Abstract
The regulation of microtubule dynamics in cystic fibrosis (CF) epithelial cells and the consequences of reduced rates of microtubule polymerization on downstream CF cellular events, such as cholesterol accumulation, a marker of impaired intracellular transport, are explored here. It is identified that microtubules in both CF cell models and in primary CF nasal epithelial cells repolymerize at a slower rate compared with respective controls. Previous studies suggest a role for cAMP in modulating organelle transport in CF cells, implicating a role for exchange protein activated by cAMP (EPAC) 1, a regulator of microtubule elongation, as a potential mechanism. EPAC1 activity is reduced in CF cell models and in Cftr−/− mouse lung compared with respective non-CF controls. Stimulation of EPAC1 activity with the selective EPAC1 agonist, 8-cpt-2-O-Me-cAMP, stimulates microtubule repolymerization to wild-type rates in CF cells. EPAC1 activation also alleviates cholesterol accumulation in CF cells, suggesting a direct link between microtubule regulation and intracellular transport. To verify the relationship between transport and microtubule regulation, expression of the protein, tubulin polymerization–promoting protein, was knocked down in non-CF human tracheal (9/HTEo−) cells to mimic the microtubule dysregulation in CF cells. Transduced cells with short hairpin RNA targeting tubulin polymerization–promoting protein exhibit CF-like perinuclear cholesterol accumulation and other cellular manifestations of CF cells, thus supporting a role for microtubule regulation as a mechanism linking CFTR function to downstream cellular manifestation.
Keywords: exchange protein activated by cAMP 1, cystic fibrosis, Rap1, microtubules, cholesterol
Clinical Relevance
This article is the first report of inherent microtubule regulatory changes in cystic fibrosis (CF). These changes to microtubule regulation increase our understanding of cellular changes associated with the absence of CF transmembrane conductance regulator function and how downstream events, such as aggressive inflammatory signaling, occur. Our mechanistic data also point to a means of pharmacologically correcting the microtubule regulation.
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, resulting in impaired expression, trafficking, or function of the protein, a cAMP-regulated chloride channel (1). The mechanisms by which disrupted CFTR function impacts cellular regulation is still unclear, and an important area of study. Here, the role of microtubules in mediating cellular changes in CF epithelial cells is explored.
Microtubule regulation of CFTR trafficking has been shown previously. Multiple early studies demonstrated that CFTR localization to the apical membrane is dependent on microtubule-dependent trafficking (2–4). Specifically, the microtubule motor protein family member, dynamin-2, associates with CFTR-associated ligand to regulate vesicle formation and CFTR expression at the plasma membrane (5). Young and colleagues (6) also demonstrated that inhibition of dynamin with the small molecule inhibitor, dynasore, increased plasma membrane levels of CFTR, showing the importance of the dynamin pathway in CFTR recycling. What is unclear is whether there is a reciprocal relationship where the absence of CFTR function would impact microtubule function.
There is indirect evidence that CFTR dysfunction has a negative impact on microtubules and related intracellular transport events. Autophagy is a process dependent on efficient microtubule-mediated transport, and dysfunctional autophagy has been clearly demonstrated in CF cells and proposed as a process key to propagating inflammatory signaling (7, 8). In CF macrophages, Bruscia and colleagues (9) demonstrate impaired intracellular movement resulting in Toll-like receptor 4 accumulation and subsequent enhanced cytokine production. We have also demonstrated disruption of intracellular movement in CF cells with the identification of perinuclear accumulation of free cholesterol due, in part, to impaired endosomal movement (10–12). Misfolded CFTR also accumulates in the perinuclear region in aggresomes (13), a process in which Kawaguchi and colleagues (14) implicated a specific role for histone deacetylase (HDAC) 6 activity.
HDAC6 is a selective microtubule deacetylase and directly impacts the regulation of intracellular transport (15). We have recently demonstrated that impaired endosomal transport and perinuclear cholesterol accumulation in CF cells is a consequence of decreased acetylation of α-tubulin in CF epithelial cells, the first direct report of microtubule modifications in CF (16). Restoring microtubule acetylation by inhibition of HDAC6 improves cholesterol processing and reduces inflammatory signaling profiles, showing the potential importance of microtubule regulation in CF cell signaling (16).
Reduced microtubule acetylation can be a marker of reduced microtubule stability (17, 18); it has been shown that microtubule acetylation increases intact microtubule lifespan from 5 minutes to over 1 hour (19, 20). However, due to changing intracellular and extracellular environments and signaling and metabolic demands of the cells, microtubule organization is constantly in flux between stable and dynamic states (21, 22). The difference between these states helps to distinguish which signaling pathways are to be activated or silenced, as well as to move and position vesicles and organelles appropriately (23, 24). Throughout interphase, centrosomes function to organize this microtubule growth and restructuring (25). These changes occur efficiently due to ordered nucleation, anchoring, and elongation events. Nucleation is initiated by the γ-tubulin ring complex, and is followed by anchoring, where tubulin and microtubule-associated proteins capture the microtubule nucleated γ-tubulin ring complex at subdistal appendages to form and maintain radial microtubule arrays, or asters. Finally, elongation extends the microtubule to the cell periphery (26–28). Functional studies suggest that the disruption of microtubule anchoring proteins and microtubule-associated proteins, such as EB1, CEP135, and p50, lead to delayed microtubule nucleation, thus compromising microtubule integrity (29–32). With compounding data suggesting altered microtubule transport and structure in CF, inherent microtubule integrity and dynamics may be also compromised.
The observation of reduced acetylated α-tubulin content in CF cells lead to the hypothesis that the absence of CFTR function negatively impacts microtubule dynamics. In this article, the observation that tubulin polymerization rates were reduced in both CF cell lines and primary CF nasal epithelial cells is reported. Mechanistically, slowed rates of tubulin polymerization were due to reduced exchange protein activated by cAMP (EPAC) 1 activity, also known as Rap guanine nucleotide exchange factor 3 (33). EPAC1 promotes microtubule polymerization by binding to (+) end microtubules (33), explaining how selective activation of EPAC1 with 8-cpt-2-O-Me-cAMP restores polymerization rates in CF cells to wild-type (WT) levels. The data provided in this article identify a novel cellular mechanism that can explain the impairment of intracellular transport reported by several groups that is related to inflammation and other aspects of CF disease.
Materials and Methods
Cells and Materials
All cells were grown at 37°C in 5% CO2 incubators, unless noted. IB3-1 cells, human epithelial cells with the F508 del mutation (CF phenotype), and S9 cells, IB3-1 cells stably transfected with the full-length WT CFTR (control) were a generous gift from Pamela L. Zeitlin (Johns Hopkins University, Baltimore, MD). Cells were cared for as previously described in LHC-8 basal medium (Biofluids, Camarillo, CA) supplemented with 5% FBS and 1 U/ml penicillin/streptomycin. Human epithelial 9/HTEo− cells were developed by Dr. Dieter Gruenert (University of California, San Francisco, CA). Cells were cared for in Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA) supplemented with 10% FBS containing 0.04 mM Hepes, 2 mM L-glutamine, and 1 U/ml penicillin/ streptomycin. Human nasal epithelial (HNE) cells were obtained from the Case Western Reserve University CF Center cell culture core facility (Cleveland, OH).
In Vitro Microtubule Formation Assay
Cells were grown on collagen-coated coverslips to 75–80% confluency. Cells were removed from the 37°C 5% CO2 incubator and placed on ice for 30–60 minutes. After a depolymerization period, prewarmed 37°C media (with vehicle or drug) were added at designated time points (0–20 min). At the end of the time course, cells were rinsed with PBS and fixed at the indicated time points. Cells were immunostained according to the protocol described subsequently here.
