To the Editor:
Findings in murine models implicate subpopulations of alveolar macrophages in the pathogenesis of lung injury and fibrosis; however, the relevance of these findings for humans with chronic lung disease is unknown in part because of a lack of proper tools to identify macrophage heterogeneity in the human lung. Here we report a flow cytometry protocol that allows unambiguous identification of alveolar macrophages, interstitial macrophages, and monocytes in the human lung and in bronchoalveolar lavage fluid. We validated this panel using normal lung tissue and tissue from patients with chronic obstructive pulmonary disease and lung fibrosis. We found evidence of heterogeneity within human alveolar macrophage populations, which suggest parallels between murine and human macrophage development and differentiation.
Lung macrophages are essential for maintaining homeostasis in the healthy lung and play an important role in pulmonary diseases (1). For decades, alveolar macrophages, which are abundant in the alveolar space of the normal lung, were considered a homogenous population of cells derived from and continuously replenished by circulating monocytes originating from the bone marrow. In careful studies of murine lung development, several groups of investigators have overturned this paradigm. They discovered that alveolar macrophages originate from fetal monocytes, which populate the lungs during the first days of life (2–4). These “tissue-resident” alveolar macrophages are capable of self-renewal, and they or their daughter cells persist in the lung over the lifespan of the host (1, 2). In addition, a healthy mouse lung contains a small fraction of the monocyte-derived interstitial macrophages and extravasated and intravascular monocytes (2, 5, 6). Various insults can induce recruitment of the monocytes into the lung. These monocytes transition through “interstitial macrophages” before differentiating into monocyte-derived alveolar macrophages, which may persist in the lung after injury (7). Previously, we found that although these monocyte-derived alveolar macrophages are phenotypically similar to tissue-resident alveolar macrophages, there were small differences in their surface marker expression after injury, suggesting that subtle changes in surface markers might distinguish monocyte-derived from tissue-resident macrophages (8).
Although human macrophages are less well studied, the murine data predict the presence of at least three distinct populations of monocytes or macrophages in the human lung: alveolar macrophages, interstitial macrophages, and monocytes. Using workflows and protocols we developed in mice, we generated a multicolor flow cytometry protocol to unambiguously identify these populations in the human lung. Using these markers we also observed heterogeneity in the alveolar macrophage population, which may reflect the relative contribution of monocyte-derived versus tissue-resident cells to the alveolar macrophage pool. Because this panel relies exclusively on the identification of cell surface markers, it is appropriate for sorting and recovering live cells for subsequent analysis.
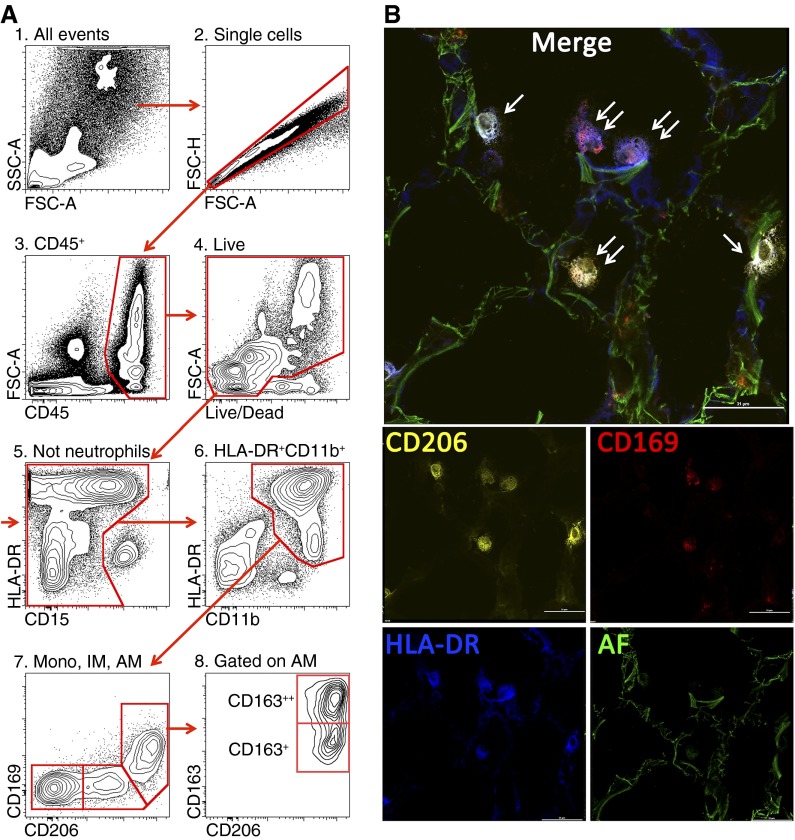
All procedures using human tissues were approved by the Northwestern University Institutional Review Board. We obtained biopsies from normal human donor lungs before transplantation (nine samples) and explanted lungs from recipients with chronic obstructive pulmonary disease (five recipients), systemic sclerosis-associated interstitial lung disease (one recipient), mixed connective tissue disease (one recipient), idiopathic lung fibrosis (one recipient), and 10 bronchoalveolar lavage (BAL) samples from patients admitted to the intensive care unit who are being evaluated for pneumonia. We also