Abstract
Tissue factor (TF) initiates the extrinsic coagulation cascade in response to tissue injury, leading to local fibrin deposition. Low levels of TF in mice are associated with increased severity of acute lung injury (ALI) after intratracheal LPS administration. However, the cellular sources of the TF required for protection from LPS-induced ALI remain unknown. In the current study, transgenic mice with cell-specific deletions of TF in the lung epithelium or myeloid cells were treated with intratracheal LPS to determine the cellular sources of TF important in direct ALI. Cell-specific deletion of TF in the lung epithelium reduced total lung TF expression to 39% of wild-type (WT) levels at baseline and to 29% of WT levels after intratracheal LPS. In contrast, there was no reduction of TF with myeloid cell TF deletion. Mice lacking myeloid cell TF did not differ from WT mice in coagulation, inflammation, permeability, or hemorrhage. However, mice lacking lung epithelial TF had increased tissue injury, impaired activation of coagulation in the airspace, disrupted alveolar permeability, and increased alveolar hemorrhage after intratracheal LPS. Deletion of epithelial TF did not affect alveolar permeability in an indirect model of ALI caused by systemic LPS infusion. These studies demonstrate that the lung epithelium is the primary source of TF in the lung, contributing 60–70% of total lung TF, and that lung epithelial, but not myeloid, TF may be protective in direct ALI.
Keywords: coagulation, fibrin, pulmonary, acute respiratory distress syndrome, alveolar capillary barrier permeability
Clinical Relevance
This study evaluates the role of tissue factor (TF) in direct acute lung injury using cell-specific genetic approaches. We identify the lung epithelium as the major source of TF in the airspace. Loss of epithelial TF increases lung injury, impairs coagulation, results in lung hemorrhage, and disrupts alveolar–capillary barrier permeability. These findings identify a potential new target for treatment of severe lung injury in humans.
A pathologic hallmark of severe acute lung injury (ALI) in humans is intra-alveolar fibrin deposition, forming hyaline membranes lining the airspace (1). Activation of the tissue factor (TF) pathway is a major driver of coagulation in the airspace (2–5). We previously demonstrated a critical role for TF in protection from ALI caused by intratracheal LPS administration (2). LTF mice, which lack murine TF and express 1% of endogenous levels of human TF to overcome embryonic lethality, developed more severe ALI in response to intratracheal LPS than littermate controls. LTF mice had a local coagulation defect in the airspace, increased histologic lung injury, increased alveolar–capillary permeability, increased alveolar hemorrhage, and a trend toward increased airspace inflammation. Administration of recombinant murine TF into the airspace in LTF mice restored local procoagulant activity and attenuated hemorrhage and permeability defects. In human patients with severe ALI, TF is elevated more than 100-fold in the alveolar space compared with plasma (3), suggesting a local source of TF production in the airspace. These data together suggest that TF may be protective in direct ALI, and that there is a local source of TF in the airspace. Improved understanding of the mechanisms that underlie the beneficial effect of TF and the specific cellular sources of TF in the airspace are needed to develop new TF-targeted therapies for ALI.
In the lung of both humans and mice, TF is expressed by the lung epithelium and by inflammatory cells (3, 6–9), including myeloid cells. In addition, TF can be strongly induced on epithelial cells, macrophages, and other cell types in vitro (10–16). The cellular sources of TF that drive the protective effects of TF during direct ALI, and the mechanisms of the protective effects of TF in the airspace, are currently unknown. Several previous studies suggested that inflammatory cells, specifically alveolar macrophages, have intense up-regulation of TF protein and activity in LPS models, bleomycin-induced lung injury, and human ALI (8, 9, 17). However, emerging data point to the alveolar epithelium as a critical mediator of intra-alveolar coagulation (3, 6, 9, 18, 19). We hypothesized that TF expression on the alveolar epithelium is a critical mediator of coagulation, inflammation, and permeability during direct lung injury caused by LPS. To test this hypothesis, we induced lung injury with intratracheal LPS administration in mice with cell-specific deletions of TF in lung epithelial cells (TF∆LEpi) or cells of myeloid lineage (TF∆mye).
Materials and Methods
Transgenic Mice
Cell-specific deletion of TF was accomplished using the Cre/Lox system (20). Homozygous floxed TF mice on a C57Bl/6 background (21) were bred with heterozygous LysM.Cre mice (22) (Jackson Laboratories, Bar Harbor, ME) or heterozygous SPC.Cre mice (23, 24). Experiments compared 8- to 16-week-old male and female mice with lung epithelial TF deletion (SPC.Cre+/−TFflox/flox, referred to as TF∆LEpi) or myeloid cell TF deletion (LysM.Cre+/−TFflox/flox, referred to as TF∆mye) with littermate controls (Cre−/−TFflox/flox, wild-type [WT]). All experiments were approved by the Vanderbilt Institute for Animal Care and Use Committee.
Models of ALI
For direct LPS-induced lung injury, mice were anesthetized with isoflurane and treated with 10 μg LPS in 100 μl (Escherichia coli, LPS; Sigma, St. Louis, MO) or 100 μl PBS (control; Mediatech, Manassas, VA) by intratracheal injection (2, 25). Indirect lung injury was induced by continuous infusion of LPS (8 μg/h for 24 h) or equal volume PBS delivered by osmotic pump (Durect, Cupertino, CA) with a single intraperitoneal dose of LPS (3 μg/mouse) or PBS at the time of pump implantation (26).
Sample Collection
At each time point, mice were killed and bronchoalveolar lavage (BAL) performed. Lungs were removed and flash frozen. All samples were stored at −80°C until further study. For histologic analysis, lungs were perfused with saline and inflated with formalin, as previously reported (2).
TF Protein Detection
Murine TF immunostaining was performed using goat polyclonal anti-TF antibody (catalog no. AF3178; R&D Systems, Minneapolis, MN) followed by a biotinylated rabbit anti-goat secondary antibody (Innogenex, San Ramon, CA) (2). For Western blotting, equal amounts of total protein in whole-lung homogenates (collected after BAL) in radioimmunoprecipitation assay buffer were separated by SDS-PAGE (2) and the amount of TF was quantified by densitometry after probing with a goat anti-TF primary antibody (R&D Systems) and Li-COR donkey anti-goat secondary antibody (Odyssey, Lincoln, NE). For quantification by ELISA, a novel assay was developed using established methods for assay development and optimization (27). For this ELISA, TF was quantified in whole-lung homogenates using a rat monoclonal anti-TF antibody for antigen capture (generously provided by D. Kirchofer, Genentech, San Francisco, CA) and a goat polyclonal anti-TF antibody for detection (R&D Systems), alongside a standard curve of known quantities of purified recombinant TF (R&D Systems).
