AF and HMW-AF Extract Suppressed Expression of CXCL10 Induced by Poly I:C or HRV16 but Did Not Suppress IL-13–Induced CCL26
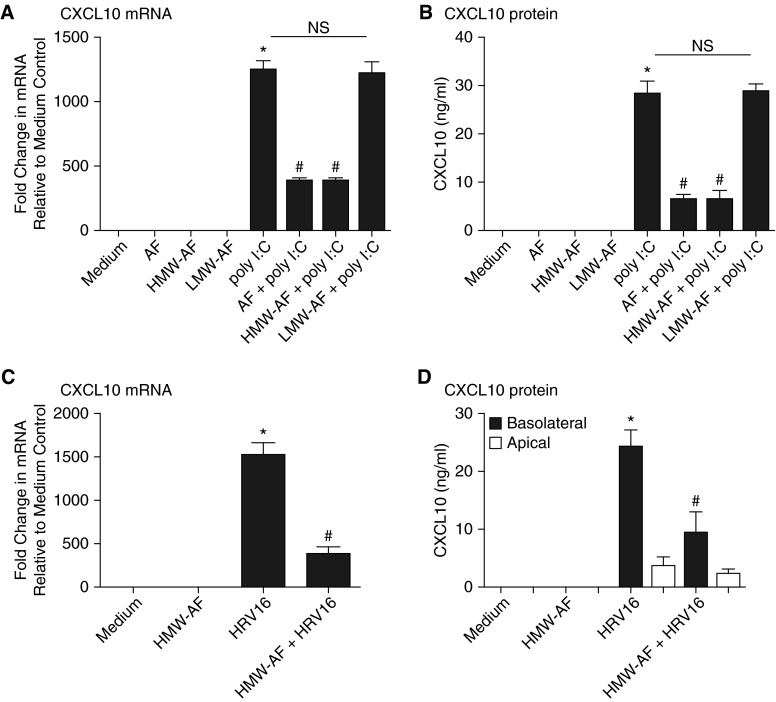
NHBE cells were pretreated with AF, HMW-AF, LMW-AF, or heat-treated AF extract (70°C for 30 min) for 1 hour before stimulation with poly I:C or IL-13 for 6 hours. CXCL10 mRNA was induced by poly I:C (1,257-fold; P < 0.01, compared with medium-only control [n = 3]) and was suppressed by whole AF and HMW-AF extract (69.0 and 69.1% reduction, respectively, compared with poly I:C stimulated cells; P < 0.01 [n = 3]) (Figure 1A). Diluent only did not suppress poly I:C–induced CXCL10 mRNA, which was consistent with our previous findings (data not shown) (6). We observed the same pattern for CXCL10 protein (i.e., inhibition by the whole extract and the HMW-AF fraction) (Figure 1B). Similarly, poly I:C stimulation induced CXCL9 mRNA (633-fold; P < 0.01 [n = 3]) and CXCL11 mRNA (752-fold; P < 0.01 [n = 3]), and these responses were suppressed by AF extract (54.0 and 68.2%, respectively, compared with poly I:C–stimulated cells; P < 0.01 [n = 3]) (data not shown). The suppressive effect of AF extract was lost when the extract was heated at 70°C for 30 minutes (see Figure E1A in the online supplement). AF extract also suppressed poly I:C–induced IFN-β mRNA (Figure E1B). IL-13 did not induce mRNA of CXCL9, CXCL10, or CXCL11. However, it did induce CCL26 mRNA (1,087-fold, compared with medium-only control; P < 0.01 [n = 3]), and this response was not suppressed by AF extract (Figure E1C). CXCL8 mRNA was induced by AF extract or IL-17A (11.3-fold and 17.7-fold, respectively; P < 0.01 [n = 3]) and was further expressed when both were combined, eliminating a global suppressive or toxic effect of AF (31.7-fold; P < 0.01 [n = 3]) (Figure E1D). We have noticed changes in cell morphology and viability induced by AF or LMW-AF extract when incubating the cells with concentrated solutions for longer than 24 hours. In contrast, HMW-AF extract did not alter cell morphology or viability (6). Therefore, we used HMW-AF extract for experiments that required longer incubations (see below). Using an ALI culture system, fully differentiated NHBE cells were infected apically with HRV16 (multiplicity of infection of 1) and showed increased CXCL10 mRNA (1,527-fold; P < 0.01 [n = 3]) and induction of basolateral expression of CXCL10 protein (24.3 pg/ml; P < 0.01 [n = 3]) (Figures 1C and 1D). Accordingly, HMW-AF extract suppressed HRV16-induced CXCL10 mRNA (74.3% reduction; P < 0.01 [n = 3]) and protein (84.2% reduction; P < 0.01 [n = 3]) (Figures 1C and 1D).
Figure 1.
