Abstract
Mechanisms of vascular endothelial cell (EC) barrier regulation during acute lung injury (ALI) or other pathologies associated with increased vascular leakiness are an active area of research. Adaptor protein krev interaction trapped-1 (KRIT1) participates in angiogenesis, lumen formation, and stabilization of EC adherens junctions (AJs) in mature vasculature. We tested a role of KRIT1 in the regulation of Rho-GTPase signaling induced by mechanical stimulation and barrier dysfunction relevant to ventilator-induced lung injury and investigated KRIT1 involvement in EC barrier protection by prostacyclin (PC). PC stimulated Ras-related protein 1 (Rap1)–dependent association of KRIT1 with vascular endothelial cadherin at AJs, with KRIT1-dependent cortical cytoskeletal remodeling leading to EC barrier enhancement. KRIT1 knockdown exacerbated Rho-GTPase activation and EC barrier disruption induced by pathologic 18% cyclic stretch and thrombin receptor activating peptide (TRAP) 6 and attenuated the protective effects of PC. In the two-hit model of ALI caused by high tidal volume (HTV) mechanical ventilation and TRAP6 injection, KRIT1 functional deficiency in KRIT1+/− mice increased basal lung vascular leak and augmented vascular leak and lung injury caused by exposure to HTV and TRAP6. Down-regulation of KRIT1 also diminished the protective effects of PC against TRAP6/HTV-induced lung injury. These results demonstrate a KRIT1-dependent mechanism of vascular EC barrier control in basal conditions and in the two-hit model of ALI caused by excessive mechanical forces and TRAP6 via negative regulation of Rho activity and enhancement of cell junctions. We also conclude that the stimulation of the Rap1-KRIT1 signaling module is a major mechanism of vascular endothelial barrier protection by PC in the injured lung.
Keywords: cyclic stretch, ventilator-induced lung injury, KRIT1, RhoA, Rap1
Clinical Relevance
Ras-related protein 1 (Rap1) GTPase effector krev interaction trapped-1 (KRIT1) gene is activated in prostacyclin-treated endothelial cells and maintains vascular barrier integrity under pathological mechanochemical stimulation associated with ventilator induced lung injury. The Rap1-KRIT1 signaling module is a new target for preventive strategies in the treatment of pathological conditions associated with acute vascular barrier dysfunction.
The acute phase of lung injury in acute respiratory distress syndrome is a devastating condition with high morbidity and an overall mortality rate of 30 to 40% (1). Acute lung injury (ALI) has been characterized by increased permeability of the blood–gas barrier, which allows an influx of protein-rich fluid into the air spaces, causing pulmonary edema. Activation of Rho GTPase signaling triggers lung endothelial barrier dysfunction associated with ALI, which may be caused by many factors, including mechanical ventilation at high tidal volume (HTV) (2, 3), exposure to edemagenic agonists associated with ALI (4–9), or a combination of mechanical ventilation and edemagenic agonists (10).
Prostaglandin I2, or prostacyclin (PC), is a product of the arachidonic acid metabolic pathway and is synthesized by many tissues, including vascular endothelial cells (11, 12). PC and its stable analogs beraprost and iloprost protect vascular endothelial cell (EC) monolayer lining all blood vessels against increased vascular leakiness caused by barrier-disruptive agonists (13–15) or excessive mechanical forces (16). Beraprost and iloprost also have demonstrated potent antiinflammatory and endothelium-dependent antiedemagenic effects in several models of ALI (17, 18).
PC binding to G-protein–coupled prostaglandin-I2 receptor (IP) receptor leads to the activation of adenylate cyclase and the elevation of intracellular cyclic adenosine monophosphate (cAMP) levels. Increased cAMP levels enhance endothelial barrier integrity (19, 20) and inhibit barrier-disruptive signaling by protein kinase A–mediated phosphorylation and inactivation of myosin light chain (MLC) kinase (21) and inactivation of the RhoA GTPase–dependent pathway of EC barrier dysfunction (22, 23). PC also triggers an alternative, protein kinase A–independent mechanism of EC barrier enhancement, which involves cAMP-activated guanine nucleotide exchange factor Epac1 and its target Ras-related protein 1 (Rap1) GTPase (24, 25). Rap1 participates in diverse processes, including integrin-mediated cell adhesion (26), cadherin-mediated cell junction formation, and regulation of the endothelial barrier (25, 27).
Krev interaction trapped-1 (KRIT1) gene has been originally identified as a Rap1-binding protein in a yeast two-hybrid screen (28). Loss-of-function mutations of KRIT1 are responsible for approximately 40% of human autosomal-dominant familial cerebral cavernous malformations (CCMs). Homozygous KRIT1 null mutations are embryonically lethal due to severe defects in vascular integrity and vascular development (29). KRIT1 displays a domain structure with several potential protein binding sites (30, 31). Although KRIT1 interaction with activated Rap1 is critical for the basal maintenance of endothelial junctional integrity (32), molecular inhibition of KRIT1 increases basal Rho activation (33). These functional properties suggest KRIT1 as a bona fide Rap1 effector with signaling functions at cell adhesions essential for regulation of EC vascular barrier in basal conditions and upon stimulation with injurious stimuli.
A role of KRIT1 in the dynamic regulation of the vascular endothelial barrier in pathologic settings associated with ALI caused by mechanical ventilation–associated excessive mechanical forces has not been yet elucidated. Given a documented role of Rho inhibition and Rap1 activation in the mechanisms of EC barrier protection in pathologic conditions, we tested the hypothesis that protective effects of PC on EC monolayers exposed to pathologic mechanical forces and disruptive agonists, as well as PC-protective effects in the experimental two-hit model of ventilator-associated lung injury, involve Rap1-induced activation of KRIT1 and KRIT1-dependent control of Rho signaling, improvement of cell junction integrity, and lung vascular EC permeability.
Materials and Methods
Further details are provided in the online supplement.
