Abstract
Lung inflammation plays a key role in the pathogenesis of bronchopulmonary dysplasia (BPD), a chronic lung disease of premature infants. The challenge in BPD management is the lack of effective and safe antiinflammatory agents. Leukadherin-1 (LA1) is a novel agonist of the leukocyte surface integrin CD11b/CD18 that enhances leukocyte adhesion to ligands and vascular endothelium and thus reduces leukocyte transendothelial migration and influx to the injury sites. Its functional significance in preventing hyperoxia-induced neonatal lung injury is unknown. We tested the hypothesis that administration of LA1 is beneficial in preventing hyperoxia-induced neonatal lung injury, an experimental model of BPD. Newborn rats were exposed to normoxia (21% O2) or hyperoxia (85% O2) and received twice-daily intraperitoneal injection of LA1 or placebo for 14 days. Hyperoxia exposure in the presence of the placebo resulted in a drastic increase in the influx of neutrophils and macrophages into the alveolar airspaces. This increased leukocyte influx was accompanied by decreased alveolarization and angiogenesis and increased pulmonary vascular remodeling and pulmonary hypertension (PH), the pathological hallmarks of BPD. However, administration of LA1 decreased macrophage infiltration in the lungs during hyperoxia. Furthermore, treatment with LA1 improved alveolarization and angiogenesis and decreased pulmonary vascular remodeling and PH. These data indicate that leukocyte recruitment plays an important role in the experimental model of BPD induced by hyperoxia. Targeting leukocyte trafficking using LA1, an integrin agonist, is beneficial in preventing lung inflammation and protecting alveolar and vascular structures during hyperoxia. Thus, targeting integrin-mediated leukocyte recruitment and inflammation may provide a novel strategy in preventing and treating BPD in preterm infants.
Keywords: LA1, integrin, hyperoxia, BPD, inflammation
Clinical Relevance
This work demonstrates that treatment with leukadherin-1 (LA1), a leukocyte surface integrin agonist that enhances leukocyte adhesion and decreases leukocyte influx, protects against hyperoxia-induced neonatal lung injury. These findings suggest that LA1 may offer a novel therapeutic strategy to alleviate bronchopulmonary dysplasia in neonates.
Bronchopulmonary dysplasia (BPD) is a significant global health problem that affects preterm infants (1, 2). Over the past four decades, the incidence of this disease has significantly increased as a result of the improved survival of extremely premature infants. BPD is a multifactorial disease that is characterized by chronic impairment of alveolar structure and pulmonary function (1). At later stages, BPD is often complicated by pulmonary hypertension (PH), and this significantly increases morbidity and mortality (3, 4). Despite recent advances in neonatal care, the pathogenesis of BPD is still poorly understood, and there is no effective therapy.
Inflammatory injury triggered by prenatal and postnatal infections, oxygen toxicity, and/or mechanical ventilation is recognized as central to the pathogenesis of BPD (5–9). The cellular inflammatory response observed in clinical and experimental BPD is dominated by an influx of neutrophils and macrophages into the lung (5, 10–12). Inflammation in the immature lung can disrupt distal lung development, leading to decreased alveolar septation and vascular growth. Excessive tissue and vascular remodeling in response to chronic inflammation can lead to parenchymal thickening, pulmonary vascular wall hypertrophy, and PH. To date, corticosteroids are the only antiinflammatory agents used to treat BPD. However, their modest effectiveness is accompanied by severe side effects on neurodevelopment and alveolar growth; therefore, their usage is limited in premature infants (13–15).
Integrins have essential roles in promoting immune cell recruitment to inflamed tissues and have attracted much interest as potential therapeutic targets (16–18). The integrins CD11b/CD18 are more restricted antigens normally expressed on monocytes, macrophages, polymorphic neutrophils (PMNs), and natural killer cells (16–19). They are the key receptors that mediate leukocyte adhesion, transendothelial migration, and influx into the injury sites. Leukadherin-1 (LA1) is a newly developed small-molecule agonist of CD11b/CD18 that contains a core furanyl thiazolidinone chemical structure motif (20, 21). LA1 enhances CD11b/CD18-dependent cell adhesion to its ligands, such as ICAM-1, and thus reduces leukocyte transendothelial migration and recruitment (20, 21). LAI has been shown to reduce leukocyte migration, tissue accumulation, and inflammatory injury in experimental models of vascular injury and nephritis (21, 22). However, it is unknown whether LA1 has therapeutic potential in experimental BPD.
Hyperoxia-induced lung injury in neonatal rodents is widely used as an experimental model for BPD (23–25). We have previously shown that chronic hyperoxia exposure induces inflammatory response, disrupts normal lung development process, and triggers a cascade of dysregulated reparative pathways that result in alveolar simplification, decreased pulmonary vascular development, pulmonary vascular remodeling, and PH (26–28). In this study, we used a newborn rat model to test our hypothesis that LA1 prevents hyperoxia-induced BPD-like pathology by blunting leukocyte influx into the lung. Our results demonstrate that administration of LA1 decreased hyperoxia-induced influx of macrophages into the lung, preserved alveolar and vascular development, and reduced pulmonary vascular remodeling. Thus, targeting integrin-mediated leukocyte recruitment and inflammation may provide a novel strategy in preventing and treating BPD in preterm infants.
Materials and Methods
Animal Model and Experimental Protocol
The study protocol was approved by the University of Miami Institutional Animal Care and Use Committee. Newborn Sprague Dawley rat pups (n = 12 per group) were randomized on Postnatal Day 2 into four groups: normoxia (21% O2) plus placebo, normoxia plus LA1, hyperoxia (85% O2) plus placebo, and hyperoxia plus LA1 (provided by V. Gupta) (21). Rat pups received LA1 (1 mg/kg, mixed in 2% DMSO) or placebo (2% DMSO) (equal volume) twice daily by intraperitoneal injection for 14 days. Pups were killed on Postnatal Day 15 for analyses.
White Blood Cell Count and Differential
Blood was collected via right ventricle puncture, and white blood cell (WBC) count and differential were performed.
Assessment of Lung Inflammation
Bronchoalveolar lavage (BAL) and differential cell count were performed as previously described (27, 29). Macrophage infiltration into alveolar airspaces was assessed by performing immunostaining with an antibody for Mac3 (a macrophage-specific marker) on lung tissue sections. The number of Mac3-positive cells in the alveolar airspaces was counted as described previously (27, 29). ELISA was used to measure the concentrations of monocyte chemoattractant protein-1 (MCP-1) (Life Technologies, Grand Island, NY) and TNF-α (R&D, Minneapolis, MN) in lung homogenates.