Cells were visualized in the appropriate range using a DM6000 upright microscope (40× oil objective; Leica, Buffalo Grove, IL) with Volocity software (Improvision, Waltham, MA). For each time point, 5–10 representative fields were captured, yielding 40–80 cells. Each cell was scored as having or not having an aster present, and quantification was determined by the ratio of cells with aster/microtubule formation to total cells at various time points. In the figure legends, n represents the number of times each experiment was repeated per condition, yielding reproducibility of the experiment.
Immunostaining
Antibodies against α-tubulin (Abcam, Cambridge, MA) and phospho-AKT (pAKT; Santa Cruz, Santa Cruz, CA) were obtained. Texas red goat anti-rabbit IgG antibodies were obtained from Invitrogen (Carlsbad, CA). Cells were rinsed three times with PBS and fixed and permeabilized with acetone for 20 minutes at −20°C. Cells were rinsed with PBS and then blocked with 5% goat serum in PBS for 30–60 minutes, rocking at room temperature. Primary antibodies were diluted in 5% goat serum in PBS and were added for 1 hour, rocking at room temperature. Cells were rinsed three times with PBS and then incubated with secondary antibodies at a final concentration of 10 mg/ml in 5% goat serum in PBS. Cells were mounted with SlowFade Gold Antifade (Invitrogen) on slides. Cells were visualized in the appropriate range using a Leica DM6000 upright microscope (40× oil objective) with Volocity software (Leica).
Mice
Mice lacking CFTR expression (S489X/S489X) and WT mice were provided by the CF animal core facility (Case Western Reserve University). All mice were used at between 10 and 12 weeks of age. CF mice were fed a liquid diet. Mice were cared for in accordance with the Case Western Reserve University Institutional Animal Care and Use Committee guidelines by the CF Animal Core Facility.
Filipin
Cells were plated at a density of 25,000–50,000 cells/well and grown to 60–70% confluency on Fisher brand coverslips (Fisher, Pittsburgh, PA). Cells were rinsed twice with PBS and then fixed with 2% paraformaldehyde for 30 minutes. Cells were rinsed twice more with PBS and then incubated with 0.05 mg/ml filipin (Sigma-Aldrich, St. Louis, MO) in PBS for 1 hour on a shaker in the dark. Filipin was dissolved freshly in DMSO before each experiment. Cells were rinsed in PBS before being mounted using SlowFade Gold Antifade reagent (Invitrogen) on slides. Cells were visualized in the ultraviolet range using a wide-field microscope with a 40× oil objective on a Leica DM6000 and analyzed using Volocity software.
Rap1 Activation
Using the cell models described above, Rap1 activation was assessed by the Rap1 activity assay using a kit obtained from Millipore (Billerica, MA) according to manufacturer instructions. Rap1-GTP and total Rap1 protein content was determined by Western blot analysis. ChemiDoc imaging system (Bio-Rad, Hercules, CA) was used to image gels and quantification of protein expression was accomplished by densitometry software (Quantity One; Bio-Rad).
Corrected Total Cellular Fluorescence Analysis
Cellular intensity of pAKT was determined calculating the corrected total cellular fluorescence (CTCF) per area of cell. Using ImageJ (National Institutes of Health, Bethesda, MD), the integrated density, area of selected cell, and mean fluorescence of the background was determined. CTCF was calculated by the following equation:
Tubulin Polymerization–Promoting Protein Knockdown
Tubulin polymerization–promoting protein (TPPP) short hairpin RNA (shRNA) was obtained from SABiosciences (Frederick, MD). Cells were seeded at 200,000 cells/well in a 6-well dish 24 hours before transfection. Transfections were performed with FuGene 6 (Roche, Indianapolis, IN), per the manufacturer’s protocol. Cells were selected by addition of hygromycin to media (80 mg/L) and subcloning. Briefly, for each transfection, 6 ml of FuGene 6 (Roche) was incubated for 10 min at room temperature in 100 ml of OptiMEM (Gibco). Concurrently, for each transfection, 0.4 mg of DNA was incubated for 10 minutes at room temperature in 100 ml of OptiMEM. Then, FuGene/OptiMEM mix was added to DNA/OptiMEM mix, mixed gently, and incubated for an additional 20 minutes at room temperature. Finally, 100 ml of diluted transfection mix was added to each well containing 400 ml of normal growth medium, and cells were incubated at 37°C in 95% O2–5% CO2 for 48 hours. TPPP expression was determined by Western blot analysis.
Statistical Analysis
The data were analyzed by two-tailed Student’s t test, unless otherwise noted. A P value of less than 0.05 was considered significant. All data represent the mean (±SEM).
Results
Effect of CFTR Function on Tubulin Polymerization
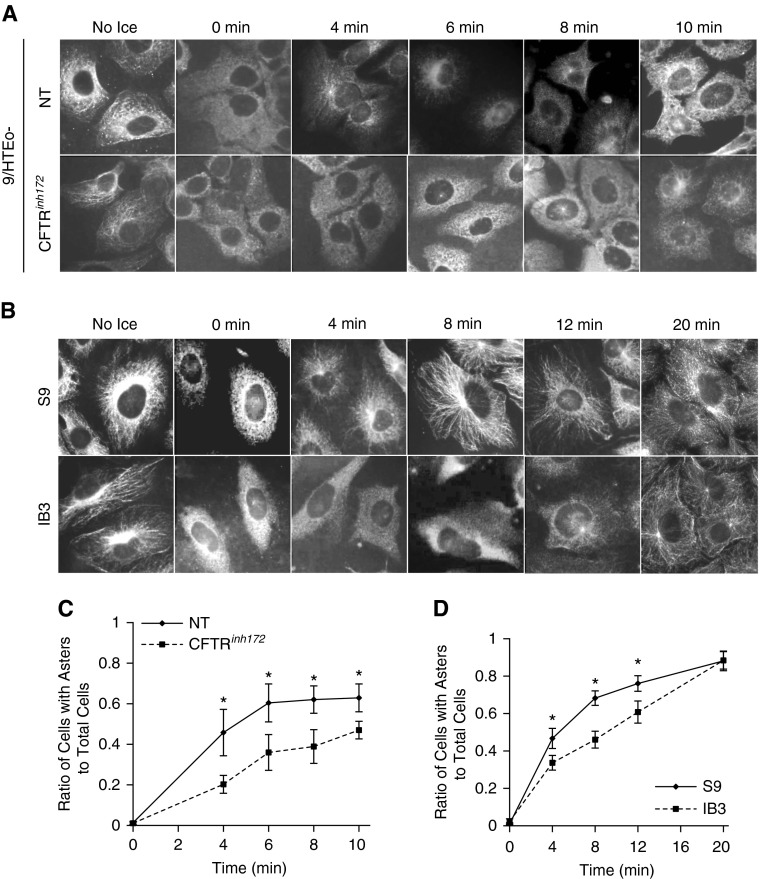
We have demonstrated that microtubule acetylation is reduced in CF cells and tissues (16). Reduced microtubule acetylation can be a marker of instability, and may suggest alterations in the balance of microtubule dynamics. Microtubule instability can arise from either an increase in catastrophe or reduced rates of elongation (18). There is circumstantial evidence of an influence of CFTR function on microtubule stability. The Roomans group (34) has observed that pharmacological CFTR inhibition resulted in an apparent shortening of microtubules. To begin testing the impact of CFTR function on microtubule dynamics, we examined how the CFTR inhibitor, CFTRinh172, impacts microtubule reformation after destabilization of microtubules by placing cells on ice and quantifying the rate of aster formation, as previously described (35). CFTR function was inhibited with CFTRinh172 (20 μM) for 72 hours in 9/HTEo− cells; 62.1 (±6.8)% of mock-treated cells were polymerized after 8 minutes compared with 38.9 (±8.3)% of CFTRinh172-treated cells (Figures 1A and 1C). These data demonstrate a clear effect of CFTRinh172 on microtubule dynamics. To test whether the effect of CFTRinh172 on microtubule repolymerization is a direct effect of the drug on CFTR function, CFTRinh172 was added acutely after microtubule depolymerization during the warming phase of the experiment. Aster formation rates were identical between the CFTRinh172-treated and vehicle-treated cells (see Figures E1A and E1C in the online supplement). These data demonstrate that CFTRinh172 effects on microtubule reformation are not due to nonspecific, acute interactions of the drug, and suggest that CFTR function may have an influence on microtubule dynamics.
