Abstract
Respiratory tract infections are a leading cause of morbidity and mortality in children under 5 years of age. Increased susceptibility to infection is associated with deficiencies in immunity during early childhood. Airway epithelium represents the first line of mucosal defense against inhaled pathogens. However, little is known about epithelial immune mechanisms in the maturing lung. IL-22 and its receptor IL-22R1 are important in host defense and repair of epithelial barriers. The objective of this study was to determine whether a quantitative difference in IL-22R1 exists between infant and adult airways using the rhesus macaque monkey as a model of childhood lung development. Immunofluorescence staining of tracheal tissue revealed minimal expression of IL-22R1 in epithelium at 1 month of age, with a progressive increase in fluorescence-positive basal cells through 1 year of age. Western blot analysis of tracheal lysates confirmed significant age-dependent differences in IL-22R1 protein content. Further, primary tracheobronchial epithelial cell cultures established from infant and adult monkeys showed differential IL-22R1 mRNA and protein expression in vitro. To begin to assess the regulation of age-dependent IL-22R1 expression in airway epithelium, the effect of histone deacetylase and DNA methyltransferase inhibitors was evaluated. IL-22R1 mRNA in adult cultures was not altered by 5-aza-2′-deoxycytidine or trichostatin A. IL-22R1 mRNA in infant cultures showed no change with 5-aza-2′-deoxycytidine but was significantly increased after trichostatin A treatment; however, IL-22R1 protein did not increase concurrently. These data suggest that IL-22R1 in airway epithelium is regulated, in part, by epigenetic mechanisms that are dependent on chronologic age.
Keywords: respiratory development, primate, epigenetic, innate immunity, trichostatin A
Clinical Relevance
Respiratory tract infections remain a leading cause of death worldwide in very young children. Novel approaches to prevention and treatment of pediatric respiratory infections will require the identification of new physiologic targets. Our data describe for the first time that IL-22R1 density on airway epithelium is developmentally regulated and that expression is markedly attenuated in early life. Given the known importance of IL-22 in epithelial defense and repair during lung injury, our findings represent a potentially important immune pathway that can be enhanced for prophylaxis and therapy.
Clinically significant immunologic differences exist between children and adults (1, 2). For example, respiratory infections represent a leading cause of death worldwide in humans <5 years of age, whereas the same infectious agents cause less severe morbidity and mortality in adults (3). This difference is partially attributed to qualitative and quantitative inadequacy in innate and adaptive immunity. Identification of novel age-related differences in immune function could represent opportunities for new therapeutics and improved clinical outcomes for pediatric patients.
Interleukins (IL) and their receptors feature prominently in mammalian immune systems. IL-22 and its receptor IL-22R1 are representative examples of immune pathways that functionally link cytokine output from cells of hematopoietic origin with physiologic responses mediated by structural cells that contribute to barrier functions. Major producers of IL-22 include innate lymphoid cells and natural killer T cells (4–7). The receptor for IL-22 is a dimer composed of IL-22R1 and IL-10RB. Whereas IL-10RB is ubiquitous, IL-22R1 is primarily found on epithelium, and this distribution limits the activity of IL-22 anatomically and supports its role in innate immunity (8). IL-22R1 is primarily expressed on structural cells such as epithelium and smooth muscle within respiratory tract, gastrointestinal tract, and skin (9–11). Dysregulation of the IL-22/IL-22R1 axis is associated with epithelial hyperproliferation and inhibition of differentiation (12, 13), chronic bacterial and fungal infections (14, 15), and aberrant barrier repair after viral or mechanical insults (14, 16, 17).
Although tissue-specific expression of IL-22R1 has been characterized, the influence of developmental age on function of the IL-22/IL-22R1 axis has not been well defined. Here, we investigated conducting airway epithelial cell expression of IL-22R1 using the rhesus macaque monkey as a model of childhood lung development. Because pediatric patients exhibit enhanced susceptibility to respiratory infections, we hypothesized that airway epithelium from infant monkeys would express less IL-22R1 as compared with adults. Based upon our initial findings of constitutively attenuated IL-22R1 in infant airway epithelium, we further explored the epigenetic regulation of IL-22R1 using inhibitors of DNA methyltransferase (DNMT) or histone deacetylase (HDAC) in vitro.
Evidence of epigenetic influences on pulmonary mucosal immunity continues to grow. For example, nasal epithelial cells from smokers have unique DNA methylation patterns as compared with nonsmokers, and this results in altered expression of antiviral genes in vitro (18). Taken together, our studies suggest one putative immune pathway by which airway epithelium of very young children may exhibit enhanced susceptibility to pathogens. Further, it appears that airway epithelial cells from infant rhesus monkeys display a degree of plasticity, as evidenced by HDAC inhibitor treatment of cultures, indicating that mucosal barriers may represent novel therapeutic targets.
Materials and Methods
A detailed version of the materials and methods is available in the online supplement.
Animals
All tracheobronchial tissues used for this study were obtained from the California National Primate Research Center Pathology Unit. Animals <12 months of age were considered “infants,” and those ≥36 months of age were considered “adults” (19). Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California at Davis. The care and housing of all animals complied with the provisions of the Institute of Laboratory Animal Resources and conformed to the practices established by the American Association for Accreditation of Laboratory Animal Care.
