Abstract
Chitinase 3–like 1 (Chi3l1), which is also called YKL-40 in humans and BRP-39 in mice, is the prototypic chitinase-like protein. Recent studies have highlighted its impressive ability to regulate the nature of tissue inflammation and the magnitude of tissue injury and fibroproliferative repair. This can be appreciated in studies that highlight its induction after cigarette smoke exposure, during which it inhibits alveolar destruction and the genesis of pulmonary emphysema. IL-18 is also known to be induced and activated by cigarette smoke, and, in murine models, the IL-18 pathway has been shown to be necessary and sufficient to generate chronic obstructive pulmonary disease–like inflammation, fibrosis, and tissue destruction. However, the relationship between Chi3l1 and IL-18 has not been defined. To address this issue we characterized the expression of Chi3l1/BRP-39 in control and lung-targeted IL-18 transgenic mice. We also characterized the effects of transgenic IL-18 in mice with wild-type and null Chi3l1 loci. The former studies demonstrated that IL-18 is a potent stimulator of Chi3l1/BRP-39 and that this stimulation is mediated via IFN-γ–, IL-13–, and IL-17A–dependent mechanisms. The latter studies demonstrated that, in the absence of Chi3l1/BRP-39, IL-18 induced type 2 and type 17 inflammation and fibrotic airway remodeling were significantly ameliorated, whereas type 1 inflammation, emphysematous alveolar destruction, and the expression of cytotoxic T lymphocyte perforin, granzyme, and retinoic acid early transcript 1 expression were enhanced. These studies demonstrate that IL-18 is a potent stimulator of Chi3l1 and that Chi3l1 is an important mediator of IL-18–induced inflammatory, fibrotic, alveolar remodeling, and cytotoxic responses.
Keywords: Chi3l1, IL-18, airway fibrosis, alveolar remodeling, COPD
Clinical Relevance
With the use of an advanced lung-specific IL-18 transgenic overexpression model, these studies are novel in defining the roles of chitinase 3-like–1 (Chi3l1)/BRP-39 in IL-18–induced pulmonary inflammatory and remodeling responses. They are also novel in defining the roles of type 1, type 2, and type 17 inflammation in the regulation of Chi3l1/BRP-39, an ancient molecule that has been conserved during evolution and plays critical roles in pulmonary injury and repair responses.
The 18 glycosyl hydrolase gene (18 GH) family is made up of chitinases, which enzymatically digest chitin and chitinase-like proteins (CLPs), which bind (but do not cleave) chitin (1). This ancient gene family exists in species as diverse as plants, insects, and humans and has evolved during speciation with a particularly impressive increase in CLP in mammals (2, 3). This retention over species and evolutionary time has led to the belief that the CLPs play essential roles in biology. To evaluate this speculation we focused on chitinase 3-like–1 (Chi3l1) (also called as YKL-40 in humans and BRP-39 in rodents), the prototypic CLP (1). These studies supported this speculation by demonstrating that Chi3l1 plays a major role in antipathogen, antigen-induced, and oxidant-induced inflammatory repair and remodeling responses by regulating a variety of essential biologic processes, including oxidant injury, apoptosis, pyroptosis, inflammasome activation, Th1/Th2 inflammatory balance, M2 macrophage differentiation, TGF-β1 elaboration, and dendritic cell accumulation and the activation of mitogen-activated protein kinase, Akt/protein kinase B, and Wnt/β-catenin signaling (4–10). Studies from our laboratory and others also identified significant correlations between dysregulated Chi3l1 and the development, severity, and/or progression of a number of diseases, including asthma and COPD (for review see Refs. 1, 11–13). However, the cytokines and other mediators that regulate Chi3l1 production in these settings have not been fully defined.
IL-18 is a member of the IL-1 cytokine superfamily (14, 15) that has an impressive ability to induce Th1/Tc1 differentiation and immune responses (14, 16). It can also contribute to the generation of type 2 immune responses (14) and plays a key role in the generation of Th17 responses in diseases characterized by autoimmunity (17, 18). A variety of lines of evidence have recently implicated IL-18 in the pathogenesis of chronic obstructive pulmonary disease (COPD). Studies from our laboratory demonstrated that IL-18 is induced and activated after cigarette smoke (CS) exposure and that IL-18 receptor alpha signaling plays a critical role in the pathogenesis of CS-induced pulmonary inflammation and emphysema (19). Increased levels of IL-18 were also noted in the serum and induced sputum from patients with COPD, where they correlate with abnormal lung function (20, 21). Our studies also demonstrated that IL-18 was sufficient to induce COPD-like tissue responses because the lung-selective overexpression of IL-18 induced tissue inflammation, emphysema, mucus metaplasia, airway fibrosis, vascular remodeling with intimal hyperplasia, and cardiac right ventricle hypertrophy, which resemble the features of human COPD (22). They also demonstrate that IL-18 simultaneously induces the signature cytokines associated with the type 1, type 2, and type 17 responses that play specific roles in the pathogenesis of the IL-18 effector repertoire (22). Importantly, studies from our laboratory also demonstrated that CS exposure augments the production of Chi3l1/BRP-39 and that Chi3l1/BRP-39 plays essential roles in the control of alveolar destruction (12). In addition, a recent study demonstrated that IL-18 overexpression induces the Chi3l1/BRP-39 molecule in lungs (13). However, the roles of Chi3l1/BRP-39 in IL-18–induced pulmonary inflammatory and tissue remodeling responses have not been adequately defined.
