Abstract
Virus-induced exacerbations often lead to further impairment of lung function in chronic obstructive pulmonary disease. IL-15 is critical in antiviral immune responses. Retinoic acid (RA) signaling plays an important role in tissue maintenance and repair, particularly in the lung. We studied RA signaling and its relation to IL-15 in the lung during cigarette smoke (CS) exposure and influenza virus infection. In vivo studies show that RA signaling is diminished by long-term CS exposure or influenza virus infection alone, which is further attenuated during infection after CS exposure. RA receptor β (RARβ) is specifically decreased in the lung of IL-15 transgenic (overexpression; IL-15Tg) mice, and a greater reduction in RARβ is found in these mice compared with wild-type (WT) mice after infection. RARβ is increased in IL-15 knockout (IL-15KO) mice compared with WT mice after infection, and the additive effect of CS and virus on RARβ down-regulation is diminished in IL-15KO mice. IL-15 receptor α (IL-15Rα) is increased and RARβ is significantly decreased in lung interstitial macrophages from IL-15Tg mice compared with WT mice. In vitro studies show that IL-15 down-regulates RARβ in macrophages via IL-15Rα signaling during influenza virus infection. These studies suggest that RA signaling is significantly diminished in the lung by CS exposure and influenza virus infection. IL-15 specifically down-regulates RARβ expression, and RARβ may play a protective role in lung injury caused by CS exposure and viral infections.
Keywords: IL-15, chronic obstructive pulmonary disease, retinoic acid receptor, cigarette smoke, influenza
Clinical Relevance
This work highlights the importance of the effects of cigarette smoke exposure and viral infection in modulating lung responses that include retinoic acid receptor pathways that are known to be important in tissue regeneration and repair. IL-15 is important in this process and affects specifically the down-regulation of retinoic acid receptor β, a potential therapeutic target for diseases such as chronic obstructive pulmonary disease.
Chronic obstructive pulmonary disease (COPD) is a major cause of chronic morbidity and mortality worldwide and is projected to become the third leading cause of death by 2020 (1). It is characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the lung to noxious particles or gases (2). Cigarette smoke (CS) exposure is the main cause of lung inflammation that induces parenchymal tissue destruction (resulting in emphysema) and disrupts normal repair and defense mechanisms (resulting in small airway fibrosis), leading to progressive airflow limitation (2, 3).
Respiratory virus infections are associated with up to 40 to 60% of COPD exacerbations (4, 5), and these exacerbations accelerate the decline in lung function, contribute to disease progression, and increase the risk of death (6, 7). As compared with nonviral exacerbations, virus-induced COPD exacerbations are associated with more severe symptoms, more frequent hospitalizations, and longer recovery periods (8, 9). A range of respiratory viruses has been shown to cause COPD exacerbations, among which the most common are rhinoviruses. However, in more severe exacerbations requiring hospitalization, influenza is more common (10). The mechanisms that mediate these viral exacerbations and their effects on the lung in CS exposure and COPD have not been adequately defined.
Previous studies from our laboratory and others have explored the effects of viral infections after CS exposure on the lung in mouse models (11–15). These studies demonstrated that CS and viruses interact in a manner to induce exaggerated pulmonary inflammation and accelerated emphysema and airway fibrosis (11). However, although almost all of these studies focused on the innate immune mechanisms (11–14), the possibility that other signaling pathways could also contribute to these effects has not been fully addressed. IL-15 is a proinflammatory cytokine that is expressed by epithelial cells and antigen-presenting cells (APCs), including macrophages and dendritic cells. IL-15 is important for the activation and proliferation of natural killer (NK) and CD8 T cells (16, 17) and is critical for the activation and function of APCs (18, 19). IL-15 is induced in respiratory virus infections and plays an important role in antiviral immune responses (20, 21); however, excessive IL-15 expression within tissue is associated with lung injury (20).
Retinoic acid (RA) signaling is critical in biological processes such as lung development (22, 23) and immune homeostasis (24, 25). It also plays an important role in tissue maintenance and repair, particularly in the lung (26, 27). RA is the main active metabolite of vitamin A, and many clinical studies have demonstrated a positive relationship between vitamin A status and lung function (28, 29). It was reported that CS exposure causes vitamin A depletion and that the deficiency of vitamin A induces the development of emphysema in rats (30, 31). Previous studies have also shown that RA treatment can promote the repair and or realveolarization of parenchymal lesions in animal models of emphysema (32–34). However, RA signaling and its relation to cytokines such as IL-15 in the lung during influenza virus infection after CS exposure have not been studied.
Here we show that RA signaling is diminished in the lung by long-term CS exposure or by influenza virus infection and is further attenuated during influenza virus infection after CS exposure. IL-15 specifically inhibits RA receptor β (RARβ) expression in vivo and in vitro in a dose-dependent manner, and RARβ may play a role in the lung inflammatory process caused by CS exposure and influenza virus infection.
Materials and Methods
Mice
C57BL/6 mice and IL-15 knockout (IL-15KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and Taconic (Hudson, NY), respectively. IL-15 transgenic (IL-15Tg) mice that use the Clara cell 10-kD protein promoter and reverse tetracycline transactivator to target IL-15 to the lung on a C57BL/6 background have been previously generated using approaches described by our laboratory (35). All animal studies were approved and were in accordance with the guidelines of the Yale Institutional Animal Care and Use Committee (IACUC).
CS Exposure
C57BL/6 mice and IL-15KO mice were exposed to room air/no smoking (NS) or to smoke from nonfiltered 3R4F research cigarettes (University of Kentucky, Lexington, KY) using the smoking apparatus and protocol previously described (11, 15, 36). During the first week, mice received a half cigarette twice a day to allow for acclimation, and thereafter they received one cigarette twice a day.
In Vivo Administration of Influenza Virus
After 1 month of NS/CS exposure or 2 to 4 weeks of oral doxycycline water treatment, the mice were lightly anesthetized, and 5.0 × 103.375 TCID50 (50% tissue culture infective doses) of A/PR8/34 (H1N1) influenza virus (equivalent to 0.05 LD50 [the individual dose required to kill 50% of a population of test animals] in C57BL/6 mice) was administered via nasal aspiration in 70 μl PBS using techniques previously described by our laboratory (37).