Immunofluorescence Microscopy
Trachea cryosections were fixed and incubated with mouse monoclonal anti–IL-22R1 antibody (Santa Cruz Biotechnologies, Santa Cruz, CA), followed by Alexa 568–conjugated secondary antibody (Invitrogen, Eugene, OR). Sections were imaged on an Olympus BX61 microscope with an Olympus DP71 color camera (Olympus, Waltham, MA).
Western Blot
Protein was isolated from fresh trachea tissues or cultured primary tracheobronchial epithelial (TBE) cells and separated on polyacrylamide gels (Bio-Rad, Richmond, CA) by electrophoresis and then transferred to blot membranes. Blots were incubated with antibodies against IL-22R1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technologies, Danvers, MA) followed by secondary antibodies conjugated to HRP. Blots were exposed to enhanced chemiluminescence substrate (Thermo-Fisher Scientific, Waltham, MA) followed by image acquisition and band density analysis for IL-22R1 and GAPDH with an automated imager and software (Protein Simple, Santa Clara, CA).
Primary Cell Culture
Primary TBE cells were isolated and cultured as previously described (20). In brief, epithelial cells were expanded with bronchial epithelial cell growth medium (Lonza, Allendale, NJ) containing 50 nM retinoic acid (Sigma-Aldrich, St. Louis, MO) and subsequently plated in Corning Transwells clear polyester inserts (Corning Life Sciences, Tewskbury, MA). Confluent cultures were transitioned to air–liquid interface conditions with a 1:1 mixture of bronchial epithelial cell growth medium and Dulbecco’s modified Eagle’s medium (Life Technologies, Carlsbad, CA) (21, 22). Cultures were grown until polarized (∼7 d).
IL-22 Treatment
Primary TBE cultures were treated with recombinant IL-22 (PeproTech, Rocky Hill, NJ) at 0, 10, or 100 ng/ml. Cells were harvested after 6 hours of treatment for quantitative RT-PCR (qRT-PCR) analysis of IL-8, lipocalin-2 (LCN2), and granulocyte/macrophage colony-stimulating factor (GM-CSF) mRNA.
Quantitative RT-PCR
Cells were collected into TRIzol (Life Technologies), total RNA was extracted, and cDNA was synthesized as previously described (22). Taqman qRT-PCR (Life Technologies) was performed to measure IL-22R1, IL-8, LCN2, GM-CSF, and GAPDH mRNA. Specific gene expression was compared with GAPDH expression to determine ∆Ct or ∆∆Ct.
Trichostatin A and 5-Aza-2′-Deoxycytidine Treatment
The effect of HDAC or DNMT inhibitors was assessed in polarized TBE cultures. The HDAC inhibitor trichostatin A (TSA) (InvivoGen, San Diego, CA) was used at 100 nM, and the DNMT inhibitor 5-aza-2′-deoxycytidine (AZA) (Sigma Aldrich) was used at 50 μM. IL-22R1 expression was assessed by qRT-PCR at 12 and 24 hours of treatment and by Western blot at 24 and 48 hours of treatment.
Statistical Analyses
Data are presented as mean ± SEM. We determined statistical significance (P ≤ 0.05) using Student’s t test, one-way ANOVA with Dunnett’s multiple comparisons test, or two-way ANOVA with Dunnett’s multiple comparisons test (Prism version 6.0; GraphPad, La Jolla, CA).
Results
IL-22R1 Expression in Airway Epithelium Is Dependent on Chronological Age
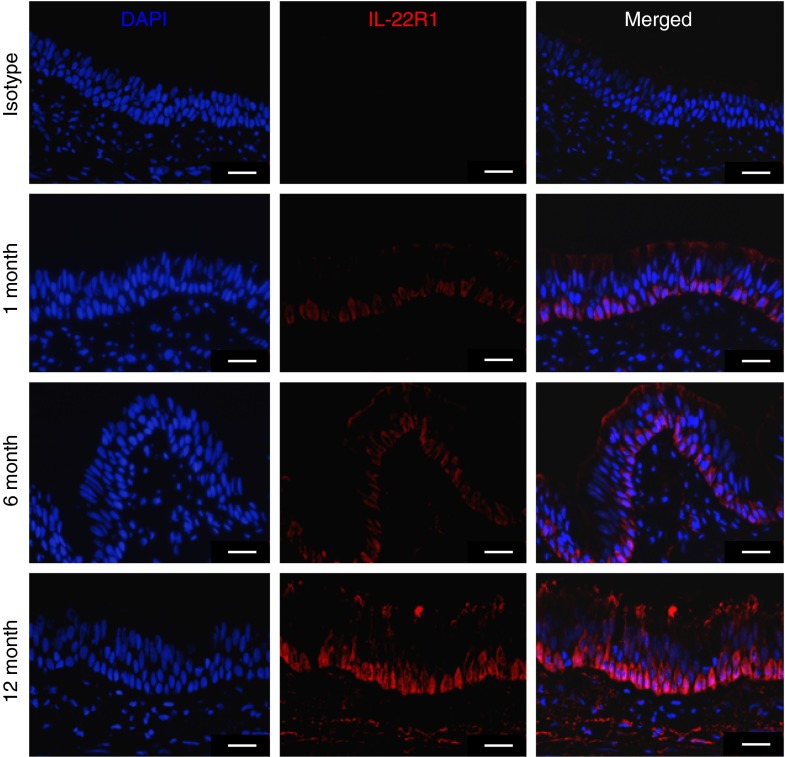
To assess whether IL-22R1 was differentially expressed in the airways during early life, we first used immunofluorescence microscopy to visualize IL-22R1 expression in monkey airways at 1, 6, and 12 months of age. At 1 month of age, there was little detectable expression of IL-22R1 in airway epithelium (Figure 1). Immunofluorescence staining was primarily detected on basal cells within the trachea, and abundance progressively increased with age. IL-22R1 protein was also detected on smooth muscle surrounding the trachea, but expression did not appear to be age dependent (data not shown).