Aspergillus fumigatus (AF) and a high-molecular-weight fraction of AF extract (HMW-AF) suppressed expression of chemokine (C-X-C motif) ligand 10 (CXCL10) induced by polyinosinic:polycytidylic acid (poly I:C) or human rhinovirus serotype 16 (HRV16). Submerged primary normal human bronchial epithelial (NHBE) cells were pretreated with 1:320 wt/vol AF, HMW-AF, or a low-molecular-weight fraction of AF extract (LMW-AF) for 1 hour before poly I:C (5 μg/ml) treatment for 6 hours. Cell lysates were collected to assess expression of CXCL10 mRNA by RT-PCR (A), and supernatants were collected for CXCL10 protein assay by ELISA (B). NHBE cells were grown in an air–liquid interface culture system, and HMW-AF was added 1 hour before the infection with HRV16 (multiplicity of infection of 1). After 48 hours, cell lysates were collected to assess expression of CXCL10 mRNA by RT-PCR (C), and supernatants were separately collected from apical and basolateral sides for CXCL10 protein detection by ELISA (D). Data represent mean ± SEM of three independent experiments. *P < 0.01 by t test when compared with medium-only control. #P < 0.01 by t test when compared with poly I:C–stimulated cells or HRV16 infected cells. NS, not significant by t test.
Suppression of Poly I:C–Induced CXCL10 by AF Extract Was Mediated by PAR-2
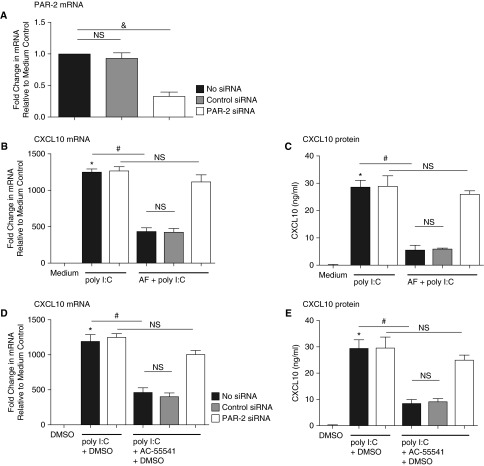
Previous reports have demonstrated that fungal extracts can signal via protease activation of PAR-2 (34, 35). We assessed the importance of PAR-2 in the AF response by knocking down PAR-2 expressed on NHBE cells using siRNA transfection (Figure 2A). The suppressive effect of AF extract on CXCL10 mRNA and protein expression induced by poly I:C was lost after PAR-2 knockdown (Figures 2B and 2C). Furthermore, when NHBE cells were stimulated with the specific PAR-2 activator AC-55541, poly I:C–induced CXCL10 mRNA was suppressed (61% reduction compared with poly I:C–stimulated cells; P < 0.01 [n = 3]) (Figure 2D). CXCL10 protein expression showed the same pattern as CXCL10 mRNA (P < 0.01 [n = 3]) (Figure 2E). These studies suggest that the suppressive effect of AF on induction of CXCL10 by the TLR3 activator is mediated via activation of PAR-2.
Figure 2.
Suppression of poly I:C–induced CXCL10 by AF extract was mediated by protease-activated receptor-2 (PAR-2). Submerged NHBE cells were transfected with 10 nM of small interfering RNA (siRNA) against nonspecific control RNA or PAR-2. (A) Knockdown efficiency of PAR-2 mRNA was analyzed by RT-PCR. After transfection with siRNA against control RNA or PAR-2, cells were incubated with 1:320 wt/vol AF extract for 1 hour and then stimulated with poly I:C (5 μg/ml) for 6 hours. (B) Expression of CXCL10 mRNA was analyzed by RT-PCR. (C) Expression of CXCL10 protein in supernatant was analyzed by ELISA. NHBE cells were transfected with 10 nM siRNA against nonspecific control RNA or PAR-2. PAR-2 activator (AC-55541 100 nM, reconstituted in DMSO) was applied 1 hour before poly I:C stimulation. (D) Expression of CXCL10 mRNA was analyzed by RT-PCR. (E) Expression of CXCL10 protein in supernatant was analyzed by ELISA. Data represent mean ± SEM of three independent experiments. *P < 0.01 by t test when compared with medium-only control. #P < 0.01 by t test when compared with poly I:C–treated cells. &P < 0.01 by t test when compared with control siRNA-transfected cells.