Measurements of ALI
BAL clot time, cell counts with differentials, microparticles, protein, hemoglobin, and cytokine concentrations were measured as previously described (2, 28, 29). For histology, formalin-fixed and paraffin-embedded lung sections were stained with hematoxylin and eosin and subsequently scored for septal thickening, edema, inflammation, and hemorrhage in a blinded fashion (2, 30).
Statistical Analysis
All statistical analyses were done with SPSS version 22 for Macintosh (IBM, Armonk, NY) with statistical consultation. A P value less than 0.05 was considered statistically significant. Three genotypes and two treatment groups were compared by two-way ANOVA, followed by Student’s t tests comparing genotypes (WT versus TF∆LEpi or WT versus TF∆mye) or treatments (PBS versus LPS) as indicated based on the ANOVA results. Three genotypes and one treatment group were compared by one-way ANOVA, followed by post hoc Tukey comparison. Nonnormally distributed data were natural log transformed before analysis.
Results
Cell-Specific TF Deletion
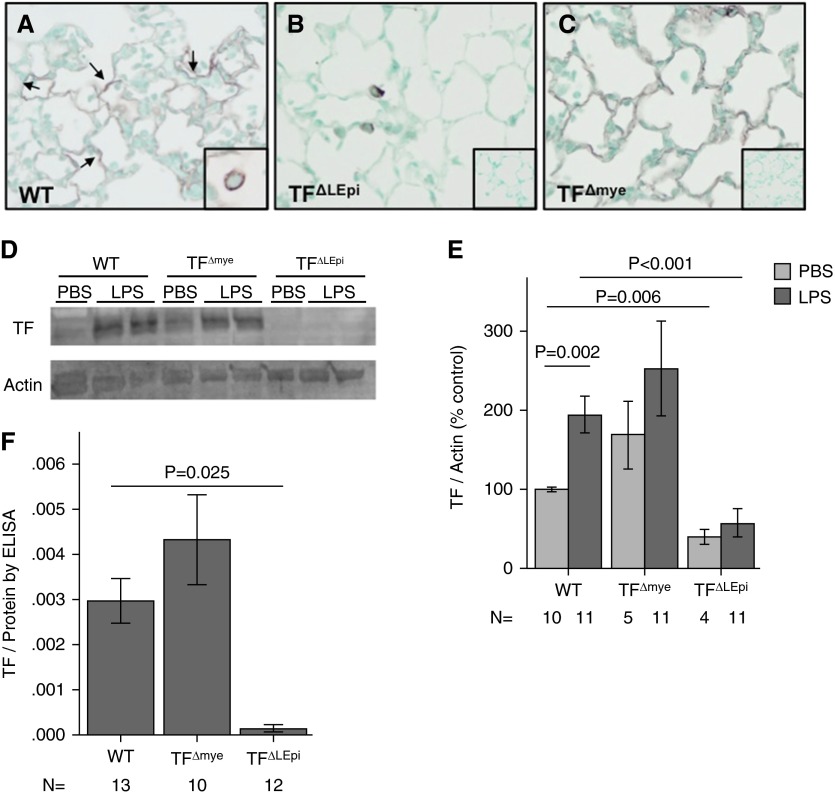
Immunohistochemistry for TF on lung sections from WT mice treated with intratracheal LPS demonstrated expression of TF in the lung epithelium and in alveolar macrophages (Figure 1A), similar to the expression pattern seen in humans with ALI (3). To study the relative contribution of TF from different cellular sources to ALI, we generated mice with constitutive cell-specific deletions of TF. Immunostaining for TF confirmed cell-specific deletion (Figures 1B and 1C). SPC.Cre+/−TFflox/flox (TF∆LEpi) mice lack TF expression in the lung epithelium, including the alveolar epithelium (Figure 1B). TF∆mye mice lack TF expression on cells of myeloid lineage, including macrophages, monocytes, and neutrophils (Figure 1C). To confirm that cell-specific TF deletion did not alter circulating inflammatory cell populations, peripheral white blood counts and differentials were assessed, and there were no significant differences between WT, TF∆LEpi, and TF∆mye mice at baseline (data not shown).
Figure 1.
Cell-specific deletion of tissue factor (TF) and quantification of the cellular sources of TF. Immunohistochemistry for TF in lungs from (A) wild-type mice (WT), (B) mice with cell-specific deletion of TF in lung epithelial cells (TF∆LEpi), and (C) mice with deletion of TF in cells of myeloid lineage (TF∆mye) treated with intratracheal LPS for 24 hours. (A) TF expression on alveolar epithelium (arrows) and macrophages (inset). (B) Elimination of epithelial TF in TF∆LEpi mice (inset is no primary antibody staining). (C) Elimination of myeloid TF in TF∆mye mice (inset is no primary antibody staining). Magnification is ×10 for larger panels and ×40 for insets. (D) Western blot for TF and actin loading controls in lung homogenates with (E) quantification of relative expression of TF protein normalized to actin. Statistics performed by two-way ANOVA followed by Student’s t tests comparing PBS versus LPS, WT versus TF∆LEpi, and WT versus TF∆mye. (F) Quantification of TF expression by ELISA after intratracheal LPS. Statistics performed by one-way ANOVA with post hoc Tukey comparison between genotypes. Bars represent mean ± SE.
The Majority of TF in the Lung Is Expressed on the Lung Epithelium
To determine the relative contributions of lung epithelial versus myeloid TF to total lung TF expression after intratracheal LPS, TF protein levels were measured in lung homogenate by Western blotting (Figures 1D and 1E) and by ELISA (Figure 1F). WT mice increase TF expression after LPS treatment. TF∆mye mice had equivalent levels of TF in the lung homogenate as compared with WT controls both at baseline (control, PBS treatment) and after intratracheal LPS, with no increase in TF after LPS treatment. In contrast, at baseline, TF∆LEpi mice had 39% TF expression compared with WT controls. After intratracheal LPS, TF∆LEpi mice were unable to increase TF expression and had 29% TF levels compared with WT controls. This confirms that the lung epithelium is the major source of TF in the lung both basally and after LPS stimulation, with minimal contribution from lung myeloid cells.
