Abstract
Our previous publication demonstrated that peroxisome proliferator–activated receptor γ (PPARγ) inhibits the pathogenesis of chronic hypoxia (CH)–induced pulmonary hypertension by targeting store-operated calcium entry (SOCE) in rat distal pulmonary arterial smooth muscle cells (PASMCs). In this study, we aim to determine the role of a membrane scaffolding protein, caveolin-1, during the suppressive process of PPARγ on SOCE. Adult (6–8 weeks) male Wistar rats (200–250 g) were exposed to CH (10% O2) for 21 days to establish CH-induced pulmonary hypertension. Primary cultured rat distal PASMCs were applied for the molecular biological experiments. First, hypoxic exposure led to 2.5-fold and 1-fold increases of caveolin-1 protein expression in the distal pulmonary arteries and PASMCs, respectively. Second, effective knockdown of caveolin-1 significantly reduced hypoxia-induced SOCE for 58.2% and 41.5%, measured by Mn2+ quenching and extracellular Ca2+ restoration experiments, respectively. These results suggested that caveolin-1 acts as a crucial regulator of SOCE, and hypoxia–up-regulated caveolin-1 largely accounts for hypoxia-elevated SOCE in PASMCs. Then, by using a high-potency PPARγ agonist, GW1929, we detected that PPARγ activation inhibited SOCE and caveolin-1 protein for 62.5% and 59.8% under hypoxia, respectively, suggesting that caveolin-1 also acts as a key target during the suppressive process of PPARγ on SOCE in PASMCs. Moreover, by using effective small interfering RNAs against PPARγ and caveolin-1, and PPARγ antagonist, T0070907, we observed that PPARγ plays an inhibitory role on caveolin-1 protein by promoting its lysosomal degradation, without affecting the messenger RNA level. PPARγ inhibits SOCE, at least partially, by suppressing cellular caveolin-1 protein in PASMCs.
Keywords: pulmonary hypertension, peroxisome proliferator–activated receptor γ, caveolin-1, store-operated calcium entry, pulmonary arterial smooth muscle cells
Clinical Relevance
During the treatment of pulmonary hypertension, peroxisome proliferator–activated receptor (PPAR) γ agonist has been reported to effectively normalize pulmonary arterial pressure and maintain pulmonary arterial structure by targeting store-operated calcium entry (SOCE) and intracellular calcium homeostasis in experimental animal models of pulmonary hypertension. This extending study deepened the molecular biological mechanism and enriched our knowledge of how PPARγ inhibits SOCE in pulmonary arterial smooth muscle cells.
Pulmonary hypertension (PH) is a severe pulmonary vascular disease characterized by sustained increase in the pulmonary arterial pressure and excessive thickening and remodeling of the distal small pulmonary arteries (PAs). These functional and structural changes then lead to right ventricular hypertrophy and, eventually, heart failure. Nowadays, it is well accepted that the dysregulation of the proliferation and migration of the PA smooth muscle cells (PASMCs) are the main reasons contributing to the abnormally excessive thickening and remodeling of the distal PAs during PH pathogenesis (1–3).
Previous studies have described that the increase of the intracellular free calcium concentration ([Ca2+]i) acts a major factor to trigger the cell proliferation by playing roles as a classic second messenger, which enters the nucleus and facilitates the transcription of a number of proproliferative genes (4). Among the three main extracellular calcium influx pathways (voltage-operated calcium entry, receptor-operated calcium entry, and store operated calcium entry [SOCE]), chronic hypoxia-triggered SOCE largely contributes to the hypoxia-enhanced [Ca2+]i and promotes the pathogenesis of CH-induced PH (CHPH). SOCE is mediated store-operated calcium channels (SOCCs), which is mainly composed by transient receptor potential cation channels (TRPCs). Our data indicated that CH-upregulated TRPC1 and TRPC6 are the key molecular basis of CH-enhanced SOCE and [Ca2+]i in PASMCs (5–7).
Peroxisome proliferator–activated receptor (PPAR) γ belongs to a kind of ligand-activated nuclear hormone receptor superfamily, which is ubiquitously expressed in pulmonary vascular endothelial and smooth muscle cells, and acts as a transcription factor to modulate the transcription of a number of genes (8). Previous studies reported that PPARγ is down-regulated in the lungs (9, 10) and distal PAs (11) of experimental PH models, whereas restoration of PPARγ by specific agonists can markedly attenuate the PH pathogenesis by normalizing the elevated right ventricle systolic pressure and distal PA remodeling (12–14). Moreover, we further demonstrated the molecular mechanisms that PPARγ inhibits PA remodeling and PASMC proliferation, mainly by targeting SOCE and TRPC proteins (11, 13).
Besides the TRPCs, recent studies further reported the membrane scaffolding protein, caveolin-1, also exerts key role in regulating the intracellular calcium homeostasis by operating the SOCE process in human PASMCs, and participates in the disease development of human idiopathic PA hypertension (IPAH) (15). Therefore, in this study, we focused on and investigated two major questions: (1) does caveolin-1 play a role in hypoxia-elevated SOCE; and (2) does caveolin-1 act in the context of PPARγ-mediated inhibition of SOCE in rat distal PASMCs.
Materials and Methods
Establishment of the CHPH Rat Model
Adult male Wistar rats (200–250 g) were purchased from Harlan Inc. (Frederick, MD) and exposed to CH (10% O2) or ambient room air for 21 days, as previously described (5, 16). This protocol was in accordance with National Institutes of Health guidelines for use of live animals, and was approved by Animal Care and Use Committee of Johns Hopkins University and the First Affiliated Hospital of Guangzhou Medical University. The surgical procedure was performed under anesthesia with sodium pentobarbital (65 mg/kg intraperitoneal), and all efforts were made to minimize animal suffering.
Primary Culture of Rat Distal PASMCs
Rat distal (>fourth generation) PASMCs were isolated and cultured as previously described (7). The cell purity of PASMCs in all the experiments were assessed by two methods: (1) calcium influx for over 50 nM in response to 60 mM KCL exposure, and (2) immunocytochemistry staining by antibodies of smooth muscle cell specific markers, smooth muscle α-actin (Sigma, St. Louis, MO) and myosin heavy chain (Abcam, Cambridge, MA). Positive smooth muscle cells were counted for greater than 90%.
Calcium Imaging and SOCE Experiments
As previously described (7), fura-2–based fluorescence imaging experiments were recorded by a fluorescence microscopy system and analyzed by InCyte2 software (Intracellular Imaging Inc., Cincinnati, OH). Two methods were used to measure the SOCE: extracellular Ca2+ restoration and Mn2+ quenching. For the extracellular Ca2+ restoration, PASMCs were perfused for 10–15 minutes with Ca2+-free Kreb solution containing 5 μM nifedipine, 10 μM cyclopiazonic acid, and 1 mM EGTA to deplete the intracellular sarcoplasmic reticulum (SR) calcium stores. We measured the [Ca2+]i at 0.2-minute intervals, and then the extracellular calcium was restored, and the SOCE measured by the delta increase in intracellular Ca2+. For the Mn2+ quenching, we measured the fura-2 fluorescence excited at 360 nm at 0.5-minute intervals before and after the addition of MnCl2 (200 μM), in the presence of nifedipine (5 μM) and cyclopiazonic acid (10 μM), which acted as Ca2+ surrogate and reduces Fura-2 fluorescence on binding to the dye to the perfusate. SOCE was evaluated by the rate at 10-minute after fura-2 fluorescence was quenched by Mn2+.
Small Interfering RNA Silencing
The small interfering RNA (siRNA) SMARTpool (Dharmacon, Lafayette, CO) against PPARγ was designed and synthesized by Thermo Scientific Inc. (Lafayette, CO), whereas the siRNA against caveolin-1 was purchased from Invitrogen Inc. (Carlsbad, CA). Nontargeting siRNA SMARTpool–treated groups served as controls. PASMCs grown to 50–70% confluence were serum starved for transfection with each specific siRNA using the Gene Silencer kit (Genlantis, San Diego, CA) according to the company’s instructions. Knockdown efficiency was determined by real-time quantitative PCR (qPCR) and Western blot.