Figure 1.
Immunofluorescence staining for IL-22R1 in rhesus monkey airway epithelium during the first year of life. Trachea cryosections from infant and adult monkeys were stained with a mouse monoclonal anti–IL-22R1 antibody (0.5 μg/ml) or a mouse IgG3 isotype control (0.5 μg/ml). Antibody binding was detected with Alexa 568–conjugated donkey anti-mouse antibodies (red). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Representative images from 1-, 6-, and 12-month-old animals are shown (n = 3–4 animals per age group). Isotype control as shown is for a 12-month-old animal. Scale bar, 25 μm.
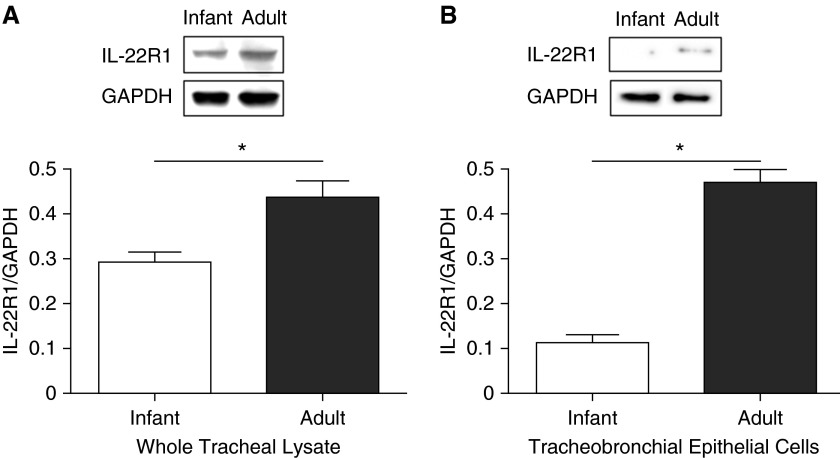
To further quantify IL-22R protein in native tissue, we performed Western blot analysis. IL-22R1 protein was identified in whole tracheal lysates from infant and adult rhesus monkeys; the molecular weight of the detected band appeared to be consistent with the expected range of 45 to 55 kD for rhesus IL-22R1 (Figure 2A). Using densitometry, IL-22R1 protein was determined to be approximately 1.5-fold greater (P = 0.0283) in lysates from adult tracheal tissue as compared with infant tissue.
Figure 2.
Western blot analysis of IL-22R1 protein from infant and adult rhesus monkey airway epithelium. Representative Western blots from whole tracheal lysate (A) and primary tracheobronchial epithelial cell cultures (B). Optical band density for IL-22R1 was analyzed with respect to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for each sample, and signals were quantified with commercial densitometry software. Data represent the mean ± SEM for each age group (n = 3–5 animals per group). *P = 0.0283 (A) and 0.0002 (B).
To confirm that differential expression of IL-22R1 in infant and adult trachea was constitutively associated with conducting airway epithelium, we assessed whether age-dependent IL-22R1 protein levels persisted in TBE cell cultures from infant and adult monkeys. As shown in Figure 2B, age-dependent expression of IL-22R1 protein was perpetuated in vitro; adult TBE cell cultures contained 4-fold more IL-22R1 than those derived from infant monkeys (P = 0.0002).
Functional Consequences of Age-Dependent IL-22R1 Expression in Airway Epithelium
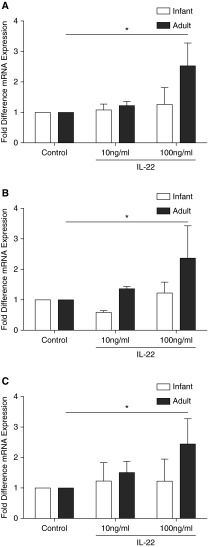
We next asked if the observed attenuated expression of IL-22R1 in infant airway epithelium corresponded with diminished responsiveness to the receptor ligand IL-22. IL-22 has been previously reported to enhance expression of IL-8, LCN2, and GM-CSF (10, 14, 15). Accordingly, we treated primary TBE cell cultures with recombinant human IL-22 and measured the induction of multiple gene transcripts known to be affected by IL-22. At 6 hours, IL-8, LCN2, and GM-CSF (Figures 3A, 3B, and 3C, respectively) mRNA were significantly induced in adult monkey–derived TBE cell cultures as compared with untreated controls (P < 0.05). In comparison, there was no significant increase in IL-8, LCN, or GM-CSF after treatment of primary TBE cell cultures derived from infant rhesus monkeys.