Figure 1.
Cystic fibrosis transmembrane conductance regulator (CFTR) activity contributes to microtubule formation rates. (A) Chronic inhibition of CFTR (20 μM CFTRinh172, 72 h) leads to decreased aster formations. Cells were immunostained for α-tubulin after microtubule formation assay. Representative images of seven experiments are shown. (B) S9 and IB3 cells were subjected to a microtubule formation assay and immunostained for α-tubulin. Representative images of seven experiments are shown. (C) Quantification of aster formation of chronic inhibition of CFTR (20 μM CFTRinh172, 72 h) was determined by the ratio of cells with asters/microtubule formations to total cells at various time points (n = 7; *P < 0.05). (D) For S9 and IB3 cells, aster formation was quantified, and the ratio of cells with asters to total cells at various time points (0–20 min) was graphed (n = 7; *P < 0.05). Data represent the mean (±SEM). NT = no treatment.
Microtubule Reformation in Cultured and Primary CF Epithelial Cells
The data described previously here suggest that CFTR function has an influence on microtubule regulation. To test the effects of CFTR function on microtubule dynamics directly, we examined microtubule repolymerization in the CF model cell line IB3 cells and control S9 cells, and in primary WT and CF HNE cells. Aster formation was greater in S9 cells at 4, 8, and 12 minutes compared with IB3 cells (Figures 1B and 1D). Although the rate of aster formation and elongation was slower in IB3 cells, repolymerization was complete and equal to S9 cells by 20 minutes. These data demonstrate that cells lacking CFTR function have reduced rates of microtubule repolymerization, consistent with CFTR inhibition studies.
We also examined microtubule depolymerization rates to determine if other measures of microtuble dynamics are altered in CF model cells by measuring aster disappearance over time after placing cells on ice. Although there was a slight increase of depolymerization rate in IB3 cells compared with S9 controls, this difference was not statistically significant (Figures E1B and E1D). These data suggest that the regulation of microtubule repolymerization is inherently altered in CF.
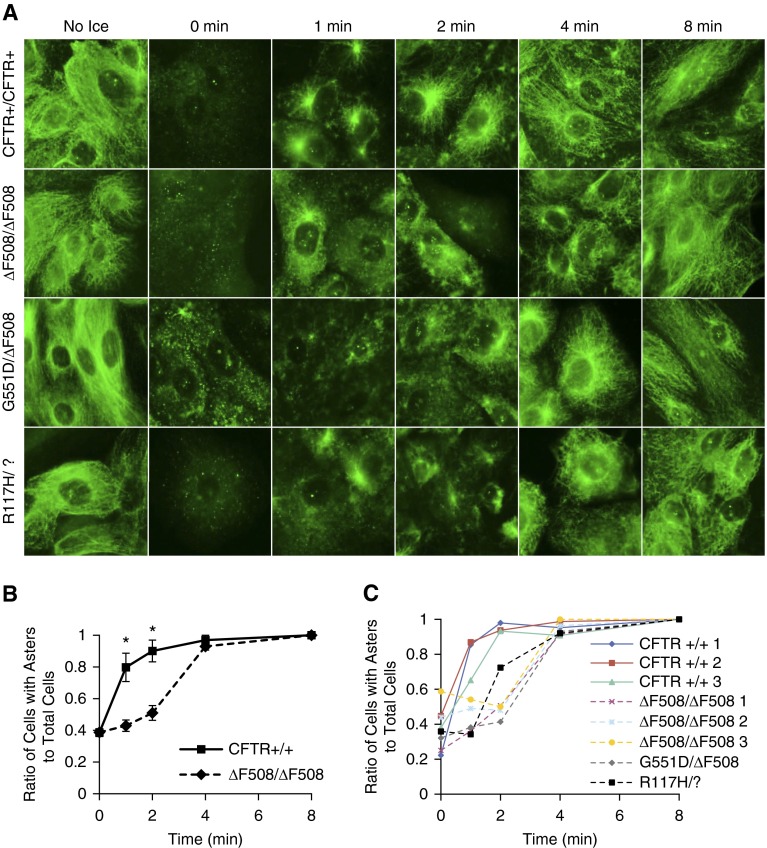
To test whether this microtubule phenotype is a manifestation of immortalized cell lines or reflective of an inherent regulatory change due to reduced CFTR function, primary HNE cells obtained by nasal brushing and expanded in culture were assessed for changes to microtubule repolymerization (Figure 2A). HNE cells from a subject without CF and a subject with F508 del were assayed three times from different passage numbers to determine reproducibility of the assay and averaged together. Microtubule repolymerization, as measured by aster formation, was significantly slower in CF cells compared with non-CF control cells. Although the overall rate of repolymerization was faster in the primary HNE cells compared with the cell lines, the relative difference between WT and CF cells is present in the primary model (Figure 2B). Repolymerization was also assessed in HNE cells from three subjects without CF and six subjects with CF of various genotypes at the same passage. Cells from each subject with CF showed the same reduced rate of repolymerization compared with control subjects without CFs (Figure 2C). These data demonstrate in three systems (pharmacological inhibition of CFTR, immortalized CF cell lines, and expanded primary HNE cells) that microtubule polymerization is slower in CF cells compared with WT.
Figure 2.
Cystic fibrosis (CF) primary human nasal epithelial (HNE) cells have slower microtubule polymerization rates compared with wild-type cells. (A) F508 del, G551D, R117H, and control HNE cells were excised and briefly cultured. Cells were subjected to microtubule polymerization assay (0–8 min) and immunostained for α-tubulin. Regardless of genotype, CF cells did not form asters as readily as control cells. Representative images are shown. (B) Reproducibility of the microtubule polymerization assay was established where one patient with CF (F508 del) and one control patient’s cells were passaged three times, each passage showing a significant decrease in aster formation rates from 1 to 2 minutes. The ratio of asters formed to total cells was quantified and plotted as a function over time (0–8 min; n = 3; *P < 0.005). (C) Quantified microtubule polymerization data are shown from the same passage of F508 del, G551D, R117H, and control HNE cell microtubule polymerization assay. Decreased polymerization is shown in all CF genotypes compared with control cells. Data represent the mean (±SEM).