Lung Histology and Morphometry
Lungs were infused with 4% paraformaldehyde via a tracheal catheter at 20 cm H2O of pressure for 5 minutes, fixed overnight, and paraffin embedded. Hematoxylin and eosin–stained tissue sections were used to determine radial alveolar count (RAC) and mean linear intercept as previously described (27, 29).
Immunostaining and Double Immunofluorescence Staining
Immunostaining and double immunofluorescence staining were performed as previously described (27, 29).
Pulmonary Vascular Morphometry
Pulmonary vascular density was determined by the average number of von Willebrand factor (vWF)-stained vessels (<50 μm in diameter) from 10 random images on each lung section (26–29). Vascular endothelial growth factor (VEGF) expression was quantified by Western blot analysis (29).
Assessment of Pulmonary Vascular Remodeling
Double immunofluorescence staining for α-SMA and vWF was performed. Twenty peripheral pulmonary vessels (<50 μm in diameter) were assessed for medial wall thickness (MWT) and the extension of muscularization (>50% of vessel circumference) as previously described (27, 29).
Assessment of PH
Right ventricular systolic pressure (RVSP) and right ventricle (RV) to left ventricle (LV) plus septum weight ratios (RV/LV+S) were determined as indices for PH (27, 29). For RVSP measurement, a 25-gauge needle fitted to a pressure transducer was inserted into the RV. Pressure levels were recorded on a Gould polygraph. Afterward, hearts were dissected for RV free wall separation from LV+S for RV/LV+S weight ratio assessment.
Western Blot Analysis
Total protein was extracted from frozen lung tissues with a RIPA buffer according to the manufacturer’s protocol (Santa Cruz, Dallas, TX). Western blot analysis was performed to assess VEGF, connective tissue growth factor (CTGF), and Wnt1-inducible signaling protein-1 (WISP-1) in lung homogenates as previously described (29).
Statistical Analysis
Data were expressed as means ± SD and analyzed by one-way ANOVA followed by Student-Newman Keuls test. A P value of <0.05 was considered significant.
Results
LA1 Decreases Lung Inflammation during Hyperoxia
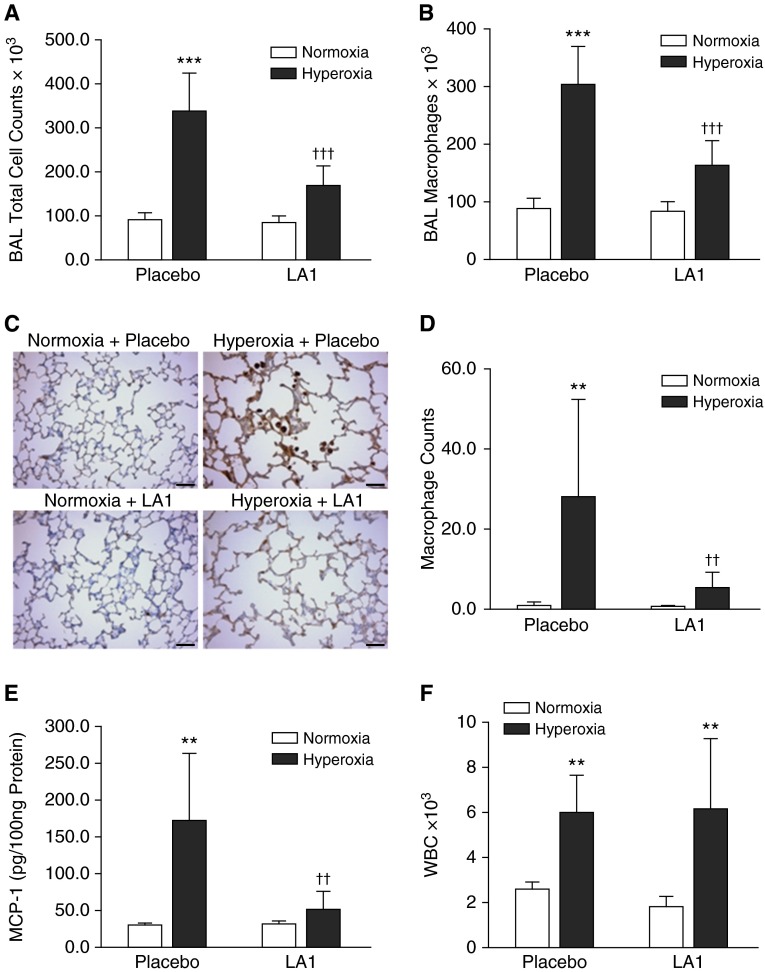
Lung inflammation was first assessed by BAL and cell count. Total cell counts and macrophages in BAL were significantly elevated in placebo-treated, hyperoxia-exposed rats as compared with the normoxia + placebo group (Figures 1A and 1B). Treatment with LA1 significantly reduced both total cell and macrophage counts during hyperoxia exposure (Figures 1A and 1B). Similarly, the neutrophil counts in BAL were significantly increased in placebo-treated and hyperoxia-exposed rats as compared with normoxia-exposed rats (19,794.4 ± 21,787.4 versus 1.4 ± 1.5, hyperoxia versus normoxia; P < 0.029). Administration of LA1 did not significantly decrease neutrophil counts in BAL during hyperoxia exposure (data not shown).
Figure 1.
Administration of leukadherin-1 (LA1) decreases leukocyte influx into the lung and monocyte chemoattractant protein-1 (MCP-1) expression. Total cell counts (A) and macrophage counts (B) in bronchoalveolar lavage (BAL) were increased in hyperoxia- and placebo-exposed animals. However, these were significantly decreased by treatment with LA1. Immunostaining for Mac3 was performed on lung tissue sections (C), and the average numbers of macrophages in alveolar airspaces were determined from 10 random images taken under the high-power view on each lung section (D). There was an increased macrophage count in the placebo-treated hyperoxia group, but this number was drastically decreased upon treatment with LA1. (E) MCP-1 in lung homogenates was increased in the hyperoxia + placebo group, but it was decreased by treatment with LA1. (F) White blood cells (WBCs) were counted in peripheral blood. Hyperoxia exposure significantly increased WBC count in both the placebo and LA1-treated groups as compared with normoxic groups. Scale bar: 50 μm (n = 4 to 5 per group). **P < 0.01 and ***P < 0.001 compared with normoxic groups; ††P < 0.01 and †††P < 0.001 compared with the hyperoxia + placebo group.