We hypothesized that IL-18 is a potent inducer of Chi3l1/BRP-39 and that Chi3l1/BRP-39 plays significant roles in IL-18–induced pulmonary inflammatory and remodeling responses. To address these speculations, we characterized the expression of Chi3l1/BRP-39 in control mice and mice in which IL-18 was overexpressed in a lung-specific manner (IL-18 Tg). We also bred these transgenic mice with mice with null mutations of Chi3l1/BRP-39 (Chi3l1−/−) and characterized the effects of transgenic IL-18 in the presence and absence of this CLP. These studies demonstrated that IL-18 is a potent stimulator of Chi3l1/BRP-39 and that this stimulation is mediated via IFN-γ–, IL-13–, and IL-17A–dependent mechanisms. They also demonstrated that Chi3l1/BRP-39 plays a critical role in the generation of IL-18–induced type 2 and type 17 inflammatory responses and pulmonary fibrosis while inhibiting IL-18–induced type 1 inflammation, cytotoxic responses, and emphysematous alveolar remodeling.
Materials and Methods
Mice
The generation of mice in which IL-18 was targeted to the lung in a doxycycline-inducible manner (IL-18 Tg+ mice) and Chi3l1/BRP-39 null mutant (−/−) mice were previously described by our laboratory (8, 23). Wild-type (WT), IFN-γ null mice (Jackson Labs, Bar Harbor, ME), IL-13 null mice (from Dr. A. McKenzie), and IL-17A null mice (from Dr. Y. Iwakura) were bred at Yale University. The WT and genetically modified mice were on a C57BL/6 genetic background. All animal experimentation was conducted with the approval of the Institutional Animal Care and Use Committee of Yale School of Medicine.
Preparation of Bronchoalveolar Lavage and Whole Lung Single Cell Suspensions
The methods that were used in the generation and evaluation of bronchoalveolar lavage (BAL) and whole lung cell preparations have been described previously by our laboratory and others (22, 24–27).
Quantification of Cytokines
The levels of IFN-γ, IL-13, IL-17A (R&D, Minneapolis, MN), and IL-18 (MBL, Nagoya, Japan) were determined using commercial ELISA kits as per the manufacturer’s instruction.
Morphometry and Lung Collagen Content
Hematoxylin and eosin staining was performed in the Research Histology Laboratory at Yale School of Medicine. Lung morphometry was used to measure the alveolar chord length (a measure of the distance between alveolar walls and thus alveolar size) (19, 28), and the collagen in the entire right lung was quantitated using the Sircol Collagen Assay Kit (Biocolor Ltd, Carrickfergus, UK) as per the manufacturer’s instructions.
Flow Cytometric Analysis
For flow cytometric analysis, antibodies against CD4, CD8, CD19, NK1.1, IFN-γ, IL-13, and IL-17A were purchased from eBioscience (San Diego, CA). For single cell preparation of lung tissues, Ficoll-Paque Premium (#17-5442-02) was purchased from GE Healthcare (Piscataway, NJ). For intracellular cytokine staining, fixation buffer and permeabilization buffer were purchased from eBioscience. Single cell suspensions were prepared as described previously by our laboratory (22). In these assessments BAL was not undertaken to be sure that intraalveolar cells were included in this analysis. To enrich for tissue infiltrating inflammatory cells and to eliminate red blood cells and cellular debris, the cell suspensions were subjected to Ficoll-Paque density gradient centrifugation. For intracellular cytokine staining, cells were stimulated with phorbol myristate acetate (50 ng/ml) and ionomycin (1 μg/ml) for 4 hours. Flow cytometric analysis was evaluated on a LSRII flow cytometer (Becton Dickinson) and analyzed with FlowJo software (Tree Star, Ashland, OR). The gating that was used excluded dead cells, and the isotype control staining has been described in our previous study (22) and is shown in Figure E1 in the online supplement.
mRNA Evaluations
Real-time RT-PCR analysis and the primers were used as previously described by our laboratory (22).
Statistical Analyses
Evaluations were undertaken with SPSS software. For the comparison of two groups, two-tailed Student’s t test or the nonparametric Mann-Whitney U test were applied as appropriate. For the comparison of more than two groups, ANOVA with post hoc Tukey’s HSD test was applied. Values are expressed as mean ± SEM. Statistical significance was defined at a level of P < 0.05.