Flow Cytometry
Lung single-cell suspensions were prepared using the Lung Dissociation Kit (Miltenyi Biotec, Auburn, CA) according to the standard protocol previously described (38). Cells were incubated with anti-mouse CD16/CD32 (eBioscience, San Diego, CA) to reduce nonspecific binding. Staining reactions were performed at 4°C with anti-mouse F4/80 PE and anti-mouse CD11c APCs (eBioscience). Alveolar macrophages (AMs) and interstitial macrophages (IMs) were sorted by flow cytometry (BD FACSAria; BD Biosciences, San Jose, CA) based on their differential F4/80 and CD11c expression as previously described (39). For the analysis of IL-15 receptor α (IL-15Rα) expression, cells were stained with anti-mouse F4/80 PE, anti-mouse CD11c eFluor 450, and anti-mouse IL-15Rα APCs (eBioscience) and acquired on a BD FACS LSRII.
Cell Culture
RAW264.7 cells were purchased from American Type Culture Collection. Cells were cultured and treated with influenza virus, recombinant mouse IL-15 (rmIL-15) (R&D Systems, Minneapolis, MN), or anti–IL-15Rα antibody (Abcam, Cambridge, MA). Details are provided in the online supplement.
Quantitative PCR
Total RNA was isolated from the lung tissue, from sorted cells including IMs and AMs, and from RAW264.7 cells. Quantitative polymerase chain reaction (PCR) was performed using the specific primers for the retinaldehyde dehydrogenases (RALDHs), RA receptors (RARs), cytochrome P450 family 26 subfamily B polypeptide 1 (Cyp26b1), IL-15, and IL-15Rα. For microRNA-29b (miR-29b) analysis, specific TaqMan probe was used. Details are provided in the online supplement.
Western Blotting
Whole lung lysates and RAW264.7 cell lysates were prepared, and the total protein concentration was determined. Equal amounts of sample proteins were used for Western blot analysis. Details are provided in the online supplement.
Statistical Analysis
Results are reported as mean (±SEM) values unless otherwise specified. Student’s unpaired two-tailed t test was performed for all statistical analyses using GraphPad Prism 6. Differences between groups were considered significant when P < 0.05.
Results
CS Exposure Regulates RA Signaling Components in the Lung
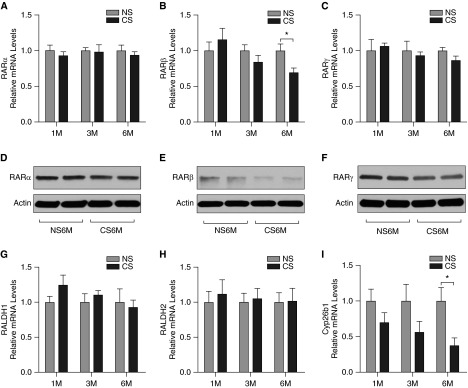
To test whether CS exposure would attenuate RA expression and signaling, we exposed C57BL/6 mice to NS or CS for 1 month, 3 months, or 6 months. The mRNA expression of the RA signaling components in the lung was measured using quantitative PCR including the key RA-synthesizing enzymes (RALDH1 and RALDH2), RARs (RARα, RARβ, and RARγ), and the major enzyme for RA metabolism (Cyp26b1). The protein level of the various RARs in the lung of mice exposed to NS or CS for 6 months was also assessed using Western blot. We found that 1-month and 3-month exposure to CS did not significantly alter expression of the RA signaling components in the lung (Figure 1). However, the mRNA expression of RARβ and Cyp26b1 was significantly decreased after 6-month exposure of CS compared with NS (Figures 1B and 1I). There were no significant changes in the expression of RARα, RARγ, RALDH1, and RALDH2 after CS exposure (Figures 1A, 1C, 1G, and 1H). Western blot analyses showed similar results in RAR expression patterns at the protein level in the lung of mice after 6-month exposure to CS (Figures 1D–1F).
Figure 1.
Cigarette smoke (CS) exposure regulates retinoic acid (RA) signaling components in the lung. C57BL/6 mice were exposed to room air/no smoking (NS) or CS for 1 month (1M), 3 months (3M), or 6 months (6M). The mRNA expression of the RA signaling components in the lung was measured using quantitative polymerase chain reaction (PCR). The relative mRNA levels of RA receptor (RAR) α (A), RARβ (B), RARγ (C), retinaldehyde dehydrogenase (RALDH) 1 (G), RALDH2 (H), and cytochrome P450 family 26 subfamily B polypeptide 1 (Cyp26b1) (I) are shown (n = 5 mice/group). The protein levels of RARα (D), RARβ (E), and RARγ (F) in the lung of mice exposed to NS or CS for 6 months were assessed using Western blotting. Data are representative of three experiments. *P < 0.05.
Influenza Virus Infection in the Setting of CS Exposure Down-Regulates RARβ
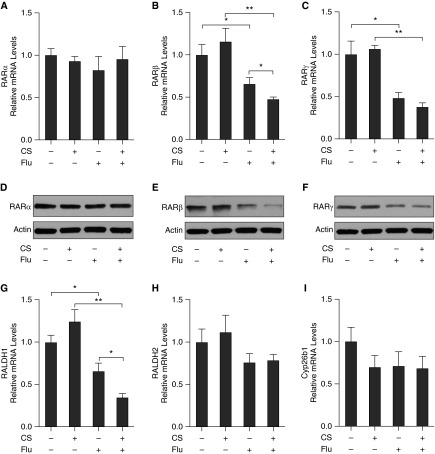
Given the importance of virus-induced exacerbations in COPD and that CS can enhance the inflammatory and remodeling effects of influenza virus (11–15), C57BL/6 mice were exposed to NS or CS for 1 month and then infected with influenza virus or vehicle control. On Day 7 after infection, total leukocyte counts in bronchoalveolar lavage fluid (BALF) and IL-15 protein levels by ELISA in lung tissue were determined. We found that the total leukocyte counts in BALF and IL-15 protein levels in lung tissue were increased after CS exposure or influenza virus infection; there was an additional increase in the levels of inflammation and IL-15 protein levels after dual exposure of CS and virus (see Figure E1 in the online supplement). The mRNA expression of the RA signaling components in the lung was measured using quantitative PCR, and the protein level of RARs in the lung was assessed using Western blot (Figure 2). We found that the mRNA expression of RARβ, RARγ, and RALDH1 in the lung was significantly decreased on Day 7 after infection with influenza virus (Figures 2B, 2C, and 2G). Importantly, dual exposure of CS and influenza virus resulted in further decrease in RARβ and RALDH1 expression (Figures 2B and 2G). There was no significant effect with dual exposure of CS and virus on RARγ expression in the lung (Figure 2C). There were also no significant changes in the expression of RARα, RALDH2, and Cyp26b1 in the lung of mice exposed to CS and/or influenza virus (Figures 2A, 2H, and 2I). Western blot analyses showed similar results in RAR expression at the protein level (Figures 2D–2F).