Figure 3.
Effect of IL-22 on innate immunity gene expression in infant and adult rhesus monkey airway epithelial cell cultures. Primary tracheobronchial epithelial cell cultures grown under air–liquid interface conditions were exposed to control media or rhIL-22 for 6 hours followed by quantitative RT-PCR for IL-8 (A), lipocalin-2 (B), and granulocyte/macrophage colony-stimulating factor (C). Data represent mean ± SEM for fold difference in expression as compared with control (n = 5–6 per age group; *P < 0.05).
IL-22R1 and IL-10RB mRNA Expression in Primary TBE Cell Cultures
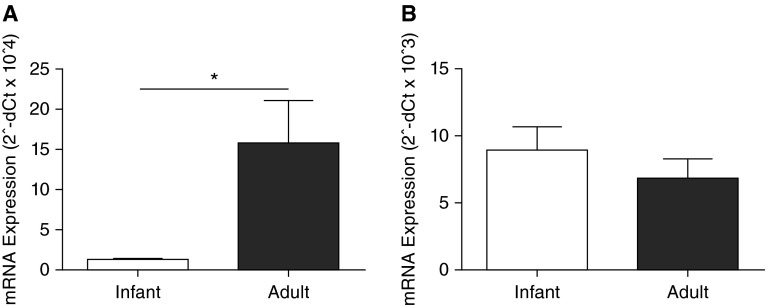
To discern if the age-dependent difference in protein expression of IL-22R1 in airway epithelium was due to transcriptional or post-transcriptional regulation, we measured IL-22R1 mRNA in primary TBE cell cultures. Adult monkey TBE cell cultures expressed nearly 15-fold higher IL-22R1 mRNA levels in comparison with cultured TBE cells derived from infant monkeys (P = 0.048) (Figure 4A). Because IL-22R1 function depends on dimerization with IL-10RB, we also quantified mRNA expression of IL-10RB to assess whether the heterodimer partner of IL-22R1 might also be developmentally regulated. As shown in Figure 4B, infant and adult primary TBE cell cultures expressed similar levels of IL-10RB mRNA.
Figure 4.
IL-22 receptor complex mRNA expression in infant and adult rhesus monkey airway epithelial cell cultures. Primary tracheobronchial epithelial cell cultures grown under air–liquid interface conditions were evaluated for IL-22R1 (A) and IL-10RB (B) mRNA levels as measured by quantitative RT-PCR. Data represent the mean ± SEM of 2−ΔCt for each target gene within both age groups (n = 8 per age group; *P = 0.048).
Epigenetic Regulation of IL-22R1 mRNA and Protein Expression In Vitro
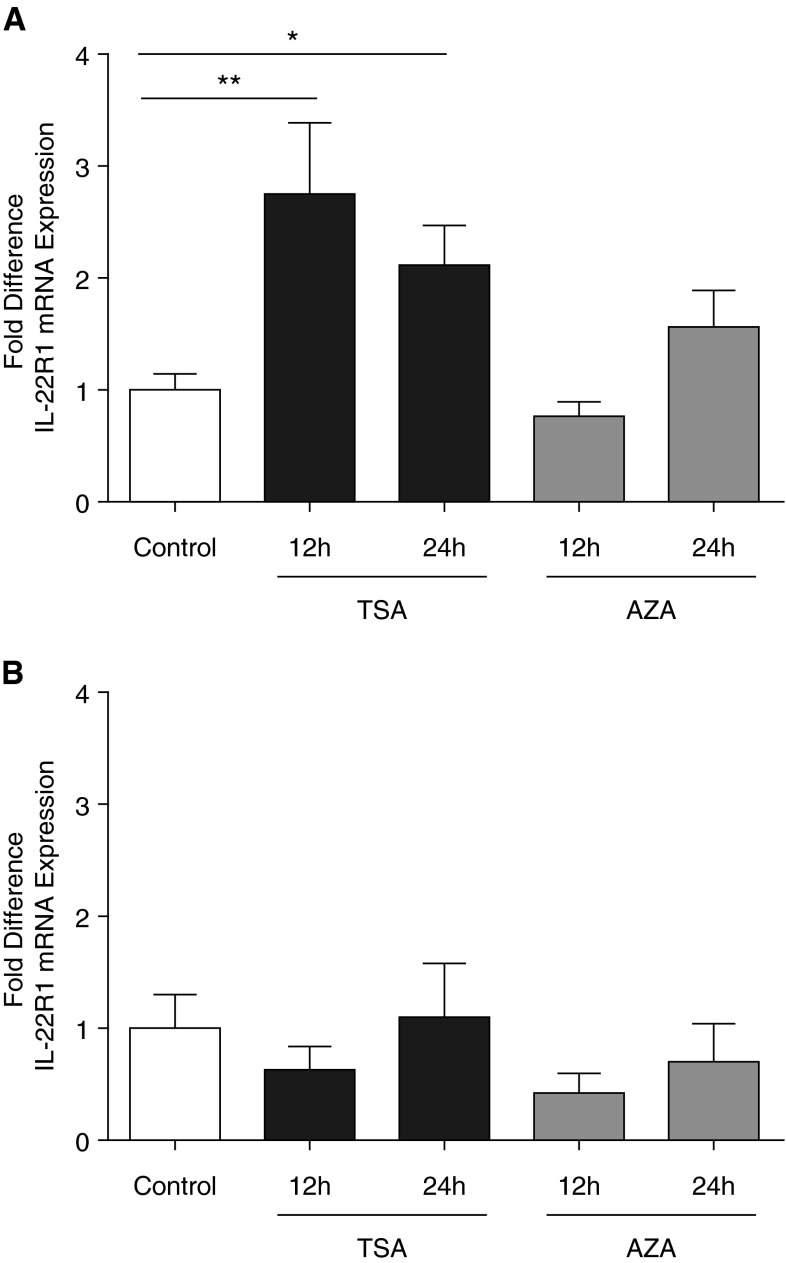