Real-Time qPCR
Total RNA was extracted from PASMCs using RNeasy Plus Mini Kit (Qiagen, Valencia, CA). Reverse transcription was performed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). cDNAs were then amplified by qPCR using QuantiTect SYBR Green PCR Master Mix (Qiagen) in an iCyclerIQ real-time PCR detection system (Bio-Rad) (17). The specific primer pairs for qPCR were:
Caveolin-1: sense, 5′-CGCACACCAAGGAGATTGAT-3′
Anti-sense, 5′-ACTGTGTGTCCCTTCTGGTT-3′
PPARγ: sense, 5′-CCTGAAGCTCCAAGAATACCAAA-3′
Anti-sense, 5′-AGAGTTGGGTTTTTTCAGAATAATAAGG-3′
18S: sense, 5′-GCAATTATTCCCCATGAACG-3′
Anti-sense, 5′-GGCCTCACTAAACCATCCAA-3′
Total, Nuclear, and Cytoplasm Fraction Protein Extraction and Western Blot
Distal PAs or PASMCs were homogenized in TPER lysis buffer (Pierce, Rockford, IL) containing 5% protease inhibitor cocktail (Sigma-Aldrich) for total protein extraction. The nuclear and cytoplasm protein fractions extraction were performed following the protocols provided with the kit (Thermo Scientific Inc.). Western blot was then performed according to previous protocols (11). The specific primary antibodies we used were caveolin-1 (R&D Systems Inc., Minneapolis, MN), PPARγ (Santa Cruz Biotechnology, Dallas, TX), histone H3 (Abcam), and β-tubulin (Sigma-Aldrich).
Immunocytochemistry–Immunofluorescence Staining
PASMCs were washed with PBS and fixed with 4% paraformaldehyde for 10 minutes. Then, after washing three times with PBS, the cells were permeabilized with 0.5% Triton-X for 10 minutes. Permeabilized cells were blocked with 20% goat serum at room temperature for 1 hour, and incubated with primary antibodies diluted in 4% goat serum for 2–4 hours at room temperature. After washing, cells were incubated with Cy3-conjugated goat anti-mouse or anti-rabbit IgG (Invitrogen) for 1 hour in the dark. The coverslips were washed and fully dried in the dark, mounted with fluoroGuard (Invitrogen) containing dye for nucleus staining with 4′,6-diamidino-2-phenylindole (Invitrogen), and sealed with nail polish for observation under a confocal microscope.
Materials and Reagents
Besides the reagents specified above, all the reagents and chemicals were obtained from Sigma-Aldrich. Fura-2 a.m. (Invitrogen) was prepared before the experiment as a 2.5-mM stock solution in 20% DMSO containing 20% pluronic F-127 (Invitrogen).
Statistical Analysis
All data are presented as mean (± SEM). The n value represents the number of animals providing PAs, the number of dishes of cells for the molecular biological experiments, and the number of cells for the intracellular calcium imaging experiments. Statistical analyses were performed using analysis of Student’s t test and one-way ANOVA. Pairwise comparison of means was conducted with t tests. For the groups containing multiple comparisons, one-way ANOVA was used for the statistical analysis. Differences were considered significant when P was less than 0.05.
Results
Hypoxic Stress Up-Regulates Caveolin-1 Expression in Rat Distal PAs and PASMCs
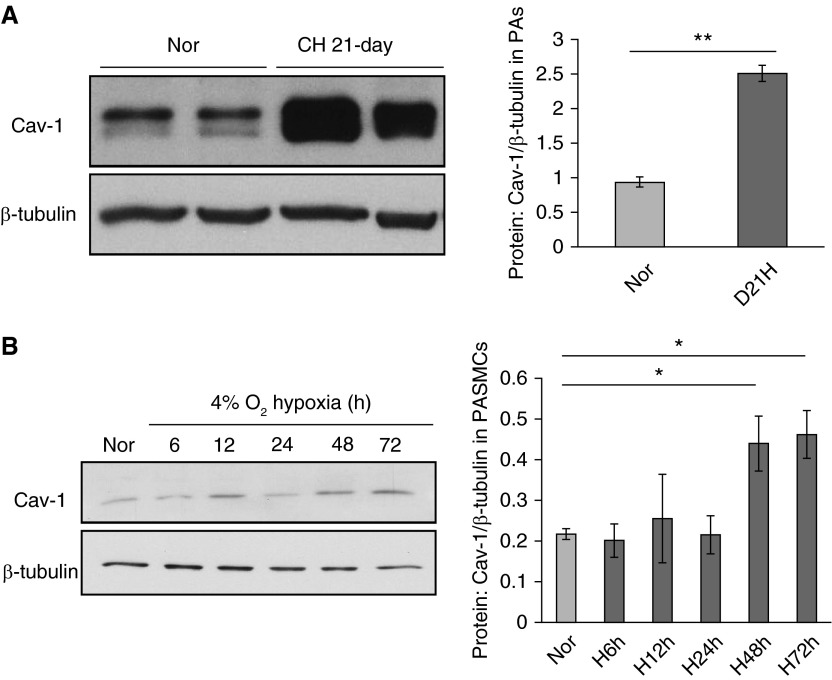
We used 21-day persistent CH exposure (10% O2) to establish the CHPH rat model following previous descriptions (5, 18). Rats exposed to room air served as controls (normoxia [Nor]). The total protein expression of caveolin-1 in the distal PAs isolated from both the CH and Nor rats was measured by Western blot. In comparison with Nor controls, hypoxia led to a 2.5-fold increase in caveolin-1 protein expression in PAs isolated from CH rats (Figure 1A). In parallel, cultured rat distal PASMCs were exposed to Nor or prolonged hypoxia (4% O2) to measure the effect of hypoxia on caveolin-1 expression in vitro. Results showed that 48-hour and 72-hour hypoxic exposure increased caveolin-1 protein level for 102.8 and 112.9%, respectively, compared with the Nor group (Figure 1B).
Figure 1.
Hypoxic stress up-regulates caveolin-1 (Cav-1) expression in rat distal pulmonary arteries (PAs) and PA smooth muscle cells (PASMCs). (A) Distal PAs isolated from rats exposed to both normoxia (Nor) and chronic hypoxia (CH; 10% O2 for 21 days) were prepared for total protein extraction and Western blot. The left panel shows the blots for Cav-1 and housekeeping protein β-tubulin; the right bar graph represents the quantified data measuring the gray density of Cav-1 and normalized to β-tubulin. (B) Rat distal PASMCs were serum starved and exposed to either Nor or different durations of prolonged hypoxia (Hyp; 4% O2). The left panel shows the blots for Cav-1 and housekeeping protein β-tubulin; the right bar graph represents the quantified data measuring the gray density of Cav-1 and normalized to β-tubulin. Data are presented as means (± SEM); n = 6 in each group. *P < 0.05 versus Nor control; **P < 0.01 versus Nor control.