Results
IL-18 Stimulates the Production of Chi3l1/BRP-39
Six-week-old WT and IL-18 Tg+ mice were placed on normal water or doxycycline water (Dox). Mice were maintained on these regimens for 4 months and killed. The levels of Chi3l1/BRP-39 protein in BAL fluid and mRNA in lung tissues were evaluated by ELISA and real-time RT-PCR, respectively. Dox did not cause significant alterations of the levels of Chi3l1/BRP-39 in the BAL or tissue responses in WT mice. In addition, BAL and tissues from IL-18 Tg+ mice on normal water were not significantly different from those from WT animals (data not shown). Similarly, the levels of Chi3l1/BRP-39 were similar in specimens from WT mice and mice with null mutations of IL-18Rα (Figure 1A). In contrast, Tg+ mice on Dox water manifest a significant increase in Chi3l1/BRP-39 in BAL fluid as well as in lung tissues (Figure 1B and data not shown). This response was most prominent after 4 months but could be appreciated after as little as 1 month of Dox administration (Figure 1B and data not shown). These changes in Chi3l1/BRP-39 protein were associated with similar changes in mRNA accumulation (Figure 1C). They were also representative of changes in a number of 18 glycosyl hydrolase (18 GH) family members because IL-18 had similar effects on the expression of acidic mammalian chitinase (AMCase) and chitotriosidase (Figures 1D and 1E). These studies demonstrate that endogenous IL-18 signaling does not play a major role in the regulation of Chi3l1/BRP-39 at baseline but that Chi3l1/BRP-39 and other 18 GH moieties are powerfully induced at sites of IL-18 induction.
Figure 1.
The regulation of chitinase 3-like–1 (Chi3l1) (BRP-39/YKL-40) and related moieties in lungs from IL-18 transgenic (Tg) mice. (A) Lungs from wild-type (WT) (IL-18Rα+) and IL-18Rα null (−/−) mice were obtained, and the levels of Chi3l1/BRP-39 in bronchoalveolar lavage (BAL) were evaluated by ELISA. (B–E) WT (Tg−) and IL-18 Tg (Tg+) mice were randomized to normal water (Dox−) or water with doxycycline (Dox+) for 4 months. All data in B, C, D, and E represent evaluations that were done after 4 months of Dox or water administration. (B and C) The effects of IL-18 on the levels of Chi3l1 protein in BAL fluid and on the accumulation of Chi3l1 mRNA in lung tissues. (D and E) The effects of IL-18 on the accumulation of AMCase mRNA and chitotriosidase mRNA. The values in A and B–E represent the mean ± SEM of evaluations in a minimum of four and eight mice, respectively. ***P < 0.001. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Type 1, Type 2, and Type 17 Cytokines Contribute to the Stimulatory Effects of IL-18
Previous studies from our laboratory demonstrated that transgenic IL-18 significantly elevated the levels of intracellular IFN-γ, IL-13, and IL-17A, the signature cytokines of type 1, type 2, and type 17 responses in the lung (22). Thus, studies were undertaken to determine if IL-18 induction of Chi3l1/BRP-39 is mediated by type 1, type 2, and type 17 cytokines. Interestingly, IL-18 induction of Chi3l1/BRP-39 was significantly reduced in lungs from mice that lack of each of these cytokines (Figure 2). Thus, optimal IL-18 stimulation of Chi3l1/BRP-39 requires type 1, type 2, and type 17 responses.
Figure 2.
Characterization of the roles of type 1, type 2, and type 17 cytokines in the induction of Chi3l1 in IL-18 Tg mice. WT (Tg−) and IL-18 Tg (Tg+) mice with the noted WT (+/+) and null (−/−) genetic loci were randomized to normal water (Dox−) or water with doxycycline (Dox+) for 4 months. The lungs were then harvested, and BAL Chi3l1 levels were characterized. The values represent the mean ± SEM of evaluations in a minimum of eight mice. **P < 0.01; ***P < 0.001.
Chi3l1/BRP-39 Plays an Important Role in the Pathogenesis of IL-18–Induced Inflammation
To evaluate the role(s) of Chi3l1/BRP-39 in IL-18–induced inflammation, we characterized the BAL responses in transgenic mice with WT or null Chi3l1 loci. Dox did not cause significant alterations in the BAL responses in WT mice. In addition, BAL from IL-18 Tg+ mice on normal water was not significantly different from BAL from WT animals. In contrast, Tg+ mice on Dox water manifest a significant increase in BAL total cell recovery (Figure 3A). Interestingly, the magnitude of BAL cell recovery was not altered in the absence of Chi3l1/BRP-39 (Figure 3A). However, Chi3l1/BRP-39 did play an important role in defining the nature of this cellular response. As previously reported, transgenic IL-18 augmented BAL macrophage, neutrophil, and lymphocyte (and to a lesser extent eosinophil) recovery in mice that contained Chi3l1/BRP-39 (Figures 3A, 3B, 3D, 3F, and 3G). Interestingly, in the absence of Chi3l1/BRP-39, the number of neutrophils was significantly enhanced (Figure 3B). In accord with this observation, the levels of mRNA encoding the neutrophil chemoattractant CXCL1 in whole lung tissues was significantly augmented by IL-18 overexpression and was further enhanced in the absence of Chi3l1/BRP-39 (Figure 3C). On the other hand, the number of eosinophils and the levels of the eosinophil inducer IL-5 were significantly decreased in the absence of Chi3l1/BRP-39 (Figures 3D and 3E). The numbers of macrophages and lymphocytes in BAL from IL-18 Tg+ mice were not significantly altered in the presence or absence of Chi3l1/BRP-39 (Figures 3F and 3G). Overall, these data suggest that Chi3l1/BRP-39 inhibits the neutrophilic inflammation and augments the eosinophilic inflammation induced by IL-18.