Cell Culture and Reagents
Human pulmonary artery endothelial cells (HPAECs) obtained from Lonza (Allendale, NJ) were used for in vitro permeability and signal transduction studies. Cyclic stretch experiments were performed using the FX-4000T Tension Plus system (Flexcell, Burlington, NC) as previously described (34, 35). Procedure details are provided in the online supplement. Phospho-specific p-cortactin-Y421, p-MYPT-Thr850, and p-MLC-Thr18/Ser19 antibodies were from Cell Signaling (Beverly, MA). Vascular endothelial (VE)-cadherin, β-catenin, and p120-catenin antibodies were from BD Transduction Laboratories (San Diego, CA). Antibodies to Rho and Rap1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Texas Red phalloidin and Alexa Flour–conjugated antibodies were from Molecular Probes (Eugene, OR). Beraprost and PC were from Cayman (Ann Arbor, MI). Thrombin receptor activating peptide (TRAP) 6 was from AnaSpec (San Jose, CA). Unless otherwise specified, biochemical reagents were obtained from Sigma (St. Louis, MO).
DNA and Small Interfering RNA Transfections
Predesigned Rap1A- and KRIT1-specific human Stealth Select small interfering RNA (siRNA) sets of standard purity were obtained from Invitrogen (Carlsbad, CA). Transfection of ECs with siRNA was performed as previously described (16). Nonspecific, nontargeting siRNA was used as a control treatment. Hemagglutinin-tagged wild-type KRIT1 and hemagglutinin-tagged KRIT1-R452E constructs subcloned into pcDNA3.1 vector for mammalian transfection were a generous gift by Mark Ginsberg (Department of Medicine, University of California, San Diego, La Jolla, CA). ECs were used for transient transfections according to the protocol described previously (36).
Transendothelial electrical resistance (TER) across confluent human pulmonary artery endothelial monolayers was measured using an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) (37).
GTPase Activation, Protein Fractionation, and Immunoprecipitation
Activation of Rho-GTPase in pulmonary EC culture was analyzed using the GTPase in vitro pulldown assay kit (Millipore, Billerica, MA). Cytosolic and membrane fractions were separated using an S-PEK kit (EMD Chemicals, Gibbstown, NJ). Coimmunoprecipitation studies and Western blot analysis were performed using confluent HPAEC monolayers as described elsewhere (38). Protein extracts were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes, and the membranes were incubated with specific antibodies of interest.
Immunofluorescence Staining
ECs were plated on glass coverslips and used for immunofluorescence staining after transfections or agonist stimulation as described (10). Slides were analyzed using a Nikon video imaging system (Nikon Instech Co., Tokyo, Japan). At least 10 microscopic fields per condition were analyzed in each independent experiment. At least three independent experiments were performed for each experimental setup Images were processed with ImageJ software (National Institutes of Health, Washington, DC) and Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
Animal Studies
All experimental protocols involving the use of animals were approved by the University of Chicago Institutional Animal Care and Use Committee for the humane treatment of experimental animals. Male C57BL/6J mice (8–10 wk old) weighing 20 to 25 g were subjected to mechanical ventilation (Harvard Apparatus, Boston, MA) at HTV (30 ml/kg) for 4 hours. Measurements of cell count, protein concentration, myeloperoxidase activity, Evans blue extravasation, and histological assessment of lung injury were conducted as described (39). Procedure details are provided in the online supplement.
Statistical Analysis
Results are expressed as means ± SD of three to six independent experiments. Stimulated samples were compared with controls by unpaired Student’s t test. For multiple-group comparisons, one-way ANOVA and Tukey’s post hoc multiple-comparison test were used. A P value < 0.05 was considered statistically significant.
Results
PC Causes Rap1-Dependent KRIT1 Recruitment to the Cell Junctions and Colocalization with VE-Cadherin
We have previously demonstrated that agonist-induced enhancement of pulmonary EC barrier properties is associated with enlargement of VE-cadherin–positive adherens junctions (AJs) (38). Although KRIT1 is critical for basal regulation of vascular EC junctions, its role in the mediation of agonist-induced signaling remains to be evaluated.
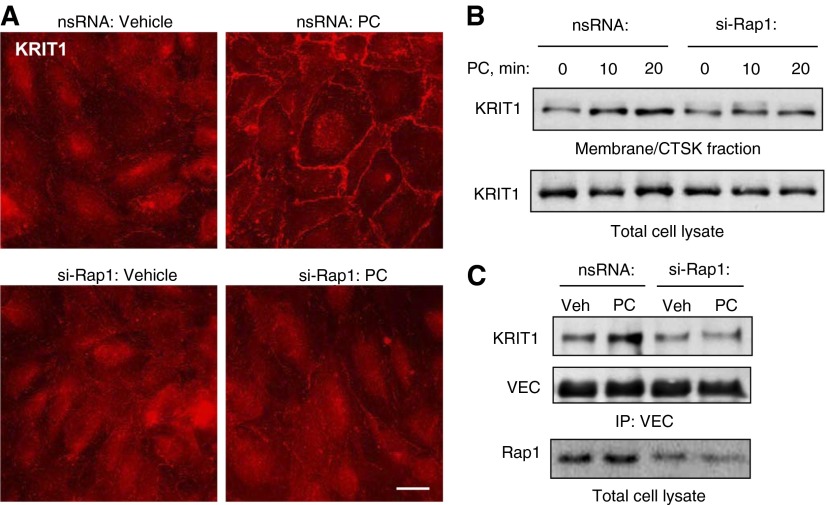
We examined the effects of PC on the subcellular localization of KRIT1 and regulation of this process by Rap1. Involvement of Rap1 in the PC-induced peripheral accumulation of KRIT1 was tested using siRNA-mediated Rap1 knockdown. Transfection with nontargeting RNA was used as a negative control. After 48 hours of transfection, ECs grown on glass coverslips were treated with PC or with vehicle, and evaluation of Rap1 effects on KRIT1 intracellular localization was monitored by immunofluorescence staining and biochemical approaches. PC caused peripheral accumulation of KRIT1 in control ECs treated with nonspecific RNA but not in cells with depleted Rap1 (Figure 1A). Subcellular fractionation assay showed a PC-induced increase in the KRIT1 content in the membrane fraction as compared with nontreated ECs (Figure 1B). Functional association of KRIT1 with VE-cadherin was tested in coimmunoprecipitation assays. PC promoted KRIT1–VE-cadherin interactions, which were reflected by increased KRIT1 content in the VE-cadherin immunoprecipitates (Figure 1C). The KRIT1–VE-cadherin association was abolished by Rap1 knockdown.
Figure 1.