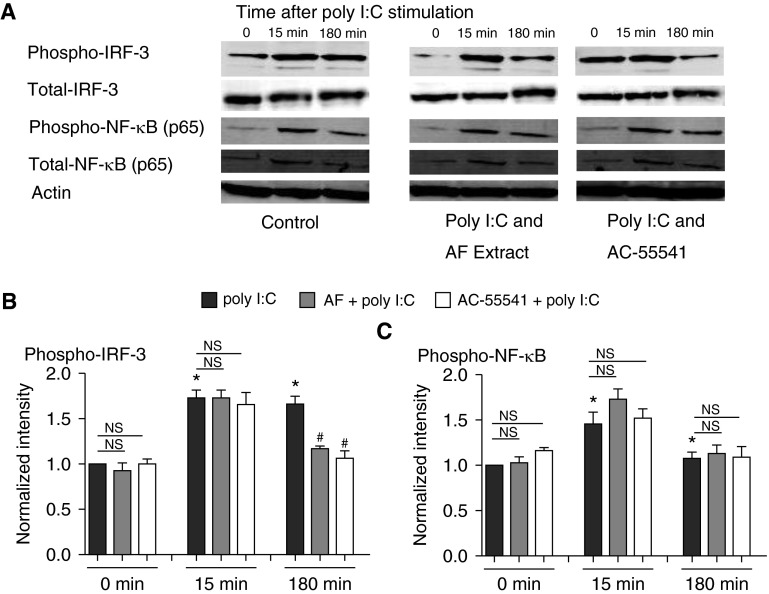
Both IRF-3 and NF-κB have been reported to be important transcriptional factors that are downstream of TLR3 in airway epithelial cells (8, 36). Poly I:C activated both IRF-3 and NF-κB in our studies; IRF-3 was suppressed by AF extract using NHBE cells (30% reduction at the 180-min time point compared with poly I:C–stimulated cells; P < 0.01 [n = 3]) (Figures 3A–3C), but activation of NF-κB was not affected. This observation is consistent with previous reports (28, 29). Recently, we have reported a rapid dephosphorylation of IFN-β–induced phospho-STAT-1 triggered by AF extract and have confirmed this in the present study (Figures E2A and E2B) (6). Because the apparent dephosphorylation of IRF-3 shown in Figure 3 was rapid, we hypothesized that PAR-2 activation may be mobilizing phosphatases that reduce TLR3 signaling through both STAT and IRF-3 and perhaps other pathways.
Figure 3.
AF extract suppressed poly I:C–activated IFN regulatory factor-3 (IRF-3), not NF-κB. (A) Submerged NHBE cells were pretreated with or without 1:320 wt/vol AF extract or the PAR-2 activator AC-55541 (100 nM) 1 hour before stimulation with poly I:C (5 μg/ml), and cellular protein was collected for each indicated time point and analyzed for phosphorylation of IRF-3 and NF-κB by Western blot. (B and C) Expressed bands for phospho–IRF-3 (B) and phospho–NF-κB (C) were quantified by using Image J software and normalized to internal control actin. Data represent mean ± SEM of three independent experiments, and Western blot images are representative of three independent experiments. *P < 0.01 by t test when compared with medium-only control. #P < 0.01 by t test when compared with cells treated with poly I:C only at the 180-minute time point.
HMW-AF Extract Induced the Expression and Activation of PTPN11
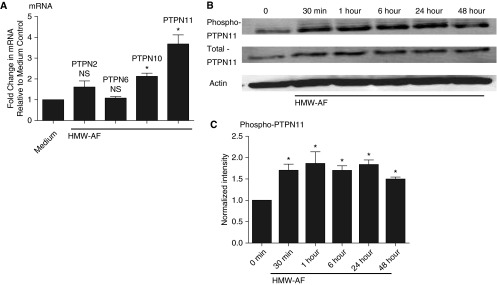
PTPN2, -6, -10, and -11 have been demonstrated to inactivate TLR3 or IFN-β signaling in various cell types (29, 37–39). We therefore screened NHBE cells stimulated with AF for induction of the various PTPN proteins. Among these signaling regulators, PTPN11 mRNA showed the strongest induction by HMW-AF extract (3.7-fold; P < 0.01 [n = 3]) (Figure 4A). Because phosphorylation of PTPN proteins is an indicator of their activation, we assessed levels of the phosphorylated form of PTPN11. Phosphorylation of PTPN11 detected by Western blot began as early as 30 minutes after the stimulation with HMW-AF and was observed for up to 48 hours (maximal activation of 1.8-fold at 15 min and 1.5-fold at 48 h; P < 0.01 [n = 3]) (Figures 4B and 4C).
Figure 4.
HMW-AF extract induced the expression and activation of tyrosine-protein phosphatase nonreceptor type 11 (PTPN11). (A) Submerged NHBE cells were stimulated with 1:320 wt/vol HMW-AF extract for 24 hours, and cell lysates were collected to analyze the expression of PTPN2, PTPN6, PTPN10, and PTPN11 mRNA by RT-PCR. (B) NHBE cells were treated with HMW-AF for the indicated time points, and cellular protein was collected for Western blot. (C) Expressed bands for phospho-PTPN11 were quantified using Image J software and normalized to internal control actin. Data represent mean ± SEM of three independent experiments, and Western blot images are representative of three independent experiments. *P < 0.01 by t test when compared with medium-only control.