Figure 2.
Influenza virus infection in the setting of CS exposure down-regulates RARβ. C57BL/6 mice were exposed to CS for 1 month and then infected with influenza virus (Flu). On Day 7 after infection, the mRNA expression of the RA signaling components in the lung was measured using quantitative PCR. The relative mRNA levels of RARα (A), RARβ (B), RARγ (C), RALDH1 (G), RALDH2 (H), and Cyp26b1 (I) are shown (n = 5 mice/group). The protein levels of RARα (D), RARβ (E), and RARγ (F) in the lung were assessed using Western blotting. Data are representative of three experiments. *P < 0.05; **P < 0.01.
Overexpression of IL-15 in the Lung Results in Significant Repression of RARβ during Influenza Virus Infection
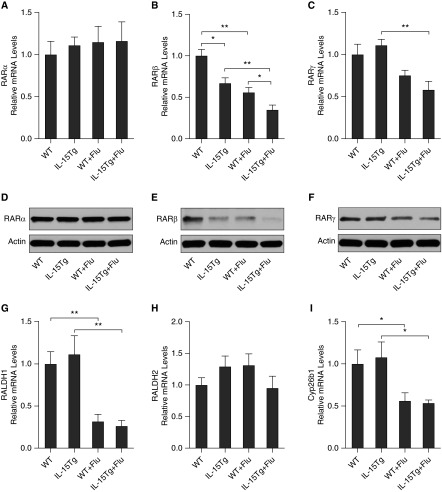
To study the role of IL-15 in the lung in our models, we used mice that overexpress IL-15 in the lung epithelium for the purpose of examining the effect of this cytokine during pulmonary viral infections. Wild-type (WT) and IL-15Tg mice were infected with influenza virus or vehicle control. On Day 7 after infection, total leukocyte counts in BALF and IL-15 levels in lung tissue were determined. We found increased leukocyte counts in BALF of IL-15Tg mice compared with control WT mice (Figure E2). Moreover, IL-15 levels in lung tissue were further increased in IL-15Tg mice compared with WT mice after influenza virus infection (Figure E2). The mRNA expression of the RA signaling components in the lung was measured using quantitative PCR, and the protein level of RARs in the lung was assessed using Western blot (Figure 3). We found that the mRNA expression of RARβ and RARγ was significantly decreased on Day 7 after infection with influenza virus (Figures 3B and 3C). RALDH1 and Cyp26b1 expressions were also decreased after infection (Figures 3G and 3I). Importantly, RARβ expression was specifically decreased in IL-15Tg lungs compared with WT controls, and there was a greater significant reduction in RARβ expression found in IL-15Tg mice compared with WT mice after influenza virus infection (Figure 3B). There were no significant changes in the expression of RARα and RALDH2 after infection in this modeling system (Figures 3A and 3H). Western blot analyses showed similar results in RARs at the protein level (Figures 3D–3F).
Figure 3.
Overexpression of IL-15 in the lung results in significant repression of RARβ during influenza virus (Flu) infection. Wild-type (WT) and IL-15 transgenic (IL-15Tg) mice were infected with Flu. On Day 7 after infection, the mRNA expression of the RA signaling components in the lung was measured using quantitative PCR. The relative mRNA levels of RARα (A), RARβ (B), RARγ (C), RALDH1 (G), RALDH2 (H), and Cyp26b1 (I) are shown (n = 3–5 mice/group). The protein levels of RARα (D), RARβ (E), and RARγ (F) in the lung were assessed using Western blotting. Data are representative of three experiments. *P < 0.05; **P < 0.01.
IL-15 Is Required for RARβ Down-Regulation in the Lung during CS Exposure and Influenza Virus Infection
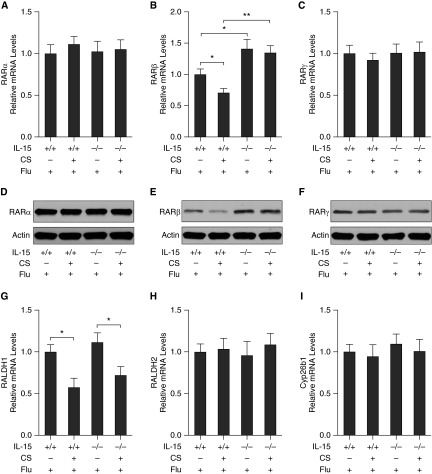
To determine the requirement of IL-15 in the RARβ down-regulation in the lung during CS exposure and influenza virus infection, WT and IL-15KO mice were exposed to NS or CS for 1 month and then infected with influenza virus. On Day 7 after infection, total leukocyte counts in BALF were determined. We found that the total leukocyte counts were significantly decreased in IL-15KO mice during influenza virus infection after NS or CS exposure compared with control WT mice (Figure E3). The mRNA expression of the RA signaling components in the lung was measured using quantitative PCR, and the protein level of RARs in the lung was assessed using Western blot (Figure 4). We found that the mRNA expression of RARβ was significantly increased in the lung of IL-15KO mice compared with WT mice on Day 7 after infection with influenza virus (Figure 4B). Importantly, the effect of dual exposure to CS and virus on RARβ down-regulation was significantly diminished in IL-15KO mice (Figure 4B). There were no significant changes in the expression of RARα, RARγ, RALDH1, RALDH2, and Cyp26b1 in the lung of IL-15KO mice compared with WT mice after infection or after dual exposure to CS and virus (Figures 4A, 4C, and 4G–4I). Western blot analyses showed similar results in RARs expression at the protein level (Figures 4D–4F).