Hypoxia-Induced Caveolin-1 Expression Contributes to Hypoxia-Enhanced SOCE in PASMCs
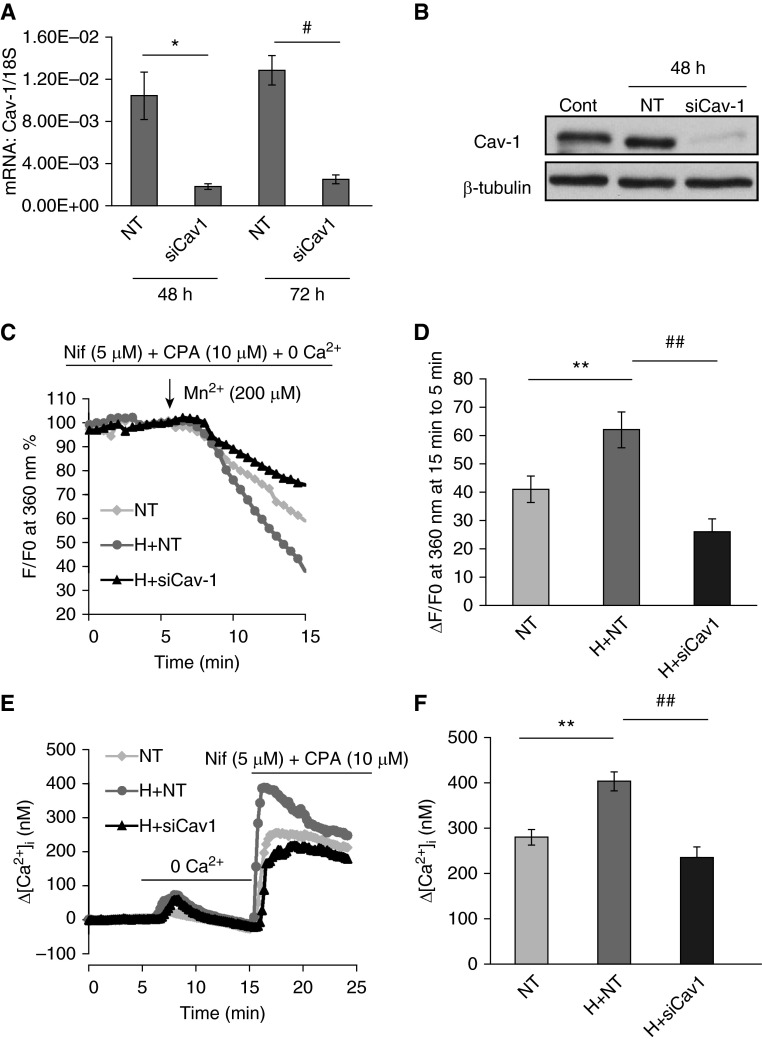
As it was observed that hypoxic stress increased caveolin-1 expression in both the PAs from CHPH rats and PASMCs, we therefore wondered if the hypoxia-increased caveolin-1 expression is a contributor to hypoxia-enhanced SOCE. Specific siRNA against caveolin-1 was applied to knockdown caveolin-1 and determine the role of caveolin-1 on SOCE in PASMCs. An over 80% knockdown efficiency of caveolin-1 at both the mRNA (Figure 2A) and protein (Figure 2B) levels was observed upon caveolin-1 siRNA treatments (25 nM, 48–72 h) compared with the nontarget siRNA treatment group. By using both Mn2+ quenching (Figures 2C and 2D) and extracellular Ca2+ restoration (Figures 2E and 2F), two methods for measuring SOCE, our results indicated that, first, prolonged hypoxia (4% O2, 48 hours) markedly elevated the SOCE from both Mn2+ quenching and extracellular Ca2+ restoration experiments, and then that caveolin-1 knockdown significantly reduced hypoxia-enhanced SOCE in both two methods. This suggests that hypoxia-increased caveolin-1 plays an important contributor to hypoxia-enhanced SOCE in PASMCs.
Figure 2.
Cav-1 contributes to store-operated calcium entry (SOCE) in PASMCs under hypoxia. (A and B) The effective knockdown of Cav-1 upon small interfering RNA (siRNA; siCav-1) and nontarget (NT) siRNA (siNT; serving as controls) treatments at both the mRNA level (A) and the protein level (B) Data are presented as means (± SEM); n = 3 in each group. *P < 0.05 versus NT; #P < 0.05 versus NT. (C and D) The effects of siCav-1 (25 nM, 48 hours) on prolonged hypoxia (4% O2)-induced SOCE. Trace graph (C) and the bar graph (D) shows the SOCE, as measured by the Mn2+ quenching experiment, in the presence of L-type voltage-operated calcium channel blocker, nifedipine (Nif; 5 μM) and the specific inhibitor of Ca2+-ATPase cyclopiazonic acid (CPA; 10 μM). Four groups were designated as NT (n = 82 cells), Hyp + NT (H + NT; n = 88 cells) and Hyp + siCav-1 (H + siCav-1; n = 80 cells), respectively. Data are presented as means (± SEM). **P < 0.01 versus NT; ##P < 0.01 versus H + NT. (E and F) The trace graph (E) and the bar graph (F) of SOCE, as measured by the extracellular restoration experiment, in the presence of nifedipine (5 μM) and CPA (10 μM). Three groups were designated as NT (n = 77 cells), Hyp + NT (H + NT; n = 88 cells), and Hyp + siCav-1 (H + siCav1; n = 61 cells), respectively. Data are presented as means (± SEM). **P < 0.01 versus NT; ##P < 0.01 versus H + NT. Cont, non-treatment control.
PPARγ Agonist GW1929 Inhibits Hypoxia-Elevated SOCE by Suppressing Caveolin-1 Protein in PASMCs
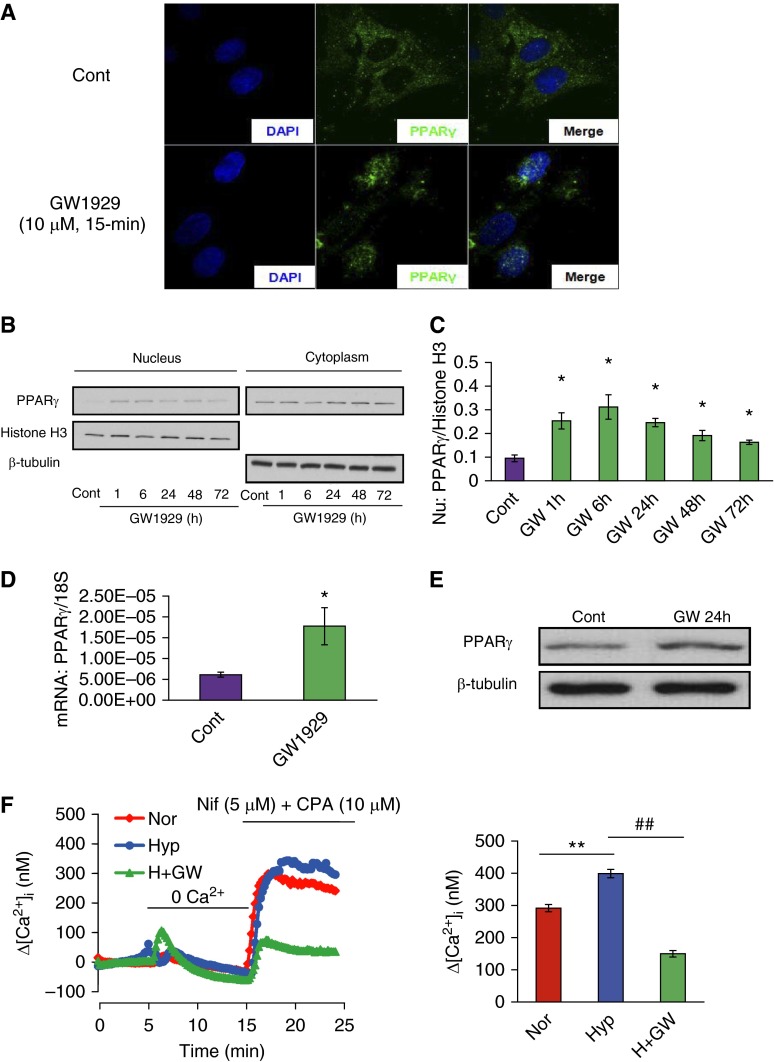
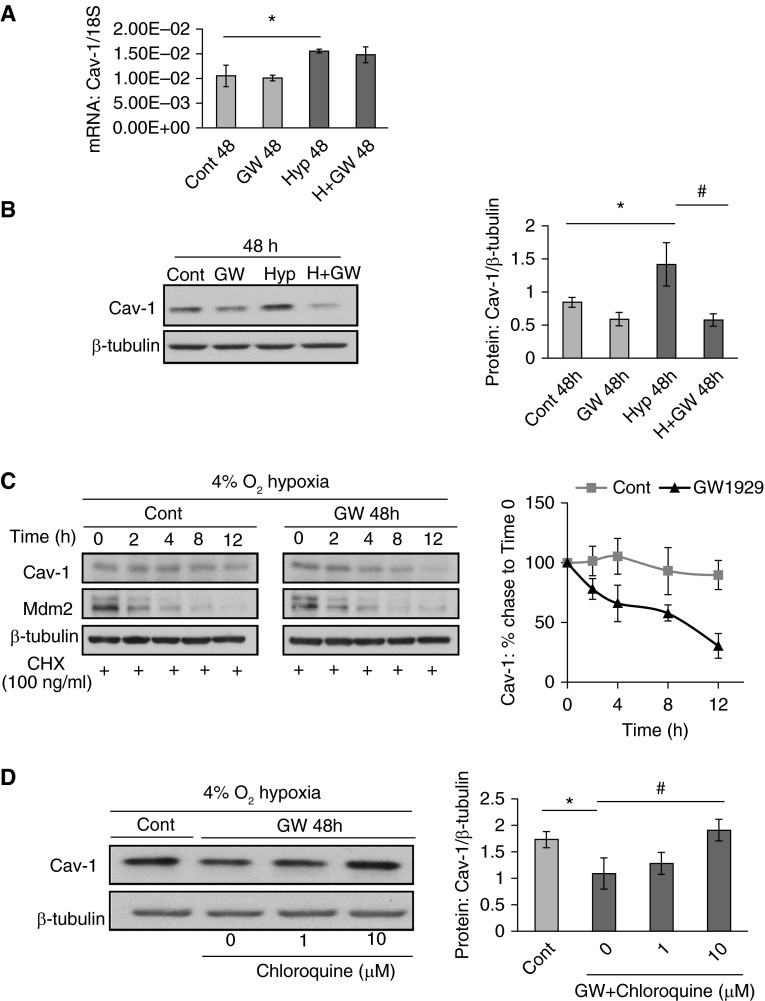
We used the high-potency selective PPARγ agonist, GW1929, for the induction of PPARγ to determine the role of PPARγ on hypoxia-elevated SOCE and hypoxia–up-regulated caveolin-1 expression. As seen in Figure 3, first, results from the immunocytochemistry–immunofluorescence staining (ICC-IF), nucleus/cytoplasm fractions extraction, and total mRNA and protein measurement experiments suggested that GW1929 (10 μM) not only promoted the nuclear translocation (Figures 3A–3C), but also up-regulated the total mRNA (Figure 3D) and protein production (Figure 3E) of PPARγ in PASMCs. GW1929 (10 μM, 48 hours) treatment significantly attenuated the hypoxia-elevated SOCE by 62.5% (Figure 3F). On one hand, hypoxic stress (4% O2, 48 hours) led to marked increases of the mRNA (Figure 4A) and protein (Figure 4B) levels of caveolin-1 for 47.6 and 68.2%, respectively. On the other hand, GW1929 suppressed such hypoxia–up-regulated caveolin-1 protein levels by 59.8% (Figure 4B), without affecting the hypoxia-elevated caveolin-1 mRNA level (Figure 4A). Despite the observation that GW1929 affected caveolin-1 at the protein level, but not the transcription level, we hypothesized that GW1929 could potentially influence the protein stability and degradation of caveolin-1. By using time-dependent treatments of the protein biosynthesis inhibitor, cycloheximide (CHX; 100 