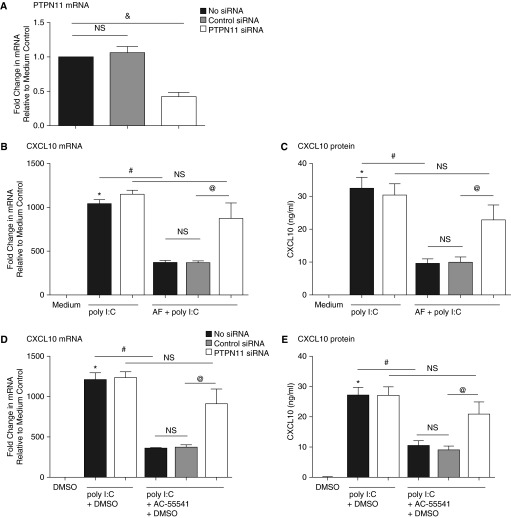
Knockdown of PTPN11 Reversed AF-Mediated Suppression of CXCL10 Induced by Poly I:C and HRV16
The suppressive effect of AF extract on poly I:C–induced CXCL10 mRNA was diminished after knockdown of PTPN11 (Figure 5A) by siRNA transfection in NHBE cells. That is, there was no significant reduction of the response to poly I:C by AF in the PTPN11 siRNA–treated cells (P = 0.20 [n = 6]) (Figure 5B). The pattern was the same when CXCL10 protein expression was analyzed by ELISA (P = 0.28 [n = 6]) (Figure 5C). Furthermore, the suppression of poly I:C–induced CXCL10 by the PAR-2 ligand AC-55541 was also reversed after knockdown of PTPN11 (P = 0.13 [n = 6] and P = 0.24 [n = 6], respectively) (Figures 5D and 5E). Finally, prior incubation with NSC-87877, a PTPN11 inhibitor, diminished the AF-mediated suppression of poly I:C–induced CXCL10 mRNA and protein expression (Figures E3A and E3B). Taken together, these results suggest that the suppressive effect of AF extract on poly I:C–induced CXCL10 was mediated by PAR-2 and, at least in part, by PTPN11 activation.
Figure 5.
Knockdown of PTPN11 reversed AF-mediated suppression of CXCL10 induced by poly I:C. Submerged NHBE cells were transfected with 10 nM siRNA against control RNA and PTPN11. (A) Knockdown efficiency of PTPN11 was analyzed by RT-PCR. NHBE cells were transfected with 10 nM siRNA against nonspecific control RNA or PTPN11. Cells were pretreated with AF extract or with the PAR-2 activator AC-55541 (100 nM) for 1 hour and then stimulated with poly I:C (5 μg/ml) for 6 hours. (B and D) The expression of CXCL10 mRNA from cell lysates was analyzed by RT-PCR. (C and E) CXCL10 protein in supernatant was analyzed by ELISA. Data represent mean ± SEM of three independent experiments. *P < 0.01 by t test when compared with medium-only control. #P < 0.01 by t test when compared with poly I:C–treated cells. &P < 0.01 by t test when compared with control siRNA-transfected cells. @P < 0.01 by t test when compared with PTPN11 siRNA-transfected cells.
Discussion
Previous studies by our laboratory and others have shown that extracts of AF suppress activation of epithelial cells by either IFN or by the TLR3 agonist poly I:C (6, 18, 28, 29). In the present study, we show that AF extract also suppressed the HRV16-induced innate immune response in fully differentiated NHBE cells using an ALI culture system (Figures 1C and 1D). Because epithelial cells exert an autocrine IFN response during induction of an antiviral state, IFN signaling is essential for innate immunity to viruses (36). This intrinsic response to IFN has also been found to be important in setting the tone of adaptive immune responses because IFN family cytokines bias adaptive immune responses toward Th1 and away from Th2 (40). Consequently, suppression of the endogenous epithelial IFN response by AF or other fungi may have the consequence of promoting Th2 by removing a pathway that ordinarily keeps Th2 (and Th17) responses in check. The present study explores the mechanism of this effect of AF and provides evidence that inactivation of poly I:C–induced TLR3 signaling by extracts of AF involves activation of PAR-2 as well as phosphorylation, and presumably activation, of PTPN11 in primary NHBE cells.