Figure 3.
Characterization of the roles of Chi3l1 in IL-18−induced pulmonary inflammation. WT (Tg−) and IL-18 Tg (Tg+) mice with the noted WT (+/+) and Chi3l1 null (−/−) genetic loci were randomized to normal water (Dox−) or water with doxycycline (Dox+) for 4 months. (A) BAL total cell recovery. (B) BAL neutrophil recovery. (C) CXCL1 mRNA in lung tissues. (D) BAL eosinophil recovery. (E) IL-5 mRNA in lung tissues. (F) BAL macrophage recovery. (G) BAL lymphocyte recovery. The values represent the mean ± SEM of evaluations in a minimum of eight mice. **P < 0.01; ***P < 0.001. CXCL1, chemokine (C-X-C motif) ligand 1.
IL-18–Induced Chi3l1/BRP-39 Augments Type 2 and Type 17 Responses and Inhibits Type 1 Responses
Lungs from Tg+ mice on Dox water demonstrated significantly elevated levels of IFN-γ, IL-13, and IL-17A (22). In accord with these observations, whole lung evaluations also revealed significantly increased levels of IFN-γ, IL-13, and IL-17A mRNA and proteins (Figures 4A–4D and data now shown). In accord with these observations, intracellular cytokine staining and FACS analysis highlighted significantly elevated levels of intracellular IFN-γ, IL-13, and IL-17A in CD4+ T-lymphocytes in lungs from Tg+ mice on Dox water compared with lungs from WT mice on normal or Dox water and Tg+ mice on normal water (Figures 4E and 4F and data not shown). Interestingly, IL-18–induced type 2 and type 17 responses were significantly reduced, whereas the IL-18–induced type 1 response was markedly enhanced in the absence of Chi3l1/BRP-39 compared with those in Chi3l1/BRP-39–sufficient IL-18 Tg+ mice (Figures 4A– 4D). Similarly, IL-18–induced Th2 and Th17 responses were significantly reduced, whereas the IL-18–induced Th1 response was markedly enhanced in the absence of Chi3l1/BRP-39 (Figures 4E and 4F and data not shown). Similar alterations were observed in CD4(−) lymphocytes, but these alterations did not reach statistical significance (Figures 4E and 4F and data not shown). Collectively, these data suggest that Chi3l1/BRP-39 enhances IL-18–induced type 2 and type 17 inflammatory responses while inhibiting IL-18–induced type 1 inflammatory responses in the murine lung.
Figure 4.
Characterization of the roles of Chi3l1 in IL-18–induced type 1, type 2, and type 17 inflammation. WT (Tg−) and IL-18 Tg (Tg+) mice with the noted WT (+/+) and Chi3l1 null (−/−) genetic loci were randomized to normal water (Dox−) or water with doxycycline (Dox+) for 4 months. (A–D) The accumulation of IL-13 mRNA, IL-17A mRNA, and IFN-γ mRNA and the production of IFN-γ protein from whole lung tissues. (E and F) Single cell suspensions were also prepared from whole lung for flow cytometry. Lymphocyte gating was undertaken, excluding dead cell populations, and FACS analysis was used to evaluate the accumulation of CD4+ cells that contain IL-17A and IL-13 in the presence (+/+) or absence (−/−) of Chi3l1/BRP-39. The values in A–D represent the mean ± SEM of evaluations in a minimum of eight mice. *P < 0.05; **P < 0.01. E and F are representative of a minimum of three similar experiments.