ng/ml), we measured the protein half-life of caveolin-1 in both the control and GW1929 treatment groups based on a time-point chase experiment. Results showed that GW1929 (10 μM, 48 hours) significantly shortened the protein half-life of caveolin-1 (Figure 4C). To ensure the CHX was effectively blocking the cellular biosynthesis process, we introduced the mouse double minute 2 homolog as a positive control. Mouse double minute 2 homolog has been previously reported with a short protein half-life (19), which actually showed similar rapid degradation upon CHX treatment in both the control and GW1929-treated groups (Figure 4C), suggesting that the CHX functionally blocked the protein biosynthesis. Furthermore, previously published papers showed that caveolin-1 protein normally undergoes degradation through the lysosome pathway (20, 21). We therefore investigated whether the lysosome inhibition can somehow rescue the suppressive effect of GW1929 on caveolin-1 protein. Inconsistent with our assumption, lysosome inhibitor, chloroquine, dose-dependently rescued the GW1929-suppressed caveolin-1 protein expression (Figure 4D), suggesting that PPARγ inhibits caveolin-1 protein level by promoting its lysosomal degradation.
Figure 3.
Peroxisome proliferator–activated receptor (PPAR) γ agonist GW1929 inhibits hypoxia-elevated SOCE. (A) In the immunocytochemistry–immunofluorescence (ICC-IF) staining, blue color shows 4′,6-diamidino-2-phenylindole (DAPI) reflecting the nucleus staining, green color shows PPARγ, and the merge pictures represent the overlap of the DAPI and PPARγ under the treatments of control and GW1929 (10 μM, 15 minutes). (B) Western blot showing the expression of PPARγ, histone H3, and β-tubulin in both the nuclear and cytoplasm fractions upon GW1929 (10 μM) treatments of different time duration. (C) Quantified data measuring the gray density of PPARγ as normalized to histone H3 in the nuclear fraction. Data are presented as means (± SEM); n = 3 in each group. *P < 0.05 versus respective controls. (D and E) Real-time quantitative PCR (qPCR) (D) and Western blot (E) data show the effects of GW1929 (10 μM, 24 hours) on the total PPARγ expression at both mRNA and protein levels. (F) Trace graph (left) and the bar graph (right) of SOCE, measured by the extracellular restoration experiment, in the presence of nifedipine (5 μM) and CPA (10 μM). Cells were treated with GW1929 (10 μM, 48 hours) in prolonged hypoxic (4% O2) exposure, and three groups were designated as Nor (n = 64 cells), Hyp (n = 78 cells), and Hyp + GW1929 (H + GW; n = 60 cells), respectively. Data are presented as means (± SEM). **P < 0.01 versus Nor; ##P < 0.01 versus Hyp. GW, GW1929.
Figure 4.
PPARγ agonist, GW1929, inhibits hypoxia-elevated Cav-1 protein level by promoting its lysosomal degradation. (A) Real-time qPCR data showing the effects of GW1929 (10 μM, 48 hours) on Cav-1 mRNA expression under Nor and Hyp conditions. Data are presented as means (± SEM); n = 3 in each group. (B) Western blots showing the expression pattern (left) of Cav-1 and β-tubulin in response to GW1929 (10 μM) treatments under Nor and Hyp and quantified data measuring the gray density of Cav-1 as normalized to β-tubulin. Data are presented as means (± SEM); n = 5 in each group. *P < 0.05 versus control at 48 hours; #P < 0.05 versus Hyp 48 hours. (C) Western blots showing the expression pattern (left) of Cav-1, mouse double minute 2 homolog (Mdm2), and β-tubulin after the treatments with/without GW1929 (10 μM, 48 hours) and underwent cycloheximide (CHX; 100 ng/ml) chase for a time series. (Right) Line graph shows the quantified data measuring the gray density of Cav-1 as normalized to β-tubulin. Data are presented as means (± SEM); n = 4 in each group. (D) Western blots showing the expression pattern (left) of Cav-1 and β-tubulin after the treatments of GW1929 (10 μM, 48 hours) in the presence of different doses of chloroquine. (Right) Line graph shows the quantified data as measuring the gray density of Cav-1 as normalized to β-tubulin. Data are presented as means (± SEM); n = 4 in each group. *P < 0.05 versus control; #P < 0.05 versus GW + chloroquine (0 μM).