obtained fixed sections of lung tissue from five stillborn infants. We developed a standardized protocol to process the lungs for flow cytometric analysis (see online supplement for details). After screening several markers, we found that HLA-DR, CD169, and CD206 allow accurate separation of the three monocyte/macrophage populations: alveolar macrophages (CD11b+HLA-DR++CD206++CD169+), interstitial macrophages (CD11b+HLA-DR++CD206+CD169−), and monocytes (CD11b+HLA-DR+CD206−CD169−) (Figure 1A and see Figure E1 in the online supplement). Interestingly, CD206, which has been suggested to indicate “M2 polarization” of macrophages, is ubiquitously expressed in macrophage populations in the normal human lung (9). Immunofluorescent microscopy confirmed the expected anatomical localization of these cells (Figure 1B). The same populations were identified in the diseased explanted lungs of patients with chronic obstructive pulmonary disease, systemic sclerosis-associated lung fibrosis, and mixed connective tissue disease undergoing lung transplantation, and all key cell markers (HLA-DR, CD169, and CD206) performed well (data not shown). We then screened several markers that might identify heterogeneity in the alveolar macrophage population. We found that the alveolar macrophage population in all studied samples (from both normal and diseased lungs) can be further subdivided into CD163++ and CD163+ subpopulations (Figure 1A, panel 8). CD163 is a scavenger receptor for hemoglobin/haptoglobin complex highly expressed on tissue macrophages, and it has been proposed as a marker of “resolving” monocyte-derived macrophages (10, 11). Because our previous studies in a mouse model of lung fibrosis demonstrated that differential expression of Siglec F allows discrimination of the tissue-resident and monocyte-derived macrophages (8), we now speculate that the heterogeneity in CD163 expression in human alveolar macrophages may also reflect their differential ontogeny. Alternatively, heterogeneity in CD163 may reflect a different activation state and/or anatomical localization; these questions need further evaluation in the large, well-described cohorts of patients. Intriguingly, BAL analysis did not reveal the presence of interstitial macrophages CD11b+HLA-DR++CD206+CD169−, nor did it show CD163 heterogeneity in alveolar macrophages observed in tissue biopsies (Figure E2). These findings parallel findings in mice in which BAL fluid incompletely described the macrophage populations in the normal or injured murine lung (12).
Figure 1.
(A) Gating strategy used to identify lung monocytes and macrophages. After excluding doublets (2), cells of hematopoietic origin were identified as CD45+ (3), followed by exclusion of the dead cells (precautions were taken not to gate out highly autofluorescent alveolar macrophages) (4). Neutrophils were identified as CD11b++CD15+CD16+HLA-DR− cells and excluded from analysis (5). We then gated on CD11b++HLA-DR+ cells (6). This allows separation from natural killer (NK) cells (CD11b+HLA-DR−CD56+) and highly autofluorescent eosinophils (CD11b+/−HLA-DR−Siglec 8+). Finally, using CD206 and CD169, cells were separated into three subpopulations: alveolar macrophages (CD11b+HLA-DR++CD206++CD169+FSChighSSChigh, AM), interstitial macrophages (CD11b+HLA-DR++CD206+CD169−, IM), and monocytes (CD11b+HLA-DR+CD206−CD169−, mono) (7). CD163 identifies two subpopulations of alveolar macrophages (8). (B) Immunofluorescent microscopy on a normal human lung. Orange is CD206 PE, red is CD169 AF647, blue is HLA-DR BV421, and green is autofluorescence in fluorescein isothiocyanate channel. Single arrows indicate HLA-DR+CD206+CD169− cells (interstitial macrophages), and double arrows indicate HLA-DR+CD206+CD169+ cells (alveolar macrophages). Scale bar is 31 μm.