We next assessed macrophage infiltration into the alveolar airspaces by immunostaining with a Mac3 antibody on lung tissue sections (Figure 1C). The macrophage counts were significantly increased in the placebo-treated hyperoxia group (Figure 1D). Treatment with LA1 significantly decreased the macrophage counts in alveolar airspaces during hyperoxia exposure (Figure 1D).
To further examine inflammatory response, we analyzed MCP-1 and TNF-α concentrations in lung homogenates. Hyperoxia exposure significantly increased MCP-1 in placebo-treated lungs (Figure 1E). However, treatment with LA1 decreased MCP-1 concentration in hyperoxia-exposed rats (Figure 1E). The concentration of TNF-α was below the detectable range in all four groups (data not shown).
To determine whether LA1 has an effect on circulating immune cells, we collected blood and measured WBC count and differential. Hyperoxia exposure increased total WBC count in the placebo-treated group as compared with normoxic groups (Figure 1F). Treatment with LA1 did not cause any significant changes in WBC count under hyperoxic or normoxic conditions (Figure 1F). Similarly to total WBC count, hyperoxia exposure in the presence of the placebo increased neutrophil and monocyte counts as compared with normoxic lungs (neutrophil: 152.25 ± 67.67 versus 52.40 ± 21.34, hyperoxia + placebo versus normoxia + placebo, P < 0.05; monocytes: 558.75 ± 55.83 versus 234.00 ± 50.45, hyperoxia + placebo versus normoxia + placebo, P < 0.001). Although administration of LA1 did not affect neutrophil or monocyte counts under hyperoxia (neutrophil: 200.80 ± 150.6 versus 152.25 ± 67.67, hyperoxia + LA1 versus hyperoxia + placebo, P = 0.57; monocyte: 664.40 ± 388.23 versus 558.75 ± 55.83, hyperoxia + LA1 versus hyperoxia + placebo, P = 0.61) or normoxia (neutrophil: 39.20 ± 21.72 versus 52.40 ± 21.33, normoxia + LA1 versus normoxia + placebo; monocyte: 162.20 ± 79.47 versus 234.00 ± 50.44, normoxia + LA1 versus normoxia + placebo, P = 0.13).
LA1 Improves Alveolarization in Hyperoxia-Exposed Animals
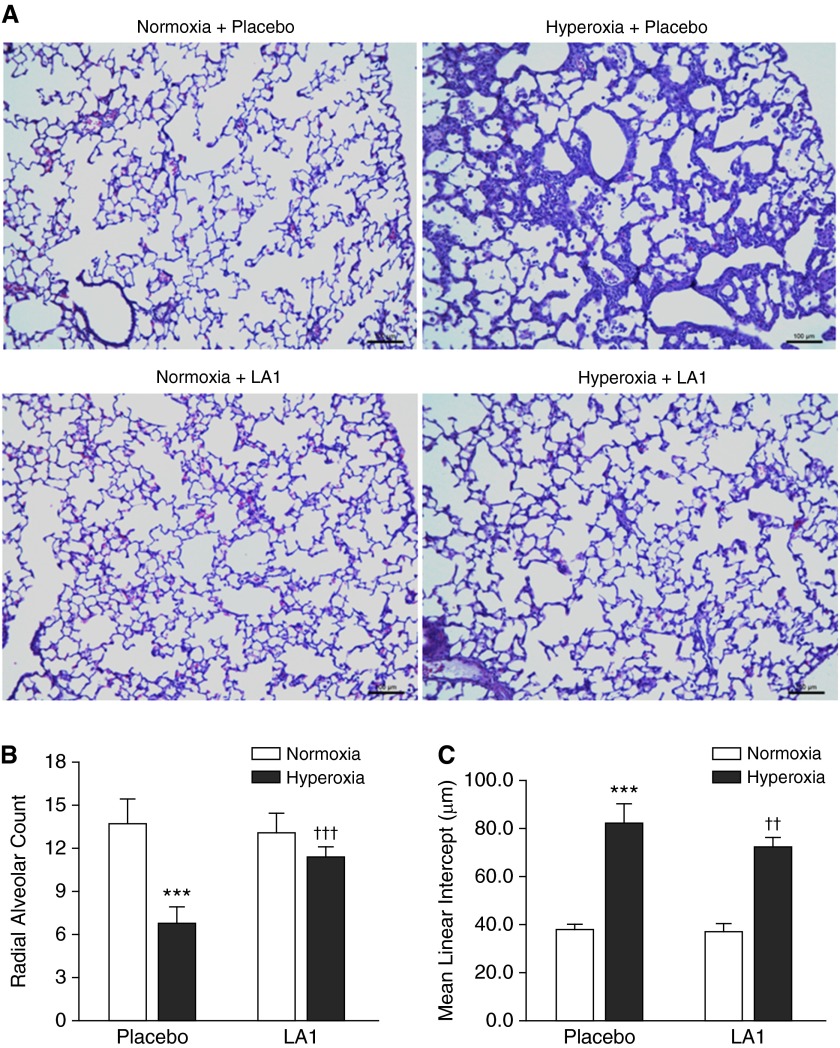
On histological examination, lungs from placebo-treated and hyperoxia-exposed animals displayed impairment of alveolarization as demonstrated by fewer, larger, and simplified alveoli (Figure 2A). Treatment with LA1 in hyperoxia-exposed rats improved alveolar structure that appeared similar to the normoxic lungs (Figure 2A). On morphometric assessment, there was a significant decrease in RAC in placebo-treated animals that were exposed to hyperoxia (6.84 ± 1.1 versus 13.8 ± 1.6, hyperoxia + placebo versus normoxia + placebo; P < 0.001). However, treatment with LA1 during hyperoxia significantly increased RAC (11.46 ± 0.7 versus 6.84 ± 1.1, hyperoxia + LA1 versus hyperoxia + placebo; P < 0.001) (Figure 2B). Mean linear intercept was significantly increased in hyperoxia + placebo lungs and decreased in hyperoxia + LA1 lungs (Figure 2C).
Figure 2.
Treatment with LA1 improves alveolar development. (A) On histological examination, lungs from placebo-treated and hyperoxia-exposed rats displayed an impairment of alveolarization with fewer, larger, and simplified alveoli. Treatment with LA1 improved alveolar structures that appeared similar to the normoxic lungs. On morphometric assessment, placebo-treated animals exposed to hyperoxia showed a decrease in radial alveolar count (RAC) (B) and increased mean linear intercept (MLI) (C) as compared with normoxic animals. Administration of LA1 during hyperoxia significantly increased RAC and decreased MLI. Scale bar: 100 μm (n = 5 per group). ***P < 0.001 compared with normoxic groups; ††P < 0.01 and †††P < 0.001 compared with the hyperoxia + placebo group.