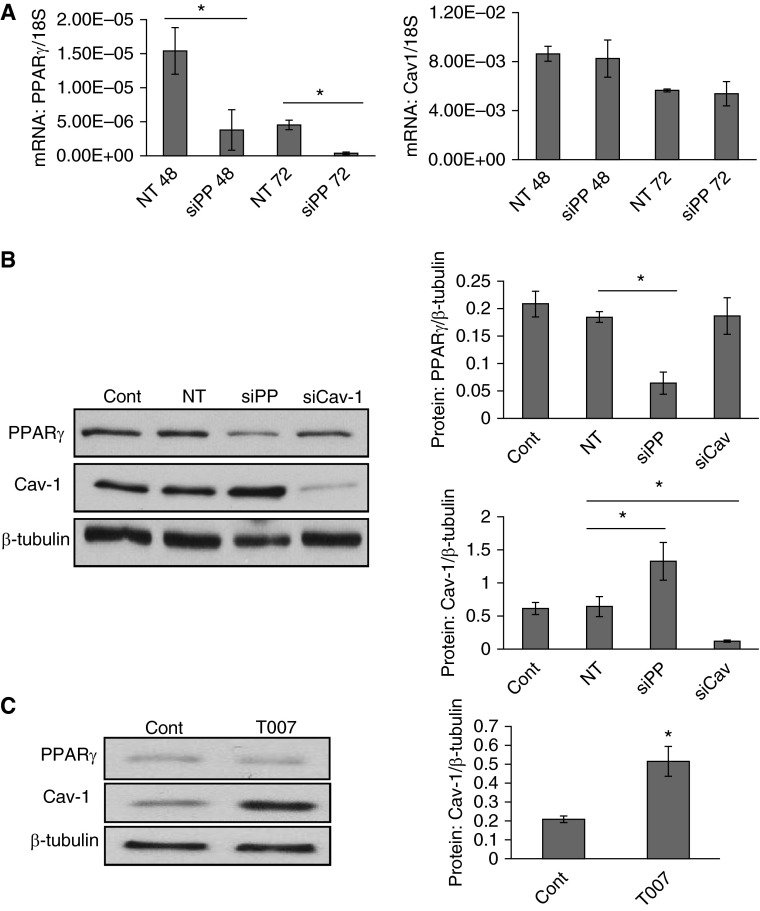
PPARγ Knockdown or Inhibition Lead to Increased Caveolin-1 Protein Expression in PASMCs
In addition to the PPARγ activation strategies, we also introduced the specific knockdown (PPARγ siRNA) and antagonist (T0070907) and measured the impacts of PPARγ loss-of-function on cellular caveolin-1 levels. Consistent with our hypothesis, results represented both PPARγ siRNA knockdown (50 nM, 48 hours, Figure 5B) and antagonist T0070907 (10 μM, 48 hours, Figure 5C) treatments induced an average of 106.7% and 146.2% increases of caveolin-1 protein expression in PASMCs, respectively. However, on the contrary, siRNA knockdown of caveolin-1 (25 nM, 48 hours) did not result in significant change of PPARγ level (Figure 5B), suggesting manipulation of cellular caveolin-1 level does not hat a feedback effect on the cellular PPARγ level in PASMCs.
Figure 5.
PPARγ siRNA or antagonist T0070907 lead to increased Cav-1 protein expression in PASMCs. (A) Real-time qPCR data showing the effects of specific PPARγ siRNA (siPP; 50 nM, 48–72 hours) on the mRNA expression of PPARγ (left) and Cav-1 (right). Data are presented as means (± SEM); n = 3 in each group. *P < 0.05 versus NT 48 hours (NT48) or NT 72 hours (NT72). (B) Western blots showing the expression pattern (left) of Cav-1, PPARγ, and β-tubulin in response to siPP (50 nM, 48 hours) and siCav-1 (25 nM, 48 hours) treatments, and the quantified data (right) measuring the gray density of PPARγ (upper) and Cav-1 (bottom) as normalized to β-tubulin. Data are presented as means (± SEM); n = 4 in each group. *P < 0.05 versus NT. (C) Western blots showing the expression pattern (left) of Cav-1 and β-tubulin in response to control or T0070907 (10 μM, 48 hours) and the quantified data (right) are represented as measuring the gray density of Cav-1 normalized to β-tubulin. Data are presented as means (± SEM); n = 4 in each group. *P < 0.05 versus control.
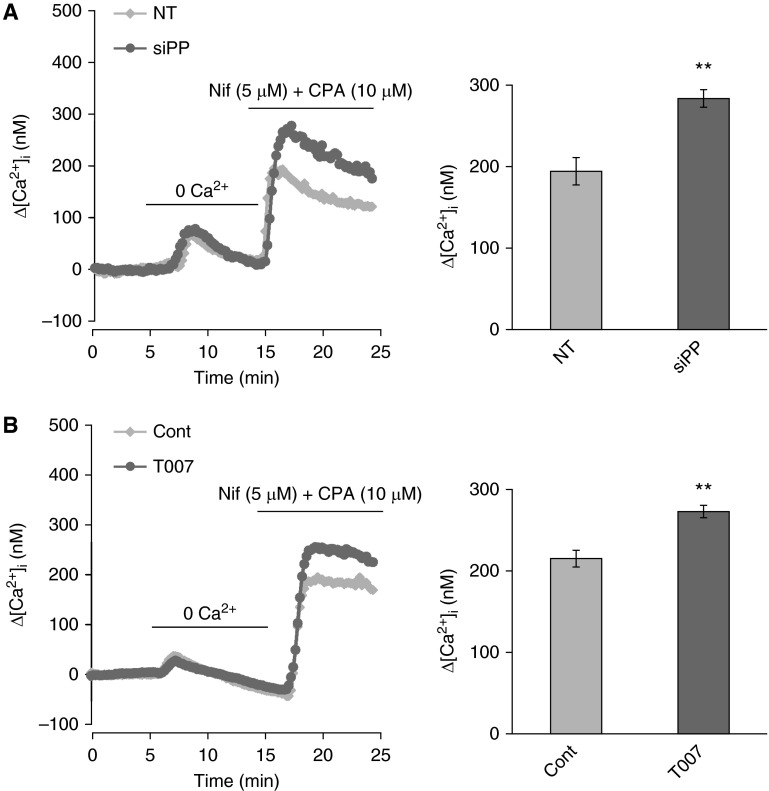
PPARγ Knockdown or Inhibition Resulted in Enhanced SOCE in PASMCs
To further investigate if the induction of cellular caveolin-1 levels upon PPARγ knockdown or inhibition can also lead to functional consequences in PASMCs, we further detected the effects of specific knockdown (PPARγ siRNA, siPP) and antagonist (T0070907) of PPARγ on SOCE. Basically, in line with the effects of PPARγ knockdown and T0070907 on caveolin-1 expression, as seen in Figure 6A, our results revealed that specific siRNA knockdown of PPARγ (50 nM, 48 h) also resulted in a marked increase of SOCE to 283.26 nM compared with the nontargeted siRNA treatment control groups (194.01 nM). In parallel, as seen in Figure 6B, T0070907 treatment (10 μM, 48 h) also led to a marked increase of SOCE to 272.93 nM, compared with the control group (215.15 nM). In combination, we summarized that, in PASMCs, knockdown or inhibition of PPARγ can manipulate cellular caveolin-1 expression, which subsequently leads to elevated SOCE and mediates the proproliferative consequences.
Figure 6.