Timing of TF Activity after Injury
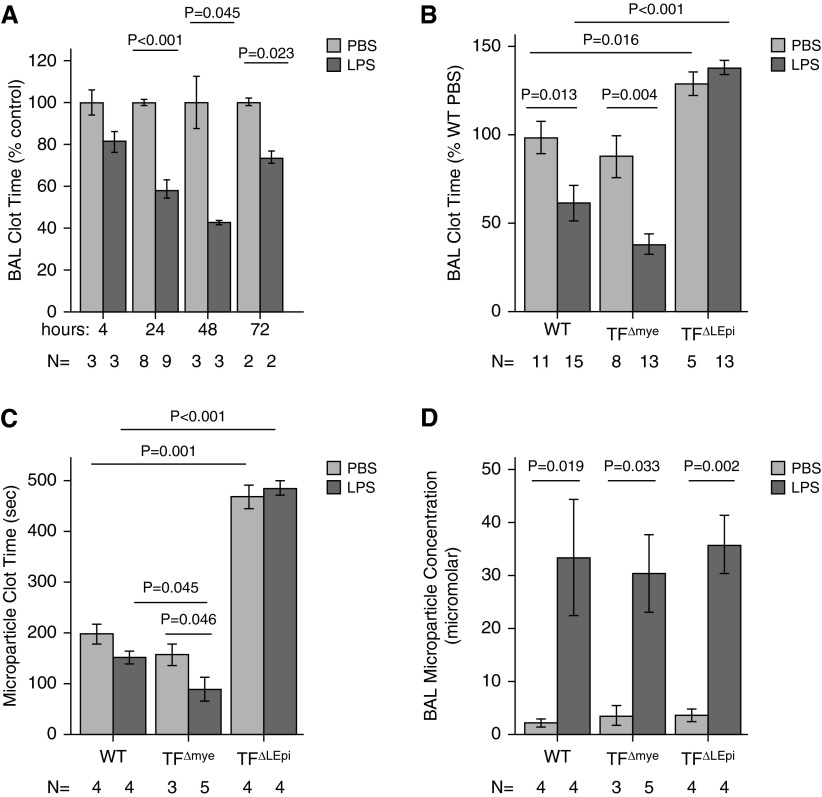
We have previously shown that the procoagulant activity in BAL fluid is TF dependent (2, 3). To determine the kinetics of TF up-regulation and activation of alveolar procoagulant activity after LPS administration, WT mice were treated with intratracheal LPS and BAL samples were collected at various times after injury. Compared with mice treated with PBS, mice treated with LPS had shortening of the BAL clot time, indicating increased procoagulant activity (Figure 2A), beginning at 24 hours and persisting for 72 hours. The 24-hour time point was selected for the remainder of the experiments.
Figure 2.
TF on the lung epithelium is required for activation of coagulation in the airspace. (A) Bronchoalveolar lavage (BAL) clot time, expressed as percent of WT PBS, measured by plasma recalcification time, decreases in response to intratracheal LPS treatment. (B) Clot times are prolonged in TF∆LEpi mice after treatment with either PBS or LPS. (C) Procoagulant activity is present in BAL microparticles in WT and TF∆mye mice but not in TF∆LEpi mice. (D) BAL microparticle concentrations are similar in all genotypes after intratracheal LPS. For BAL clot times, subsequent Student’s t tests compared PBS versus LPS in each genotype, and WT versus TF∆LEpi and WT versus TF∆mye in each treatment. For microparticle concentration, subsequent t tests compared PBS versus LPS in each genotype. Bars represent mean ± SE; statistical analysis performed by two-way ANOVA.
Epithelial TF, but Not Myeloid TF, Is Responsible for Procoagulant Activity in the Airspace
Clot times, as a reflection of TF procoagulant activity, were measured in BAL samples from TF∆LEpi and TF∆mye mice 24 hours after treatment with intratracheal PBS or LPS (Figure 2B). Similar to WT mice, TF∆mye mice showed shortening of BAL clot time in response to LPS. In contrast, TF∆LEpi had prolonged clot times at baseline and failed to reduce clot time after LPS exposure, suggesting that lung epithelial TF was the critical mediator of intra-alveolar procoagulant activity. TF∆LEpi mice also had prolonged clot times in whole-lung homogenates compared with WT or TF∆mye mice after LPS administration (data not shown), confirming that the lung epithelium was the dominant source of TF procoagulant activity in the lung. Because alveolar epithelial cells produce TF-bearing microparticles during ALI (29), we next tested whether the procoagulant activity in the airspace of mice treated with intratracheal LPS was dependent on microparticle generation. BAL fluid from WT and TF∆mye mice, but not from TF∆LEpi mice, contained procoagulant microparticles both at baseline and after LPS administration (Figure 2C). The lack of microparticle procoagulant activity in TF∆LEpi mice was not due to a defect in microparticle release (Figure 2D), as total microparticle levels were similar, but because microparticles from TF∆LEpi mice lacked procoagulant activity. These findings are consistent with the lung epithelium being the major source of TF procoagulant activity in the airspace during ALI.