Treatment with LA1 Improves Pulmonary Vascular Development during Hyperoxia
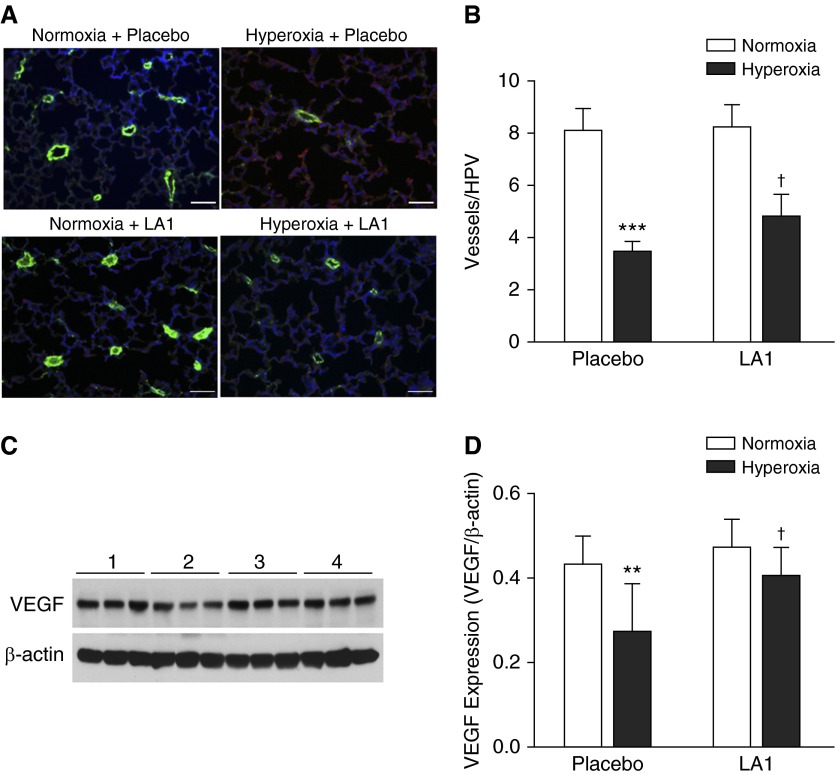
To determine the effects of LA1 on pulmonary vascular development, we performed immunofluorescence for vWF on lung tissue sections and quantified vascular density (Figure 3A). The vascular density was significantly decreased in the placebo-treated hyperoxia group compared with the normoxia group (3.42 ± 0.43 versus 8.08 ± 0.82, hyperoxia + placebo versus normoxia + placebo; P < 0.001). Administration of LA1 to hyperoxia-exposed animals modestly increased vascular density (4.80 ± 0.86 versus 3.42 ± 0.43, hyperoxia + LA1 versus hyperoxia + placebo; P < 0.05) (Figure 3B).
Figure 3.
LA1 administration improves vascular development. (A) Double immunofluorescence staining with an anti–von Willebrand factor (vWF) antibody (green signals), anti–α-smooth muscle actin (α-SMA) antibody (red signal), and 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (blue signals) was performed on lung tissue sections. (B) Vascular density was determined by counting vWF-positive vessels (<50 μm) on 10 random images from each lung section. The vascular density was significantly decreased in hyperoxia + placebo lungs compared with normoxia lungs. Treatment with LA1 modestly increased vascular density in hyperoxia-exposed animals. (C) Representative photo images of Western blots for vascular endothelial growth factor (VEGF) and β-actin expression. (D) Densitometry analysis demonstrated that VEGF expression was significantly decreased in hyperoxia + placebo lungs but was increased by treatment with LA1. Scale bar: 50 μm. **P < 0.01, and ***P < 0.001 compared with normoxic groups (n = 5 per group). †P < 0.05 compared with hyperoxia plus placebo group. HPV, high-power view.
Because VEGF is one of the most important angiogenic factors, we assessed the effects of LA1 on VEGF expression. As illustrated in Figures 3C and 3D, VEGF expression was decreased in hyperoxia + placebo lungs. Treatment with LA1 significantly increased VEGF expression as compared with placebo during hyperoxia.
LA1 Decreases Pulmonary Vascular Remodeling
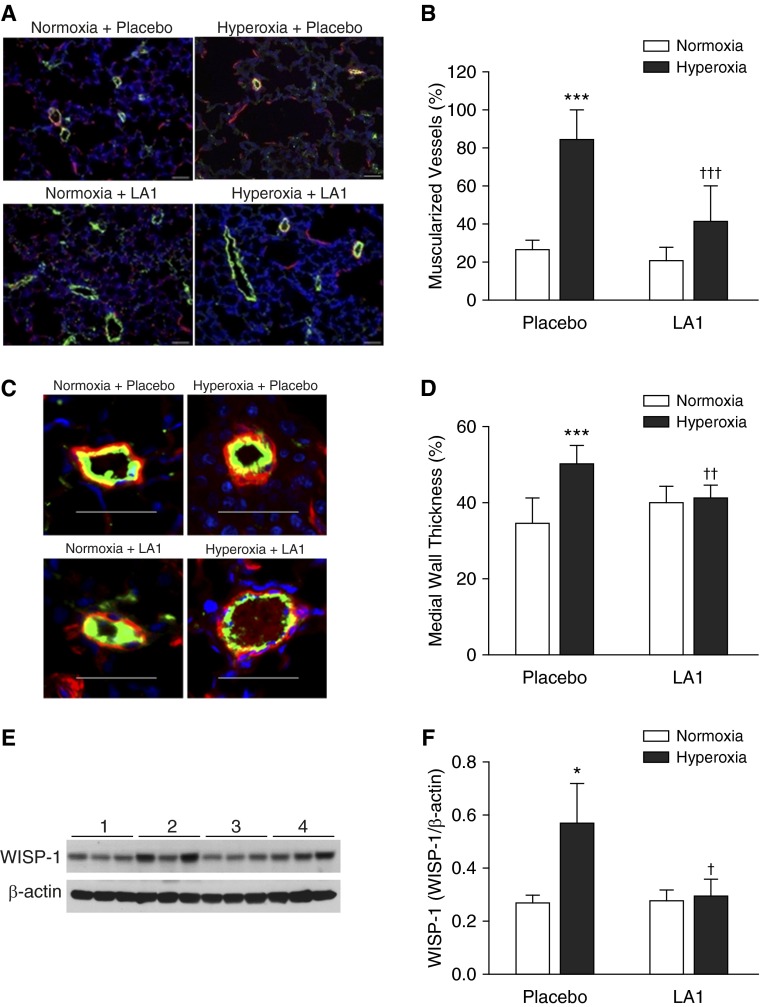
To determine the effects of LA1 on pulmonary vascular remodeling, double immunofluorescence for vWF and α-SMA was performed (Figure 4A). The percentage of muscularized peripheral pulmonary arterioles (>50% of vessel circumference) was measured. Hyperoxia-exposed rats treated with the placebo had significantly increased muscularized peripheral pulmonary arterioles compared with normoxia-exposed rats (84.9 ± 14.9 versus 26.4 ± 5.2, hyperoxia + placebo versus normoxia + placebo; P < 0.001) (Figure 4B). In hyperoxia-exposed rats, treatment with LA1 significantly decreased vascular remodeling, as evident by decreased the percentage of muscularized peripheral pulmonary arterioles (41.6 ± 18.5 versus 84.9 ± 14.9, hyperoxia + LA1 versus hyperoxia + placebo; P < 0.001) (Figure 4B).