PPARγ siRNA or antagonist T0070907 lead to elevated SOCE in PASMCs. (A) The effects of PPARγ knockdown by siRNA (siPP; 50 nM, 48 hours) on SOCE. Left graph shows the intracellular calcium traces of both NT and siPP treatment groups; Right bar graph shows the mean data after calculating the Δ-calcium change representing the SOCE. (B) The effects of T0070907 (T007, 10 μM, 48 hours) on SOCE. Left graph shows the intracellular calcium traces of both control and T007 treatment groups; right bar graph shows the mean data after calculating the Δ-calcium change representing SOCE. Data are presented as means (± SEM); n = 4 in each group. **P < 0.01 versus NT (A) Cont (B).
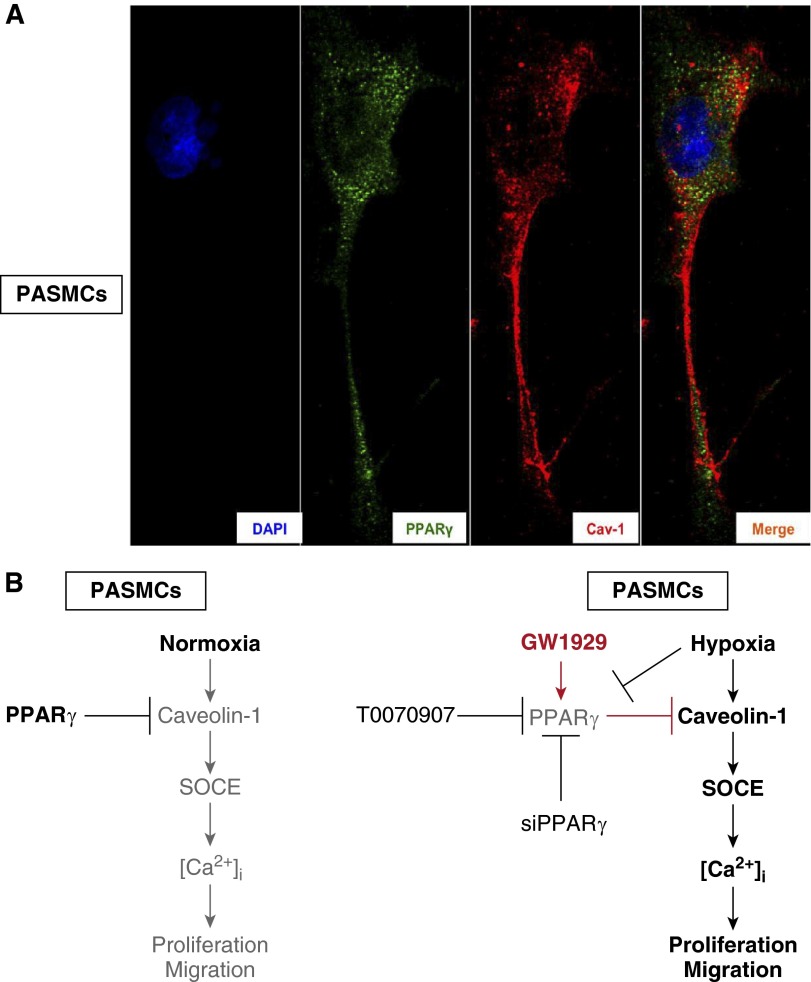
PPARγ and Caveolin-1 Protein Share an Indirect Binding Manner in PASMCs
Given that PPARγ activation led to dramatic decreases of caveolin-1 protein, we went through the published literature and found some evidence that indicated direct binding and regulation between PPARγ and caveolin-1 in cancer cell lines (22, 23); however, no evidence was reported about the direct protein–protein interaction between these two proteins in PASMCs. Therefore, we further tested the colocalization and protein–protein interaction between PPARγ and caveolin-1 in primary cultured PASMCs. As our results demonstrate, neither ICC-IF (Figure 6A) nor Co-Immunoprecipitation (Co-IP) (data not shown) showed specific colocalization or direct protein–protein interaction between PPARγ and caveolin-1 in the cellular compartments of PASMCs.
Discussion
In this study, we determined that hypoxic exposure up-regulates caveolin-1 expression in both distal PAs isolated from established rat CHPH model and cultured rat distal PASMCs. We further found that the hypoxia-upregulated caveolin-1 largely accounts for the hypoxia-elevated SOCE and intracellular free calcium concentration in PASMCs. Moreover, by using pharmacological agonist, antagonist, and siRNA knockdown strategies, we found that caveolin-1 also acts as an important target in the context of PPARγ-mediated inhibition of the hypoxia-elevated SOCE. In mechanism, PPARγ suppresses caveolin-1 level by promoting the lysosomal degradation of caveolin-1 protein in rat distal PASMCs.
Caveolae are cholesterol- and sphingosine-rich invaginations of the plasma membrane, of which the main principal structural components are caveolins (24). Recent studies have demonstrated the importance of caveolae in intracellular calcium mobilization by facilitating intracellular calcium release and extracellular calcium influx (25–27). Caveolae are described as the compartment involved in the regulation of SOCE, where Ca2+ enters the cell via caveolae accompany with the activation of endothelial nitric oxide synthase (eNOS) (28). So far, three isoforms of caveolins (caveolin-1, -2, and -3) have been identified, among which, caveolin-1 and -2 have relatively ubiquitous distribution pattern (29). Notably, caveolin-1 has been recently proven to participate in and play important roles during the pathogenesis of PH; however, the roles were controversial. Initially, Razani and colleagues (30) and Zhao and colleagues (31) reported that mice lacking caveolin-1 established PH with a hyperproliferative phenotype, vascular abnormalities, persistent increase of PA pressure, and right ventricle hypertrophy. The study by Achcar and colleagues (32) reported that caveolin-1 protein was down-regulated in the plexiform lesions, but not in the total lung tissue of patients with severe PH. In the past few years, researchers have observed and discussed the dual roles of caveolin-1 on the proliferation and migration of different vascular cell types during PH development (33). On the one hand, in the endothelial cells of monocrotaline-induced PH rat models, caveolin-1 expression was markedly decreased (34), whereas re-expression of caveolin-1 in the endothelium (35) or a short-term administration of a cell-permeable caveolin-1 peptide (36) can effectively reverse the PH characteristics in either caveolin-1 knockout or monocrotaline-induced PH models. Caveolin-1 shares tight coupling with eNOS, which acts as a natural inhibitor and controls the activity of eNOS (37, 38). In hypoxic endothelium, the decreased caveolin-1 protein results in eNOS uncoupling and chronic persistent activation, which leads to oxidative and nitrosative stress and results in vascular contraction and remodeling (33, 39, 40). However, on the other hand, Patel and colleagues (15) reported a remarkable increase of caveolin-1 in the PASMCs isolated from patients with IPAH, associated with increased SOCE and cell proliferation, which indicated that a proproliferative role of caveolin-1 might occur in the smooth muscle cells. Caveolin-1 can contribute to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1 in the caveolae compartment (41–43). Recent studies have further indicated that caveolin-1 not only directly interacts with TRPC1 (44, 45), but also participates in the membrane localization and the cluster interaction of the main SOCC molecules, TRPC stromal interaction molecule (STIM)-ORAI calcium release activated calcium modulator (ORAI), upon intracellular calcium store depletion and the intracellular calcium sensor stromal interaction molecule 1 (STIM1) translocation, which is believed to be a key event during intracellular calcium mobilization (46).