Prostacyclin causes Ras-related protein 1 (Rap1)-dependent recruitment of krev interaction trapped-1 (KRIT1) gene to the cell cortical layer, localization to adherens junctions, and association with vascular endothelial (VE)-cadherin. (A) Human pulmonary endothelial cell monolayers grown on glass coverslips were transfected with nonspecific (top panels) or Rap1-specific small interfering RNA (siRNA) (bottom panels) for 48 hours and treated with vehicle or prostacyclin (PC) (200 ng/ml, 15 min) followed by immunofluorescence staining for KRIT1. Scale bar = 10 μm. (B) Translocation of KRIT1 to the membrane/cytoskeletal fraction was detected with specific antibodies. (C) Coimmunoprecipitation assay using antibody to VE-cadherin was performed, and VE-cadherin and KRIT1 content in the immunoprecipitates was detected using appropriate antibody. siRNA-induced Rap1 knockdown was monitored by Western blot with Rap1 antibody. Results are representative of three independent experiments. CTSK, cytoskeletal; IP, immunoprecipitation; nsRNA, negative strand RNA; si-Rap1, Rap1-specific siRNA; VEC, VE-cadherin; Veh, vehicle.
KRIT1 Mediates PC-Induced EC Barrier Enhancement, Peripheral Actin Cytoskeleton, and AJ Remodeling
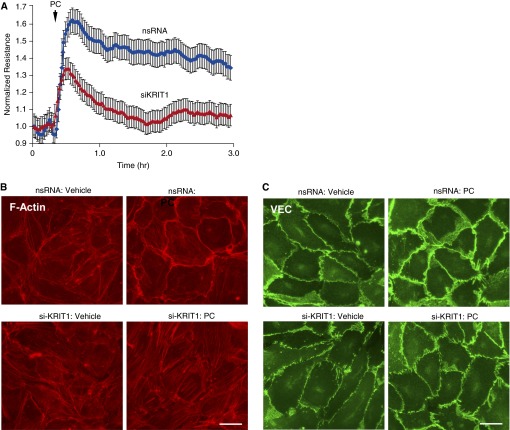
The role of KRIT1 in the mediation of barrier-protective effects by PC was evaluated using siRNA-induced KRIT1 knockdown. After 48 hours of transfection with nonspecific or KRIT1-specific siRNA duplexes, human pulmonary ECs were treated with PC, and transendothelial electrical resistance (TER) was monitored. Because complete KRIT1 knockdown may lead to global destabilization of cell–cell junctions and disruption of the EC monolayer, in these and in the following experiments we used approximately 50% inhibition of KRIT1 expression. Depletion of KRIT1 significantly attenuated EC barrier enhancement in response to PC (Figure 2A). Effects of KRIT1 knockdown on PC-induced remodeling of AJs and peripheral actin cytoskeleton essential for EC barrier enhancement were further tested by cell imaging and biochemical analysis.
Figure 2.
KRIT1 knockdown attenuates PC-induced endothelial barrier enhancement, cortical actin remodeling, and p120-catenin–VE-cadherin interactions. (A) Pulmonary endothelial cells (ECs) were transfected with KRIT1-specific siRNA (siKRIT1) or with nonspecific RNA for 48 hours. EC permeability response to PC treatment (200 ng/ml) was monitored by measurements of transendothelial electrical resistance. (B) PC-induced actin cytoskeleton remodeling in control and KRIT1-depleted ECs was evaluated by fluorescence staining of F-actin using Texas Red phalloidin. Scale bar = 10 μm. (C) Effect of KRIT1 knockdown on PC-induced enhancement of adherens junctions was evaluated by staining for VEC. Results are representative of three independent experiments. nsRNA, control treatment with nontargeting RNA; si-KRIT1, treatment with KRIT1-specific siRNA.
Pulmonary ECs transfected with nonspecific RNA responded to PC by enhancement of the F-actin rim at the cell periphery (Figure 2B, top panels), which was abolished in the cells with KRIT1 knockdown (Figure 2B, bottom panels). PC treatment also induced peripheral accumulation of VE-cadherin reflecting enhancement of AJs in control ECs but not in the cells with depleted KRIT1 (Figure 2C).
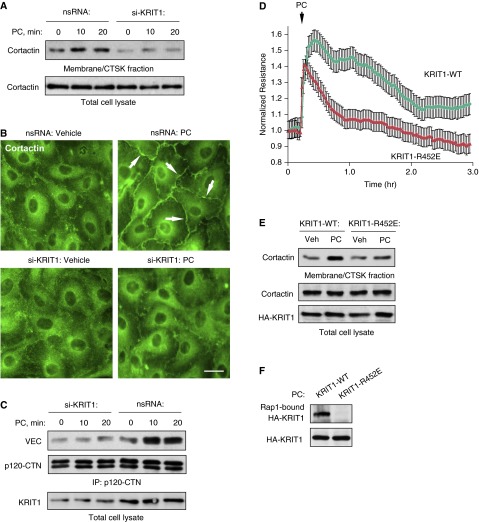
Cortactin plays an essential role in cortical cytoskeletal dynamics by stimulating formation of peripheral lamellipodia-like structures involved in cell spreading (40) and leading to the establishment of intercellular junctions. Cortactin translocation to the cell membrane/cytoskeletal fraction was observed in PC-stimulated pulmonary ECs but was abolished by KRIT1 knockdown (Figure 3A). In imaging studies, PC induced formation of cortactin-enriched, lamellipodia-like structures at the cell periphery (Figure 3B). KRIT1 knockdown suppressed these effects. siRNA-induced depletion of endogenous KRIT1 also attenuated PC-induced association of AJ proteins, p120-catenin, and VE-cadherin (Figure 3C). These data demonstrate the important role of KRIT1 in the regulation of PC-induced strengthening of AJ and EC barrier enhancement.
Figure 3.