Figure 4.
Treatment with LA1 attenuates hyperoxia-induced pulmonary vascular remodeling. (A) Double-immunofluorescence staining for vWF (green signals) and α-SMA (red signals) and DAPI nuclear staining (blue signals). (B) The percentage of muscularized peripheral pulmonary vessels (≥50% of circumference) was significantly increased in lungs from the hyperoxia + placebo group. Administration of LA1 significantly decreased vascular muscularization in hyperoxia-exposed animals. (C) Medial wall thickness (MWT) was assessed on 20 peripheral vessels (<50 μm in diameter) on each lung section. (D) Hyperoxia significantly increased MWT in lungs of placebo-treated animals. However, treatment with LA1 significantly decreased MWT during hyperoxia. (E) Representative photo images of Western blots for Wnt1-inducible signaling protein-1 (WISP-1) and β-actin expression. (F) Densitometry analysis demonstrated that WISP-1 expression was significantly increased in the hyperoxia + placebo lungs and was decreased by administration of LA1. Scale bar: 50 μm. *P < 0.05 and ***P < 0.001 compared with normoxic groups; †P < 0.05, ††P < 0.01, and †††P < 0.001 compared with hyperoxia + placebo group (n = 5 per group).
We also assessed MWT of peripheral pulmonary arterioles (<50 μm in diameter) and found that hyperoxia exposure significantly increased MWT in placebo-treated rats compared with normoxic rats (50.00 ± 4.57 versus 35.00 ± 6.72, hyperoxia + placebo versus normoxia + placebo; P < 0.001). Treatment with LA1 significantly decreased MWT of peripheral pulmonary arterioles during hyperoxia exposure (40.00 ± 4.57 versus 50.00 ± 2.92, hyperoxia + LA1 versus hyperoxia + placebo; P < 0.01) (Figures 4C and 4D).
We further explored the effects of LA1 on the expression of growth factors that are known to be involved in vascular remodeling and lung fibrosis, such as CTGF and WISP-1. Hyperoxia exposure increased CTGF and WISP-1 expression, whereas treatment with LA1 significantly decreased WISP-1 expression (Figures 4E and 4F) but did not affect CTGF expression (data not shown).
Effects of LA1 on Hyperoxia-Induced PH
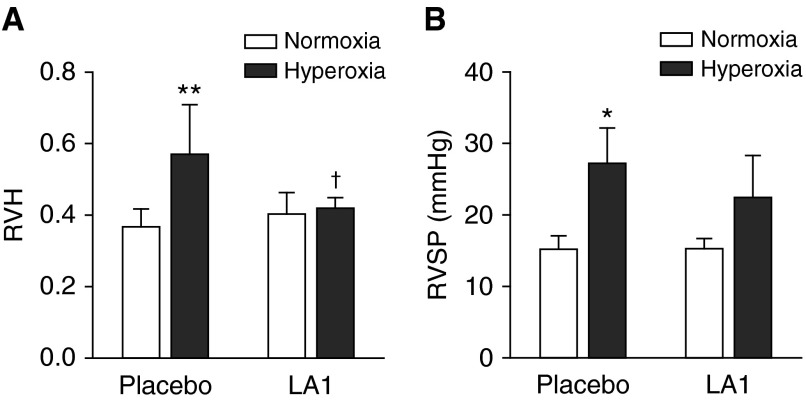
The weight ratio of RV/LV+S, an index of right ventricular hypertrophy (RVH) and RVSP, was assessed to evaluate the effects of LA1 on PH. In hyperoxia-exposed rats, RVH was significantly increased when compared with the normoxia group (0.57 ± 0.06 versus 0.37 ± 0.02, hyperoxia + placebo versus normoxia + placebo; P < 0.01) (Figure 5A). Treatment with LA1 significantly decreased RVH in the hyperoxia-exposed group when compared with placebo (0.42 ± 0.01 versus 0.57 ± 0.06, hyperoxia + LA1 versus hyperoxia + placebo; P < 0.05) (Figure 5A). RVSP was significantly elevated in hyperoxia- and placebo-exposed rats compared with the normoxia groups. Although there was a slight decrease in RVSP in LA1-treated hyperoxic rats as compared with the placebo-treated hyperoxia group, this decrease did not reach statistical significance.
Figure 5.
Effects of LA1 on hyperoxia-induced pulmonary hypertension (PH). Right ventricular hypertrophy (RVH) (A) and right ventricular systolic pressure (RVSP) (B) were assessed as indices of PH. Hyperoxia exposure resulted in RVH in placebo-treated rats. Administration of LA1 significantly decreased RVH during hyperoxia. Hyperoxia also increased RVSP in placebo-exposed rats. Treatment with LA1 resulted in a slightly decreased RVSP during hyperoxia, but this did not reach statistical significance. *P < 0.05 and **P < 0.01 compared with normoxic groups; †P < 0.05 compared with hyperoxia + placebo group (n = 5 per group).
Discussion
This study demonstrates that LA1, a novel CD11b/CD18 agonist, prevents hyperoxia-induced leukocyte infiltration, which leads to improved alveolar development and vascularization and reduced pulmonary vascular remodeling in neonatal rats. To the best of our knowledge, this is the first report on the therapeutic efficacy of LA1 in hyperoxia-induced neonatal lung injury. Given that oxygen toxicity and inflammation play key roles in the pathogenesis of BPD, our study highlights that leukocyte integrin agonists may be used as a novel strategy to alleviate lung inflammation and preserve alveolar and vascular development in neonates at risk for BPD.