Chi3l1/BRP-39 Regulates the Pulmonary Remodeling Responses Induced by IL-18
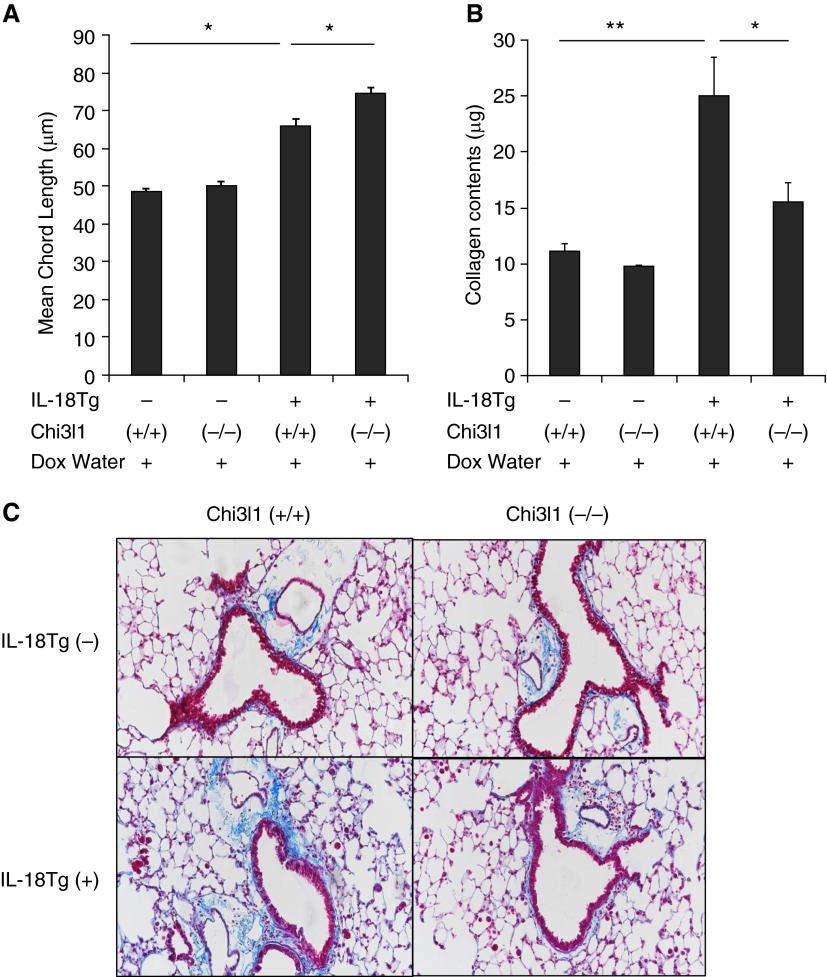
As reported previously, transgenic IL-18 is a potent stimulator of alveolar and airway remodeling that induces emphysematous alveolar septal destruction and airway fibrosis when expressed in the murine lung (22). Interestingly, the alveolar destructive responses were significantly enhanced in the absence of Chi3l1/BRP-39 (Figure 5A). In contrast, the pulmonary fibrotic response was significantly ameliorated in the absence of Chi3l1/BRP-39 (Figures 5B and 5C and data not shown). Overall, these results demonstrate that Chi3l1/BRP-39 inhibits emphysematous alveolar destruction and augments the fibrotic responses that are characteristic of lung-specific IL-18 Tg+ mice.
Figure 5.
Characterization of the roles of Chi3l1 in IL-18–induced tissue pathology. WT (Tg−) and IL-18 Tg (Tg+) mice with the noted WT (+/+) and Chi3l1 null (−/−) genetic loci were randomized to normal water (Dox−) or water with doxycycline (Dox+) for 4 months. (A) Emphysematous alveolar destruction was evaluated with assessments of mean chord length. (B) Pulmonary fibrosis was evaluated with measurements of lung collagen content. (C) A representative trichrome stain histologic evaluation, which compares the collagen accumulation in IL-18 Tg (Tg+) mice with WT (+/+) and Chi3l1 null (−/−) loci. The values in A and B represent the mean ± SEM of evaluations in a minimum of eight mice. *P < 0.05; **P < 0.01. C is representative of at least three similar evaluations.
Chi3l1/BRP-39 Contributes to IL-18 Regulation of Molecules Associated with Cell Cytotoxicity
In keeping with recent studies that demonstrate that cytotoxic lymphocytes can play a role in the pathogenesis of emphysematous alveolar destruction (29), the levels of mRNA encoding NK cell group 2D, retinoic acid early transcript 1 (Raet-1), perforin, and granzyme B were significantly increased in lungs from IL-18 Tg+ mice compared with those from WT controls (Figures 6A–6D). Interestingly, comparisons of IL-18 Tg+ mice with WT and null Chi3l1 loci demonstrated that the levels of mRNA encoding these genes were significantly enhanced in the absence of Chi3l1/BRP-39 (Figures 6A–6D). Taken together, these studies demonstrate that Chi3l1/BRP-39 inhibits pulmonary cytotoxic mediators, which are known to play important roles in the IL-18–induced alveolar remodeling response.
Figure 6.
Characterization of the roles of Chi3l1 in IL-18–induced induction of cytotoxic molecules. WT (Tg−) and IL-18 Tg (Tg+) mice with the noted WT (+/+) and Chi3l1 null (−/−) genetic loci were randomized to normal water (Dox−) or water with doxycycline (Dox+) for 4 months. (A–D) The levels of whole lung mRNA encoding NK cell group 2D (NKG2D), perforin, retinoic acid early transcript 1 (Raet-1), and granzyme-B. The values in A through D represent the mean ± SEM of evaluations in a minimum of eight mice. *P < 0.05; **P < 0.01.