Figure 4.
IL-15 is required for RARβ down-regulation in the lung during CS exposure and influenza virus infection. WT and IL-15 knockout mice were exposed to NS or CS for 1 month and then infected with Flu. On Day 7 after infection, the mRNA expression of the RA signaling components in the lung was measured using quantitative PCR. The relative mRNA levels of RARα (A), RARβ (B), RARγ (C), RALDH1 (G), RALDH2 (H), and Cyp26b1 (I) are shown (n = 5 mice/group). The protein levels of RARα (D), RARβ (E), and RARγ (F) in the lung were assessed using Western blotting. Data are representative of three experiments. *P < 0.05; **P < 0.01.
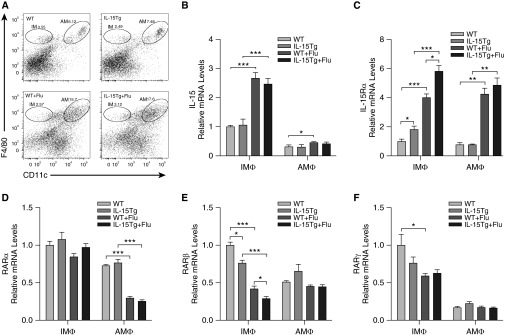
Increased IL-15 and IL-15Rα in Lung Interstitial Macrophages Promotes Down-Regulation of RARβ
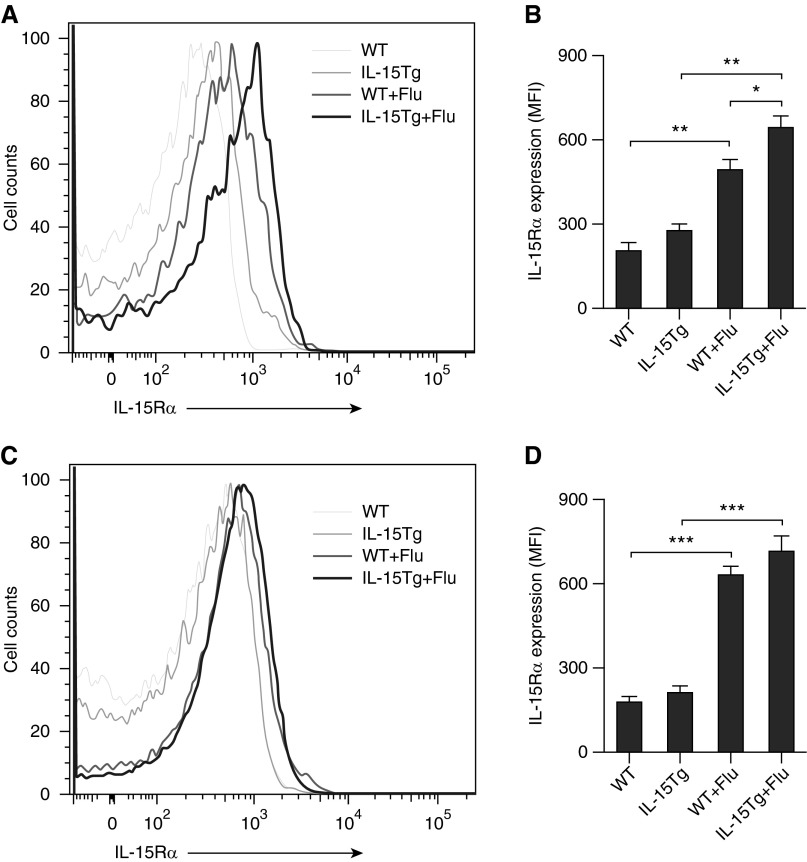
Given that the predominant cell population after viral infections is macrophages, we focused on studying the role of IL-15 and IL-15Rα and their effects on RARs in subpopulations of lung macrophages. WT and IL-15Tg mice were infected with influenza virus or vehicle control. On Day 7 after infection, lung IMs (F4/80+ CD11c−) and AMs (F4/80+ CD11c+) were isolated using flow cytometry, and the mRNA expression of IL-15, IL-15Rα, and RARs in these two cell populations was measured using quantitative PCR (Figure 5). Despite the fact there was a net increase in the absolute numbers of total lung macrophages (AMs and IMs) in infected IL-15Tg compared with infected WT mice (data not shown), only the proportion of AMs was increased after influenza virus infection in the IL-15Tg lungs, whereas there were no significant changes in the proportion of IMs in the lung (Figure 5A). The proportion of AMs in the lung of IL-15Tg mice was higher compared with WT after infection (18.5 ± 1.0% versus 15.0 ± 0.7%; P < 0.05). IL-15 and IL-15Rα expression was significantly increased in the lung IMs from WT or IL-15Tg mice after influenza virus infection, and IL-15Rα expression in IMs from IL-15Tg mice was higher compared with WT after infection (Figures 5B and 5C). On the contrary, the expression of RARβ and RARγ was significantly decreased after infection, and specifically RARβ expression in IMs from IL-15Tg mice was lower compared with WT after infection (Figures 5E and 5F). There were no significant changes in the expression of RARα in the lung IMs after influenza virus infection (Figure 5D). In the AMs, IL-15 and IL-15Rα expression was also significantly increased after infection; however, there was no significant difference in IL-15Rα expression between IL-15Tg and WT mice after infection (Figures 5B and 5C). The expression of RARα was significantly decreased in the lung AMs after infection, whereas there were no significant changes in RARβ and RARγ expression (Figures 5D–5F). In addition, IL-15Rα expression on IMs and AMs in the lung was also analyzed by flow cytometry. We found similar results as the mRNA expression (Figures 6A–6D).