KRIT1 activation mediates PC-induced peripheral cortactin accumulation, assembly of adherens junction protein complex, and EC barrier enhancement. (A) ECs were stimulated with PC (200 ng/ml) for 10 and 20 minutes. Membrane/cytoskeletal fractions were isolated, and the content of cortactin was determined by Western blot analysis. Equal protein content in whole cell lysates was confirmed by detection of cortactin in control and PC-treated samples. (B) PC-induced cortactin redistribution in control and KRIT1-depleted ECs was evaluated by immunofluorescence staining with cortactin antibody. Cortactin peripheral translocation is shown by arrows. (C) Control and KRIT1-knockdown ECs were treated with PC (200 ng/ml), and coimmunoprecipitation assay with p120-catenin (CTN) antibody was performed. VE-cadherin and p120-catenin content in the immunoprecipitates was detected using the appropriate antibody. siRNA-induced KRIT1 knockdown was confirmed by Western blot with Rap1 antibody. Results are representative of three independent experiments. Scale bar = 10 μm. (D) Wild-type (WT) KRIT1 or its KRIT1-R452E mutant were ectopically expressed in pulmonary ECs, and permeability response to PC treatment (200 ng/ml) was monitored by measurements of transendothelial electrical resistance. (E) The effect of wild-type KRIT1 and KRIT1-R452E mutant expression on PC-induced cortactin translocation to membrane/cytoskeletal fraction was monitored by Western blot analysis. Equal cortactin content in whole cell lysates was used as a normalization control. Probing of total lysates with hemagglutinin (HA)-tagged antibody was performed to verify expression of recombinant KRIT1. Shown are representative results of three independent experiments. (F) Interaction of wild-type KRIT1 or its KRIT1-R425E mutants with Ral-agarose containing activated Rap1.
The role of Rap1-mediated KRIT1 activation in the modulation of agonist-induced EC permeability was further investigated in experiments with the KRIT1-R452E mutant that exhibited an approximately 40-fold–reduced affinity for Rap1A (32). Pulmonary ECs were transfected with plasmids encoding wild-type KRIT1 or KRIT1-R452E. The recombinant KRIT1 protein levels in EC cultures were comparable to the levels of endogenous KRIT1 protein (data not shown). Expression of KRIT1-R452E significantly attenuated pulmonary PC-induced EC barrier enhancement (Figure 3D) and suppressed peripheral translocation of cortactin in response to PC (Figure 3E). Wild-type KRIT1, but not KRIT1-R452E mutant, interacted with Ral-agarose after pulldown of activated Rap1 from PC-stimulated EC lysates (Figure 3F).
KRIT1 Protects Monolayer Integrity of ECs Exposed to 18% Cyclic Stretch and TRAP6
Concurrent stimulation of pulmonary ECs exposed to pathologically relevant levels of cyclic stretch with the thrombin receptor activating peptide TRAP6 known to activate Rho signaling was used to reproduce a “double-hit” model of ventilator-induced lung injury (15). Cell monolayers were exposed to high-magnitude (18% linear elongation, sinusoidal wave, 25 cycles/min) cyclic stretch to recapitulate the mechanical stresses experienced by the alveolar endothelium at HTV mechanical ventilation (further details are provided in the online supplement). Using this model, we evaluated the protective effects of PC in control cells and ECs with depleted KRIT1.
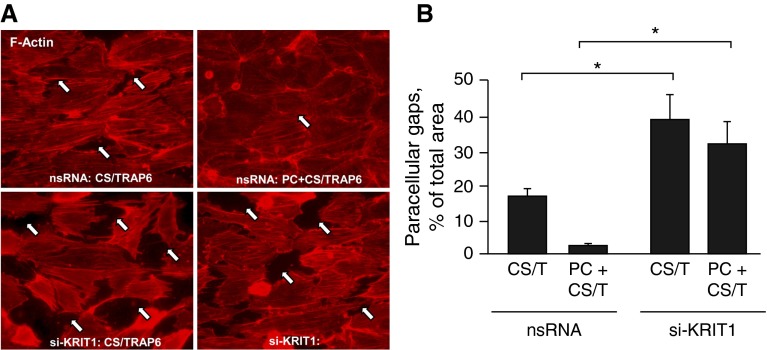
Consistent with our published studies (41), combined 18% cyclic stretch (CS) and TRAP6 stimulation caused pronounced disruption of the EC monolayer accompanied by stress fiber formation. These effects were attenuated by cell cotreatment with PC (Figure 4A, top panels). Importantly, KRIT1 knockdown augmented EC barrier disruption caused by 18% CS and TRAP6 and suppressed the barrier protective effect of PC (Figure 4A, bottom panels). Quantitative image analysis of gap formation in mechanically stimulated lung endothelium showed that, whereas PC significantly attenuated the formation of intercellular gaps (Figure 4B), KRIT1 knockdown exacerbated 18% CS/TRAP6-induced gap formation and suppressed the protective effect of PC.
Figure 4.
KRIT1 knockdown attenuates the barrier-protective effect of PC in pulmonary ECs exposed to high-magnitude cyclic stretch and thrombin receptor activating peptide (TRAP) 6. (A) ECs grown on Bioflex plates with elastic bottoms were transfected with KRIT1-specific siRNA or with nonspecific RNA for 48 hours. Cells were then subjected to 18% cyclic stretch (CS) (2 h) in the presence of 50 ng/ml TRAP6 with or without PC cotreatment in the last 15 minutes. Immunofluorescence staining of CS-preconditioned ECs was performed with Texas Red–conjugated phalloidin to detect actin filaments. Scale bar = 10 μm. (B) Bar graph depicts quantitative image analysis of gap formation. Results are represented as mean ± SD. *P < 0.05 (n = 5). CS/T, 18% cyclic stretch + TRAP6.