Discussion
Chi3l1 is an injury- and inflammation-induced mediator that decreases injury and cell death while augmenting tissue repair and regulating the nature of the local inflammatory response (8, 30, 31). In the lung this can be nicely seen in adaptive Th2 responses and in the injury and repair responses induced by agents such as bleomycin, infection with pneumococcus, and CS exposure (8, 10, 12). IL-18 is also a pleotropic mediator that regulates injury; repair; and type 1, type 2, and type 17 cytokine balance (14, 16, 32, 33). In keeping with these similarities, studies were undertaken to define the relationship(s) between Chi3l1 and IL-18. These studies demonstrate that IL-18 is a potent stimulator of Chi3l1/BRP-39 in the murine lung. Interestingly, similar effects were seen with other GH 18 moieties, suggesting that IL-18 is an important regulator of many members of this gene family. These studies also demonstrate that Chi3l1 is a key mediator of many of the tissue effects of IL-18, where it contributes to the genesis of Th2 and Th17 inflammation and tissue fibrosis while inhibiting type 1 inflammation, cell cytotoxicity, and alveolar remodeling. These findings add significantly to our understanding of the stimuli that control the elaboration of Chi3l1. They also raise the exciting possibility that Chi3l1 is an important regulator of injury, repair, and inflammation at sites of and in disease characterized by IL-18 excess.
IL-18 is surprising in its ability to stimulate type 1, type 2, and type 17 responses. The importance of this pleiotropy was recently demonstrated in studies from our laboratory, which demonstrated that these pathways play different roles in the generation of COPD-like tissue response in the murine lung (22). Specifically, IFN-γ–dependent pathways inhibited macrophage, lymphocyte, and eosinophil accumulation while stimulating alveolar destruction and moieties associated with cell cytotoxicity. In contrast, IL-13 and IL-17A were intimately associated with IL-18, optimally inducing IL-13 via an IL-17A–dependent mechanism and the IL-17/IL-13 axis–stimulating mucus metaplasia and vascular and airway fibrosis (22). They also highlight important reciprocal interactions between these responses, with the type 1 and type 17/type 2 cytokine responses feeding back to antagonize one another. The present studies add to this body of data by demonstrating that IL-18 also stimulates Chi3l1 and that optimal Chi3l induction requires type 2, type 1, and type 17 responses. This finding is compatible with the concept that Chi3l1 is induced during the course of a variety of inflammatory and injury responses, where it feeds back to decrease injury and drive repair. In COPD, these findings raise the possibility that the levels of serum or BAL Chi3l1 can be a surrogate for the degree of ongoing inflammation and/or injury or a marker that predicts disease progression.
One of the major conceptual advances in immunology is the appreciation that there are T cell subsets with unique cytokine profiles and inflammatory responses that are characterized by the excess accumulation of specific cytokines, such as type 2, type 1, and type 17 moieties, differ significantly from one another. Studies of T cell differentiation have highlighted the importance of dendritic cells and the cytokine microenvironment in the generation of these cells and cell populations. Our studies add to this understanding by demonstrating that the ability of IL-18 to generate these different responses also depends on the induction of Chi3l1. Specifically, in the absence of Chi3l1, IL-18 induction of type 2 and type 17 responses was diminished, whereas type 1 responses were augmented. These findings are in accord with studies from our laboratory highlighting the Chi3l1 dependence of aeroallergen-induced adaptive type 2 responses, pneumococcus-induced responses, and autoimmune encephalitis (8, 10, 34). The augmented type 1 response is in accord with the exaggerated experimental autoimmune encephalomyelitis that is seen in mice with Chi3l deficiency (34). The mechanisms by which Chi3l1 regulates these responses, however, have not been defined and require additional investigation.
CS exposure causes epithelial cell stress and immune activation (29). The cytotoxic lymphocyte activation receptor NK cell group 2D on circulating and tissue T cells links these responses by inducing cell lysis and enhancing innate and adaptive immunity after binding to stress-induced ligands, such as Raet-1 (29). We previously demonstrated that IL-18 augments pulmonary expression of cells and molecules that are involved in cell cytotoxicity, with IL-18 Tg mice manifesting increased levels of CD8 and NK1.11 cells and enhanced expression of Raet-1, perforin, and granzyme B (22). Our studies also demonstrated that these responses are induced via an IFN-γ–dependent mechanism and are inhibited by IL-18–induced IL-17A and IL-13 responses. In accord with these findings, the present studies demonstrate that the ability of IL-18 to induce these cell cytotoxicity alterations is at least partially Chi3l dependent, with Chi3l1 inhibiting these responses. To our knowledge, this is the first time that Chi3l1 or any other GH18 moiety has been linked to cell cytotoxicity. However, the demonstration that Chi3l1 inhibits these innate immune responses is in accord with studies from our laboratory, which demonstrated that Chi3l1 also inhibits a number of other innate immune responses, such as inflammasome activation (8, 10, 34). The link between Chi3l1 and cell cytotoxicity has interesting implications for visceral malignancies and other diseases where cell cytotoxicity plays an important role and Chi3l1 levels are augmented.