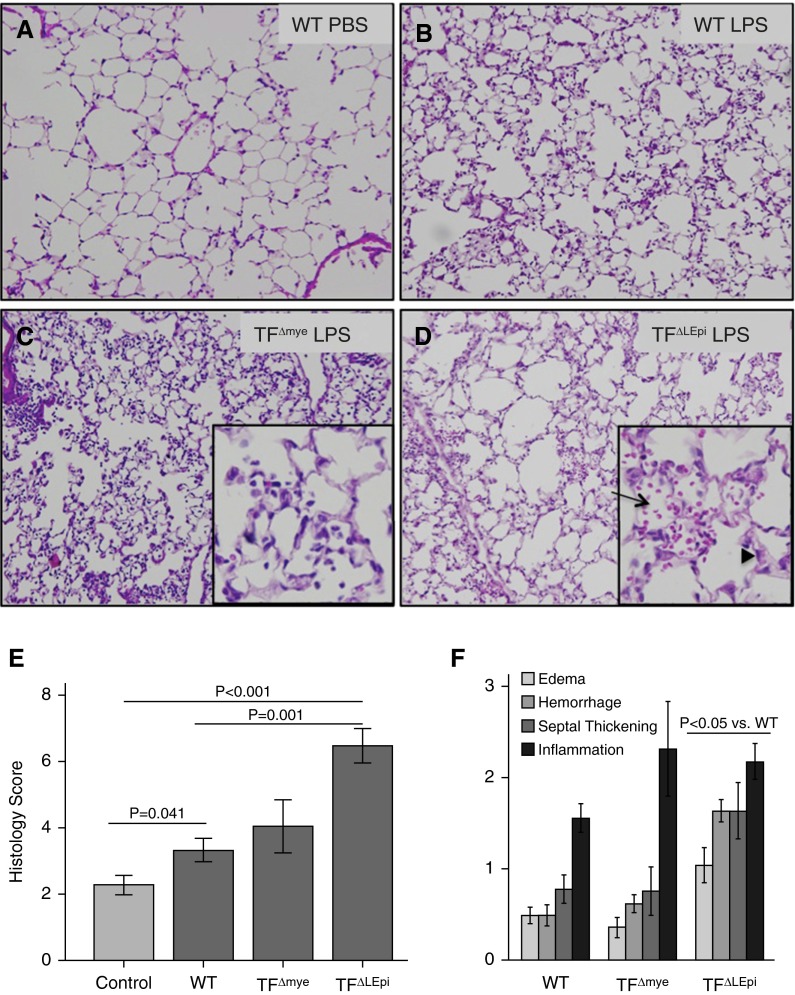
Deletion of Lung Epithelial TF Leads to Increased Histologic Lung Injury
After intratracheal LPS administration, histologic analysis of injured lungs showed that TF∆LEpi mice had increased indices of lung injury compared with WT or TF∆mye mice (Figures 3A–3D). The total lung injury score was increased in TF∆LEpi mice compared with WT (Figure 3E). Edema, septal thickening, hemorrhage, and inflammation were each increased in TF∆LEpi mice compared with WT, but there were no significant differences between WT and TF∆mye mice (Figure 3F).
Figure 3.
Deletion of lung epithelial TF leads to increased histologic lung injury. (A–D) Ten representative photomicrographs from each treatment group were scored for edema, hemorrhage, septal thickness, and inflammation. Insets show ×40 magnification of a portion of the larger panels (×10). (D) The arrow indicates alveolar hemorrhage, and the arrowhead shows septal thickening. (E) The total lung injury score was calculated for each animal. (F) Individual injury score components. Bars represent mean ± SE; statistical analysis performed by two-way ANOVA followed by Student’s t tests comparing PBS versus LPS, WT versus TF∆LEpi, and WT versus TF∆mye.
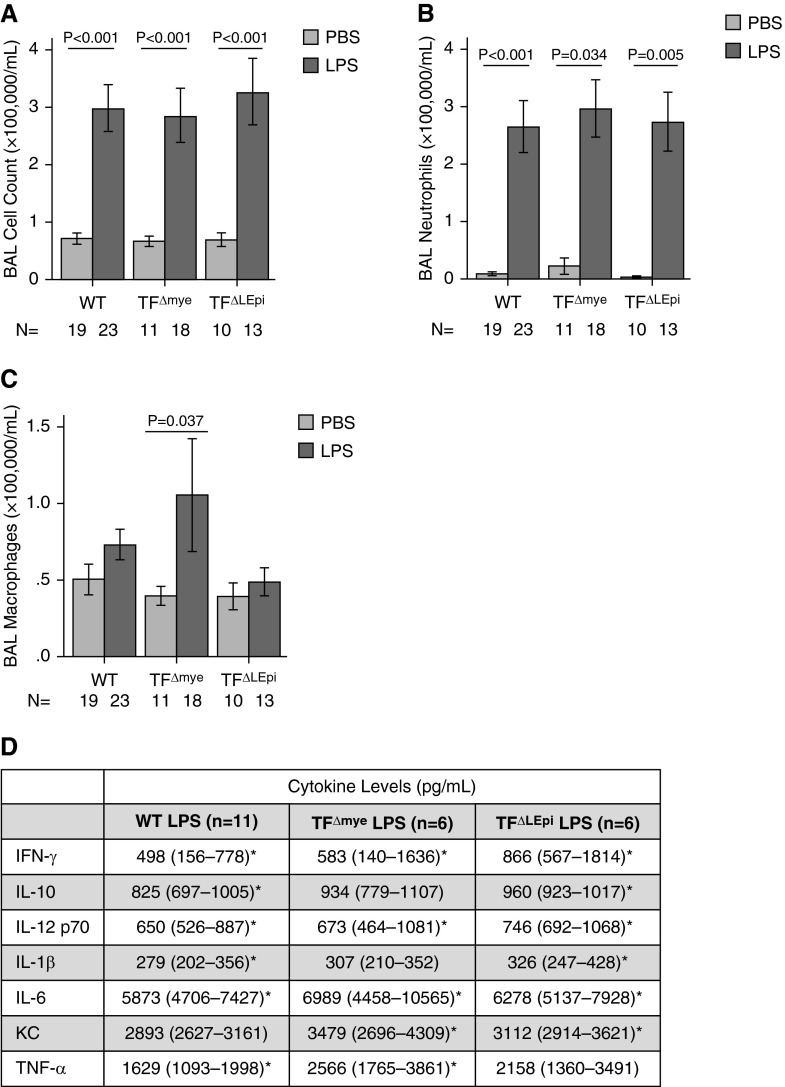
Neither Epithelial nor Myeloid TF Modulates BAL Lung Inflammation
In the airspace, WT, TF∆mye, and TF∆LEpi mice each had increased numbers of inflammatory cells in BAL fluid after LPS treatment compared with mice treated with PBS (Figure 4A) with no differences among the mouse genotypes. The majority of the infiltrating cells were neutrophils with the remainder being macrophages (Figures 4B–4C). Consistent with these data, concentrations of several proinflammatory cytokines and chemokines in BAL fluid were increased after intratracheal LPS as compared with intratracheal PBS, with only subtle differences between TF genotypes (Figure 4D).
Figure 4.