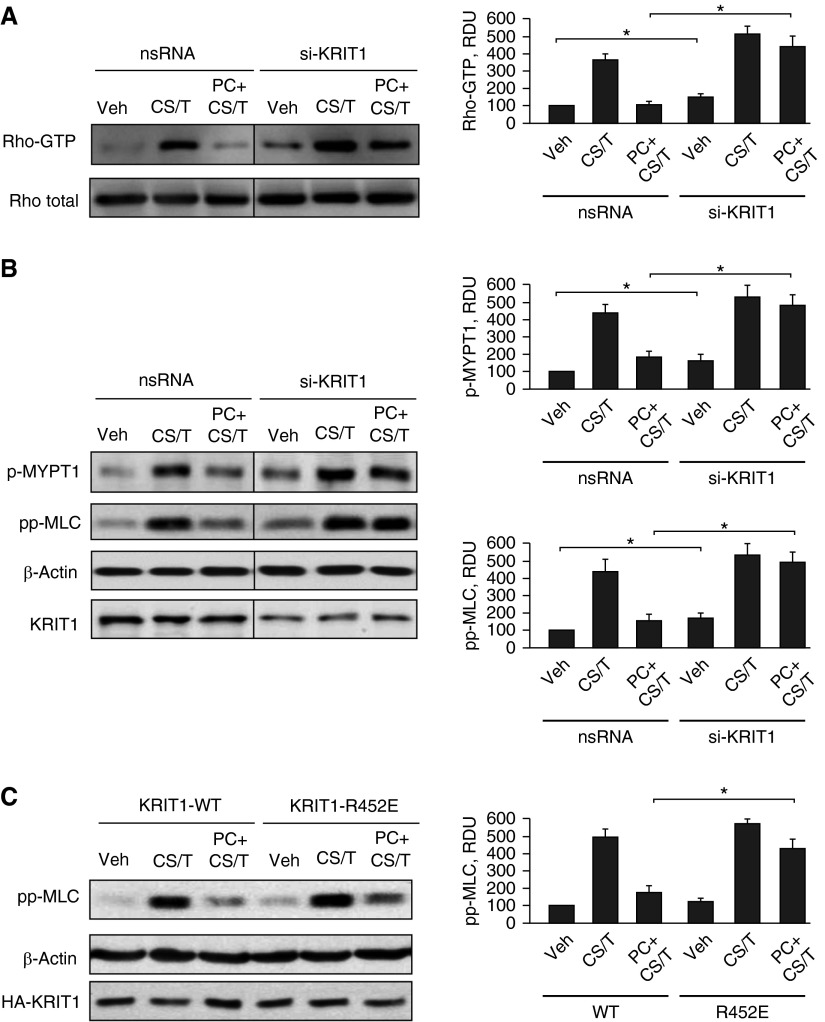
KRIT1 Mediates the PC-Activated Negative Regulation of the Rho Pathway Induced by 18% CS and TRAP6
The barrier-disruptive effects of high-magnitude cyclic stretch and thrombin are associated with activation of Rho GTPase (41). Our previous studies show the involvement of Rap1 GTPase in the iloprost-induced negative regulation of Rho signaling (16). However, the specific mechanism of Rho down-regulation downstream of Rap1 remains elusive. Next, experiments tested a role of Rap1-dependent KRIT1 activation in PC-induced inhibition of the Rho pathway. PC markedly suppressed Rho activation caused by 18% CS and TRAP6, but this effect was abolished by KRIT1 knockdown (Figure 5A). Moreover, KRIT1 knockdown augmented Rho activation in control and 18% CS/TRAP6-stimulated ECs in the absence of PC cotreatment. The Rho pathway of endothelial permeability involves phosphorylation of the myosin-binding subunit of myosin-associated phosphatase type 1 (MYPT1) at the Rho kinase–specific site Thr-850, leading to increased MLC phosphorylation and activated actomyosin contraction (42). We examined the effects of PC on MYPT1 and MLC phosphorylation levels in control and KRIT1-depleted cells exposed to 18% CS and TRAP6. KRIT1 depletion induced larger increases in MYPT1 and MLC phosphorylation in cells exposed to 18% CS and TRAP6. In turn, cotreatment with PC inhibited MYPT1 and MLC phosphorylation in control ECs transfected with nonspecific RNA but failed to inhibit 18% CS/TRAP6-induced MYPT1 and MLC phosphorylation in KRIT1-depleted cells (Figure 5B).
Figure 5.
Rho-dependent mechanism of KRIT1-mediated barrier preservation of ECs exposed to 18% CS and TRAP6. ECs grown on Bioflex plates and transfected with nonspecific or KRIT1-specific siRNA were subjected to 18% CS for 2 hours followed by TRAP6 (50 ng/ml, 15 min) stimulation with or without PC (200 ng/ml) cotreatment. (A) Rho activation was evaluated by RhoGTP pulldown assay and normalized to total Rho content in cell lysates. (B) Activation of Rho pathway was evaluated by Western blot analysis of phospho-myosin light chain phosphatase (pMYPT1) and diphospho–myosin light chain (ppMLC) levels. Reprobing with β-actin antibody was used as a normalization control. KRIT1 knockdown was verified by Western blot and represented approximately 50% of the original KRIT1 level. (C) ECs were transiently transfected with plasmids encoding wild-type KRIT1 and KRIT1-R452E mutant, and activation of Rho pathway was evaluated by Western blot analysis of ppMLC levels. Bar graphs depict results of membrane densitometry analysis. Data are expressed as mean ± SD. *P < 0.05 (n = 5). RDU, relative density units.
The role of KRIT1 activation by Rap1 in the protective effects of PC against the EC barrier disruptive Rho pathway was further tested in experiments with wild-type KRIT1 and the KRIT1-R452E mutant. Twenty-four hours after transfection, HPAECs were exposed to 18% CS and TRAP6 with or without PC cotreatment. Activation of Rho signaling was monitored by levels of diphosphorylated MLC. In comparison to wild-type KRIT1, expression of KRIT1-R452E increased MLC phosphorylation in pulmonary ECs caused by 18% CS and TRAP6 (Figure 5C). Expression of KRIT1-R452E also attenuated the inhibitory effects of PC on MLC phosphorylation in 18% CS/TRAP6-stimulated cells. Taken together, these results suggest that the intrinsic inhibitory Rap1-KRIT1 mechanism present in ECs prevents them from hyperactivation of RhoA signaling.