Figure 5.
Increased IL-15 and IL-15 receptor α (IL-15Rα) in lung interstitial macrophages promotes down-regulation of RARβ. WT and IL-15Tg mice were infected with Flu. (A) On Day 7 after infection, lung interstitial macrophages (IMs) and alveolar macrophages (AMs) were isolated using flow cytometry. (B–F) The mRNA expression of IL-15 (B), IL-15Rα (C), RARα (D), RARβ (E), and RARγ (F) in the lung IMs and AMs was measured using quantitative PCR. Data are representative of three experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 6.
IL-15Rα expression on lung macrophages is increased during influenza virus infection. WT and IL-15Tg mice were infected with Flu. On Day 7 after infection, lung single-cell suspensions were prepared. Cells were incubated with anti-mouse F4/80, anti-mouse CD11c, and anti-mouse IL-15Rα and analyzed by flow cytometry. Representative flow cytometry histograms and mean fluorescence intensity (MFI) of IL-15Rα expression on lung interstitial macrophages (A and B) and alveolar macrophages (C and D) are shown. Data are representative of three experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
IL-15Rα Is Required for RARβ Down-Regulation in Macrophages by Influenza Virus or Recombinant IL-15
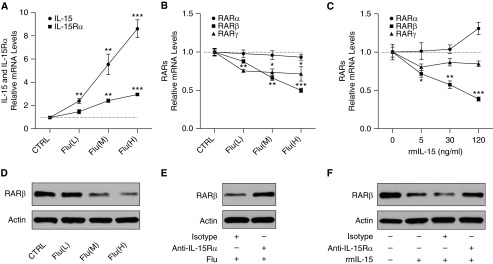
To determine the dose–response effects of influenza virus on RARs, RAW264.7 macrophages were treated with influenza virus at three different dosages for 24 hours. The mRNA expression of IL-15, IL-15Rα, and RARs was measured using quantitative PCR, and the protein levels of RARβ were also assessed using Western blot. We found that the expression of IL-15 and IL-15Rα was significantly increased with the stimulation of influenza virus in a dose-dependent manner (Figure 7A). The RARβ and RARγ expression was significantly decreased after virus treatment, whereas no changes were observed in RARα expression (Figure 7B). Importantly, influenza virus inhibited the expression of RARβ in a dose-dependent manner (Figures 7B and 7D).
Figure 7.
IL-15Rα is required for RARβ down-regulation in macrophages by influenza virus or recombinant IL-15. RAW264.7 macrophages were cultured without influenza virus (control [CTRL]) or with virus at low multiplicity of infection (MOI) [Flu(L)], medium MOI [Flu(M)], and high MOI [Flu(H)] for 24 hours. The mRNA expression of IL-15 and IL-15Rα (A) and RARs (B) was measured using quantitative PCR, and the protein levels of RARβ (D) were assessed using Western blot. Cells were cultured with recombinant mouse IL-15 (rmIL-15) at 0, 5, 30, and 120 ng/ml for 24 hours. The mRNA expression of RARs (C) was measured. Cells were preincubated with 20 μg/ml anti–IL-15Rα antibody or isotype control for 30 minutes and then treated with influenza virus at high MOI or rmIL-15 at 120 ng/ml for 24 hours. RARβ (E and F) was assessed using Western blot. Data are representative of three experiments. *P < 0.05; **P < 0.01; ***P < 0.001 compared with controls.
To determine the dose–response effects of IL-15 on RARs, RAW264.7 macrophages were treated with rmIL-15 at three different dosages for 24 hours. The expression of RARs in these cells was measured using quantitative PCR. We found that the expression of RARβ was inhibited by rmIL-15 in a dose-dependent manner, whereas there were no significant changes in RARα and RARγ expression after treatment with rmIL-15 (Figure 7C). IL-15 and IL-15Rα signaling is critical for the activation of APCs, including macrophages and dendritic cells, upon microbial infection (18, 19). Blocking IL-15Rα by using anti–IL-15Rα antibody decreased IL-15–mediated RANTES (chemokine ligand 5) and cytokines production in these cells (19). RANTES and TNF-α levels in the culture supernatant were also detected via ELISA. Both of these cytokines were increased by the stimulation of rmIL-15 in a dose-dependent manner (Figures E4A and E4B).
To determine the requirement of IL-15Rα in the down-regulation of RARβ by IL-15 or influenza virus, RAW264.7 macrophages were preincubated with IL-15Rα–blocking antibody and then treated with influenza virus or rmIL-15. The protein levels of RARβ were assessed using Western blot. We found that IL-15Rα blocking resulted in increased RARβ expression in these cells treated with influenza virus or rmIL-15 (Figures 7E and 7F).
Previous studies have suggested that RARβ reduction is mainly caused by DNA hypermethylation (40) and that IL-15 can regulate expression of DNA methyltransferase 3b (Dnmt3b) via the repression of miR-29b (41). Therfore, we also measured the miR-29b expression using quantitative PCR in RAW264.7 macrophages treated with rmIL-15. We found that miR-29b expression was also inhibited by rmIL-15 in a dose-dependent manner (Figure E4C). In addition, IL-15Rα blocking resulted in increased miR-29b expression in these cells treated with rmIL-15 (Figure E4D).
Discussion
COPD is characterized by an imbalance between tissue inflammation, injury, and repair that ultimately results in the progressive destruction of pulmonary parenchyma (42).
The disruption of normal repair mechanisms also causes airway fibrosis in COPD (2). Previous studies have suggested that RA signaling plays an important role in tissue maintenance and repair processes in the lung (26). In this study, we show that RARβ expression is significantly reduced in the lung after long-term CS exposure, which is consistent with the findings from previous related studies (43, 44). These results support the hypothesis that the inhibition of alveolar repair by CS is one of the mechanisms for the development of emphysema (42).