Our previous studies and those of others have demonstrated the protective roles of PPARγ ligands on CHPH disease progression (12, 13). These beneficial roles are likely mediated through inhibiting SOCE and the expression of SOCCs channel protein TRPCs, which then lead to suppressed proliferation and migration of PASMCs (11, 13). Previous studies have reported that, in cancer cell lines, caveolin-1 acts as a direct transcription target of the main hypoxic regulator, hypoxia-inducible factors 1 and 2, and facilitates the proproliferative consequences during the process of tumorigenesis (47, 48). Interestingly, the effects of PPARγ on caveolin-1 vary considerably in different cell types. Reports have shown that PPARγ ligands or active components can up-regulate caveolin-1 expression in human macrophage THP-1 cells (49) and a list of human carcinoma cells (22, 50, 51), but not in human colon cancer cell HCT-116 cells (52), and down-regulates caveolin-1 expression in prostate cancer cells (53) in either PPARγ-dependent or -independent mechanisms. However, in the lung tissue, Tian and colleagues (54) demonstrated that down-regulation of PPARγ expression is associated with up-regulation of caveolin-1 expression in an ovine PH model. Using the gene array screening method, they further reported that pharmacological inhibition of PPARγ leads to increased caveolin-1 gene transcription, suggesting an inhibitory role of PPARγ on caveolin-1 in mammalian lungs.
Therefore, in this study, we focused on the smooth muscle cells and aim to figure out the following questions: (1) despite the fact that increased caveolin-1 expression was observed and contributed to elevated SOCE in PASMCs from patients with IPAH, is caveolin-1 also regulated by hypoxic stress and involved in the hypoxia-triggered SOCE in rat distal PASMCs? (2) As a key factor in the modulation of SOCE, does caveolin-1 act as a treatment target and fit into PPARγ-mediated suppression of hypoxia-elevated SOCE and proliferation in PASMCs? Regarding the first question, we initially found that hypoxic stress (both in vivo CH exposure and in vitro prolonged hypoxic exposure) significantly up-regulates caveolin-1 expression in the distal PAs and cultured PASMCs (Figure 1). Then, by using specific siRNA targeting caveolin-1, we observed that caveolin-1 contributes to hypoxia-enhanced SOCE (Figure 2), suggesting that the hypoxia-elevated caveolin-1 expression largely accounts for the hypoxia-triggered SOCE in PASMCs. We knew that, upon activation, transcription factor PPARγ and its partner retinoid receptor retinoid X receptor form heterodimerization (55). Then, the PPARγ/retinoid X receptor heterodimer translocates into the nucleus and binds to PPAR response elements located in the promoter region of responsive genes to regulate the transcription of target genes (56). To answer the second question, similar to previous reports, PPARγ activation markedly attenuated hypoxia-elevated SOCE in PASMCs (Figure 3F). More interestingly, on the one hand, we did not see statistically significant alteration of either PPARγ agonist, GW1929 (Figure 4A), or PPARγ siRNA (Figure 5A) treatments on caveolin-1 mRNA level, suggesting no transcriptional regulation of PPARγ on caveolin-1. However, on the other hand, GW1929 significantly inhibited (Figure 4B), whereas PPARγ siRNA (Figure 5B) and PPARγ antagonist, T0070907 (Figure 5C), markedly increased caveolin-1 protein levels and SOCE (Figure 6) in cultured rat distal PASMCs. Moreover, by using the treatment of the protein biosynthesis inhibitor, cycloheximide, and the lysosome inhibitor, chloroquine, we further observed that PPARγ activation markedly affected the protein stabilization and shortened the caveolin-1 protein half-life in comparison with the control group (Figure 4C). Treatment of the specific lysosome inhibitor, chloroquine, dose-dependently rescued the GW1929-decreased caveolin-1 protein level (Figure 4D). These results comprehensively indicate that PPARγ could inhibit caveolin-1 protein level by promoting its lysosomal degradation. On the other side, specific siRNA knockdown of caveolin-1 did not affect the cellular PPARγ level (Figure 5B), suggesting a nonmutual regulation between PPARγ and caveolin-1. Moreover, although some previous publications have reported the direct protein–protein interaction and regulation between PPARγ and caveolin-1 in cancer cell lines, such as HT-29 and MCF-7 (22, 23), our results from both ICC-IF (Figure 7A) and Co-IP (data not shown) experiments did not show obvious colocalization or protein–protein binding between PPARγ and caveolin-1 in primary cultured rat distal PASMCs. We presumed that this variation might be explained as the differences across cell types, especially between the immortalized cancer cell lines and the physiological primary cultured PASMCs.
Figure 7.
PPARγ and Cav-1 protein share negative colocalization in PASMCs. (A) ICC-IF staining shows the distribution of DAPI (blue), PPARγ (green), Cav-1 (red), and the merge (merge) in PASMCs (n = 3 in each group). (B) Schematic representing the hypothesized regulative signaling axis under both normoxic and hypoxic conditions.
Taken together, as summarized in Figure 7B, we concluded that: (1) under normoxic conditions, a physiological level of PPARγ retained natural suppression of caveolin-1 and TRPC expression, inhibited caveolin-1 and TRPCs-mediated SOCE and intracellular calcium homeostasis, and repressed the proliferation and migration; but (2) under hypoxic stresses, hypoxia–down-regulated PPARγ levels lost the control of caveolin-1/TRPC proteins and resulted in sustained elevation of SOCE and [Ca2+]i, which led to excessive proliferation and migration; whereas (3) the treatment of PPARγ agonist can effectively rescue the suppressed cellular PPARγ level, which subsequently maintained the inhibitory effects on intracellular calcium homeostasis via the caveolin-1/TRPCs–SOCE–basal [Ca2+]i axis. As far as we know, this is the first report that links PPARγ with caveolin-1 in PASMCs during the pathogenesis of CHPH. This study provides new insights into the molecular mechanism of how PPARγ inhibits the SOCE and intracellular calcium homeostasis in a caveolin-1–dependent mechanism, which is essential for identifying novel therapeutic targets in the treatment of PH.
Footnotes
This work was supported by National Institutes of Health grant R01-HL093020, National Natural Science Foundation of China grants 81173112, 81470246, 81170052, and 81220108001, Guangdong Natural Science Foundation team grant 1035101200300000, Guangzhou Department of Education Yangcheng Scholarship grant 12A001S, Guangzhou Department of Natural Science grant 2014Y2-00167, and Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme 2014, China (W.L.). Also supported by an award from the State Scholarship Fund and China Scholarship Council grant 201208440091 (K.Y.).