TF expressed on lung epithelium or myeloid cells does not significantly affect BAL inflammation. (A) Total cell counts and (B and C) differentials in BAL fluid were assessed manually 24 hours after intratracheal PBS or LPS administration. Bars represent mean ± SE; statistical analysis performed by two-way ANOVA followed by Student’s t tests comparing PBS versus LPS in each genotype. (D) Levels of proinflammatory cytokines and chemokines in BAL were measured by ELISA. Data are median (interquartile range). *P < 0.05 comparing PBS and LPS for each genotype. KC, keratinocyte chemoattractant.
Epithelial TF, but Not Myeloid TF, Regulates Lung Permeability
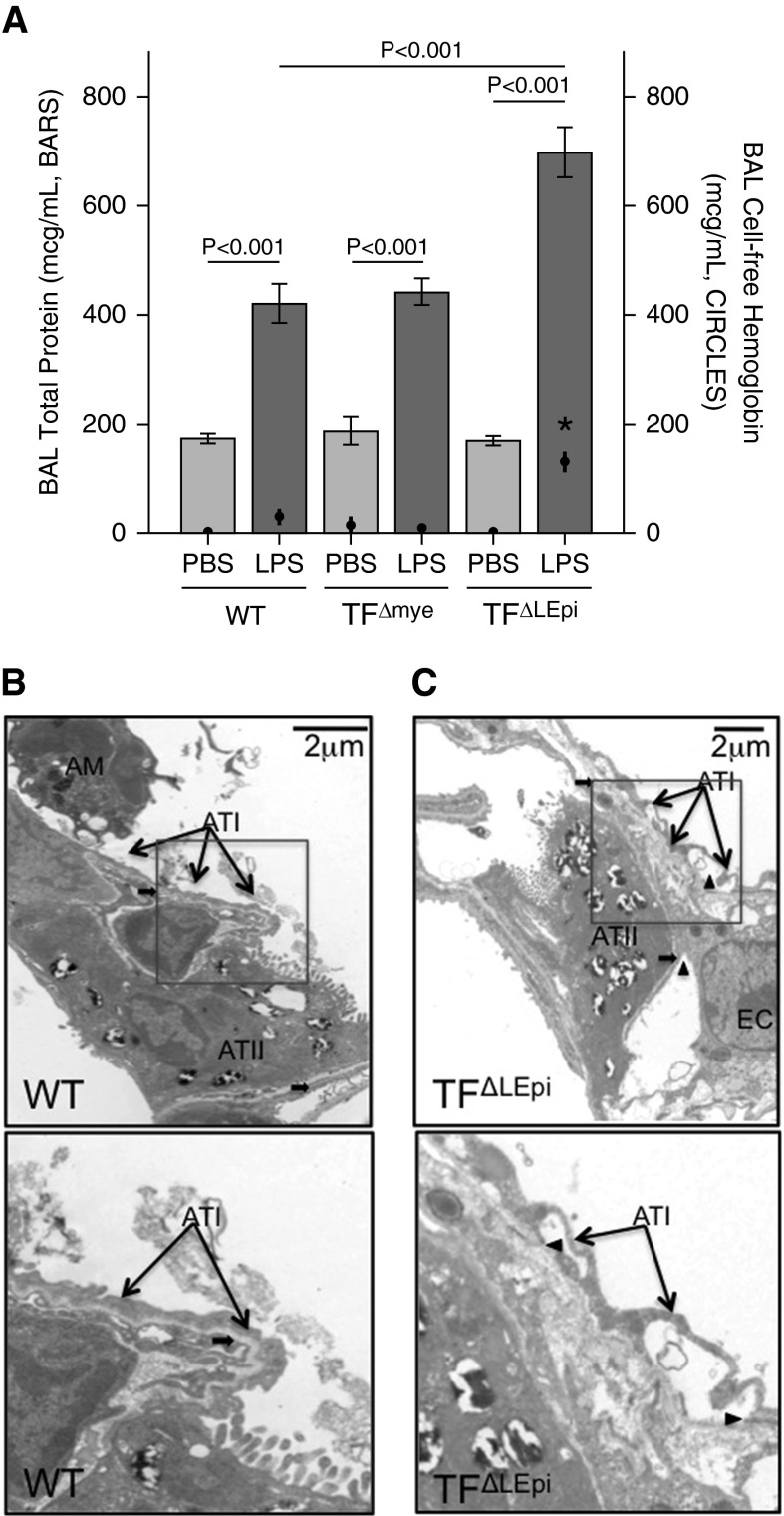
Total levels of protein in the BAL fluid were increased in all mice treated with LPS compared with those treated with intratracheal PBS (Figure 5A). TF∆LEpi mice had increased BAL protein levels after LPS administration compared with WT mice, demonstrating that epithelial TF was a major regulator of alveolar permeability. TF∆LEpi mice also had significantly higher cell-free hemoglobin levels in the BAL after LPS treatment than WT mice, consistent with the increased alveolar hemorrhage seen by histology (Figures 5A and 3F). In TF∆LEpi mice specifically, the increase in BAL protein is not explained by increased cell-free hemoglobin, as hemoglobin is 18% of the total protein measured. To determine whether increased permeability and hemorrhage were due to ultrastructural changes in the alveolar–capillary barrier, transmission electron microscopy was performed on mice treated with intratracheal LPS. While WT mice had preserved epithelial integrity after LPS treatment, TF∆LEpi mice showed detachment of both type I and type II alveolar epithelial cells from the basement membrane (Figures 5B and 5C). There were no ultrastructural abnormalities detected in the endothelium.
Figure 5.
Lack of TF on lung epithelium disrupts the alveolar–capillary barrier. (A) Total protein (bars) and cell-free hemoglobin (circles) levels in BAL fluid assessed by protein assay or ELISA, respectively, 24 hours after intratracheal PBS or intratracheal LPS. Bars and circles represent mean ± SE for each variable. *P < 0.001 comparing hemoglobin levels between WT LPS and TF∆LEpi LPS, and between TF∆LEpi PBS and TF∆LEpi LPS; statistical analysis performed by two-way ANOVA followed by Student’s t tests comparing PBS versus LPS, WT versus TF∆LEpi, and WT versus TF∆mye. For protein measurement, n = 5–10 for each PBS group, n = 11–17 for each LPS group. For hemoglobin measurement, n = 10–15 for each PBS group, n = 14–24 for each LPS group. Transmission electron microscopy of lungs of (B) WT mice or (C) TF∆LEpi mice treated with LPS. Bottom panels represent close-up view of insets in top panels. Thin arrows indicate alveolar epithelial type I cells; thick arrows indicate basement membrane; arrowheads indicate loss of attachment of type I alveolar epithelium (ATI) and type II alveolar epithelium (ATII) cells from basement membrane. AM, alveolar macrophage; EC, endothelial cell.