Enhanced morbidity in virus-infected and second-hand smoke–exposed children and enhanced disease severity in virus-infected normal smokers have been described clinically (45, 46). These clinical findings suggest that the interactions between CS exposure and viral infections play important roles in clinical scenarios that include virus-induced COPD exacerbations, which have become important clinical parameters in understanding the pathogenesis of COPD. Studies from our laboratory and others have demonstrated that CS and viruses interact in a manner to induce exaggerated inflammatory, emphysema-like, and airway fibrotic changes in animal CS exposure and infection models (11–15). Studying the mechanisms of how CS exposure and viral infections interact in the lung and affect these pulmonary tissue changes will provide potentially important therapeutic target for diseases such as COPD. Our work described here shows that RARβ and RALDH1 expression are diminished in the lung after influenza virus infection and are further attenuated after dual exposure to CS and influenza virus, a relevant and common scenario in COPD. This pattern of down-regulation was not observed in the other subtypes of RARs. Our results additionally suggest that the RA signaling–mediated repair pathway is further inhibited by the combination of CS exposure and influenza virus infection, which could worsen the imbalance between tissue injury and repair in the COPD lung and could ultimately lead to the exaggerated emphysema and airway fibrosis during virus-induced COPD exacerbations.
Here we show that IL-15 is induced in the lung by CS exposure or influenza virus infection alone and is further increased by the combination of CS and influenza virus in the mouse models and in vitro cell exposure systems and that the increase in IL-15 level that is observed with CS exposure and virus infection is associated with increased lung inflammation. Moreover, similar exaggerated lung inflammation can be observed in IL-15Tg mice after influenza virus infection. In the present study, by using a IL-15Tg mouse model of influenza virus infection, we also show that RARβ expression is specifically and significantly decreased in the lung of IL-15Tg mice compared with WT mice. A greater reduction in RARβ expression is found in IL-15Tg mice compared with WT mice after influenza virus infection. In addition, by using IL-15KO mice, we show that RARβ is significantly increased in the lung of IL-15KO mice compared with WT mice after infection and that the effect of dual exposure to CS and virus on RARβ down-regulation is significantly diminished in IL-15KO mice. These results suggest that IL-15 regulates RA signaling in the lung, as shown by its ability to significantly inhibit RARβ expression during CS exposure and influenza virus infection. The impairment of this repair signal is associated with the exaggerated inflammatory lung injury.
Macrophages are highly heterogeneous based on their anatomical location, specialized function, and activation state (47). Lung macrophages play a central role in the development and disease progression of COPD (48). AMs and IMs represent the two main lung macrophage subsets, which are localized in distinct anatomical compartments in the lung, the air spaces, and lung connective tissue, respectively (49, 50). AMs have been described in detail (47), but IMs have not yet been fully characterized, and their in vivo function remains unknown. A number of studies have suggested that IMs are an intermediary stage in the maturation of AMs. There is also evidence that AMs and IMs are distinct cell populations with differing functions and that each population contributes to different inflammatory and immune responses in the lung (47). However, because IMs are in direct contact with the lung matrix and other pulmonary connective tissue components, the release of mediators or enzymes by these cells may have greater biological and/or pathological effects than those released by macrophages in the alveolar compartment.
Previous studies have demonstrated that RA signaling decreases the matrix metalloproteinases and increases the tissue inhibitors of metalloproteinases in macrophages and peripheral blood mononuclear cells (51, 52). In the present study, IL-15 and IL-15Rα expression was significantly induced in lung IMs from WT and IL-15Tg mice after influenza virus infection. IL-15Rα expression was further increased in the lung IMs from IL-15Tg mice compared with WT mice after infection. Expression of IL-15 in AMs is not as robust, but IL-15Rα can be induced with influenza virus infection in both WT and IL-15Tg mice. Interestingly, the expression of RARβ is down-regulated in lung IMs after infection, and a greater reduction in RARβ expression is found in the lung IMs from IL-15Tg mice compared with WT mice after infection. These results suggest that the RARβ-mediated RA signal is further diminished in the lung IMs from IL-15Tg mice after influenza virus infection. Given the important role of RARβ in tissue repair, a decrease in the RARβ-mediated RA signal with IL-15 and IL-15Rα expression and influenza virus infection could lead to the further release of matrix metalloproteinases from macrophages and could contribute to lung tissue destruction, as is often seen in COPD.
In the in vitro studies, we demonstrate that influenza virus infection increases the IL-15 and IL-15Rα expression and inhibits the RARβ expression in macrophages in a dose-dependent manner. Previous studies have shown that IL-15 and IL-15Rα signaling is critical for the activation of APCs, including macrophages and dendritic cells, upon microbial infection (18, 19). IL-15Rα knockdown or blocking with antibody decreases IL-15–mediated RANTES production in these cells (19). Here we show that the RANTES and TNF-α are induced in macrophages treated with IL-15 in a dose-dependent manner and that IL-15 treatment specifically reduces the RARβ expression in a dose-dependent manner. We also demonstrate that blocking IL-15Rα results in increased RARβ expression in macrophages treated with influenza virus or IL-15. These results suggest that IL-15 down-regulates RARβ expression in macrophages via IL-15Rα during influenza virus infection.
RARβ is unique among its family members because its gene expression is lost during early development in a variety of tumors. A number of studies have demonstrated its unique physiological role among the RAR subtypes as a tumor repressor protein (53). The aberrant methylation of CpG islands is an epigenetic change that induces the transcriptional silencing of tumor suppressor genes, such as the RARβ gene. It has been reported that RARβ expression is lost or reduced in a large percentage of patients with lung cancer and in a population at high risk of lung cancer (54, 55). The hypermethylation of RARβ gene is considered a major cause of the loss of RARβ expression (40), and Dnmt3b has been reported to be a direct, negatively regulated target of miR-29b (56). Previous studies have also demonstrated that IL-15 represses miR-29b via induction of Myc/NF-κBp65/Hdac-1, resulting in Dnmt3b overexpression and DNA hypermethylation (41). Here we show that miR-29b is inhibited in a dose-dependent manner by IL-15 in macrophages that are dependent on IL-15Rα, suggesting that IL-15 may down-regulate RARβ expression in this fashion.