Next, we asked if epigenetic mechanisms might contribute to attenuation of IL-22R1 transcription in infant airway epithelium. Primary TBE cultures derived from infant monkeys were treated with TSA and subsequently evaluated for IL-22R1; TSA treatment resulted in a 2- to 3-fold increase in IL-22R1 mRNA after 12 (P = 0.021) and 24 hours (P = 0.032) as compared with time-matched control cultures (Figure 5A). In comparison, adult TBE cell cultures did not show a significant change in IL-22R1 mRNA levels after treatment with TSA (Figure 5B). Similar treatment with AZA resulted in no significant change in IL-22R1 mRNA levels for either infant or adult TBE cultures relative to time-matched controls (Figures 5A and 5B).
Figure 5.
Epigenetic regulation of IL-22R1 transcription by histone deacetylase or DNA methyltransferase inhibition. Primary tracheobronchial epithelial cell cultures were treated with control media, trichostatin A (TSA), or 5-aza-2′-deoxycytidine (AZA) for 12 or 24 hours, followed by quantitative RT-PCR for IL-22R1 mRNA. IL-22R1 mRNA quantity is expressed as 2−ΔCt. Controls (at 12 and 24 h) were normalized to a value of 1 for fold difference IL-22R1 expression in infant (A) and adult (B) cultures treated with either TSA or AZA. Data represent the mean ± SEM for each age group (n = 8 infants and 8 adults). *P = 0.032 and **P = 0.021.
To assess for toxicity for either TSA or AZA treatment, we further compared cycle threshold for the GAPDH housekeeping gene in cultures and found no significant difference with respect to time or treatment regardless of age groups over the course of the experiment (data not shown). Likewise, the effect of TSA or AZA was also examined with respect to IL-1α, which is known to be differentially expressed in infant and adult epithelium (21); there was no apparent change in IL-1α mRNA levels regardless of treatment or age group (data not shown).
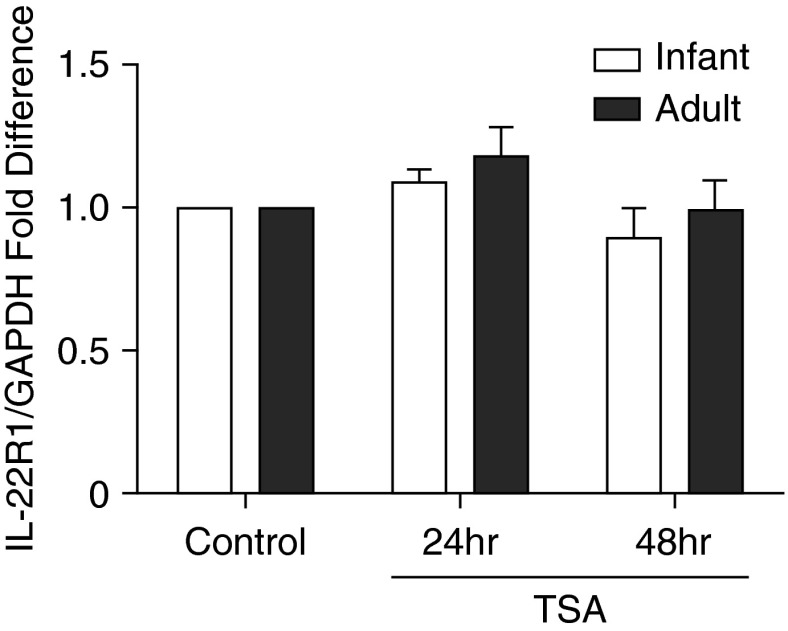
Finally, we determined if the age-dependent effects of TSA on IL-22R1 mRNA levels in primary TBE cell cultures were accompanied by an increase in IL-22R1 protein. In contrast with our finding for IL-22R1 mRNA expression, we did not find a corresponding increase in IL-22R1 protein for either age group at 24 or 48 hours after TSA treatment of TBE cell cultures (Figure 6).