KRIT1 Deficiency Exacerbates HTV/TRAP6-Induced Lung Injury and Vascular Leakiness and Attenuates the Protective Effects of PC
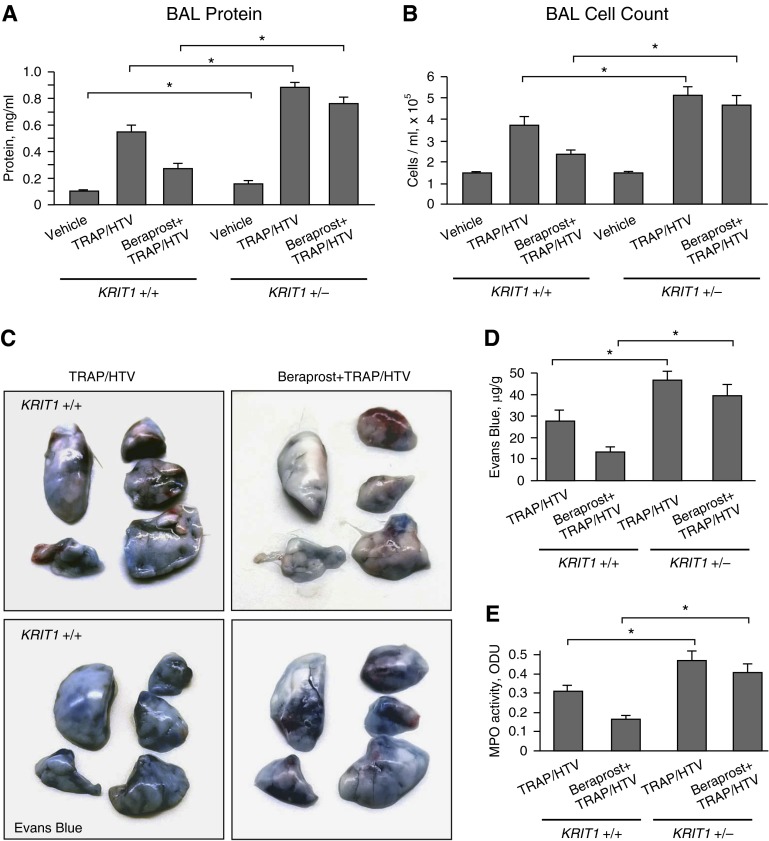
The role of KRIT1 in the control of lung injury and associated vascular leak was tested in the animal two-hit model of lung injury. Mice were exposed to mechanical ventilation at HTV (30 ml/kg) and TRAP6, the thrombin-derived, nonthrombogenic peptide that serves as a PAR1 receptor ligand (43). Because Krit−/− homozygous mice develop massive cerebral cavernous malformations at an early stage and generally are not viable (29), in this study we used Krit1+/− hypomorphic heterozygous mice. In vivo, heterozygous Krit1+/− mice expressed half the WT levels of KRIT1 protein in pulmonary and brain tissues and exhibited impaired pulmonary and cerebral vessel barrier function in basal conditions (33). To examine whether KRIT1 is involved in PC-mediated protective effects in vivo, Krit+/+ and Krit+/− mice were treated with TRAP6 and subjected to 4-hour HTV with or without cotreatment with the stable PC analog beraprost. Exposure to TRAP6 and HTV significantly increased protein content (Figure 6A) and cell counts (Figure 6B) in the bronchoalveolar lavage of Krit+/− mice, as compared with their Krit+/+ counterparts. The protective effects of beraprost against TRAP6/HTV-induced lung dysfunction were also significantly attenuated in Krit+/− mice. We also observed a statistically significant increase in bronchoalveolar lavage protein content in the Krit1+/− group under nonventilated conditions, which was consistent with previous studies (33).
Figure 6.
KRIT1 deficiency increases HTV/TRAP6-induced lung vascular leak and attenuates protective effects of beraprost. Krit+/+ and Krit−/+ mice were pretreated with vehicle of beraprost (20 μg/kg, intravenously) followed by TRAP6 injection (1.5 × 10–5 mol/kg, intratracheally) and mechanical ventilation at high tidal volume (HTV) (30 ml/kg, 4 h) (TRAP/HTV). (A) Measurements of protein concentration in bronchoalveolar lavage (BAL) fluid. *P < 0.05 (n = 6). (B) Total cell count in BAL fluid. *P < 0.05 (n = 6). (C) Vascular leak was analyzed by Evans blue–labeled albumin extravasation into the lung tissue. Shown are images of lungs excised from the chest and perfused with PBS. (D) The quantitative analysis of Evans blue extravasation was performed by spectrophotometric evaluation of Evans blue extracted from the lung tissue samples. *P < 0.05 (n = 4). (E) Myeloperoxidase (MPO) activity in Krit+/+ and Krit−/+ mice exposed to TRAP6/HTV with and without beraprost treatment. *P < 0.05 (n = 4). ODU, optical density units.
TRAP6/HTV induced lung vascular leak, which was evident in mice after 4 hours of stimulation and detected by Evans blue dye accumulation in the lung parenchyma (Figure 6C). Evans blue extravasation was increased in TRAP6/HTV-exposed Krit+/− mice as compared with their wild-type counterparts. In turn, the protective effect of beraprost on TRAP6/HTV-induced Evans blue accumulation in the lungs was largely suppressed in TRAP6/HTV-exposed Krit+/− mice. Quantitative analysis of Evans blue–labeled albumin extravasation further confirmed these results (Figure 6D). Measurement of myeloperoxidase activity in the lung tissue, as an additional parameter of neutrophilic infiltration and inflammation, confirmed exacerbated inflammation caused by HTV/TRAP6 and attenuation of beraprost protective effects against lung injury in Krit+/− mice (Figure 6E). Taken together, these results delineate the important role of KRIT1 in the mediation of PC-protective effects.
Discussion
Vascular abnormalities and the high incidence of seizures and hemorrhagic stroke have been described in patients with CCMs with mutations in the KRIT1/CCM2 genetic locus (44, 45) and are associated with weakening of brain microvascular EC junctions and elevation of Rho activity (33). Compromised vascular lumen organization in CCM also suggests a KRIT1 role in the control of the endothelial polarity complex (46).
The current study is the first demonstration of KRIT1 involvement in lung barrier enhancing effects caused by the pharmacological treatment with PC. Our results show that PC-induced KRIT1 colocalization with VE-cadherin at the AJ leads to enhancement of the EC barrier. KRIT1 knockdown abolished PC-induced assembly of AJ protein complexes, activation of cortical actin remodeling, and EC barrier enhancement. KRIT1 effects required KRIT1 activation by Rap1 because expression of the KRIT1-R452E mutant deficient in binding to the active Rap1 and becoming fully activated suppressed PC effects on EC barrier enhancement and peripheral cytoskeleton remodeling reflected by enhanced peripheral actin rim and accumulation of cortactin. In agreement with our data, intracellular localization of recombinant wild-type KRIT1 was restricted to cell–cell junctions, whereas the isolated R452E mutation failed to target KRIT1 to cell–cell junctions (32). These results suggest that Rap1-induced activation of KRIT1 is critical for the PC-induced remodeling of cell junctions and enhancement of the pulmonary EC barrier.