BPD continues to be one of the most common long-term pulmonary complications associated with preterm birth, causing significant morbidity and mortality (1, 2). The new “BPD” is recognized as a developmental arrest of the immature lung in response to the inflammatory process generated by prenatal or postnatal infections, oxygen toxicity, and/or mechanical ventilation (30–32). It is well known that inflammation is predominantly mediated by leukocytes and their influx into the site of injury (5, 33, 34). Clinical studies have demonstrated the presence of increased neutrophils, activated macrophages, and high concentrations of inflammatory mediators in tracheal aspirates from preterm infants who subsequently developed BPD (5, 35, 36). Leukocyte trafficking persists in infants who developed chronic lung disease, and increased CD11b/CD18-expressing neutrophils were seen in infants with chronic lung disease versus no disease (37). Animal models further support the key role of inflammation in the pathogenesis of BPD. In experimental models of BPD induced by endotoxin, hyperoxia, or mechanical ventilation, an influx of neutrophils and macrophages is uniformly seen in the acute phase of inflammatory injury (11, 27, 38). These cells produce proinflammatory cytokines, proteases, and reactive oxygen species that can subsequently cause alveolar epithelial and endothelial cell injury and apoptosis. Persistent inflammation can disrupt alveolar and vascular development and induce tissue remodeling and fibrosis that ultimately result in chronic alterations of alveolar structure and lung function.
The challenge in BPD management is the lack of effective and safe antiinflammatory agents. Corticosteroids, in particular dexamethasone, as antiinflammatory agents were commonly used in the prevention and treatment of BPD. Concerns about their long-term side effects on growth and neurodevelopment have limited their systemic use to extremely high-risk populations with cautions on the dose and length of therapy (13–15). Taking a fresh approach, this study assessed the effects of LA1, a novel small molecule agonist of CD11b/CD18, on preventing hyperoxia-induced lung injury in neonatal rats. Neutrophils, macrophages, monocytes, and natural killer cells bear CD11b/CD18 integrins on their cell surface in an inactive form (16–19). β2 Integrins, such as CD11b/CD18, play a key role in leukocyte adhesion and transendothelial migration. A series of in vitro experiments has demonstrated that LA1 activates CD11b/CD18, enhances leukocyte adhesion to ligands, and decreasess leukocyte transendothelial cell migration (20, 21). Recent in vivo studies showed that LA1 has a clear therapeutic advantage over CD11b/CD18 antibodies in reducing the influx of macrophage into injured vessels (22). We showed that administration of LA1 significantly decreased hyperoxia-induced influx of macrophages into the lung at the end of 14 days of exposure to hyperoxia. Furthermore, LA1 treatment decreased MCP-1 production in hyperoxia-exposed lungs. Previous studies have showed that treatment with antibodies against cytokine-induced neutrophil chemoattractant-1 and macrophage inflammatory protein-2 reduced the PMN count in BAL in hyperoxia-exposed newborn rats (39, 40). Also in hyperoxia-exposed newborn rats, anti–MCP-1 antibody therapy effectively reduced PMN and macrophage infiltration and reduced protein oxidation (41). Taken together, these data indicate that targeting leukocyte adhesion and trafficking is effective in preventing hyperoxia-induced leukocyte infiltration in the neonatal lung. In contrast to these previous studies, we also assessed how hyperoxia and LA1 affect the circulating immune cell response. Our results demonstrated that hyperoxia significantly increased total WBC, neutrophil, and monocyte counts in the presence or absence of LA1. Furthermore, treatment with LA1 does not affect these cell counts under normoxic conditions. These are important findings that highlight the potential of LA1 as a future antiinflammatory agent in clinical applications.
We demonstrated in this study that treatment with LA1 is not only effective in preventing hyperoxia-induced lung inflammation; it is also beneficial in preventing hyperoxia-induced alveolar structural damage. LA1 administration improved alveolarization during hyperoxia exposure. Alveolar development and angiogenesis in lung go hand in hand, and impairment of alveolarization has a negative effect on angiogenesis and vice versa (3). In our study we saw improved vascular density along with alveolarization in LA1-treated hyperoxic rats. We speculate that the protective effects of LA1 on alveolar and vascular development are secondary to its antiinflammatory property because there is no known direct effect of LA1 on cell growth, survival, and apoptosis. There are increasing reports on the beneficial effects of novel antiinflammatory therapies on preserving alveolar structure in experimental BPD. Treatment with cytokine-induced neutrophil chemoattractant-1 and macrophage inflammatory protein-2 antibody preserved alveolar volume and surface density (39, 40). Nold and colleagues have recently shown that IL-1 receptor antagonist increased alveolar number, decreased alveolar size, and increased the alveolar surface area to volume ratio in perinatal inflammation and postnatal hyperoxia-induced murine BPD (38). Multiple studies have investigated the effects of mesenchymal stem cell therapy in the prevention and treatment of hyperoxia-induced rodent models of BPD. These studies have all demonstrated that stem cell therapy is beneficial in improving alveolar and vascular development during hyperoxia (42–44). Although the exact mechanisms remain to be fully understood, the protective effects of stem cell therapy were correlated with the prevention of neutrophil and macrophage infiltration (43, 44). It is suggested that these beneficial effects on alveolar structure may be caused by the immunomodulatory proteins produced by stem cells because the culture medium has similar effects. Nevertheless, our results further support the notion that targeting inflammatory responses is key in preserving alveolar and vascular development in the immature lung.
The development of PH remains a significant cause of morbidity and mortality in infants with severe BPD. In both clinical and hyperoxia-induced rodent models of BPD, PH is characterized by significant RVH and elevation of RVSP. The PH may be caused by decreased pulmonary vasculature, which limits vascular surface area, leading to elevation of pulmonary vascular resistance (4). The excessive pulmonary vascular remodeling may further contribute to high pulmonary vascular resistance through narrowing of the vessel diameter and decreased vascular compliance (4). We showed in this study that treatment with LA1 resulted in a significant decrease in RVH and a trend toward a decrease in RVSP. LA1 administration modestly increased vascular density and significantly decreased pulmonary vascular remodeling, as demonstrated by reduced muscularization of peripheral pulmonary vessels during hyperoxia. These histological changes were associated with increased VEGF expression and decreased WISP-1 expression. VEGF is an important angiogenic factor in lung development and injury repair. It is mainly produced by alveolar type II epithelial cells and acts on its receptors expressed by capillary endothelial cells (45, 46). Interaction of VEGF with its receptors is not only important for promoting endothelial cell growth and remodeling; it is also essential for the appropriate development of alveolar structure (45, 46). Previous studies have demonstrated that VEGF therapy with recombinant protein or adenoviral-delivered gene promotes angiogenesis and alveolarization in hyperoxia-exposed neonatal rats (47–49). Although the exact mechanism by which LA1 treatment increases VEGF expression during hyperoxia is unknown, given that LA1 reduces lung inflammation and improves alveolar structure under hyperoxia, we speculate that the increased VEGF expression in the LA1-treated hyperoxic group is secondary to reduced lung epithelial cell injury. The increased VEGF expression could be beneficial for improved vascular and alveolar development.