Reduced EPAC1 Activation in CF as a Mechanism Leading to Reduced Tubulin Polymerization Rates in CF Cells
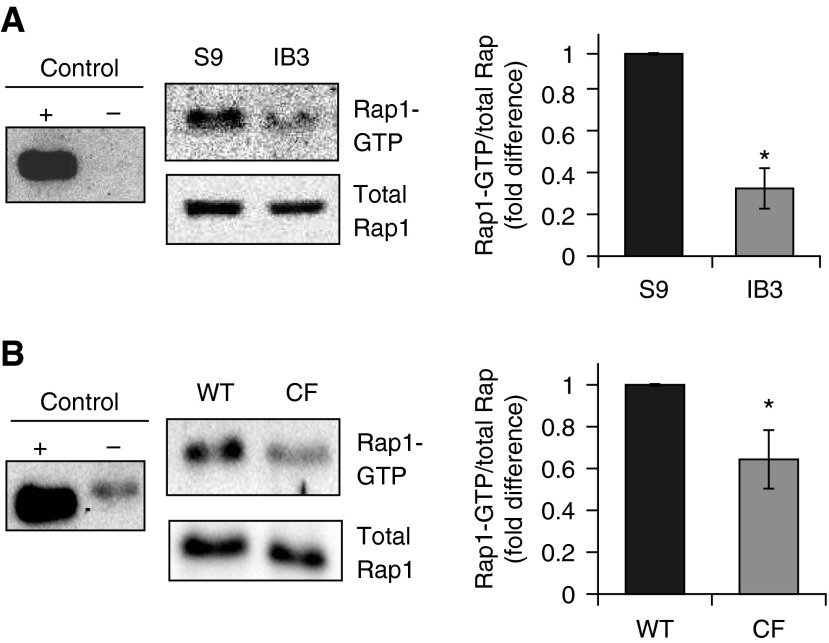
We have previously demonstrated that cAMP signaling could impact cholesterol transport and intracellular organelle movement (11, 12). One cAMP-signaling component known to impact tubulin polymerization is EPAC1 (33). Decreased EPAC1 activation in CF cells would be consistent with our findings of reduced rates of tubulin polymerization. As EPAC1 is also an activator of the small GTPase, Rap1, we can examine Rap1-GTP content as an indicator of EPAC1 activation. To determine the activation of EPAC1/Rap1 signaling in CF, we examined Rap1-GTP content in CF IB3 cells and in S9 controls (Figure 3A). Rap1-GTP content normalized to total Rap1 levels was significantly reduced in CF cells compared with controls (0.33- ± 0.10-fold of control; n = 3; P < 0.01). To confirm these cellular studies, whole-lung lysate from Cftr +/+ and Cftr−/− mice were also examined. Rap1-GTP/total Rap1 content was significantly reduced (0.64- ± 0.15-fold of WT, n = 4 for each genotype, P = 0.03) in CF mouse lungs compared with WT lungs (Figure 3B). These data demonstrate in primary mouse tissue a basal decrease in Rap1 activation that is indicative of a decrease in EPAC1 activity.
Figure 3.
Ras-related protein 1 (Rap1)-GTP content is reduced in both IB3 cells and in CF mouse whole lung compared with respective non-CF controls. (A) IB3 cell Rap1-GTP/total Rap1 content is reduced to 0.33 (±0.09)-fold (n = 3; *P = 0.01) compared with control S9 cell content. (B) Whole-lung lysates were examined for Rap1-GTP content normalized to total Rap1 amount isolated from Cftr+/+ and Cftr−/− mice. The Rap1-GTP/total Rap1 ratio is 0.64 (±0.15)-fold of wild-type (WT) levels (n = 4 for each genotype; *P = 0.03) in CF mouse lungs. Data represent the mean (±SEM).
We also had the opportunity to examine EPAC1/Rap1 basal activity in primary bronchial epithelial cells from a subject without CF (WT HBE) and a subject with CF with the F508 del (CFBE). Rap1-GTP content was reduced in CFBE samples compared with WT HBE cells (0.68- ± 0.12-fold of WT; n = 3; P = 0.03; Figure E2). Rap1-GTP assays were performed on three separate filters for each sample to ensure assay reproducibility. However, cells from only one subject of each genotype were available for examination. Although no specific conclusion can be made from this single experiment, data are included to show consistency with mouse and cultured cell data.
EPAC1-Mediated Signaling Regulates Microtubule Polymerization Rates
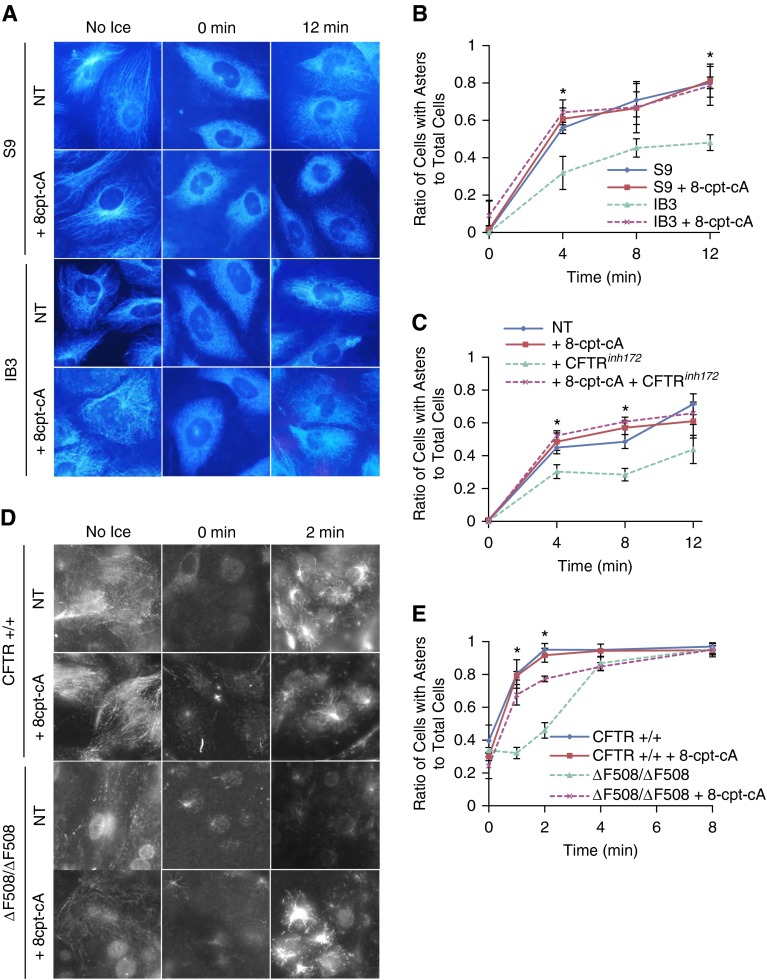
Microtubule polymerization is enhanced through EPAC1, where direct binding to tubulin promotes microtubule formation, and our data presented previously here demonstrate reduced EPAC1 activation in CF models. To test whether EPAC1 activation could restore tubulin polymerization rates, IB3 and S9 control cells were treated with the EPAC1-selective activator, 8-cpt-2-O-Me-cAMP (8-cpt-cA; 25 μM for 24 h). With 8-cpt-cA treatment, IB3 aster formation rates increased to control S9 levels. At 12 minutes, 78.5 (±10.4)% cells had aster formation in the presence of 8-cpt-cA compared with only 48.1 (±4.2)% of cells in mock-treated IB3 cells (Figures 4A and 4B). Addition of 8-cpt-cA to S9 control cells did not further accelerate microtubule polymerization, indicating the specificity and importance of the EPAC1 pathway in CF microtubule polymerization.
Figure 4.