TF on the Lung Epithelium Mediates Airspace Procoagulant Activity, but Not Permeability, during Indirect ALI
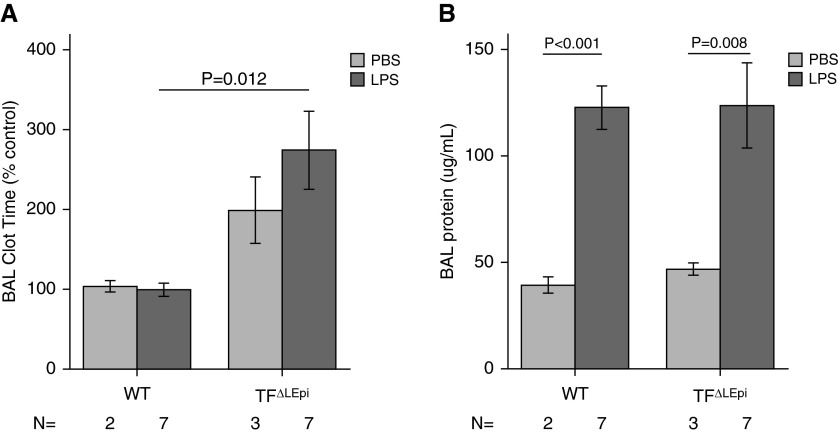
Because of the major effects of epithelial TF on coagulation and permeability after direct ALI caused by intratracheal LPS, we next tested whether epithelial TF had a similar function during indirect lung injury caused by systemic LPS delivery. There was no difference in BAL clot times between WT and TF∆LEpi mice after implantation of PBS-containing osmotic pumps. In contrast, TF∆LEpi mice given systemic LPS via osmotic pump showed reduced airspace procoagulant activity as compared with WT mice (Figure 6A). All groups of mice treated with systemic LPS demonstrated increased alveolar–capillary permeability, as measured by increased BAL protein (Figure 6B). However, in contrast to direct ALI, TF∆LEpi mice had similar permeability to WT mice.
Figure 6.
Epithelial TF mediates airspace coagulation, but not permeability, during indirect acute lung injury. (A) BAL clot time is prolonged in TF∆LEpi mice after systemic LPS delivery. (B) BAL protein levels are increased after systemic LPS administration, with no differences between genotypes. Bars represent mean ± SE; statistical analysis performed by two-way ANOVA. For BAL clot time, subsequent Student’s t tests compared WT versus TF∆LEpi and WT versus TF∆mye in each treatment. For BAL protein, subsequent t tests compared PBS versus LPS in each genotype.
Discussion
The data presented in this article support a critical role for TF expressed on the lung epithelium, but not on myeloid cells, in the response to direct ALI from intratracheal LPS administration. The majority of the abnormalities that we previously observed in LTF mice after LPS-mediated injury (2) are recapitulated by absence of TF on the lung epithelium. These findings add to a growing literature showing that coagulation in the airspace is regulated by lung epithelial cells rather than inflammatory cells (3, 9, 29, 31). Our group has previously reported increased levels of TF in pulmonary edema fluid of patients with acute respiratory distress syndrome compared with patients with hydrostatic pulmonary edema (3). Another study showed that TF on microparticles had procoagulant activity in the airspace of patients with acute respiratory distress syndrome (29). Others have shown that the alveolar space contains procoagulant proteins after intra-alveolar injury (9). The current study adds to these data by showing that the lung epithelium is the major source of TF during direct ALI and identifies the local source of TF that modulates both procoagulant activity and alveolar permeability.
Conversely, TF expressed on myeloid cells did not have a major impact on lung injury, inflammation, or permeability in this injury model. The lack of effect of deletion of myeloid TF in ALI is an unexpected finding, and provides new insight into the regulation of local coagulation in the airspace. It is well established that alveolar macrophages express TF (8–10, 18). Two studies of bleomycin-induced lung injury demonstrated strong up-regulation of TF mRNA (8) and protein expression (18) on macrophages during ALI. TF mRNA was at least sixfold more pronounced in macrophages than changes in TF in epithelial cells (8). Two other studies suggested a procoagulant role for hematopoietic sources of TF in a model of endotoxemia (9, 10). It is important to note that these studies of myeloid TF were done on myeloid cells isolated from mice and studied in culture, so, although TF up-regulation on individual macrophages may be robust, the overall contribution to total TF activity in the airspace is minimal. Our data demonstrate that, although loss of myeloid TF has limited impact on the total TF expression in the lung after intratracheal LPS, myeloid TF has no demonstrable effect on the severity of ALI. Furthermore, our data define the cell-specific roles of TF in vivo for the first time, and demonstrate that TF on the lung epithelium not only comprises the majority of total lung TF, but also has an important role in lung epithelial barrier integrity in direct lung injury. Finally, we show that loss of epithelial TF impairs airspace coagulation after systemic LPS administration, but that TF does not regulate epithelial permeability in this model.
Neither epithelial nor myeloid TF deletion significantly altered inflammatory cell recruitment or cytokine expression in the airspace. This result was unexpected, because we had anticipated that TF-bearing macrophages would be important mediators of inflammation. There are extensive data that link activation of coagulation with activation of inflammation (32). Thrombin and fibrin can each stimulate cytokine production in monocytes and endothelial cells in vitro (33–35). In addition, TF modulates inflammation in models of systemic sepsis, and inhibition of coagulation has been shown to reduce lung inflammation in these models (36–39). Our data support the concept that the role of TF in inflammation in the airspace is distinct from its effects in the circulation. This conclusion is supported by the lack of impact of protease-activated receptor 2, a key link between TF coagulation and inflammatory signaling, in multiple models of direct ALI (40). Furthermore, two studies have shown that inhibition of coagulation using nebulized anticoagulants or fibrinolytics failed to attenuate lung inflammation (41, 42). These studies and our results support the notion that inhibition of coagulation in the airspace may not influence the inflammatory response in the lung. Another potential reason that our results differ from previous data is that the current study is the first to use cell-specific germline deletion of TF, rather than antibodies or inhibitors, to assess the role of TF in ALI. Soluble forms of TF and splice variants are known to have variable physiologic function compared with full-length, cell-associated TF (43), and these other forms of TF may not have been affected in previous studies. In addition, TF expressed on other cell types, such as the endothelium or lymphocytes, may be sufficient to stimulate inflammation during ALI. It is also possible that lung TF may regulate the timing of lung inflammation. In this study, we chose to focus on the differences between epithelial and myeloid TF deletion at the peak of injury after LPS at 24 hours. Whether TF regulates earlier or later stages of inflammation, or modulates resolution of inflammation, is an important question that requires further study.