We observed phosphorylation of PTPN11 (Y542) in bronchial epithelial cells upon activation of PAR-2 either by AF or by a synthetic PAR-2 agonist. Downstream, exposure to AF inhibited the phosphorylation of STAT1 (Y701) and IRF-3 after poly I:C activation; these are two key transcription factors in the antiviral response. Suppression of these signaling pathways was associated with reduction of TLR3 induction of mRNA and protein for the marker of antiviral responses, CXCL10. Previously it was shown that protease activities of allergens could induce type 2 immunological allergic responses in animal models (41). One interpretation of our findings is that proteases found in AF may activate PAR-2, induce PTPN11, and suppress IRF-3 and STAT1 signaling and thereby suppress the expression of Th1-promoting chemokines induced by these transcription factors, such as CXCL10.
In allergic airway disease, viral pathogens, such as HRV and respiratory syncytial virus, and aeroallergens are thought to play major roles in the initiation of sensitization and in the exacerbation of allergic airway disorders. There are several findings indicating that some commonality in the underlying mechanisms may link these two events. Bronchial epithelial cells from patients with asthma were shown to have defects in expression of type I and III IFNs when exposed to viral infection (23, 24). Consistent with this observation, supplemental IFN-β inhalation therapy was effective in the treatment of patients with severe asthma (42). Importantly, IFN-β inhalation therapy up-regulated serum levels of CXCL10 protein and enhanced IFN-inducible genes, such as OAS-1, Mx1, and CXCL10, in the sputum (42). Fungi, fungal proteases, PAR receptors, and epithelial phosphatases are likely to regulate differentiation of adaptive immune cells. Collison and colleagues showed an important role of protein phosphatase 2A in enhanced expression of cytokines, such as IL-25 and IL-33, from airway epithelial cells derived from subjects with asthma (31). To date, it is not known whether the apparent defect in IFN expression or signaling in epithelium from indivduals with asthma is a cell-intrinsic or cell-extrinsic effect. The ability of fungi to inhibit IFN signaling in epithelium should be considered in the context of potential extrinsic mechanisms. Recently, Zhu and colleagues showed that A. alternata extract inhibited poly I:C–induced IRF-3 phosphorylation, resulting in reduced induction of IFN-β in the A549 alveolar epithelial cell line (28). Interestingly, Zhu and colleagues also showed that the effect was protease independent, in contrast to our findings. Because Zhu and colleagues used Alternaria and we used Aspergillus, it may be that the different fungi use distinct mechanisms to inhibit IFN signaling. However, our previous study (6), the study by Zhu and colleagues (28), and the present study all show that fungal extracts can inhibit IFN signaling in epithelial cells. Because allergic airways diseases are often associated with fungal infections, it is possible that fungi may contribute to the reduced IFN signaling in asthma and CRS.
Our current results implicate PAR-2 as one of the major signaling pathways leading to suppression of viral-induced TLR3 signaling in airway epithelial cells. Furthermore, our data show that AF-inhibited CXCL10 induced by poly I:C and HRV (Figures 1 and E3) while leaving IL-13–induced CCL26 unaffected (Figure E1). Whereas CXCL10 is important in Th1 and antiviral responses, CCL26 is an eosinophil attractant chemokine important in the initiation and/or exacerbation of allergic airway responses (26, 43). Selective suppression of the Th1-promoting chemokine (CXCL10) without inhibition of the Th2 promoting chemokine (CCL26) exemplifies the Th2 bias of epithelium that AF confers and supports the rationale of this study to elucidate the underlying mechanism.
Several other studies have shown that specific allergens such as HDM, cockroach, Alternaria species, and AF possess strong protease activity and can activate PAR-2 (9, 10, 28, 34, 35). In addition, PAR-2 knockout mice showed reduced induction of eotaxin by ovalbumin, resulting in diminished eosinophil recruitment to the lung when compared with control mice (44). In our in vitro studies, PAR-2 knockdown abolished AF-mediated suppression of TLR3 signaling (Figure 2). Because HDM and cockroach antigen preparations are known to possess proteolytic activity, we have also tested extracts of these allergens in our knockdown model and found results consistent with those with the AF extract (Figures E1E and E1F). From these results, we speculate that PAR-2 signaling is common to many allergens and may be a pivotal pathway that regulates antiviral innate immune responses of airway epithelial cells to inhaled allergens.