Figure 6.
Effect of TSA on IL-22R1 protein expression in primary tracheobronchial epithelial cell cultures. Primary tracheobronchial epithelial cell cultures were treated with control media or TSA for 24 or 48 hours. IL-22R1 and GAPDH band densities were measured by Western blot analysis of culture lysates. The ratio of IL-22R1/GAPDH was then compared with the media control for each monkey. Data represent the mean ± SEM for fold difference in protein expression (n = 4–6 per age group).
Discussion
Immunological differences between adults and children are associated with enhanced morbidity and mortality in the face of respiratory infections in early life. A multifactorial explanation is most likely but remains incompletely described. Here, we focused on the epithelial barrier’s role in innate immunity. We examined airway epithelial cell cultures and airway tissue sections from infant and adult rhesus monkeys for the presence of IL-22R1. We found that IL-22R1 expression in tracheobronchial airway epithelium progressively increases with age in rhesus monkeys. Attenuated expression of IL-22R1 in infant airway epithelium was perpetuated in isolated epithelial cells after proliferation and differentiation in vitro, suggesting an epigenetic mechanism of inhibition. In concurrence with the observed reduction in IL-22R1 expression in infant monkey airway epithelium, treatment of TBE cell cultures with the IL-22R1 ligand IL-22 resulted in an attenuated innate immune cytokine response in TBE cultures from infant monkeys as compared with adults. We further showed that IL-10RB, which participates in the IL-22 receptor complex, was not differentially expressed in primary TBE cell cultures from either infant or adult monkeys, suggesting that IL-22R1 is the limiting component with respect to age and IL-22 functionality. Finally, treatment with TSA partially abrogated the difference in IL-22R1 mRNA, increasing expression 2- to 3-fold in infant, but not adult, airway epithelial cultures. To the best of our knowledge, this is the first report of age-dependent regulation for the IL-22/IL-22R1 axis.
Dysregulation of the IL-22/IL-22R1 axis has been associated with many diseases and has been identified as a potential therapeutic target in some cases (7, 23). The effects of IL-22 in the respiratory tract can be summarized as antibacterial and reparative in nature (14, 17). Mice deficient in IL-22 have more severe lung injury than wild-type mice in influenza infection models (4, 24), and in a rat model of ventilator-induced lung injury, nebulized IL-22 improved survival (16). To date, functional studies have focused on deficiency of IL-22; the potential regulatory role of IL-22R1 levels has not yet been explored. If a deficiency in receptor levels recapitulates the effects of cytokine deficiency, our findings have important implications. Thus, IL-22R1 density in airways may be a factor in the higher morbidity and mortality seen in respiratory infections of infants and children as compared with adults.
The magnitude of age-related IL-22R1 expression was not consistent when comparing mRNA from TBE cell cultures versus protein from whole tracheal homogenate. As shown for human airway smooth muscle (25), we observed IL-22R1 expression in airway smooth muscle of rhesus monkeys, and this compartment was included in the homogenate analysis. Because smooth muscle IL-22R1 expression levels appeared to be constitutive for all age groups, it would be expected that the magnitude of age-dependent IL-22R1 expression in whole tracheal homogenates would be less than isolated airway epithelial cell cultures. Not surprisingly, when we measured IL-22R1 protein in TBE cultures by Western blot, the difference between adult and infant monkeys was exaggerated as compared with the whole tracheal homogenates that contain the smooth muscle component. The functional role of the IL-22/IL-22R1 axis in airway smooth muscle is complex, but there is evidence that it can contribute to remodeling and increased smooth muscle mass in asthma (26, 27).
We found epithelial IL-22R1 expression to be primarily associated with the basal cells of the trachea. Basal cells in this location are responsible for reparative processes after injury of the airways, and it has been speculated that this cell phenotype may contribute to the pathogenesis of COPD (28). In addition, IL-22 signaling is required to maintain epithelial integrity in the face of respiratory infections (4, 14, 24). Therefore, our finding of localized expression of IL-22R1 on airway basal cells is not unexpected. One important question to address in future studies is how IL-22R1low–expressing epithelial cells in infant airways are ultimately replaced by IL-22R1high–expressing cells as animals mature. Based upon our finding that infant TBE cells perpetuate the IL-22R1low phenotype in vitro despite proliferation and expansion in culture, it may be speculated that the IL-22R1high phenotype requires an environmental signal for induction.
In addition to epithelial integrity, a multitude of cytokines, chemokines, and antimicrobial peptides are induced by IL-22 (15, 29). In our study, we evaluated the impact of IL-22 treatment on primary TBE cell cultures, focusing on IL-8, LCN2, and GM-CSF (10, 14, 15). No significant response was elicited by IL-22 in infant monkey TBE cell cultures, whereas adult cultures showed increased mRNA expression for all three targets evaluated. It has been previously reported that costimulation with IL-17 is required for maximal IL-22 responses (14, 30); therefore, it is possible that our findings may have been different with IL-17 costimulation. Although it is beyond the scope of the current study, the contribution of IL-17 on functionality of the IL-22/IL-22R1 axis in infant airway epithelium would be important to assess in the future.