Although COPD is referred to as a “disease,” it can be more accurately thought of as a constellation of heterogeneous syndromes (35–38). As a result, the clinical manifestations of the disease and the degree to which emphysema versus airway (chronic bronchitis) and vascular pathologies predominate can differ from individual to individual. This complexity is so profound that the National Institutes of Health is sponsoring ongoing studies of subpopulations and intermediate outcome measures in the disorder (37). To account for this heterogeneity, some have proposed that these differences are the result of disparate disease causes (39). Only time and study will determine if this is true. However, our data allow for another non–mutually exclusive hypothesis. Specifically, our studies demonstrate that IL-18 can induce many of the classic abnormalities that are seen in COPD and simultaneously induce the production of Chi3l1. They also demonstrate that the levels of expression of Chi3l1 alter the phenotypes that are induced by IL-18. A number of naturally occurring Chi3l1 polymorphisms have been described that alter its levels of expression (40). This allows for the exciting hypothesis that environmental or genetic influences that alter an individual’s propensity for IL-18–stimulated activation of the Chi3l1 axis (the Chi3l1 ligand, its stimulators, and or its receptors) could contribute to the heterogeneity of COPD and other IL-18–mediated disorders. These findings also have important implications regarding therapeutic approaches that can be used in COPD. In our prior studies we suggested that optimal treatment of COPD needs to be directed at “central” mediators, such as IL-18, or formulated as combination therapies to simultaneously control the multiple pathways that it induces. The present studies suggest that manipulations of the Chi3l1 axis might be a useful way to accomplish the latter. Further investigation of the regulation and roles of Chi3l1 and related moieties in COPD is warranted.
Footnotes
This work was supported by National Institutes of Health grants HL-079328, R01-HL-093017, and U01-HL-108638 (J.A.E.) and Hl-084225 (C.G.L.); by Flight Attendance Medical Research Institute grant 113258 (M.-J.K.); by Connecticut Department of Public Health grant 2013-0196 (M.-J.K.); and by National Research Foundation of Korea grant-2013R1A1A2A10060048 (J.-M.C.).
Author Contributions: Conceived the idea and designed the experiments: M.-J.K. and J.A.E. Performed experiments: M.-J.K., C.M.Y., M.N., D.-H.K., and J.-M.C. Provided important reagent/tools: C.G.L. Analyzed data: M.-J.K., C.M.Y., M.N., D.-H.K., J.-M.C., C.G.L., and J.A.E. Drafted the manuscript: M.-J.K. and J.A.E. Provided scientific insight: J.-M.C. and C.G.L. All of the authors reviewed the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0366OC on May 8, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aerts JM, van Breemen MJ, Bussink AP, Ghauharali K, Sprenger R, Boot RG, Groener JE, Hollak CE, Maas M, Smit S, et al. Biomarkers for lysosomal storage disorders: identification and application as exemplified by chitotriosidase in Gaucher disease. Acta Paediatr Suppl. 2008;97:7–14. doi: 10.1111/j.1651-2227.2007.00641.x. [DOI] [PubMed] [Google Scholar]
- 3.Funkhouser JD, Aronson NN., Jr Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol. 2007;7:96. doi: 10.1186/1471-2148-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Areshkov PO, Avdieiev SS, Balynska OV, Leroith D, Kavsan VM. Two closely related human members of chitinase-like family, CHI3L1 and CHI3L2, activate ERK1/2 in 293 and U373 cells but have the different influence on cell proliferation. Int J Biol Sci. 2012;8:39–48. doi: 10.7150/ijbs.8.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CC, Llado V, Eurich K, Tran HT, Mizoguchi E. Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin Immunol. 2011;140:268–275. doi: 10.1016/j.clim.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruchała-Niedoszytko M, Małgorzewicz S, Niedoszytko M, Gnacińska M, Jassem E. The influence of obesity on inflammation and clinical symptoms in asthma. Adv Med Sci. 2013;58:15–21. doi: 10.2478/v10039-012-0082-y. [DOI] [PubMed] [Google Scholar]
- 7.Kim MN, Lee KE, Hong JY, Heo WI, Kim KW, Kim KE, Sohn MH. Involvement of the MAPK and PI3K pathways in chitinase 3-like 1-regulated hyperoxia-induced airway epithelial cell death. Biochem Biophys Res Commun. 2012;421:790–796. doi: 10.1016/j.bbrc.2012.04.085. [DOI] [PubMed] [Google Scholar]
- 8.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Chen NY, Lee CG, Elias JA. The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am J Respir Crit Care Med. 2010;182:918–928. doi: 10.1164/rccm.200912-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dela Cruz CS, Liu W, He CH, Jacoby A, Gornitzky A, Ma B, Flavell R, Lee CG, Elias JA. Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe. 2012;12:34–46. doi: 10.1016/j.chom.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffman FD. Chitinase 3-Like-1 (CHI3L1): a putative disease marker at the interface of proteomics and glycomics. Crit Rev Clin Lab Sci. 2008;45:531–562. doi: 10.1080/10408360802334743. [DOI] [PubMed] [Google Scholar]