In newborn mice, tissue-resident alveolar macrophages are absent at birth but differentiate from lung fetal monocytes in the first days of life (3). We reasoned that if a similar developmental pathway occurs in humans, monocytes and interstitial macrophages, but not alveolar macrophage markers, should be found in human lungs before birth. Immunostaining of lung sections from stillborn infants were negative for both CD169+ cells and CD206+ cells located in the alveoli, whereas CD206+ cells were present in the interstitium (Figure E3). These results are consistent with earlier work suggesting that alveolar macrophages are not found in stillborn infants in the absence of infection but are routinely found in neonates (13). Although our laboratories are completely independent, our findings, protocol, and conclusions are similar to those described by Yu and colleagues in this issue of the Journal (pp. 13–24)., highlighting the utility and reproducibility of this approach for future studies of alveolar macrophage populations in the human lung (14).
Footnotes
A.B. is supported by the John H. Gibbon Jr. Research Scholarship from the American Association of Thoracic Surgery. K.R. is supported by National Institutes of Health (NIH) grants HL079190 09 and HL124664 01. K.K.M. is supported by NIH grant K23 HL093302. H.P. is supported by NIH grants AR050250, AR064546, AI092490, and HL108795 and funds provided by Solovy/Arthritis Research Society. G.R.S.B. is supported by NIH grants ES013995, HL071643, and AG049665 and the Veterans Administration. A.V.M. is supported by NIH grant AR061593, an American Thoracic Society/Scleroderma Foundation Research grant, and a BD Bioscience Immunology Research grant. The Northwestern University Flow Cytometry Facility and Center for Advanced Microscopy are supported by National Cancer Institute Cancer Center Support grant P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center.
Author Contributions: A.B. designed the study, developed the institutional review board (IRB) protocol, undertook patient recruitment, obtained lung tissue, interpreted data, and wrote the manuscript. S.M.B. developed the IRB protocol, undertook patient recruitment, interpreted data, and wrote the manuscript. L.M.-N. processed lung tissue for immunofluorescent microscopy, performed staining, acquired and interpreted data, and wrote the manuscript. A.C.M.-P. processed lung tissue for flow cytometry and immunofluorescent microscopy, performed staining, acquired and interpreted data, and wrote the manuscript. S.S. processed lung tissue for immunohistochemistry, performed staining, acquired and interpreted data, and wrote the manuscript. K.R. provided lung tissue, interpreted data, and wrote the manuscript. M.M.D. provided lung tissue, interpreted data, and wrote the manuscript. K.K.M. provided lung tissue from stillborn infants, interpreted data, and wrote the manuscript. H.P. designed the study, interpreted data, and wrote the manuscript. G.R.S.B. designed the study, provided bronchoalveolar lavage samples, interpreted data, and wrote the manuscript. A.V.M. designed the study; performed sample processing, flow cytometric acquisition, analysis, and cell sorting; interpreted data; and and wrote manuscript.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 2.Scott CL, Henri S, Guilliams M. Mononuclear phagocytes of the intestine, the skin, and the lung. Immunol Rev. 2014;262:9–24. doi: 10.1111/imr.12220. [DOI] [PubMed] [Google Scholar]
- 3.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, et al. Immunological Genome Consortium. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 8.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, Friedrich K, Müller-Quernheim J, Zissel G. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47:1650–1660. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Evans BJ, Haskard DO, Sempowksi G, Landis RC. Evolution of the macrophage CD163 phenotype and cytokine profiles in a human model of resolving inflammation. Int J Inflam. 2013;2013:780502. doi: 10.1155/2013/780502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol. 2013;49:180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alenghat E, Esterly JR. Alveolar macrophages in perinatal infants. Pediatrics. 1984;74:221–223. [PubMed] [Google Scholar]
- 14.Yu YA, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW, Kraft M, Hollingsworth JW, Gunn MD, Tighe RM. Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. Am J Respir Cell Mol Biol. 2016;54:13–24. doi: 10.1165/rcmb.2015-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]