The mechanism by which activation of PAR-2 may suppress the IFN-mediated antiviral response remains uncertain. Our studies implicate PTPN11, an inhibitor of phosphorylation-dependent signaling, in this response. This conclusion is supported by other reports in the literature. Yu and colleagues showed that PAR-2 activation by the agonist peptide SLIGRL induced expression of PTPN11 (30). In the present study, we show that AF extract activated PTPN11 in airway epithelial cells. Until the present study, a direct link between PAR-2–induced PTPN11 and TLR3 signaling had not been made. However, PTPN11 is widely expressed in various tissues and cell types and has been implicated in diverse signaling pathways, including those initiated by growth factors, such as epidermal growth factor and insulin-like growth factor-1, and cytokines, such as IFNs, IL-5, IL-6, and IL-1β (45, 46). In addition, PTPN11 was shown to regulate a variety of signal transduction processes, such as mitogen-activated protein kinase (MAPK), JAK-STAT, and PI3 kinase signaling. We thus tested the hypothesis that the inhibition of JAK-STAT signaling by AF may result from induction of PTPN11. In our current study, PTPN11 was important in regulating both poly I:C–activated IRF-3 (Figure 2) and IFN-β–activated STAT1 signaling (6) but did not affect IL-13–induced STAT6 signaling (Figure E1). Using a system other than AF and triggering distinct from PAR-2, An and colleagues reported negative regulation of TLR3-activated IFN-β production based on studies in a PTPN11-deficient mouse, and they have also shown that PTPN11 suppressed IRF-3 activation by inhibiting TBK1, a kinase that phosphorylates IRF-3 (18). TBK1 is known to be phosphorylated at serine moieties, and PTPN11 was originally shown to dephosphorylate at tyrosine moieties. It is still unclear how the interaction of TBK1 and PTPN11 leads to suppression of poly I:C–activated signaling; therefore, further investigation is warranted (47). You and colleagues showed inhibition of IFN-α– and IFN-γ–induced STAT1 by PTPN11 (22). Recently, Nhu and colleagues demonstrated that activation of PAR-2 leads to negative regulation of TLR3 signaling by blunting the phosphorylation of IRF-3 and STAT1 in bronchial epithelial cells but did not implicate PTPN11 (29). Taken together with our present findings, it can be hypothesized that PAR-2 activation induces PTPN11 expression and phosphorylation and suppresses TLR3 or IFN-β signaling. Although PTPN11 plays a central role in our system, we also noticed that inhibition of PTPN11 (Figure E3) failed to completely abolish the inhibitory effect of AF extract. This suggests that other phosphatases, such as PTPN10, which was also induced by HMW-AF extract, may be playing a role in our system as well. Alternatively, AF may be inducing other inhibitory pathways that work via distinct mechanisms. Further study is required to address the role of other phosphatase family members. One novel aspect of the present report is that it may help to define the mechanisms by which epithelial cell exposure to AF by mucosal colonization or inhalation plays a role in allergic sensitization.
Previous studies have shown that TLR3 signaling leads downstream to activation of both IRF-3 and NF-κB in airway epithelial cells (7, 8, 32). PAR-2 has been shown to activate NF-κB and/or MAPK pathways (48–50). In our present study, we failed to detect the activation of NF-κB by either HMW-AF extract or PAR-2 activator (Figure 2). Also, neither AF, HMW-AF extract, nor PAR-2 activator alone induced CXCL10 (Figure 1). IL-17A–induced CXCL8 mRNA was enhanced by AF extract (Figure E1), and it has been reported that IL-17A mainly activates the MAPK pathway (51, 52); we therefore speculate that the main signaling of PAR-2 could be via the MAPK pathway. It will be necessary to carefully examine the downstream pathway of PAR-2 signaling leading to activation of phosphatases, especially PTPN11, in future investigations.
This study demonstrates that AF extract, especially HMW-AF extract, activated PAR-2 and PTPN11; these events in turn suppressed poly I:C– and HRV16-induced CXCL10 mRNA and protein expression in airway epithelial cells. These findings give new insight into the mechanism by which AF allergen modulates the antiviral/IFN response of airway epithelial cells. Suppression of this response, which is important in immunity to both viral and fungal pathogens, can increase susceptibility to such pathogens and may lead to an epithelial bias of subsequent adaptive immune responses promoting Th2 or Th17 responses in favor of Th1 responses. Further insight into the regulation of epithelial activation by PAR-2 and PTPN11 may lead to new strategies to interfere with virus-induced pathogenesis or allergic sensitization.
Footnotes
This study was supported by National Institutes of Health grants R37HL068546, R01HL078860, R01AI104733, and U19AI106683; by the Ernest S. Bazley Foundation; and by a Showa University Research Grant for Young Researchers (Showa University Research Fund).