The Wnt/β-catenin signaling pathway is a developmentally active pathway that has been linked to the pathogenesis of BPD (26, 28, 50, 51). WISP-1 is a target gene of Wnt/β-catenin signaling that plays a critical role in lung epithelial to mesenchymal transition, ventilator-induced lung injury, and lung fibrosis (52–54). We have previously shown that hyperoxia exposure increases WISP-1 expression in neonatal rat lungs, and inhibition of Wnt/β-catenin signaling down-regulates WISP-1 expression and decreases pulmonary vascular remodeling and PH in these rats (55). In this study, LA1 treatment decreased WISP-1 expression in hyperoxia-exposed lungs, and this may underlay the effect of LA1 on protecting against hyperoxia-induced pulmonary vascular remodeling.
In summary, this study demonstrates the beneficial effects of LA1 on preventing the lung inflammatory response, improving alveolarization and vascular development, and reducing pulmonary vascular remodeling and PH in a hyperoxia-induced experimental model of BPD. We also showed that LA1 does not affect the circulating immune cell response upon hyperoxia exposure. LA1, a small molecule agonist of leukocyte surface integrins CD11b/CD18, has thus far been studied in very few experimental models of inflammatory disorders. It is more effective in blocking leukocyte influx as compared with CD11b/CD18 antibodies (22). Leukocyte influx plays an important role in lung inflammation, which is key in the pathogenesis of BPD. Therefore, LA1 may be used as a novel therapeutic modality for managing preterm infants with BPD. However, future work is needed to explore the exact mechanisms by which LA1 prevents and treats experimental BPD and to reveal the potential side effects.
Footnotes
This work was supported by National Institutes of Health grants DK084195 and HL109582 (V.G.), by Project Newborn from the University of Miami (S.W.), and by a Micah Batchelor Award from the Batchelor Foundation (S.W.).
Author Contributions: Conception and design of the study: J.J., M.H.F., V.G., and S.W. Acquisition, analysis, and interpretation of data: J.J., J.K., M.R., S.C., D.H., S.H., and S.W.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0422OC on April 24, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari A, Bhandari V. Pitfalls, problems, and progress in bronchopulmonary dysplasia. Pediatrics. 2009;123:1562–1573. doi: 10.1542/peds.2008-1962. [DOI] [PubMed] [Google Scholar]
- 3.Thebaud B, Abmen SH. Bronchpulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenmark KR, Abman SH. Lung vascular development: implications for pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 5.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006;11:354–362. doi: 10.1016/j.siny.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Bose CL, Dammann CE, Laughon MM. BPD and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93:F455–F461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 7.Wright CJ, Kirpalani H. Targeting inflammation to prevent bronchopulmonary dysplasia: can new insights be translated into therapies. Pediatrics. 2011;128:111–126. doi: 10.1542/peds.2010-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambalavanan N, Schelonka RL, Dimmitt RA, Carlo WA, Munoz-Hernandez B, Das A, McDonald SA, Thorsen P, Skogstrand K, Hougaard DM, et al. Eunice Kennedy Shriver National Institute of Child Health and Human development Neonatal Research Network. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–1141. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jónsson B, Tullus K, Brauner A, Lu Y, Noack G. Early increase of TNF alpha and IL-6 in tracheobronchial aspirate fluid indicator of subsequent chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997;77:F198–F201. doi: 10.1136/fn.77.3.f198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1099–L1110. doi: 10.1152/ajplung.00217.2007. [DOI] [PubMed] [Google Scholar]
- 11.Hilgendorff A, Parai K, Ertsey R, Juliana Rey-Parra G, Thébaud B, Tamosiuniene R, Jain N, Navarro EF, Starcher BC, Nicolls MR, et al. Neonatal mice genetically modified to express the elastase inhibitor elafin are protected against the adverse effects of mechanical ventilation on lung growth. Am J Physiol Lung Cell Mol Physiol. 2012;303:L215–L227. doi: 10.1152/ajplung.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis. 1984;130:817–821. doi: 10.1164/arrd.1984.130.5.817. [DOI] [PubMed] [Google Scholar]
- 13.LeFlore JL, Salhab WA, Broyles RS, Engle WD. Association of antenatal and postnatal dexamethasone exposure with outcome in extremely low bith weight neoanates. Pediatrics. 2002;110:275–279. doi: 10.1542/peds.110.2.275. [DOI] [PubMed] [Google Scholar]
- 14.Halliday H, Ehrenkranz R, Doyle L. Early (< 8d) postnatal corticosteroids for preventing BPD in preterm infants. Cochrane Database Syst Rev. 2009;(1):CD001146. doi: 10.1002/14651858.CD001146.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, Shankaran S, Finer NN, Van Meurs KP, Engle WA, Das A Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months' adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics. 2009;123:E430–E437. doi: 10.1542/peds.2008-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 17.Li R, Arnaout MA. Functional analysis of the beta 2 integrins. Methods Mol Biol. 1999;129:105–124. doi: 10.1385/1-59259-249-X:105. [DOI] [PubMed] [Google Scholar]
- 18.Mayadas TN, Cullere X. Neutrophil beta2 integrins: moderators of life or death decisions. Trends Immunol. 2005;26:388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Simon DI, Dhen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faridi MH, Maiguel D, Barth CJ, Stoub D, Day R, Schürer S, Gupta V. Identification of novel agonists of the integrin CD11b/CD18. Bioorg Med Chem Lett. 2009;19:6902–6906. doi: 10.1016/j.bmcl.2009.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiguel D, Faridi MH, Wei C, Kuwano Y, Balla KM, Hernandez D, Barth CJ, Lugo G, Donnelly M, Nayer A, et al. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci Signal. 2011;4:ra57. doi: 10.1126/scisignal.2001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faridi MH, Altintas MM, Gomez C, Duque JC, Vazquez-Padron RI, Gupta V. Small molecule agonists of integrin CD11b/CD18 do not induce global conformational changes and are significantly better than activating antibodies in reducing vascular injury. Biochim Biophys Acta. 2013;1830:3696–3710. doi: 10.1016/j.bbagen.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. 2008;13:6653–6661. doi: 10.2741/3179. [DOI] [PubMed] [Google Scholar]