Exchange protein activated by cAMP (EPAC) 1/Rap1 activator 8-cpt-2-O-Me-cAMP (8-cpt-cA) improves microtubule polymerization rates. (A) S9 and IB3 cells were treated with 25 μM 8-cpt-cA for 24 hours, subjected to a microtubule formation assay, and immunostained for α-tubulin. Representative images of aster formation are shown for various time points (no ice, 0, and 12 min). (B) Quantification of microtubule formation assay for S9 and IB3 cells with and without 8-cpt-cA was determined by the ratio of cells with asters to total cells at various time points (n = 3; *P < 0.05). (C) 9/HTEo− cells were treated with vehicle, 20 μM CFTRinh172, and/or 25 μM 8-cpt-cA for 24 hours and subjected to microtubule polymerization assay. Cells were immunostained with α-tubulin. Quantification of microtubule polymerization assay for 9/HTEo− cells was determined by the ratio of cells with asters to total cells at various time points (n = 4; *P < 0.05). (D) Primary F508 del and control HNE cells were excised and briefly cultured. Cells were treated with 25 μM 8-cpt-cA or vehicle for 24 hours and subjected to microtubule polymerization assay (0–8 min) and immunostained for α-tubulin. Representative images of aster formation are shown for various time points (no ice, 0 and 2 min). (E) Quantification of F508 del and control HNE cells treated with 25 μM 8-cpt-cA or vehicle was performed, and the ratio of asters formed to total cells was plotted as a function over time (0–8 min) (n = 3; *P < 0.05). Data represent the mean (±SEM).
Furthermore, to verify that CFTR is responsible for reduced microtubule polymerization rates through the EPAC1 pathway, CFTR was chronically inhibited with 20 μM CFTRinh172 for 72 hours in wild-type 9/HTEo− epithelial cells and stimulated EPAC1 with 8-cpt-cA for the final 24 hours of treatment. Consistent with the IB3 data, aster formation rates improved 50%, increasing microtubule polymerization rate from 44.0 (±9.1)% when CFTR was inhibited to 66.3 (±7.4)% when treated with CFTRinh172 and the EPAC1-selective agonist, 8-cpt-cA, at 12 minutes (Figure 4C). This rate was not significantly different from untreated 9/HTEo cells, where its polymerization rate was 71.3 (±6.1)% at 12 minutes. EPAC1 signaling is thus a key intermediate linking CFTR to microtubule regulation.
Because primary HNE cells from subjects with CF exhibit the same slower polymerization rate compared with control subjects as seen in cultured cell models, the impact of 8-cpt-cA on polymerization in primary cells was examined. The percentage of cells with asters improved from 45.9 (±4.7)% to 77.3 (±1.7)% (P < 0.05) in F508 del/F508 del HNE cells with 8-cpt-cA treatment at 2 minutes. This improvement was significant, but did not reach the level of WT HNE cells, where 95.1 (±3.7)% of cells show asters at 2 minutes (Figures 4D and 4E). 8-cpt-cA had no impact on WT HNE cell polymerization rates. These data demonstrate that 8-cpt-cA–sensitive regulation of microtubule polymerization is conserved in primary CF HNE cells.
Mobilization of Intracellular Cholesterol in Response to EPAC1 Activation
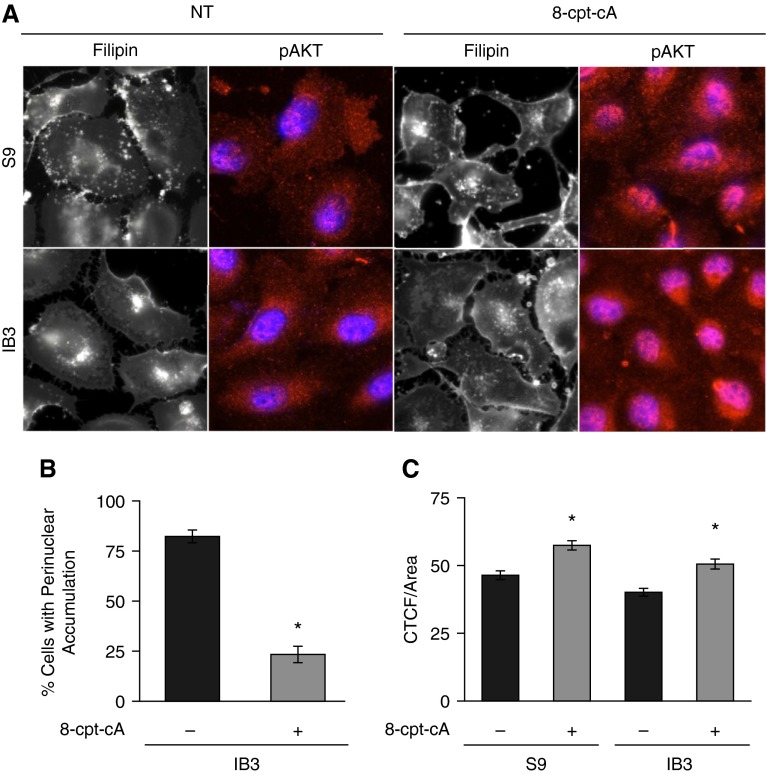
The above data demonstrate that EPAC1/Rap1 signaling is deficient in CF cells and tissues leading to reduced rates of microtubule polymerization, as measured by aster formation. Our previous work demonstrated a relationship between microtubule regulation and cholesterol accumulation as a marker of intracellular transport (16). Therefore, it was hypothesized that EPAC1 activation with 8-cpt-cA should disperse accumulated cholesterol in CF cells due to restored microtubule polymerization rates. CF IB3 cells and control S9 cells were treated with 8-cpt-cA (25 μM; 24 h) and assessed for cholesterol accumulation by imaging of endogenous cholesterol with filipin stain. Treatment of IB3 cells with 8-cpt-cA completely reversed CF-related cholesterol accumulation (Figures 5A and 5B), showing that EPAC1 signaling is likely a key intermediate in the regulation of this phenotype through microtubule regulation. As a control for the efficacy of 8-cpt-cA treatment, the activation and nuclear translocation of AKT was monitored by staining for pAKT and its nuclear localization. 8-cpt-cA treatment significantly increased AKT activation (Figures 5A and 5C), as determined by cellular fluorescence.
Figure 5.
Effect of EPAC1-selective agonist, 8-cpt-cA, on cholesterol distribution in IB3 cells. (A) IB3 cells were either treated with vehicle or with 25 μM 8-cpt-cA for 24 hours and assessed for cholesterol by filipin staining. To ensure efficacy of 8-cpt-cA, cells were immunostained with phospho-AKT (pAKT) antibodies and 4′,6-diamidino-2-phenylindole. Representative images are shown from four separate experiments. (B) Filipin imaging was quantified by assessing cells for perinuclear cholesterol accumulation by a grader blinded to experimental group. Data are presented as percentage of cells with perinuclear accumulation. IB3 cells exhibited 82.3 (±3.2)% of cells with accumulation compared with 23.4 (±6.1)% of IB3 cells treated with 8-cpt-cA (n = 20 separate images for each condition; *P < 0.001). (C) pAKT intensity was determined by assessing cells for corrected total cellular fluorescence (CTCF). Data are presented as intensity (n = 100 separate images for each condition; *P < 0.001). Data represent the mean (±SEM).