Author Contributions: Conception and design: T.H., A.K., B.B., J.E.N., D.S.G., and R.P.S. Analysis and interpretation: T.H., L.A.S., R.G.C., and R.P.S. Drafting the manuscript for important intellectual content: T.H. and R.P.S.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0062OC on June 13, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.O’Sullivan SM. Asthma death, CD8+ T cells, and viruses. Proc Am Thorac Soc. 2005;2:162–165. doi: 10.1513/pats.200502-016AW. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pleis JR, Ward BW, Lucas JW. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. Vital Health Stat 10. 2010;249:1–207. [PubMed] [Google Scholar]
- 4.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by Toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 5.Porter PC, Lim DJ, Maskatia ZK, Mak G, Tsai CL, Citardi MJ, Fakhri S, Shaw JL, Fothergil A, Kheradmand F, et al. Airway surface mycosis in chronic TH2-associated airway disease. J Allergy Clin Immunol. 2014;134:325–331. doi: 10.1016/j.jaci.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhushan B, Homma T, Norton JE, Sha Q, Siebert J, Gupta DS, Schroeder JW, Jr, Schleimer RP. Suppression of epithelial STAT1 activation by extracts of Aspergillus fumigatus. Am J Respir Cell Mol Biol. 2015;53:87–95. doi: 10.1165/rcmb.2014-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsukura S, Kokubu F, Kurokawa M, Kawaguchi M, Ieki K, Kuga H, Odaka M, Suzuki S, Watanabe S, Takeuchi H, et al. Synthetic double-stranded RNA induces multiple genes related to inflammation through Toll-like receptor 3 depending on NF-kappaB and/or IRF-3 in airway epithelial cells. Clin Exp Allergy. 2006;36:1049–1062. doi: 10.1111/j.1365-2222.2006.02530.x. [DOI] [PubMed] [Google Scholar]
- 8.Homma T, Matsukura S, Hirose T, Ohnishi T, Kimura T, Kurokawa M, Ieki K, Odaka M, Suzuki S, Watanabe S, et al. Cooperative activation of CCL5 expression by TLR3 and tumor necrosis factor-alpha or interferon-gamma through nuclear factor-kappaB or STAT-1 in airway epithelial cells. Int Arch Allergy Immunol. 2010;152:9–17. doi: 10.1159/000312120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;49:741–750. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquet A. Interactions of airway epithelium with protease allergens in the allergic response. Clin Exp Allergy. 2011;41:305–311. doi: 10.1111/j.1365-2222.2010.03661.x. [DOI] [PubMed] [Google Scholar]
- 11.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 12.Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, Thompson PJ, Stewart GA. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea MR, Rogahn CJ, Andrade-Gordon P. Localization of protease-activated receptors-1 and -2 in human mast cells: indications for an amplified mast cell degranulation cascade. Biotech Histochem. 2000;75:85–90. doi: 10.3109/10520290009064152. [DOI] [PubMed] [Google Scholar]
- 14.Colognato R, Slupsky JR, Jendrach M, Burysek L, Syrovets T, Simmet T. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. 2003;102:2645–2652. doi: 10.1182/blood-2002-08-2497. [DOI] [PubMed] [Google Scholar]
- 15.Howells GL, Macey MG, Chinni C, Hou L, Fox MT, Harriott P, Stone SR. Proteinase-activated receptor-2: expression by human neutrophils. J Cell Sci. 1997;110:881–887. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- 16.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, Matsuwaki Y, Asaka D, Hama T, Otori N, Moriyama H. The expression of protease-activated receptors in chronic rhinosinusitis. Int Arch Allergy Immunol. 2013;161:138–146. doi: 10.1159/000350386. [DOI] [PubMed] [Google Scholar]
- 18.An H, Zhao W, Hou J, Zhang Y, Xie Y, Zheng Y, Xu H, Qian C, Zhou J, Yu Y, et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25:919–928. doi: 10.1016/j.immuni.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Matthews RJ, Bowne DB, Flores E, Thomas ML. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992;12:2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 21.Bennett AM, Tang TL, Sugimoto S, Walsh CT, Neel BG. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You M, Yu DH, Feng GS. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol. 1999;19:2416–2424. doi: 10.1128/mcb.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 25.Hussein YM, Alzahrani SS, Alharthi AA, Ghonaim MM, Alhazmi AS, Eed EM, Shalaby SM. Association of serum cytokines levels, interleukin 10 -1082G/A and interferon-γ +874T/A polymorphisms with atopic asthma children from Saudi Arabia. Cell Immunol. 2014;289:21–26. doi: 10.1016/j.cellimm.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Heller NM, Matsukura S, Georas SN, Boothby MR, Rothman PB, Stellato C, Schleimer RP. Interferon-gamma inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31:573–582. doi: 10.1165/rcmb.2004-0195OC. [DOI] [PubMed] [Google Scholar]