The other important finding of this study is the KRIT1 role in the modulation of lung vascular endothelial barrier dysfunction caused by pathologic mechanical forces. Pulmonary vasculature may experience excessive mechanical forces even during mechanical ventilation of patients with acute respiratory distress syndrome at a regimen considered to be safe. This paradoxical effect appears due to a high degree of regional heterogeneity in the severely inflamed lungs containing completely flooded nonventilated alveolar regions and overinflated regions with maintained ventilation. The combination of elevated CS amplitude and proinflammatory agonist used in this study best represents the clinically relevant microenvironment of pulmonary endothelium in disease. Barrier disruption of EC monolayers exposed to high-magnitude cyclic stretch and barrier-disruptive agonist TRAP6 in vitro is driven by the Rho pathway (10) and is consistent with Rho-dependent mechanism of lung barrier dysfunction in animal models of ventilator-induced lung injury (5, 16).
A study by Stockton and colleagues (33) demonstrated that KRIT1 in association with another member of CCM family, CCM2, acts as a negative regulator of RhoA, thereby limiting Rho kinase activity downstream. Hemizygous deficiency of KRIT1 or CCM2 increased vascular leak in basal conditions and in the lungs of LPS-stimulated mice by a Rho/Rho-kinase–dependent mechanism. Our data show increased Rho signaling in ECs with depleted KRIT1 under basal conditions but also demonstrate exacerbated activation of Rho signaling in the lung ECs subjected to mechanical stimulation.
How may KRIT1 be potentially involved in Rho regulation by mechanical forces? Focal adhesion protein complexes containing transmembrane cell adhesion receptors integrins serve as mechanosensors and mechanotransducers and stimulate signaling pathways in response to cell mechanical stimulation (47). Mechanical loading of integrins activates Rho-specific the guanine nucleotide exchange factors (GEFs) LARG and GEF-H1 associated with focal adhesions and stimulates Rho activity (48). Pathologic cyclic stretch also activates the focal adhesion-associated paxillin–GEF-H1–mitogen-activated protein kinase signaling module and triggers the Rho pathway of EC barrier dysfunction (49). In turn, KRIT1 activated by cell treatment with PC may inhibit stretch-induced integrin activation by binding the integrin cytoplasmic associated protein-1 (ICAP1), a negative regulator of β1 integrin activation (50). As a result, KRIT1-suppressed integrin activation may dampen stretch-induced focal adhesion assembly and formation of paxillin–mitogen-activated protein kinase–GEF-H1 signalosome and thus reduce barrier-disruptive RhoA signaling. We speculate that PC treatment of TRAP6/CS-exposed ECs may attenuate this stretch-induced mechanotransduction pathway via Rap1–KRIT1–ICAP1–mediated inhibition of integrin signaling and prevent activation of the stretch-induced focal adhesion signalosome.
This study demonstrates the role of KRIT1 in the maintenance of barrier integrity of pulmonary ECs exposed to high-magnitude cyclic stretch. KRIT1 deficiency elevated lung vascular leak under basal conditions and exacerbated vascular barrier dysfunction caused by HTV and attenuated protective effects of PC. These findings suggest that KRIT1 plays an essential role in permanent control and reparation of lung vascular endothelial integrity in physiologic and pathologic conditions, which may be further enhanced by PC treatment. Furthermore, the data show that the barrier protective effects of PC-induced KRIT1 activation observed in mechanically stimulated pulmonary ECs were due to the direct effect of KRIT1 on PC-induced Rho suppression, as shown by experiments with KRIT1 knockdown. KRIT1 is expressed in ECs and epithelial cells as well as in other tissues (51). Although our in vivo data (Evans blue extravasation) show the direct role of KRIT1 in the permeability control of the vascular endothelium, we cannot exclude additional effects by Krit1 down-regulation in the lung epithelium, which may also weaken the epithelial barrier and contribute to increased lung barrier dysfunction observed in KRTI1+/− mice.
Increased sensitivity of KRIT1+/− mice to excessive mechanical ventilation also suggests that the vasculature of these mice may be also prone to injury caused by other excessive hemodynamic disturbances, such as rapid fluctuations of intravascular pressure, blood flow, etc. These excessive mechanical forces may play a key role in the unexpected rupture of brain capillaries, local brain hemorrhage, stroke, and seizures reported in patients with familial mutations in the KRIT1/CCM2 genetic locus (44).
In conclusion, this study demonstrates a novel role of KRIT1 in the barrier protection of ECs under pathologic mechanical stretch and describes the KRIT1 involvement in the EC barrier enhancement induced by PC. The KRIT1-dependent mechanism described in this study may also contribute to the beneficial effects of other agonists capable of stimulating Rap1 activity. For example, the barrier-protective effects of oxidized phospholipids involve Rap1-dependent enhancement of cell junctions and functional interactions between AJs and tight junctions (52, 53), which may involve signaling by KRIT1. The results of this study also offer a novel insight into the mechanism of CCM development in the brain microvasculature by a dysregulated mechanotransduction mechanism of Rho activation caused by KRIT1 functional deficiency.
Acknowledgments
Acknowledgments
The authors thank Mark Ginsberg (Department of Medicine, University of California, San Diego, La Jolla, CA) for the generous gift of the wild-type and R452E mutant KRIT1 plasmids and Douglas Marchuk (Duke University, Durham, NC) for sharing Krit+/− mice.
Footnotes
This work was supported by National Heart, Lung, and Blood Institutes grants HL87823, HL076259, and HL107920.