Epigenetic regulation of gene expression in airway epithelium is evident in inflammatory conditions like asthma, smoking, and infection; TSA has been instrumental in uncovering the role of histone acetylation in some of these studies (18, 31–33). Although TSA is an inhibitor of most of the class I and II HDAC proteins, its activity is restricted outside of the HDAC family of proteins (34). The result of histone deacetylation is a closed chromatin pattern and transcriptional repression. As such, HDAC inhibitors usually act to increase mRNA expression. Here we have demonstrated that treatment with TSA specifically increased IL-22R1 mRNA expression in infant TBE cell cultures, but it did not have the same effect in adult cultures. Moreover, there was no apparent effect of treatment of infant TBE cells with a DNMT inhibitor, suggesting that methylation of the IL-22R1 promoter or transcriptional factor promoters that regulate IL-22R1 expression is not a primary mechanism for suppression of IL-22R1 during early life.
One potential mechanism by which TSA may selectively enhance IL-22R1 mRNA expression in TBE cells is that infants may have an inherently different histone acetylation pattern of the IL-22R1 promoter as compared with adults. Alternatively, infant and adult cells may have different levels of histone acetyl transferase and/or HDAC activities. It is also possible that the effect of TSA is not directly related to histone acetylation/deacetylation; both histone acetyl transferase and HDAC can modify nonhistone proteins, resulting in increased or decreased activity of ubiquitous transcription factors (34–36). The latter explanation is less likely because TSA did not increase IL-22R1 expression in adult TBE cultures. Further, the increase in gene transcription was not universal; we have found no effect of TSA on IL-1α or GAPDH mRNA expression in infant TBE cell cultures.
There are several possible explanations for the lack of correlation between IL-22R1 mRNA and protein expression after TSA treatment of infant TBE cell cultures. One possibility is that IL-22R1 protein was induced after TSA treatment of infant TBE cells, but proteasomal degradation prevented stabilization and membrane expression (37). Alternatively, there may be post-transcriptional control of IL-22R1; for example, in human keratinocytes, miR-197 negatively regulates IL-22R1 (38). The TSA-induced increase in IL-22R1 mRNA for infant TBE cells also did not reach the levels observed in adult TBE cells; therefore, a larger increase may be required to detect a significant impact on protein translation.
In summary, we have shown intrinsic suppression of IL-22R1 expression in airway epithelium using a nonhuman primate model of lung development. Developmental regulation of IL-22R1 in the conducting airways was limited to the epithelial compartment, as evidenced by the finding that smooth muscle appeared to express IL-22R1 at comparable levels starting at 1 month of age in monkeys. TSA (but not a DNMT inhibitor) increased IL-22R1 mRNA expression in infant TBE cell cultures, suggesting an epigenetic mechanism for attenuated transcription. However, additional post-translational mechanisms may also participate in the developmental regulation of IL-22R1 protein expression. The pathophysiologic consequences IL-22R1 deficiency in the infant airway remain an important question that will be explored further in future studies.
Acknowledgments
Acknowledgments
The authors thank Theodore Wang and Brandon Ma for technical assistance, Carolyn Black and Dr. Candice Clay for editorial advice, the Pathology Unit at the California National Primate Research Center for collecting tissues for our experiments, and Dr. Steven Laing for assistance with image acquisition and interpretation for the immunofluorescence portion of this manuscript.
Footnotes
This work was supported by National Institutes of Health grants R01HL097087 (L.A.M.), P51OD011107, and T32AI060555 (D.T.D.).