- 24.Bonikos DS, Bensch KG, Ludwin SK, Northway WH. Oxygen toxicity in the newborn: the effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab Invest. 1975;32:619–635. [PubMed] [Google Scholar]
- 25.Buczynski B, Maduekwe ET, O'Reilly MA. The role of hyperoxia in the pathogenesis of experimental BPD. Semin Perinatol. 2013;37:69–78. doi: 10.1053/j.semperi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alapati D, Rong M, Chen S, Hehre D, Rodriguez MM, Lipson KE, Wu S. Connective tissue growth factor antibody therapy attenuates hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol. 2011;45:1169–1177. doi: 10.1165/rcmb.2011-0023OC. [DOI] [PubMed] [Google Scholar]
- 27.Hummler SC, Rong M, Chen S, Hehre D, Alapati D, Wu S. Targeting GSK-3β to prevent hyperoxia-induced lung injury in neonatal rats. Am J Respir Cell Mol Biol. 2013;48:578–588. doi: 10.1165/rcmb.2012-0383OC. [DOI] [PubMed] [Google Scholar]
- 28.Alapati D, Rong M, Chen S, Hehre D, Hummler SC, Wu S. Inhibition of β-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental BPD. Am J Respir Cell Mol Biol. 2014;51:104–113. doi: 10.1165/rcmb.2013-0346OC. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Rong M, Platteau A, Hehre D, Ruiz P, Whitsett J, Bancalari B, Wu S. CTGF disrupts alveolarization and induces pulmonary hypertension in neonatal mice: implication in the pathogenesis of severe bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2011;300:L330–L340. doi: 10.1152/ajplung.00270.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 31.Jobe AL. The new BPD: an arrest of lung development. Pediatr Res. 1999;46:641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 33.Ryan RM, Ahmed Q, Lakshminrusimha S. Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol. 2008;34:174–190. doi: 10.1007/s12016-007-8031-4. [DOI] [PubMed] [Google Scholar]
- 34.Hilgendroff A, Reiss I, Ehrardt H, Eickelberg O, Alvira CM. Chronic lung disease in the preterm infant: lessons learned from animal models. Am J Respir Cell Mol Biol. 2014;50:233–245. doi: 10.1165/rcmb.2013-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groneck P, Gotze-Speer B, Oppermann M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm infants. Pediatrics. 1994;93:712–718. [PubMed] [Google Scholar]
- 36.Kim BI, Lee HE, Choi CW, Jo HS, Choi EH, Koh YY, Choi JH. Increased in cord blood soluble E-selectin and tracheal aspirate neutropils at birth and the development of new bronchopulmonary dysplasia. J Perinat Med. 2004;3293:282–287. doi: 10.1515/JPM.2004.053. [DOI] [PubMed] [Google Scholar]
- 37.Kotecha S, Silverman M, Shaw RJ, Klein N. Soluble L-selectin concentration in bronchoalveolar lavage fluid obtained from infants who develop chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 1998;78:F143–F147. doi: 10.1136/fn.78.2.f143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nold MF, Mangan NM, Rudloff I, Cho SX, Shariatian N, Samarasinghe TD. Skuza EM, Pedersen J, Veldman A, Berger PJ, et al. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proc Natl Acad Sci USA. 2013;110:14384–14389. doi: 10.1073/pnas.1306859110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auten RL, Mason SN, Tanaka DT, Welty-Wolf K, Whorton MH. Anti-neutropil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L336–L344. doi: 10.1152/ajplung.2001.281.2.L336. [DOI] [PubMed] [Google Scholar]
- 40.Deng H, Mason SN, Auton RL. Lung inflammation in hyperoxia can be prevented by antichemokine treatment in newborn rats. Am J Respir Crit Care Med. 2000;162:2316–2323. doi: 10.1164/ajrccm.162.6.9911020. [DOI] [PubMed] [Google Scholar]
- 41.Vozzelli MA, Mason SN, Whorton MH, Auton RL. Antimacrophage chemokine treatment prevents neutropil and macrophage influx in hyperoxia-exposed newborn rats. Am J Physiol Lung Cell Mol Physiol. 2004;286:L488–L493. doi: 10.1152/ajplung.00414.2002. [DOI] [PubMed] [Google Scholar]
- 42.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutsko RP, Young KC, Ribeiro A, Torres E, Rodriguez M, Hehre D, Devia C, McNiece I, Suguihara C. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res. 2013;73:46–53. doi: 10.1038/pr.2012.152. [DOI] [PubMed] [Google Scholar]
- 45.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 46.McGrath-Morrow SA, Cho C, Cho C, Zhen L, Hicklin DJ, Tuder RM. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol. 2005;32:420–427. doi: 10.1165/rcmb.2004-0287OC. [DOI] [PubMed] [Google Scholar]
- 47.Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol. 1997;16:557–567. doi: 10.1165/ajrcmb.16.5.9160838. [DOI] [PubMed] [Google Scholar]
- 48.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1068–L1078. doi: 10.1152/ajplung.00093.2006. [DOI] [PubMed] [Google Scholar]
- 49.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia- induced lung injury: evidence that angiogenesis participates alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 50.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rahan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-(beta) and Wnt signaling and is protected by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1031–L1041. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popova AP, Bentley JK, Anyanwu AC, Richardson MN, Linn MJ, Lei J, Wong EJ, Goldsmith AM, Pryhuber GS, Hershenson MB. Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;303:L439–L448. doi: 10.1152/ajplung.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heise RL, Stober V, Chelucaraju C, Hollingsworth JW, Garrantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011;286:17435–17444. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li HH, Li Q, Liu P, Liu Y, Wasserloos K, Chao W, You M, Oury TD, Chhinder S, Hackam DJ, et al. WNT1-inducible signaling pathway protein 1 contributes to ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2012;47:528–535. doi: 10.1165/rcmb.2012-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seegar W, Schaefer L, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alapathi D, Rong M, Chen S, Lin C, Li Y, Wu S. Inhibition of LRP5/6-mediated Wnt/β-catenin signaling by Mesd attenuates hyperoxia-induced pulmonary hypertension in neonatal rats. Pediatr Res. 2013;73:719–725. doi: 10.1038/pr.2013.42. [DOI] [PubMed] [Google Scholar]