Given the beneficial effects of RA in animal models of emphysema, the therapeutic potential of RA and RARγ agonist were evaluated in human patients with emphysema that were mostly focused on patients with α1-antitrypsin deficiencies (57, 58). Unfortunately, both of these trials failed to show significance of the benefit on the primary outcomes of lung function and density. Our results indicate that the potentially protective RARβ is significantly down-regulated during CS exposure and with influenza virus infection. Given the importance of RARβ in mediating the repair signaling during lung injury, we report that RARβ is significantly inhibited during CS exposure, influenza virus infection, and/or IL-15 expression and may play an important protective role in the virus-induced COPD exacerbations. Because RARβ is suppressed in COPD, particularly during viral exacerbations, agents that could reverse the silencing of RARβ could prove to be important at restoring this protective lung response.
Footnotes
This work was supported by National Institutes of Health grant HL-103770, by Flight Attendant Medical Research Institute (FAMRI) YCSA, by the Clinical and Translational Science Award grant UL1 RR024139 from the National Center for Research Resources, and by a component of the National Institutes of Health and National Institutes of Health roadmap for Medical Research.
Author Contributions: Conception and design: J.W., C.G.L., J.A.E., and C.S.D.C. Experiments: J.W., W.L., C.M., R.S., and N.A. Analysis and interpretation: J.W. and C.S.D.C. Drafting the manuscript for important intellectual content: J.W. and C.S.D.C.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0448OC on April 29, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 3.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 4.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 5.Mallia P, Johnston SL. How viral infections cause exacerbation of airway diseases. Chest. 2006;130:1203–1210. doi: 10.1378/chest.130.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade TW, Jeffries DJ, Johnston SL, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 9.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 10.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 11.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins CS, Bauer CM, Vujicic N, Gaschler GJ, Lichty BD, Brown EG, Stämpfli MR. Cigarette smoke impacts immune inflammatory responses to influenza in mice. Am J Respir Crit Care Med. 2006;174:1342–1351. doi: 10.1164/rccm.200604-561OC. [DOI] [PubMed] [Google Scholar]
- 13.Motz GT, Eppert BL, Wortham BW, Amos-Kroohs RM, Flury JL, Wesselkamper SC, Borchers MT. Chronic cigarette smoke exposure primes NK cell activation in a mouse model of chronic obstructive pulmonary disease. J Immunol. 2010;184:4460–4469. doi: 10.4049/jimmunol.0903654. [DOI] [PubMed] [Google Scholar]
- 14.Wortham BW, Eppert BL, Motz GT, Flury JL, Orozco-Levi M, Hoebe K, Panos RJ, Maxfield M, Glasser SW, Senft AP, et al. NKG2D mediates NK cell hyperresponsiveness and influenza-induced pathologies in a mouse model of chronic obstructive pulmonary disease. J Immunol. 2012;188:4468–4475. doi: 10.4049/jimmunol.1102643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Kang MJ, Jha BK, Silverman RH, Lee CG, Elias JA. Role of ribonuclease L in viral pathogen-associated molecular pattern/influenza virus and cigarette smoke-induced inflammation and remodeling. J Immunol. 2013;191:2637–2646. doi: 10.4049/jimmunol.1300082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 18.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 19.Chenoweth MJ, Mian MF, Barra NG, Alain T, Sonenberg N, Bramson J, Lichty BD, Richards CD, Ma A, Ashkar AA. IL-15 can signal via IL-15Rα, JNK, and NF-κB to drive RANTES production by myeloid cells. J Immunol. 2012;188:4149–4157. doi: 10.4049/jimmunol.1101883. [DOI] [PubMed] [Google Scholar]
- 20.Zdrenghea MT, Mallia P, Johnston SL. Immunological pathways in virus-induced COPD exacerbations: a role for IL-15. Eur J Clin Invest. 2012;42:1010–1015. doi: 10.1111/j.1365-2362.2012.02672.x. [DOI] [PubMed] [Google Scholar]
- 21.Dela Cruz CS, Kang MJ, Liu W, Lee CG, Elias JA. Interleukin-15 and influenza virus infection in a mouse model of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2012;9:85. [Google Scholar]
- 22.Chen F, Cao Y, Qian J, Shao F, Niederreither K, Cardoso WV. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J Clin Invest. 2010;120:2040–2048. doi: 10.1172/JCI40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, Marquez H, Kim YK, Qian J, Shao F, Fine A, Cruikshank WW, Quadro L, Cardoso WV. Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Invest. 2014;124:801–811. doi: 10.1172/JCI70291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pino-Lagos K, Benson MJ, Noelle RJ. Retinoic acid in the immune system. Ann N Y Acad Sci. 2008;1143:170–187. doi: 10.1196/annals.1443.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belloni PN, Garvin L, Mao CP, Bailey-Healy I, Leaffer D. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest. 2000;117(1 suppl):235S–241S. doi: 10.1378/chest.117.5_suppl_1.235s. [DOI] [PubMed] [Google Scholar]
- 27.Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci. 2004;359:799–808. doi: 10.1098/rstb.2004.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aird FK, Greene SA, Ogston SA, Macdonald TM, Mukhopadhyay S. Vitamin A and lung function in CF. J Cyst Fibros. 2006;5:129–131. doi: 10.1016/j.jcf.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Grievink L, Smit HA, Ocké MC, van ’t Veer P, Kromhout D. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax. 1998;53:166–171. doi: 10.1136/thx.53.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li T, Molteni A, Latkovich P, Castellani W, Baybutt RC. Vitamin A depletion induced by cigarette smoke is associated with the development of emphysema in rats. J Nutr. 2003;133:2629–2634. doi: 10.1093/jn/133.8.2629. [DOI] [PubMed] [Google Scholar]