Effect of Microtubule Dysregulation on CF Cellular Phenotypes
We propose that altered microtubule dynamics in CF cells are directly related to downstream signaling events previously identified in CF models. To test this hypothesis, expression of TPPP was reduced in immortalized human tracheal 9/HTEo− cells. We have previously published that microtubules in CF cell tissues exhibit reduced acetylation compared with controls (16). In this article, we present data showing reduced rates of microtubule formation. To test the effect of these microtubule alterations on CF phenotypes, TPPP was targeted, because it directly regulates the two microtubule alterations that we have observed in CF cells. In addition to promoting tubulin polymerization, TPPP is also known to act as an HDAC6 inhibitor (36). HDAC6 is a cytosolic deacetylase that regulates tubulin acetylation. It was hypothesized that knockdown of TPPP would both reduce microtubule formation rates and decrease microtubule acetylation, replicating the CF cellular condition regarding microtubule regulation. Two important characteristics of CF that we have previously identified are perinuclear accumulation of free cholesterol and increased protein expression of signaling protein, Ras homolog gene family, member A (RhoA), in CF cells and tissues (10–12, 37). If these microtubule polymerization processes are key to downstream cellular events characteristic of CF cells, then these two phenotypes should be replicated. A modest 30% reduction of TPPP protein expression resulted in a significant increase in CF-like perinuclear cholesterol accumulation and an approximately twofold increase in RhoA expression (Figure 6A), comparable to increases of RhoA that we previously reported in CF models (37). Acetylated tubulin levels and rates of polymerization were also reduced as the expected result of lowered TPPP expression (Figures 6B and 6C). These data support that changes to microtubule regulation can lead to downstream phenotypes characteristic of CF.
Figure 6.
Decreased tubulin polymerization–promoting protein (TPPP) 1 expression leads to CF phenotypes. Expression of TPPP1 was successfully knocked down in non-CF human tracheal epithelial (9/HTEo−) cells, which were stably transfected with short hairpin RNA (shRNA) targeting TPPP1 (shTPPP1). (A) Lysates from control 9/HTEo− cells with scrambled sequence (9/HTEo− Neg Ctrl) and 9/HTEo− with TPPP1 shRNA (9/HTEo− shTPPP1) were analyzed via Western blot for acetylated tubulin (Ac-tub), TPPP1, and Ras homolog gene family, member A (RhoA). Representative blots are shown along with quantification to the right (TPPP1/actin, RhoA/actin, and Ac-tub/α-tub). Significance was determined by t test (RhoA/actin, n = 11, *P < 0.01; TPPP1/actin, n = 11, *P < 0.01; Ac-tub/α-tub, n = 11, *P < 0.01). (B) 9/HTEo− Neg Ctrl and 9/HTEo− shTPPP1 cells were subjected to a microtubule polymerization assay and immunostained for α-tubulin. Quantification of microtubule polymerization assay for 9/HTEo− Neg Ctrl and 9/HTEo− shTPPP1 cells was determined by the ratio of cells with asters to total cells at various time points. Significance was determined by t-test comparing 9/HTEo− Neg Ctrl to 9/HTEo− shTPPP1 ratios at various time points (0–20 min; n = 4; *P < 0.05). (C) 9/HTEo− Neg Ctrl and 9/HTEo− shTPPP1 cells were immunostained with Ac-tub antibodies (left). Cells were stained with filipin and quantified by determining percentage of cells with perinuclear cholesterol accumulation (*P < 0.01; right). Representative images are shown from 40 separate images for each cell model. Data represent the mean (±SEM).
Discussion
Understanding the molecular mechanisms linking CFTR function in epithelial cells to cellular changes associated with CF has been a long-standing goal. The hypothesis of this study is that impaired microtubule dynamics, due to reduced EPAC1 signaling, links CFTR function to CF cellular events. We focus on cholesterol accumulation as a key intermediate linking CFTR function with a number of downstream signaling events, such as RhoA activation, impaired signal transducer and activator of transcription-1 (STAT1) stimulation, and reduced nitric oxide synthase-2 (NOS2) expression (37, 38).
We have previously demonstrated that microtubule acetylation is reduced in CF cells and tissues, leading to downstream signaling changes, including increased NF-κB signaling and cholesterol accumulation (16). Reduced microtubule acetylation can be a marker of microtubule instability, so we examined microtubule dynamics in cultured and primary nasal epithelial CF cells. As shown, we have found a distinct reduction in polymerization rate in CF cell models, primary tissue, and in response to pharmacological CFTR inhibition. The predicted consequence of this change to microtubule dynamics is an impairment of intracellular transport that we measure by examining intracellular cholesterol transport. In addition to our previous reports of perinuclear cholesterol accumulation as a marker of impaired organelle transport mechanisms in CF, other groups have also reported alterations to intracellular transport. For example, Toll-like receptor 4 accumulation in early endosomes of CF macrophages indicative of impaired endosomal transport have been reported (9), along with deficiencies in autophagy (7).
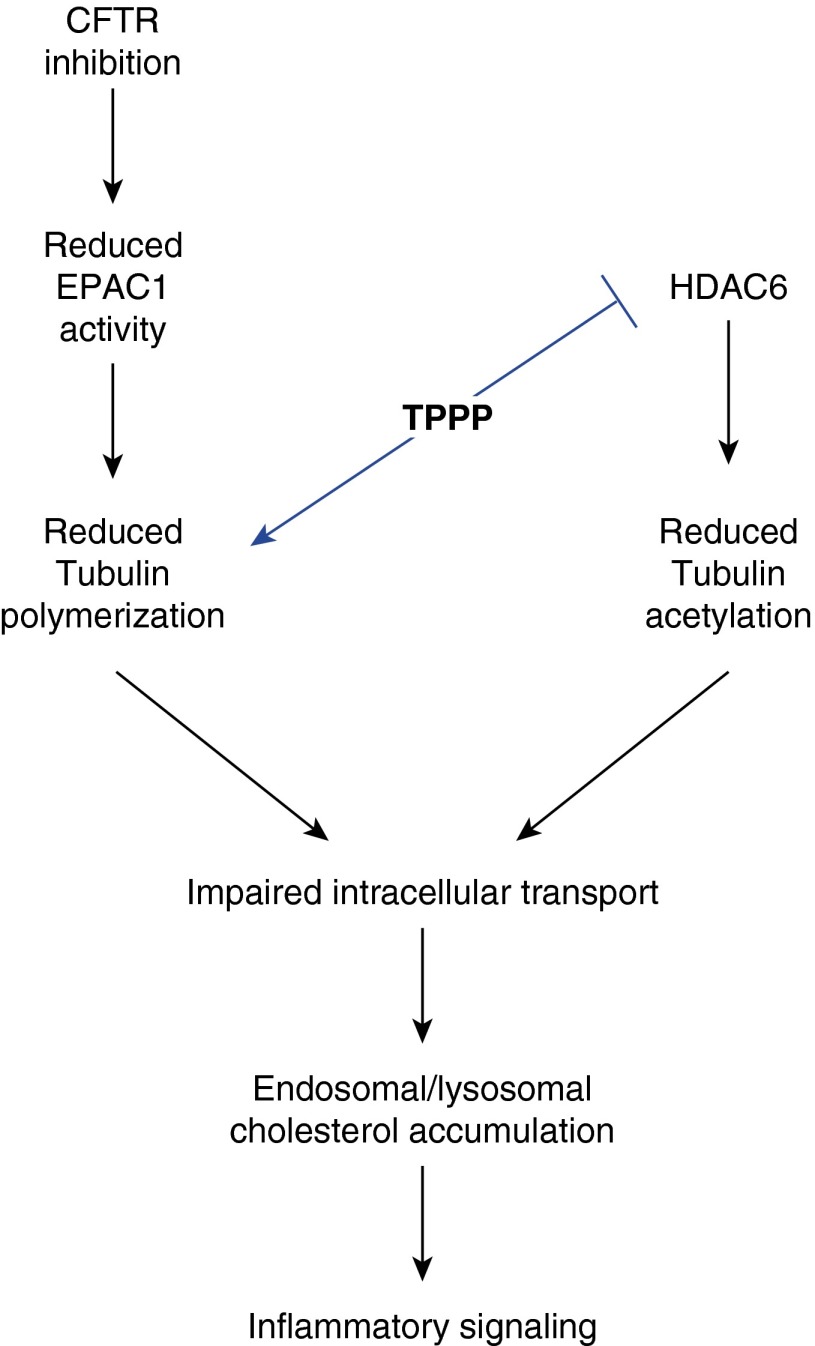
To further verify that microtubule alterations are responsible for cellular changes characteristic of CF, we knocked down expression of the protein, TPPP. TPPP is a regulator of tubulin polymerization and also an HDAC6 inhibitor (36). Knocking down expression was predicted to mimic the microtubule changes in CF cells. A modest reduction in TPPP expression of 30% resulted in the development of multiple CF phenotypes, including cholesterol accumulation. In addition to helping verify the role of microtubule regulation in CF cellular phenotypes, TPPP is of particular interest to CF research, because its gene is located on chromosome 5 in a region that has been identified to contain modifiers of CF airway disease severity (39). Although it is only one candidate among others in the region, it is intriguing that TPPP directly influences the two microtubule changes that we have identified in CF cells: tubulin polymerization and acetylation. The relationship between TPPP and identified CF-related changes to microtubules is shown in Figure 7. It was predicted that knocking down TPPP expression should replicate the reduced rate of microtubule formation and reduce acetylation of tubulin identically to what is observed in CF cells and lead to the same downstream cellular events. The finding that these downstream events do occur shows a strong correlation between microtubule regulation and CF phenotypes. The case for microtubule importance in modulating aspects of CF disease is supported by the recent paper that identified the dynactin subunit, dynactin 4 (DCTN4), as a modifier of the timing of Psuedomonas aeruginosa infection in patients with CF using exome sequencing (40). These two genetics studies, along with our cellular studies, support a potentially critical role for microtubule regulation and intracellular transport in modulating the progression of CF airway disease.