Author Contributions: Conception and design: A.A.B. and K.G.B. Analysis and interpretation: A.M., F.M., Y.T., A.A.S., A.A.B., and K.G.B. Drafting the manuscript for important intellectual content: A.M., A.A.B., and K.G.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0376OC on April 29, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 2.Desai LP, Sinclair SE, Chapman KE, Hassid A, Waters CM. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol. 2007;293:L769–L778. doi: 10.1152/ajplung.00127.2007. [DOI] [PubMed] [Google Scholar]
- 3.Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care. 2008;12:R27. doi: 10.1186/cc6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-β1-induced endothelial barrier dysfunction involves Smad2-dependent p38 activation and subsequent RhoA activation. J Appl Physiol. 2006;101:375–384. doi: 10.1152/japplphysiol.01515.2005. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances α(v)β6 integrin-dependent TGF-β activation and promotes acute lung injury. J Clin Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, McAnulty RJ, Sheppard D, Jenkins G. Lysophosphatidic acid induces αvβ6 integrin-mediated TGF-β activation via the LPA2 receptor and the small G protein Gαa(q) Am J Pathol. 2009;174:1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol. 2000;164:6543–6549. doi: 10.4049/jimmunol.164.12.6543. [DOI] [PubMed] [Google Scholar]
- 9.Guo F, Tang J, Zhou Z, Dou Y, Van Lonkhuyzen D, Gao C, Huan J. GEF-H1-Rhoa signaling pathway mediates LPS-induced NF-κB transactivation and IL-8 synthesis in endothelial cells. Mol Immunol. 2012;50:98–107. doi: 10.1016/j.molimm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Birukova AA, Fu P, Xing J, Yakubov B, Cokic I, Birukov KG. Mechanotransduction by GEF-H1 as a novel mechanism of ventilator-induced vascular endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298:L837–L848. doi: 10.1152/ajplung.00263.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Gee MH, Tahamont MV, Flynn JT, Cox JW, Pullen RH, Andreadis NA. Prostaglandin E1 prevents increased lung microvascular permeability during intravascular complement activation in sheep. Circ Res. 1987;61:420–428. doi: 10.1161/01.res.61.3.420. [DOI] [PubMed] [Google Scholar]
- 14.Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res. 2010;155:44–54. doi: 10.1016/j.trsl.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birukova AA, Fu P, Xing J, Birukov KG. Rap1 mediates protective effects of iloprost against ventilator induced lung injury. J Appl Physiol. 2009;107:1900–1910. doi: 10.1152/japplphysiol.00462.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard LS, Morrell NW. New therapeutic agents for pulmonary vascular disease. Paediatr Respir Rev. 2005;6:285–291. doi: 10.1016/j.prrv.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Ueno Y, Koike H, Annoh S, Nishio S. Anti-inflammatory effects of beraprost sodium, a stable analogue of PGI2, and its mechanisms. Prostaglandins. 1997;53:279–289. doi: 10.1016/s0090-6980(97)89601-3. [DOI] [PubMed] [Google Scholar]
- 19.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 20.Birukov KG, Zebda N, Birukova AA. Barrier enhancing signals in pulmonary edema. Compr Physiol. 2013;3:429–484. doi: 10.1002/cphy.c100066. [DOI] [PubMed] [Google Scholar]
- 21.Verin AD, Gilbert-McClain LI, Patterson CE, Garcia JG. Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am J Respir Cell Mol Biol. 1998;19:767–776. doi: 10.1165/ajrcmb.19.5.3126. [DOI] [PubMed] [Google Scholar]
- 22.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284:L972–L980. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- 23.Birukova AA, Burdette D, Moldobaeva N, Xing J, Fu P, Birukov KG. Rac GTPase is a hub for protein kinase A and Epac signaling in endothelial barrier protection by cAMP. Microvasc Res. 2010;79:128–138. doi: 10.1016/j.mvr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 25.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7:585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 28.Serebriiskii I, Estojak J, Sonoda G, Testa JR, Golemis EA. Association of Krev-1/rap1a with Krit1, a novel ankyrin repeat-containing protein encoded by a gene mapping to 7q21–22. Oncogene. 1997;15:1043–1049. doi: 10.1038/sj.onc.1201268. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead KJ, Plummer NW, Adams JA, Marchuk DA, Li DY. Ccm1 is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development. 2004;131:1437–1448. doi: 10.1242/dev.01036. [DOI] [PubMed] [Google Scholar]
- 30.Zawistowski JS, Serebriiskii IG, Lee MF, Golemis EA, Marchuk DA. KRIT1 association with the integrin-binding protein ICAP-1: a new direction in the elucidation of cerebral cavernous malformations (CCM1) pathogenesis. Hum Mol Genet. 2002;11:389–396. doi: 10.1093/hmg/11.4.389. [DOI] [PubMed] [Google Scholar]
- 31.Kleaveland B, Zheng X, Liu JJ, Blum Y, Tung JJ, Zou Z, Sweeney SM, Chen M, Guo L, Lu MM, et al. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nat Med. 2009;15:169–176. doi: 10.1038/nm.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu JJ, Stockton RA, Gingras AR, Ablooglu AJ, Han J, Bobkov AA, Ginsberg MH. A mechanism of Rap1-induced stabilization of endothelial cell–cell junctions. Mol Biol Cell. 2011;22:2509–2519. doi: 10.1091/mbc.E11-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med. 2010;207:881–896. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285:L785–L797. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 35.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304:40–49. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Birukova AA, Zebda N, Cokic I, Fu P, Wu T, Dubrovskyi O, Birukov KG. p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp Cell Res. 2011;317:859–872. doi: 10.1016/j.yexcr.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-{beta}-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2007;293:L199–L211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- 39.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292:L924–L935. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- 40.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 41.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol. 2006;168:1749–1761. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via rho and its target rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 43.Storck J, Zimmermann ER. Regulation of the thrombin receptor response in human endothelial cells. Thromb Res. 1996;81:121–131. doi: 10.1016/0049-3848(95)00220-0. [DOI] [PubMed] [Google Scholar]
- 44.Awad IA. Unfolding knowledge on cerebral cavernous malformations. Surg Neurol. 2005;63:317–318. doi: 10.1016/j.surneu.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Fisher OS, Boggon TJ. Signaling pathways and the cerebral cavernous malformations proteins: lessons from structural biology. Cell Mol Life Sci. 2014;71:1881–1892. doi: 10.1007/s00018-013-1532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci. 2010;123:1073–1080. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MA, Desimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guilluy C, Swaminathan V, Garcia-Mata R, O'Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gawlak G, Tian Y, O'Donnell JJ, III, Tian X, Birukova AA, Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. FASEB J. 2014;28:3249–3260. doi: 10.1096/fj.13-245142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W, Draheim KM, Zhang R, Calderwood DA, Boggon TJ. Mechanism for KRIT1 release of ICAP1-mediated suppression of integrin activation. Mol Cell. 2013;49:719–729. doi: 10.1016/j.molcel.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denier C, Gasc JM, Chapon F, Domenga V, Lescoat C, Joutel A, Tournier-Lasserve E. Krit1/cerebral cavernous malformation 1 mRNA is preferentially expressed in neurons and epithelial cells in embryo and adult. Mech Dev. 2002;117:363–367. doi: 10.1016/s0925-4773(02)00209-5. [DOI] [PubMed] [Google Scholar]
- 52.Birukova AA, Fu P, Wu T, Dubrovskyi O, Sarich N, Poroyko V, Birukov KG. Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids. J Cell Physiol. 2012;227:1883–1890. doi: 10.1002/jcp.22916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol. 2011;226:2052–2062. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]