- 27.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 28.Zhu L, Lee B, Zhao F, Zhou X, Chin V, Ling SC, Chen Y. Modulation of airway epithelial antiviral immunity by fungal exposure. Am J Respir Cell Mol Biol. 2014;50:1136–1143. doi: 10.1165/rcmb.2013-0357OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nhu QM, Shirey K, Teijaro JR, Farber DL, Netzel-Arnett S, Antalis TM, Fasano A, Vogel SN. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010;3:29–39. doi: 10.1038/mi.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Z, Ahmad S, Schwartz JL, Banville D, Shen SH. Protein-tyrosine phosphatase SHP2 is positively linked to proteinase-activated receptor 2-mediated mitogenic pathway. J Biol Chem. 1997;272:7519–7524. doi: 10.1074/jbc.272.11.7519. [DOI] [PubMed] [Google Scholar]
- 31.Collison A, Hatchwell L, Verrills N, Wark PA, de Siqueira AP, Tooze M, Carpenter H, Don AS, Morris JC, Zimmermann N, et al. The E3 ubiquitin ligase midline 1 promotes allergen and rhinovirus-induced asthma by inhibiting protein phosphatase 2A activity. Nat Med. 2013;19:232–237. doi: 10.1038/nm.3049. [DOI] [PubMed] [Google Scholar]
- 32.Chustz RT, Nagarkar DR, Poposki JA, Favoreto S, Jr, Avila PC, Schleimer RP, Kato A. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2011;45:145–153. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi SK, Bianco A, Allen JT, Knight RA, Spiteri MA. Interferon-gamma (IFN-gamma) down-regulates the rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) on human airway epithelial cells. Clin Exp Immunol. 1997;110:362–369. doi: 10.1046/j.1365-2249.1997.4221440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oguma T, Asano K, Tomomatsu K, Kodama M, Fukunaga K, Shiomi T, Ohmori N, Ueda S, Takihara T, Shiraishi Y, et al. Induction of mucin and MUC5AC expression by the protease activity of Aspergillus fumigatus in airway epithelial cells. J Immunol. 2011;187:999–1005. doi: 10.4049/jimmunol.1002257. [DOI] [PubMed] [Google Scholar]
- 36.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ten Hoeve J, de Jesus Ibarra-Sanchez M, Fu Y, Zhu W, Tremblay M, David M, Shuai K. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol Cell Biol. 2002;22:5662–5668. doi: 10.1128/MCB.22.16.5662-5668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyake A, Murata Y, Okazawa H, Ikeda H, Niwayama Y, Ohnishi H, Hirata Y, Matozaki T. Negative regulation by SHPS-1 of Toll-like receptor-dependent proinflammatory cytokine production in macrophages. Genes Cells. 2008;13:209–219. doi: 10.1111/j.1365-2443.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 39.Salojin KV, Owusu IB, Millerchip KA, Potter M, Platt KA, Oravecz T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol. 2006;176:1899–1907. doi: 10.4049/jimmunol.176.3.1899. [DOI] [PubMed] [Google Scholar]
- 40.van Schaik SM, Obot N, Enhorning G, Hintz K, Gross K, Hancock GE, Stack AM, Welliver RC. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J Med Virol. 2000;62:257–266. doi: 10.1002/1096-9071(200010)62:2<257::aid-jmv19>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 41.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djukanović R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, Niven R, Singh D, Reddel HK, Davies DE, et al. INTERCIA Study Group. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections: a randomized trial. Am J Respir Crit Care Med. 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bochner BS, Hudson SA, Xiao HQ, Liu MC. Release of both CCR4-active and CXCR3-active chemokines during human allergic pulmonary late-phase reactions. J Allergy Clin Immunol. 2003;112:930–934. doi: 10.1016/j.jaci.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Takizawa T, Tamiya M, Hara T, Matsumoto J, Saito N, Kanke T, Kawagoe J, Hattori Y. Abrogation of bronchial eosinophilic inflammation and attenuated eotaxin content in protease-activated receptor 2-deficient mice. J Pharmacol Sci. 2005;98:99–102. doi: 10.1254/jphs.scz050138. [DOI] [PubMed] [Google Scholar]
- 45.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neel BG, Tonks NK. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 47.O’Neill LA. When signaling pathways collide: positive and negative regulation of Toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Goon Goh F, Sloss CM, Cunningham MR, Nilsson M, Cadalbert L, Plevin R. G-protein-dependent and -independent pathways regulate proteinase-activated receptor-2 mediated p65 NFκB serine 536 phosphorylation in human keratinocytes. Cell Signal. 2008;20:1267–1274. doi: 10.1016/j.cellsig.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and extracellular-signal-regulated kinase. J Allergy Clin Immunol. 2003;112:1112–1118. doi: 10.1016/j.jaci.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 50.Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006;281:6910–6923. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 51.Iyoda M, Shibata T, Kawaguchi M, Hizawa N, Yamaoka T, Kokubu F, Akizawa T. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-α and IL-1β. Am J Physiol Renal Physiol. 2010;298:F779–F787. doi: 10.1152/ajprenal.00198.2009. [DOI] [PubMed] [Google Scholar]
- 52.Kawaguchi M, Fujita J, Kokubu F, Huang SK, Homma T, Matsukura S, Adachi M, Hizawa N. IL-17F-induced IL-11 release in bronchial epithelial cells via MSK1-CREB pathway. Am J Physiol Lung Cell Mol Physiol. 2009;296:L804–L810. doi: 10.1152/ajplung.90607.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]