Author Contributions: K.Y. and J.W. initiated and designed the project, analyzed data, and wrote the manuscript; K.Y. performed the animal, functional, and molecular experiments; H.J. performed the animal and molecular experiments; Q.J., X.Y., and M.Z. performed the molecular experiments and edited the manuscript; W.L. provided consultation and advice on the project and edited the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2015-0002OC on May 28, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med (Berl) 2013;91:297–309. doi: 10.1007/s00109-013-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimoda LA, Laurie SS. HIF and pulmonary vascular responses to hypoxia. J Appl Physiol (1985) 2014;116:867–874. doi: 10.1152/japplphysiol.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 4.Jernigan NL, Resta TC. Calcium homeostasis and sensitization in pulmonary arterial smooth muscle. Microcirculation. 2014;21:259–271. doi: 10.1111/micc.12096. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 6.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia–induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L848–L858. doi: 10.1152/ajplung.00319.2003. [DOI] [PubMed] [Google Scholar]
- 8.Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, et al. International union of pharmacology. LXI. Peroxisome proliferator–activated receptors. Pharmacol Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 9.Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, Nozell SE, Oparil S, Chen YF. Hypoxia induces downregulation of PPAR-γ in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-β signaling. Am J Physiol Lung Cell Mol Physiol. 2011;301:L899–L907. doi: 10.1152/ajplung.00062.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator–activated receptor gamma (PPARγ) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Yang K, Xu L, Zhang Y, Lai N, Jiang H, Zhong N, Ran P, Lu W. Sildenafil inhibits hypoxia-induced transient receptor potential canonical protein expression in pulmonary arterial smooth muscle via cGMP-PKG-PPARγ axis. Am J Respir Cell Mol Biol. 2013;49:231–240. doi: 10.1165/rcmb.2012-0185OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia–induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol. 2010;42:482–490. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Lu W, Yang K, Zhang J, Jia J, Yun X, Tian L, Chen Y, Jiang Q, Zhang B, et al. Peroxisome proliferator–activated receptor gamma inhibits pulmonary hypertension targeting store-operated calcium entry. J Mol Med (Berl) 2015;93:327–342. doi: 10.1007/s00109-014-1216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia-induced pulmonary arterial hypertension in rats. Respirology. 2010;15:659–668. doi: 10.1111/j.1440-1843.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamsetti SS, et al. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970–2979. doi: 10.1096/fj.07-8424com. [DOI] [PubMed] [Google Scholar]
- 16.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L104–L113. doi: 10.1152/ajplung.00058.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Jiang Q, Wan L, Yang K, Zhang Y, Chen Y, Wang E, Lai N, Zhao L, Jiang H, et al. Sodium tanshinone IIA sulfonate inhibits canonical transient receptor potential expression in pulmonary arterial smooth muscle from pulmonary hypertensive rats. Am J Respir Cell Mol Biol. 2013;48:125–134. doi: 10.1165/rcmb.2012-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itahana K, Mao H, Jin A, Itahana Y, Clegg HV, Lindstrom MS, Bhat KP, Godfrey VL, Evan GI, Zhang Y. Targeted inactivation of Mdm2 ring finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell. 2007;12:355–366. doi: 10.1016/j.ccr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9:2423–2433. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 21.Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 22.Burgermeister E, Tencer L, Liscovitch M. Peroxisome proliferator–activated receptor-gamma upregulates caveolin-1 and caveolin-2 expression in human carcinoma cells. Oncogene. 2003;22:3888–3900. doi: 10.1038/sj.onc.1206625. [DOI] [PubMed] [Google Scholar]
- 23.Burgermeister E, Friedrich T, Hitkova I, Regel I, Einwachter H, Zimmermann W, Rocken C, Perren A, Wright MB, Schmid RM, et al. The Ras inhibitors caveolin-1 and docking protein 1 activate peroxisome proliferator–activated receptor gamma through spatial relocalization at helix 7 of its ligand-binding domain. Mol Cell Biol. 2011;31:3497–3510. doi: 10.1128/MCB.01421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Yamamura H, Ohya S, Imaizumi Y. Caveolin-1 facilitates the direct coupling between large conductance Ca2+-activated K+ (BKCa) and CAV1.2 Ca2+ channels and their clustering to regulate membrane excitability in vascular myocytes. J Biol Chem. 2013;288:36750–36761. doi: 10.1074/jbc.M113.511485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isshiki M, Nishimoto M, Mizuno R, Fujita T. Fret-based sensor analysis reveals caveolae are spatially distinct Ca2+ stores in endothelial cells. Cell Calcium. 2013;54:395–403. doi: 10.1016/j.ceca.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Minshall RD, Sessa WC, Stan RV, Anderson RG, Malik AB. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1179–L1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 28.Isshiki M, Ying YS, Fujita T, Anderson RG. A molecular sensor detects signal transduction from caveolae in living cells. J Biol Chem. 2002;277:43389–43398. doi: 10.1074/jbc.M205411200. [DOI] [PubMed] [Google Scholar]
- 29.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 30.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 31.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J, Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Achcar RO, Demura Y, Rai PR, Taraseviciene-Stewart L, Kasper M, Voelkel NF, Cool CD. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest. 2006;129:696–705. doi: 10.1378/chest.129.3.696. [DOI] [PubMed] [Google Scholar]
- 33.Mathew R. Cell-specific dual role of caveolin-1 in pulmonary hypertension. Pulm Med. 2011;2011:573432. doi: 10.1155/2011/573432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB. Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation. 2004;110:1499–1506. doi: 10.1161/01.CIR.0000141576.39579.23. [DOI] [PubMed] [Google Scholar]
- 35.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006;114:912–920. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 37.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem. 2002;277:44085–44092. doi: 10.1074/jbc.M205934200. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin: caveolin binding negatively regulates the auto-activation of src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem. 2007;282:16631–16643. doi: 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 40.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, Brovkovych V, Yuan JX, Wharton J, Malik AB. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of TRP1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 42.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 43.Brazer SC, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70:1174–1183. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 45.Remillard CV, Yuan JX. Transient receptor potential channels and caveolin-1: good friends in tight spaces. Mol Pharmacol. 2006;70:1151–1154. doi: 10.1124/mol.106.029280. [DOI] [PubMed] [Google Scholar]
- 46.Pani B, Liu X, Bollimuntha S, Cheng KT, Niesman IR, Zheng C, Achen VR, Patel HH, Ambudkar IS, Singh BB. Impairment of TRPC1-stim1 channel assembly and AQP5 translocation compromise agonist-stimulated fluid secretion in mice lacking caveolin1. J Cell Sci. 2013;126:667–675. doi: 10.1242/jcs.118943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie L, Xue X, Taylor M, Ramakrishnan SK, Nagaoka K, Hao C, Gonzalez FJ, Shah YM. Hypoxia-inducible factor/MAZ-dependent induction of caveolin-1 regulates colon permeability through suppression of occludin, leading to hypoxia-induced inflammation. Mol Cell Biol. 2014;34:3013–3023. doi: 10.1128/MCB.00324-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Roche O, Xu C, Moriyama EH, Heir P, Chung J, Roos FC, Chen Y, Finak G, Milosevic M, et al. Hypoxia promotes ligand-independent EGF receptor signaling via hypoxia-inducible factor–mediated upregulation of caveolin-1. Proc Natl Acad Sci USA. 2012;109:4892–4897. doi: 10.1073/pnas.1112129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llaverias G, Vazquez-Carrera M, Sanchez RM, Noe V, Ciudad CJ, Laguna JC, Alegret M. Rosiglitazone upregulates caveolin-1 expression in THP-1 cells through a PPAR-dependent mechanism. J Lipid Res. 2004;45:2015–2024. doi: 10.1194/jlr.M400049-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Tencer L, Burgermeister E, Ebert MP, Liscovitch M. Rosiglitazone induces caveolin-1 by PPARgamma-dependent and PPRE-independent mechanisms: the role of EGF receptor signaling and its effect on cancer cell drug resistance. Anticancer Res. 2008;28:895–906. [PubMed] [Google Scholar]
- 51.Chintharlapalli S, Papineni S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPARγ-dependent and PPARγ-independent pathways. Mol Cancer Ther. 2006;5:1362–1370. doi: 10.1158/1535-7163.MCT-06-0002. [DOI] [PubMed] [Google Scholar]
- 52.Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator–activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and nonsteroidal anti-inflammatory drug-activated gene-1. Mol Pharmacol. 2005;68:1782–1792. doi: 10.1124/mol.105.017046. [DOI] [PubMed] [Google Scholar]
- 53.Chintharlapalli S, Papineni S, Safe S. 1,1-bis(3′-indolyl)-1-(p-substitutedphenyl)methanes inhibit growth, induce apoptosis, and decrease the androgen receptor in LNCaP prostate cancer cells through peroxisome proliferator–activated receptor gamma-independent pathways. Mol Pharmacol. 2007;71:558–569. doi: 10.1124/mol.106.028696. [DOI] [PubMed] [Google Scholar]
- 54.Tian J, Smith A, Nechtman J, Podolsky R, Aggarwal S, Snead C, Kumar S, Elgaish M, Oishi P, Goerlach A, et al. Effect of PPARgamma inhibition on pulmonary endothelial cell gene expression: gene profiling in pulmonary hypertension. Physiol Genomics. 2009;40:48–60. doi: 10.1152/physiolgenomics.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson JA. Interaction of the peroxisome-proliferator–activated receptor and retinoid X receptor. Proc Natl Acad Sci USA. 1993;90:1440–1444. doi: 10.1073/pnas.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiazolidinediones YJH. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]