Author Contributions: D.T.D., J.E.G., and L.A.M. designed the experiments. D.T.D. and J.E.G. conducted the experiments. D.T.D. and L.A.M. analyzed the data and drafted the manuscript. J.E.G. contributed editorial input into the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0452RC August 26, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55:688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health OrganizationWorld health statistics 2014. Geneva, Switzerland: WHO; 2014 [Google Scholar]
- 4.Paget C, Ivanov S, Fontaine J, Renneson J, Blanc F, Pichavant M, Dumoutier L, Ryffel B, Renauld JC, Gosset P, et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem. 2012;287:8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar P, Thakar MS, Ouyang W, Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 2013;6:69–82. doi: 10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo H, Topham DJ. Interleukin-22 (IL-22) production by pulmonary Natural Killer cells and the potential role of IL-22 during primary influenza virus infection. J Virol. 2010;84:7750–7759. doi: 10.1128/JVI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13:21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 8.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. doi: 10.1074/jbc.M005304200. [DOI] [PubMed] [Google Scholar]
- 9.Whittington HA, Armstrong L, Uppington KM, Millar AB. Interleukin-22: a potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol. 2004;31:220–226. doi: 10.1165/rcmb.2003-0285OC. [DOI] [PubMed] [Google Scholar]
- 10.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 2009;87:523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 14.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eidenschenk C, Rutz S, Liesenfeld O, Ouyang W. Role of IL-22 in microbial host defense. Curr Top Microbiol Immunol. 2014;380:213–236. doi: 10.1007/978-3-662-43492-5_10. [DOI] [PubMed] [Google Scholar]
- 16.Hoegl S, Bachmann M, Scheiermann P, Goren I, Hofstetter C, Pfeilschifter J, Zwissler B, Muhl H. Protective properties of inhaled IL-22 in a model of ventilator-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:369–376. doi: 10.1165/rcmb.2009-0440OC. [DOI] [PubMed] [Google Scholar]
- 17.Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn JF. IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol. 2013;182:1286–1296. doi: 10.1016/j.ajpath.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rager JE, Bauer RN, Müller LL, Smeester L, Carson JL, Brighton LE, Fry RC, Jaspers I. DNA methylation in nasal epithelial cells from smokers: identification of ULBP3-related effects. Am J Physiol Lung Cell Mol Physiol. 2013;305:L432–L438. doi: 10.1152/ajplung.00116.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plopper C, St George J, Cardoso W, Wu R, Pinkerton K, Buckpitt A. Development of airway epithelium: patterns of expression for markers of differentiation. Chest. 1992;101(Suppl):2S–5S. [PubMed] [Google Scholar]
- 20.Wu R, Sato GH, Whitcutt MJ. Developing differentiated epithelial cell cultures: airway epithelial cells. Fundam Appl Toxicol. 1986;6:580–590. doi: 10.1016/0272-0590(86)90170-3. [DOI] [PubMed] [Google Scholar]
- 21.Clay CC, Reader JR, Gerriets JE, Wang TT, Harrod KS, Miller LA. Enhanced viral replication and modulated innate immune responses in infant airway epithelium following H1N1 infection. J Virol. 2014;88:7412–7425. doi: 10.1128/JVI.00188-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maniar-Hew K, Clay CC, Postlethwait EM, Evans MJ, Fontaine JH, Miller LA. Innate immune response to LPS in airway epithelium is dependent on chronological age and antecedent exposures. Am J Respir Cell Mol Biol. 2013;49:710–720. doi: 10.1165/rcmb.2012-0321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, et al. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol. 2013;87:6911–6924. doi: 10.1128/JVI.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang Y, Al-Alwan L, Risse PA, Roussel L, Rousseau S, Halayko AJ, Martin JG, Hamid Q, Eidelman DH. Th17 cytokines induce human airway smooth muscle cell migration. J Allergy Clin Immunol. 2011;127:1046–1053. doi: 10.1016/j.jaci.2010.12.1117. [DOI] [PubMed] [Google Scholar]
- 26.Chang Y, Al-Alwan L, Risse PA, Halayko AJ, Martin JG, Baglole CJ, Eidelman DH, Hamid Q. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB J. 2012;26:5152–5160. doi: 10.1096/fj.12-208033. [DOI] [PubMed] [Google Scholar]
- 27.Hirose K, Takahashi K, Nakajima H. Roles of IL-22 in allergic airway inflammation. J Allergy. 2013;2013:260518. doi: 10.1155/2013/260518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crystal RG. Airway basal cells: the “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:1355–1362. doi: 10.1164/rccm.201408-1492PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- 30.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soto-Ramírez N, Arshad SH, Holloway JW, Zhang H, Schauberger E, Ewart S, Patil V, Karmaus W. The interaction of genetic variants and DNA methylation of the interleukin-4 receptor gene increase the risk of asthma at age 18 years. Clin Epigenetics. 2013;5:1. doi: 10.1186/1868-7083-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dombrowsky H, Barrenschee M, Kunze M, Uhlig S. Conserved responses to trichostatin A in rodent lungs exposed to endotoxin or stretch. Pulm Pharmacol Ther. 2009;22:593–602. doi: 10.1016/j.pupt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Shames DS, Minna JD, Gazdar AF. DNA methylation in health, disease, and cancer. Curr Mol Med. 2007;7:85–102. doi: 10.2174/156652407779940413. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida M, Matsuyama A, Komatsu Y, Nishino N. From discovery to the coming generation of histone deacetylase inhibitors. Curr Med Chem. 2003;10:2351–2358. doi: 10.2174/0929867033456602. [DOI] [PubMed] [Google Scholar]
- 35.Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25:552–563. doi: 10.1183/09031936.05.00117504. [DOI] [PubMed] [Google Scholar]
- 36.Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- 37.Weathington NM, Snavely CA, Chen BB, Zhao J, Zhao Y, Mallampalli RK. Glycogen synthase kinase-3β stabilizes the interleukin (IL)-22 receptor from proteasomal degradation in murine lung epithelia. J Biol Chem. 2014;289:17610–17619. doi: 10.1074/jbc.M114.551747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lerman G, Sharon M, Leibowitz-Amit R, Sidi Y, Avni D. The crosstalk between IL-22 signaling and miR-197 in human keratinocytes. PLoS One. 2014;9:e107467. doi: 10.1371/journal.pone.0107467. [DOI] [PMC free article] [PubMed] [Google Scholar]