- 31.Baybutt RC, Hu L, Molteni A. Vitamin A deficiency injures lung and liver parenchyma and impairs function of rat type II pneumocytes. J Nutr. 2000;130:1159–1165. doi: 10.1093/jn/130.5.1159. [DOI] [PubMed] [Google Scholar]
- 32.Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med. 1997;3:675–677. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 33.Hind M, Maden M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur Respir J. 2004;23:20–27. doi: 10.1183/09031936.03.00119103. [DOI] [PubMed] [Google Scholar]
- 34.Stinchcombe SV, Maden M. Retinoic acid induced alveolar regeneration: critical differences in strain sensitivity. Am J Respir Cell Mol Biol. 2008;38:185–191. doi: 10.1165/rcmb.2007-0252OC. [DOI] [PubMed] [Google Scholar]
- 35.Kang MJ, Choi JM, Kim BH, Lee CM, Cho WK, Choe G, Kim DH, Lee CG, Elias JA. IL-18 induces emphysema and airway and vascular remodeling via IFN-γ, IL-17A, and IL-13. Am J Respir Crit Care Med. 2012;185:1205–1217. doi: 10.1164/rccm.201108-1545OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Zhao MQ, Xu L, Ramana CV, Declercq W, Vandenabeele P, Enelow RI. Requirement for tumor necrosis factor-receptor 2 in alveolar chemokine expression depends upon the form of the ligand. Am J Respir Cell Mol Biol. 2005;33:463–469. doi: 10.1165/rcmb.2005-0204OC. [DOI] [PubMed] [Google Scholar]
- 38.Jungblut M, Oeltze K, Zehnter I, Hasselmann D, Bosio A. Standardized preparation of single-cell suspensions from mouse lung tissue using the gentleMACS dissociator. J Vis Exp. 2009;29:1266. doi: 10.3791/1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bedoret D, Wallemacq H, Marichal T, Desmet C, Quesada Calvo F, Henry E, Closset R, Dewals B, Thielen C, Gustin P, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J Clin Invest. 2009;119:3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virmani AK, Rathi A, Zöchbauer-Müller S, Sacchi N, Fukuyama Y, Bryant D, Maitra A, Heda S, Fong KM, Thunnissen F, et al. Promoter methylation and silencing of the retinoic acid receptor-beta gene in lung carcinomas. J Natl Cancer Inst. 2000;92:1303–1307. doi: 10.1093/jnci/92.16.1303. [DOI] [PubMed] [Google Scholar]
- 41.Mishra A, Liu S, Sams GH, Curphey DP, Santhanam R, Rush LJ, Schaefer D, Falkenberg LG, Sullivan L, Jaroncyk L, et al. Aberrant overexpression of IL-15 initiates large granular lymphocyte leukemia through chromosomal instability and DNA hypermethylation. Cancer Cell. 2012;22:645–655. doi: 10.1016/j.ccr.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc. 2006;3:703–708. doi: 10.1513/pats.200605-121SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 44.Liu C, Wang XD, Bronson RT, Smith DE, Krinsky NI, Russell RM. Effects of physiological versus pharmacological beta-carotene supplementation on cell proliferation and histopathological changes in the lungs of cigarette smoke-exposed ferrets. Carcinogenesis. 2000;21:2245–2253. doi: 10.1093/carcin/21.12.2245. [DOI] [PubMed] [Google Scholar]
- 45.Wilson KM, Pier JC, Wesgate SC, Cohen JM, Blumkin AK. Secondhand tobacco smoke exposure and severity of influenza in hospitalized children. J Pediatr. 2013;162:16–21. doi: 10.1016/j.jpeds.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 46.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 47.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70:163–170. [PubMed] [Google Scholar]
- 48.Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Fathi M, Johansson A, Lundborg M, Orre L, Sköld CM, Camner P. Functional and morphological differences between human alveolar and interstitial macrophages. Exp Mol Pathol. 2001;70:77–82. doi: 10.1006/exmp.2000.2344. [DOI] [PubMed] [Google Scholar]
- 50.Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004;1:59–70. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- 51.Frankenberger M, Hauck RW, Frankenberger B, Häussinger K, Maier KL, Heyder J, Ziegler-Heitbrock HW. All trans-retinoic acid selectively down-regulates matrix metalloproteinase-9 (MMP-9) and up-regulates tissue inhibitor of metalloproteinase-1 (TIMP-1) in human bronchoalveolar lavage cells. Mol Med. 2001;7:263–270. [PMC free article] [PubMed] [Google Scholar]
- 52.Mao JT, Tashkin DP, Belloni PN, Baileyhealy I, Baratelli F, Roth MD. All-trans retinoic acid modulates the balance of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with emphysema. Chest. 2003;124:1724–1732. doi: 10.1378/chest.124.5.1724. [DOI] [PubMed] [Google Scholar]
- 53.Alvarez S, Germain P, Alvarez R, Rodríguez-Barrios F, Gronemeyer H, de Lera AR. Structure, function and modulation of retinoic acid receptor beta, a tumor suppressor. Int J Biochem Cell Biol. 2007;39:1406–1415. doi: 10.1016/j.biocel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, Martinet N, Vignaud JM. Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:1059–1066. doi: 10.1093/jnci/91.12.1059. [DOI] [PubMed] [Google Scholar]
- 55.Ayoub J, Jean-François R, Cormier Y, Meyer D, Ying Y, Major P, Desjardins C, Bradley WE. Placebo-controlled trial of 13-cis-retinoic acid activity on retinoic acid receptor-beta expression in a population at high risk: implications for chemoprevention of lung cancer. J Clin Oncol. 1999;17:3546–3552. doi: 10.1200/JCO.1999.17.11.3546. [DOI] [PubMed] [Google Scholar]
- 56.Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CE, Callegari E, Schwind S, Pang J, Yu J, Muthusamy N, et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roth MD, Connett JE, D’Armiento JM, Foronjy RF, Friedman PJ, Goldin JG, Louis TA, Mao JT, Muindi JR, O’Connor GT, et al. FORTE Study Investigators. Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130:1334–1345. doi: 10.1378/chest.130.5.1334. [DOI] [PubMed] [Google Scholar]
- 58.Stolk J, Stockley RA, Stoel BC, Cooper BG, Piitulainen E, Seersholm N, Chapman KR, Burdon JG, Decramer M, Abboud RT, et al. Randomised controlled trial for emphysema with a selective agonist of the γ-type retinoic acid receptor. Eur Respir J. 2012;40:306–312. doi: 10.1183/09031936.00161911. [DOI] [PubMed] [Google Scholar]