Our current data support a major role for lung epithelial TF in maintenance of alveolar–capillary barrier integrity during direct, but not indirect, ALI. Mice lacking lung epithelial TF expression demonstrated marked increases in BAL protein and lung hemorrhage after direct injury with intratracheal LPS, suggesting major disruption of the alveolar barrier. This finding is particularly interesting, as intratracheal administration of LPS does not typically cause severe changes in alveolar permeability (44, 45). It is important to note that, although TF∆LEpi mice have increased alveolar hemorrhage in the airspace, hemoglobin itself had a limited impact on the total BAL proteins measured, suggesting that the increased BAL protein is not explained by increased alveolar hemorrhage. Whether extracellular hemoglobin itself may contribute to alveolar permeability through mechanisms independent of epithelial TF remains unknown. The protective effect of TF on alveolar permeability was not apparent during indirect ALI from systemic LPS administration, perhaps because indirect ALI is predominantly driven by endothelial disruption (46). The beneficial effect of TF during direct ALI may be related to its procoagulant functions, with fibrin deposition at sites of alveolar injury forming a physical barrier over the leaky alveolar–capillary interface. The disruption of alveolar epithelial cell attachments to the basement membrane seen in microscopy of TF∆LEpi mice raises the possibility that additional noncoagulant mechanisms are involved in permeability maintenance. For example, TF is known to interact with β1-integrin and actin during cancer metastasis (47, 48). Whether TF interacts with β1-integrin or other cytoskeletal structures in lung epithelial cells is unknown, and is the focus of ongoing studies. In addition, our data extend current knowledge of the role of TF in the lung by demonstrating that the impact of TF on permeability and coagulation in the airspace is independent from inflammation.
In summary, the current study demonstrates that the lung epithelial cells, rather than myeloid cells, are the major source of TF activity in the airspace in ALI, and that lung epithelial TF is protective during direct ALI pathogenesis. Unexpectedly, TF expressed on myeloid cells had no significant impact on the degree of lung injury, permeability, or inflammation in this model system. These findings are of major importance, because they suggest that therapeutic inhibition of TF in the airspace in ALI may be harmful. Through its expression of TF, the alveolar epithelium is a critical modulator of alveolar permeability, either through activation of coagulation or potentially through other functions, such as cell–matrix interactions that are not yet understood. Because TF in the lung epithelium has a protective role in the airspace of mice with direct ALI, it is possible that supplementation of TF locally in the airspace, or specifically on lung epithelial cells, could be a new therapeutic approach for human ALI.
Acknowledgments
Acknowledgments
The authors thank Gillian Sills, Nancy Wickersham, Dawn Newcomb, Gregory Shemancik, and Justin Woods for technical assistance, and Erin Plosa and Jay Jerome for help with electron microscopy. They appreciate the assistance of the Vanderbilt Biostatistics Clinic Program for guidance on statistical analysis.
Footnotes
This work was supported by National Institutes of Health grants NIH HL087738-07 (C.M.S.), NIH HL103836 (L.B.W.), NIH HL090785 (J.A.B.), and NIH HL 105,479 (W.E.L.), Department of Veterans Affairs grant BX001988 (W.E.L.), American Heart Association Established Investigator Award (L.B.W.), and an American Heart Association Clinical Research Award (J.A.B.).
Author Contributions: Conception and design—C.M.S., J.K.C., W.E.L., L.B.W., and J.A.B.; data acquisition—C.M.S., B.S.G., N.D.P., R.H.C., J.K.C., and J.A.B.; analysis and interpretation—C.M.S., J.K.C., N.M., L.B.W., and J.A.B.; manuscript preparation—C.M.S., N.M., L.B.W., and J.A.B. Review of final manuscript: all authors.
Originally Published in Press as DOI: 10.1165/rcmb.2014-0179OC on April 17, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med. 1982;3:35–56. [PubMed] [Google Scholar]
- 2.Bastarache JA, Sebag SC, Clune JK, Grove BS, Lawson WE, Janz DR, Roberts JL, II, Dworski R, Mackman N, Ware LB. Low levels of tissue factor lead to alveolar haemorrhage, potentiating murine acute lung injury and oxidative stress. Thorax. 2012;67:8. doi: 10.1136/thoraxjnl-2012-201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastarache JA, Wang L, Geiser T, Wang Z, Albertine KH, Matthay MA, Ware LB. The alveolar epithelium can initiate the extrinsic coagulation cascade through expression of tissue factor. Thorax. 2007;62:608–616. doi: 10.1136/thx.2006.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31(4) Suppl:S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 5.Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest. 1989;84:695–705. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erlich J, Fearns C, Mathison J, Ulevitch RJ, Mackman N. Lipopolysaccharide induction of tissue factor expression in rabbits. Infect Immun. 1999;67:2540–2546. doi: 10.1128/iai.67.5.2540-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drake TA, Cheng J, Chang A, Taylor FB., Jr. Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142:1458–1470. [PMC free article] [PubMed] [Google Scholar]
- 8.Wygrecka M, Markart P, Ruppert C, Petri K, Preissner KT, Seeger W, Guenther A. Cellular origin of pro-coagulant and (anti)-fibrinolytic factors in bleomycin-injured lungs. Eur Respir J. 2007;29:1105–1114. doi: 10.1183/09031936.00097306. [DOI] [PubMed] [Google Scholar]
- 9.Wygrecka M, Markart P, Ruppert C, Kuchenbuch T, Fink L, Bohle RM, Grimminger F, Seeger W, Gunther A. Compartment- and cell-specific expression of coagulation and fibrinolysis factors in the murine lung undergoing inhalational versus intravenous endotoxin application. Thromb Haemost. 2004;92:529–540. doi: 10.1160/TH04-02-0126. [DOI] [PubMed] [Google Scholar]