Figure 7.
Schematic diagram of the relationship between TPPP and CF-related alterations to microtubule regulation. Down-regulation of TPPP would reduce tubulin polymerization and lead to loss of inhibition of histone deacetylase (HDAC) 6. Increased HDAC6 activity would result in reduced microtubule acetylation, a finding we have reported previously (16).
Mechanistically, we identify that deficient EPAC1 signaling is responsible for reduced microtubule elongation rates and, subsequently, cholesterol accumulation. We previously demonstrated an important role of cAMP signaling in the control of cholesterol accumulation in CF cell models (11); however, a specific mechanism for its action was not identified. Here, we demonstrate a significant role for EPAC1 function in the control of microtubule regulation and, consequently, cholesterol processing in CF cells. No other study, to our knowledge, has identified a defect of EPAC1/Rap1 activation in CF cells and tissues. EPAC1 and Rap1 signaling can encompass a variety of pathways, and a deficiency of EPAC1 function could explain the breadth of effect of lost CFTR function on cellular functions. The implication of reduced EPAC1/Rap1 functions in CF phenotypes needs to be further explored. For example, EPAC1 deficiency is associated with insulin resistance, and could be a factor in the development of CF-related diabetes (41).
Determining the ultimate link between CFTR function and these cellular changes remains a challenge. In this article, we have demonstrated reduced activation of the cAMP-responsive protein, EPAC1. How the loss of CFTR function would impact EPAC1 activation is unknown, because broad changes to cAMP signaling have not been found. However, Schmid and colleagues (42) have recently demonstrated that impaired CFTR function leads to reduced soluble adenylate cyclase (sAC) activation due to altered bicarbonate transport regulation. Reduced sAC activation could result in impaired EPAC1/Rap1 signaling and account for reduce microtubule polymerization rates. Reduced cAMP production by impaired sAC activation could also impact microtubule stability in CF through protein kinase A (PKA) regulation. PKA-mediated phosphorylation of the microtubule-destabilizing protein, stathmin, inhibits this function and results in stabilized microtubules (43). Although our data do not suggest a change in depolymerization, reduced sAC/PKA signaling could impact stathmin regulation and increase microtubule instability in CF cells. The relationship among CFTR function, cAMP signaling, and microtubule regulation needs to be further explored.
It is concluded from this study that CF cells exhibit a deficiency of EPAC1/Rap1 signaling that leads to impaired microtubule reformation rates and, subsequently, to cholesterol accumulation. These findings are significant in that this is the first report, to our knowledge, of reduced EPAC1/Rap1 signaling in CF cells and altered microtubule dynamics. We also demonstrate the direct relationship between microtubule regulation and downstream cellular events, such as cholesterol accumulation, by reducing TPPP expression in a model cell line. With the advent of new CFTR correctors and potentiators being used or tested clinically for the treatment of CF, it will become more important to understand how CFTR function influences cellular events to assess treatment efficacy and to identify complimentary sites of therapeutic intervention.
Acknowledgments
Acknowledgments
The authors thank P. Bead (Case Western Reserve University) for technical assistance.
Footnotes
This work was supported by National Institutes of Health (NIH) grants R01EB009481, R01HL109362, and 2P30DK027651, and Cystic Fibrosis Foundation Research Development Program R447-CR11. The Leica microscopes in the Genetics Department Imaging Facility at Case Western Reserve University were made available through NIH–National Center for Research Resources Shared Instrumentation grant S10 RR021228.
Author Contributions: Concept and design—S.M.R. and T.J.K.; data acquisition—S.M.R., T.I., D.A.C., and C.U.C.; data analysis and interpretation—S.M.R., J.D.B., and T.J.K.; drafting—S.M.R., T.I., D.A.C., J.D.B., and T.J.K.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0462OC on May 8, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Anderson MP, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991;251:679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- 2.Morris RG, Tousson A, Benos DJ, Schafer JA. Microtubule disruption inhibits AVT-stimulated Cl− secretion but not Na+ reabsorption in A6 cells. Am J Physiol. 1998;274:F300–F314. doi: 10.1152/ajprenal.1998.274.2.F300. [DOI] [PubMed] [Google Scholar]
- 3.Morris AP, Cunningham SA, Tousson A, Benos DJ, Frizzell RA. Polarization-dependent apical membrane CFTR targeting underlies cAMP-stimulated Cl− secretion in epithelial cells. Am J Physiol. 1994;266:C254–C268. doi: 10.1152/ajpcell.1994.266.1.C254. [DOI] [PubMed] [Google Scholar]
- 4.Tousson A, Fuller CM, Benos DJ. Apical recruitment of CFTR in T-84 cells is dependent on cAMP and microtubules but not Ca2+ or microfilaments. J Cell Sci. 1996;109:1325–1334. doi: 10.1242/jcs.109.6.1325. [DOI] [PubMed] [Google Scholar]
- 5.Cheng J, Wang H, Guggino WB. Modulation of mature cystic fibrosis transmembrane regulator protein by the PDZ domain protein CAL. J Biol Chem. 2004;279:1892–1898. doi: 10.1074/jbc.M308640200. [DOI] [PubMed] [Google Scholar]
- 6.Young A, Gentzsch M, Abban CY, Jia Y, Meneses PI, Bridges RJ, Bradbury NA. Dynasore inhibits removal of wild-type and DeltaF508 cystic fibrosis transmembrane conductance regulator (CFTR) from the plasma membrane. Biochem J. 2009;421:377–385. doi: 10.1042/BJ20090389. [DOI] [PubMed] [Google Scholar]
- 7.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, et al. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 8.Abdulrahman BA, Khweek AA, Akhter A, Caution K, Kotrange S, Abdelaziz DH, Newland C, Rosales-Reyes R, Kopp B, McCoy K, et al. Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis. Autophagy. 2011;7:1359–1370. doi: 10.4161/auto.7.11.17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruscia EM, Zhang PX, Satoh A, Caputo C, Medzhitov R, Shenoy A, Egan ME, Krause DS. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol. 2011;186:6990–6998. doi: 10.4049/jimmunol.1100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;292:L476–L486. doi: 10.1152/ajplung.00262.2006. [DOI] [PubMed] [Google Scholar]
- 11.Manson ME, Corey DA, White NM, Kelley TJ. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-Pick type C cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L809–L819. doi: 10.1152/ajplung.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manson ME, Corey DA, Bederman I, Burgess JD, Kelley TJ. Regulatory role of β-arrestin-2 in cholesterol processing in cystic fibrosis epithelial cells. J Lipid Res. 2012;53:1268–1276. doi: 10.1194/jlr.M021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 15.Boyault C, Sadoul K, Pabion M, Khochbin S. HDAC6, at the crossroads between cytoskeleton and cell signaling by acetylation and ubiquitination. Oncogene. 2007;26:5468–5476. doi: 10.1038/sj.onc.1210614. [DOI] [PubMed] [Google Scholar]