- 12.Matsuura H, Hartl D, Kang MJ, Dela Cruz CS, Koller B, Chupp GL, Homer RJ, Zhou Y, Cho WK, Elias JA, et al. Role of breast regression protein-39 in the pathogenesis of cigarette smoke-induced inflammation and emphysema. Am J Respir Cell Mol Biol. 2011;44:777–786. doi: 10.1165/rcmb.2010-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakazaki Y, Hoshino T, Takei S, Sawada M, Oda H, Takenaka S, Imaoka H, Matsunaga K, Ota T, Abe Y, et al. Overexpression of chitinase 3-like 1/YKL-40 in lung-specific IL-18-transgenic mice, smokers and COPD. PLoS One. 2011;6:e24177. doi: 10.1371/journal.pone.0024177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 15.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27:98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren’s syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008;181:2898–2906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- 18.Boraschi D, Dinarello CA. IL-18 in autoimmunity: review. Eur Cytokine Netw. 2006;17:224–252. [PubMed] [Google Scholar]
- 19.Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, Rochester C, Cain H, Chupp G, Yoon HJ, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol. 2007;178:1948–1959. doi: 10.4049/jimmunol.178.3.1948. [DOI] [PubMed] [Google Scholar]
- 20.Imaoka H, Hoshino T, Takei S, Kinoshita T, Okamoto M, Kawayama T, Kato S, Iwasaki H, Watanabe K, Aizawa H. Interleukin-18 production and pulmonary function in COPD. Eur Respir J. 2008;31:287–297. doi: 10.1183/09031936.00019207. [DOI] [PubMed] [Google Scholar]
- 21.Rovina N, Dima E, Gerassimou C, Kollintza A, Gratziou C, Roussos C. Interleukin-18 in induced sputum: association with lung function in chronic obstructive pulmonary disease. Respir Med. 2009;103:1056–1062. doi: 10.1016/j.rmed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Kang MJ, Choi JM, Kim BH, Lee CM, Cho WK, Choe G, Kim DH, Lee CG, Elias JA. IL-18 induces emphysema and airway and vascular remodeling via IFN-γ, IL-17A, and IL-13. Am J Respir Crit Care Med. 2012;185:1205–1217. doi: 10.1164/rccm.201108-1545OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang MJ, Lee CG, Elias JA. Interleukin-18 induces pulmonary inflammation and remodeling via an interleukin-17-dependent pathway [abstract] Am J Respir Crit Care Med. 2010;181:A5089. [Google Scholar]
- 24.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham RE. Tissue disaggregation. Methods Mol Biol. 1994;34:225–228. doi: 10.1385/0-89603285-x:225. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Dong Z, Zhou R, Luo D, Wei H, Tian Z. Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cell Mol Immunol. 2005;2:271–280. [PubMed] [Google Scholar]
- 27.Jaatinen T, Laine J.2007Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient Curr Protoc Stem Cell BiolChapter 2: Unit 2A 1 [DOI] [PubMed] [Google Scholar]
- 28.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borchers MT, Wesselkamper SC, Curull V, Ramirez-Sarmiento A, Sánchez-Font A, Garcia-Aymerich J, Coronell C, Lloreta J, Agusti AG, Gea J, et al. Sustained CTL activation by murine pulmonary epithelial cells promotes the development of COPD-like disease. J Clin Invest. 2009;119:636–649. doi: 10.1172/JCI34462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Peng H, Sun H, Peng X, Tang C, Gan Y, Chen X, Mathur A, Hu B, Slade MD, et al. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in Mammalian lung fibrosis. Sci Transl Med. 2014;6:240ra276. doi: 10.1126/scitranslmed.3007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CG, Dela Cruz CS, Ma B, Ahangari F, Zhou Y, Halaban R, Sznol M, Elias JA. Chitinase-like proteins in lung injury, repair, and metastasis. Proc Am Thorac Soc. 2012;9:57–61. doi: 10.1513/pats.201112-056MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawayama T, Okamoto M, Imaoka H, Kato S, Young HA, Hoshino T. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443–449. doi: 10.1089/jir.2012.0029. [DOI] [PubMed] [Google Scholar]
- 33.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonneh-Barkay D, Wang G, Laframboise WA, Wiley CA, Bissel SJ. Exacerbation of experimental autoimmune encephalomyelitis in the absence of breast regression protein 39/chitinase 3-like 1. J Neuropathol Exp Neurol. 2012;71:948–958. doi: 10.1097/NEN.0b013e31826eaee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celli BR. Roger S. Mitchell lecture. Chronic obstructive pulmonary disease phenotypes and their clinical relevance. Proc Am Thorac Soc. 2006;3:461–465. doi: 10.1513/pats.200603-029MS. [DOI] [PubMed] [Google Scholar]
- 36.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, Fabbri LM, Goldin JG, Jones PW, Macnee W, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foundation for the National Institutes of Health. SubPopulations and InteRmediate Outcome Measures in COPD Study(SPIROMICS)Bethesda, MD: FNIH; 2015 [accessed 2015 Jul 6]. Available from:http://www.fnih.org/work/related-programs/subpopulations-and-intermediate-outcome-measures-copd-study-spiromics
- 38.Lee JH, Lee YK, Kim EK, Kim TH, Huh JW, Kim WJ, Lee JH, Lee SM, Lee S, Lim SY, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respir Med. 2010;104:542–549. doi: 10.1016/j.rmed.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Rennard SI. COPD heterogeneity: what this will mean in practice. Respir Care. 2011;56:1181–1187. doi: 10.4187/respcare.01419. [DOI] [PubMed] [Google Scholar]
- 40.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, Radford S, Parry RR, Heinzmann A, Deichmann KA, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]