- 16.Rymut SM, Harker A, Corey DA, Burgess JD, Sun H, Clancy JP, Kelley TJ. Reduced microtubule acetylation in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2013;305:L419–L431. doi: 10.1152/ajplung.00411.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci. 1992;103:953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- 18.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002;21:6820–6831. doi: 10.1093/emboj/cdf682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulinski JC. Microtubule modification: acetylation speeds anterograde traffic flow. Curr Biol. 2007;17:R18–R20. doi: 10.1016/j.cub.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Bulinski JC, Gundersen GG. Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays. 1991;13:285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- 21.Burchill BR, Oliver JM, Pearson CB, Leinbach ED, Berlin RD. Microtubule dynamics and glutathione metabolism in phagocytizing human polymorphonuclear leukocytes. J Cell Biol. 1978;76:439–447. doi: 10.1083/jcb.76.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Brabander M, Geuens G, Nuydens R, Willebrords R, De Mey J. Microtubule stability and assembly in living cells: the influence of metabolic inhibitors, taxol and pH. Cold Spring Harb Symp Quant Biol. 1982;46:227–240. doi: 10.1101/sqb.1982.046.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–245. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- 24.Rowning BA, Wells J, Wu M, Gerhart JC, Moon RT, Larabell CA. Microtubule-mediated transport of organelles and localization of beta-catenin to the future dorsal side of Xenopus eggs. Proc Natl Acad Sci USA. 1997;94:1224–1229. doi: 10.1073/pnas.94.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsenti E, Kobayashi S, Mitchison T, Kirschner M. Role of the centrosome in organizing the interphase microtubule array: properties of cytoplasts containing or lacking centrosomes. J Cell Biol. 1984;98:1763–1776. doi: 10.1083/jcb.98.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA. Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schek HT, III, Gardner MK, Cheng J, Odde DJ, Hunt AJ. Microtubule assembly dynamics at the nanoscale. Curr Biol. 2007;17:1445–1455. doi: 10.1016/j.cub.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan J, Calder GM, Doonan JH, Lloyd CW. EB1 reveals mobile microtubule nucleation sites in Arabidopsis. Nat Cell Biol. 2003;5:967–971. doi: 10.1038/ncb1057. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho-Santos Z, Machado P, Alvarez-Martins I, Gouveia SM, Jana SC, Duarte P, Amado T, Branco P, Freitas MC, Silva ST, et al. BLD10/CEP135 is a microtubule-associated protein that controls the formation of the flagellum central microtubule pair. Dev Cell. 2012;23:412–424. doi: 10.1016/j.devcel.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Patel SR, Richardson JL, Schulze H, Kahle E, Galjart N, Drabek K, Shivdasani RA, Hartwig JH, Italiano JE., Jr Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood. 2005;106:4076–4085. doi: 10.1182/blood-2005-06-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comartin D, Gupta GD, Fussner E, Coyaud É, Hasegan M, Archinti M, Cheung SW, Pinchev D, Lawo S, Raught B, et al. CEP120 and SPICE1 cooperate with CPAP in centriole elongation. Curr Biol. 2013;23:1360–1366. doi: 10.1016/j.cub.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Kerssemakers JWJ, Munteanu EL, Laan L, Noetzel TL, Janson ME, Dogterom M. Assembly dynamics of microtubules at molecular resolution. Nature. 2006;442:709–712. doi: 10.1038/nature04928. [DOI] [PubMed] [Google Scholar]
- 33.Sehrawat S, Cullere X, Patel S, Italiano J, Jr, Mayadas TN. Role of Epac1, an exchange factor for Rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell. 2008;19:1261–1270. doi: 10.1091/mbc.E06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilsson HE, Dragomir A, Lazorova L, Johannesson M, Roomans GM. CFTR and tight junctions in cultured bronchial epithelial cells. Exp Mol Pathol. 2010;88:118–127. doi: 10.1016/j.yexmp.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Dafinger C, Liebau MC, Elsayed SM, Hellenbroich Y, Boltshauser E, Korenke GC, Fabretti F, Janecke AR, Ebermann I, Nürnberg G, et al. Mutations in KIF7 link Joubert syndrome with Sonic Hedgehog signaling and microtubule dynamics. J Clin Invest. 2011;121:2662–2667. doi: 10.1172/JCI43639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokési N, Lehotzky A, Horváth I, Szabó B, Oláh J, Lau P, Ovádi J. TPPP/p25 promotes tubulin acetylation by inhibiting histone deacetylase 6. J Biol Chem. 2010;285:17896–17906. doi: 10.1074/jbc.M109.096578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreiselmeier NE, Kraynack NC, Corey DA, Kelley TJ. Statin-mediated correction of STAT1 signaling and inducible nitric oxide synthase expression in cystic fibrosis epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1286–L1295. doi: 10.1152/ajplung.00127.2003. [DOI] [PubMed] [Google Scholar]
- 38.Kelley TJ, Drumm ML. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J Clin Invest. 1998;102:1200–1207. doi: 10.1172/JCI2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright FA, Strug LJ, Doshi VK, Commander CW, Blackman SM, Sun L, Berthiaume Y, Cutler D, Cojocaru A, Collaco JM, et al. Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nat Genet. 2011;43:539–546. doi: 10.1038/ng.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emond MJ, Louie T, Emerson J, Zhao W, Mathias RA, Knowles MR, Wright FA, Rieder MJ, Tabor HK, Nickerson DA, et al. National Heart, Lung, and Blood Institute (NHLBI) GO Exome Sequencing Project. Exome sequencing of extreme phenotypes identifies DCTN4 as a modifier of chronic Pseudomonas aeruginosa infection in cystic fibrosis. Nat Genet. 2012;44:886–889. doi: 10.1038/ng.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kai AK, Lam AK, Chen Y, Tai AC, Zhang X, Lai AK, Yeung PK, Tam S, Wang J, Lam KS, et al. Exchange protein activated by cAMP 1 (Epac1)-deficient mice develop β-cell dysfunction and metabolic syndrome. FASEB J. 2013;27:4122–4135. doi: 10.1096/fj.13-230433. [DOI] [PubMed] [Google Scholar]
- 42.Schmid A, Sutto Z, Schmid N, Novak L, Ivonnet P, Horvath G, Conner G, Fregien N, Salathe M. Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J Biol Chem. 2010;285:29998–30007. doi: 10.1074/jbc.M110.113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yip YY, Yeap YY, Bogoyevitch MA, Ng DC. cAMP-dependent protein kinase and c-Jun N-terminal kinase mediate stathmin phosphorylation for the maintenance of interphase microtubules during osmotic stress. J Biol Chem. 2014;289:2157–2169. doi: 10.1074/jbc.M113.470682. [DOI] [PMC free article] [PubMed] [Google Scholar]