- 10.Pawlinski R, Wang JG, Owens AP, III, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116:806–814. doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues: implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenmakers SH, Groot AP, Florquin S, Reitsma PH, Spek CA. Blood cell–derived tissue factor influences host response during murine endotoxemia. Blood Cells Mol Dis. 2004;32:325–333. doi: 10.1016/j.bcmd.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Egorina EM, Sovershaev MA, Bjorkoy G, Gruber FX, Olsen JO, Parhami-Seren B, Mann KG, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory SA, Morrissey JH, Edgington TS. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol Cell Biol. 1989;9:2752–2755. doi: 10.1128/mcb.9.6.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand K, Fowler BJ, Edgington TS, Mackman N. Tissue factor mRNA in THP-1 monocytic cells is regulated at both transcriptional and posttranscriptional levels in response to lipopolysaccharide. Mol Cell Biol. 1991;11:4732–4738. doi: 10.1128/mcb.11.9.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maugeri N, Brambilla M, Camera M, Carbone A, Tremoli E, Donati MB, de Gaetano G, Cerletti C. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost. 2006;4:1323–1330. doi: 10.1111/j.1538-7836.2006.01968.x. [DOI] [PubMed] [Google Scholar]
- 17.Lyberg T, Nakstad B, Hetland O, Boye NP. Procoagulant (thromboplastin) activity in human bronchoalveolar lavage fluids is derived from alveolar macrophages. Eur Respir J. 1990;3:61–67. [PubMed] [Google Scholar]
- 18.Olman MA, Mackman N, Gladson CL, Moser KM, Loskutoff DJ. Changes in procoagulant and fibrinolytic gene expression during bleomycin-induced lung injury in the mouse. J Clin Invest. 1995;96:1621–1630. doi: 10.1172/JCI118201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross TJ, Simon RH, Sitrin RG. Tissue factor procoagulant expression by rat alveolar epithelial cells. Am J Respir Cell Mol Biol. 1992;6:397–403. doi: 10.1165/ajrcmb/6.4.397. [DOI] [PubMed] [Google Scholar]
- 20.Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 21.Pawlinski R, Tencati M, Holscher T, Pedersen B, Voet T, Tilley RE, Marynen P, Mackman N. Role of cardiac myocyte tissue factor in heart hemostasis. J Thromb Haemost. 2007;5:1693–1700. doi: 10.1111/j.1538-7836.2007.02649.x. [DOI] [PubMed] [Google Scholar]
- 22.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 23.Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- 24.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005;175:2598–2605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- 26.Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J Immunol. 2006;176:4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- 27.Markham NO, Cooper T, Goff M, Gribben EM, Carnahan RH, Reynolds AB. Monoclonal antibodies to DIPA: a novel binding partner of p120-catenin isoform 1. Hybridoma (Larchmt) 2012;31:246–254. doi: 10.1089/hyb.2012.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastarache JA, Koyama T, Wickersham ME, Ware LE. Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples. J Immunol Methods. 2014;408:13–23. doi: 10.1016/j.jim.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB. Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1035–L1041. doi: 10.1152/ajplung.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med. 2002;165:242–249. doi: 10.1164/ajrccm.165.2.2108087. [DOI] [PubMed] [Google Scholar]
- 31.Chapman HA, Stahl M, Allen CL, Yee R, Fair DS. Regulation of the procoagulant activity within the bronchoalveolar compartment of normal human lung. Am Rev Respir Dis. 1988;137:1417–1425. doi: 10.1164/ajrccm/137.6.1417. [DOI] [PubMed] [Google Scholar]
- 32.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2) suppl:S26–S34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 33.Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002;99:1053–1059. doi: 10.1182/blood.v99.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson K, Choi Y, DeGroot E, Samuels I, Creasey A, Aarden L. Potential mechanisms for a proinflammatory vascular cytokine response to coagulation activation. J Immunol. 1998;160:5130–5135. [PubMed] [Google Scholar]
- 35.Sower LE, Froelich CJ, Carney DH, Fenton JW, II, Klimpel GR. Thrombin induces IL-6 production in fibroblasts and epithelial cells: evidence for the involvement of the seven-transmembrane domain (STD) receptor for alpha-thrombin. J Immunol. 1995;155:895–901. [PubMed] [Google Scholar]
- 36.Welty-Wolf KE, Carraway MS, Ortel TL, Ghio AJ, Idell S, Egan J, Zhu X, Jiao JA, Wong HC, Piantadosi CA. Blockade of tissue factor-factor X binding attenuates sepsis-induced respiratory and renal failure. Am J Physiol Lung Cell Mol Physiol. 2006;290:11. doi: 10.1152/ajplung.00155.2005. [DOI] [PubMed] [Google Scholar]
- 37.Carraway MS, Welty-Wolf KE, Miller DL, Ortel TL, Idell S, Ghio AJ, Petersen LC, Piantadosi CA. Blockade of tissue factor: treatment for organ injury in established sepsis. Am J Respir Crit Care Med. 2003;167:1200–1209. doi: 10.1164/rccm.200204-287OC. [DOI] [PubMed] [Google Scholar]
- 38.Miller DL, Welty-Wolf K, Carraway MS, Ezban M, Ghio A, Suliman H, Piantadosi CA. Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 2002;26:650–658. doi: 10.1165/ajrcmb.26.6.4688. [DOI] [PubMed] [Google Scholar]
- 39.Welty-Wolf KE, Carraway MS, Miller DL, Ortel TL, Ezban M, Ghio AJ, Idell S, Piantadosi CA. Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am J Respir Crit Care Med. 2001;164:1988–1996. doi: 10.1164/ajrccm.164.10.2105027. [DOI] [PubMed] [Google Scholar]
- 40.Su X, Matthay MA. Role of protease activated receptor 2 in experimental acute lung injury and lung fibrosis. Anat Rec. 2009;292:580–586. doi: 10.1002/ar.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofstra JJ, Cornet AD, Declerck PJ, Dixon B, Aslami H, Vlaar AP, Roelofs JJ, van der Poll T, Levi M, Schultz MJ. Nebulized fibrinolytic agents improve pulmonary fibrinolysis but not inflammation in rat models of direct and indirect acute lung injury. PLoS One. 2013;8:e55262. doi: 10.1371/journal.pone.0055262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofstra JJ, Vlaar AP, Cornet AD, Dixon B, Roelofs JJ, Choi G, van der Poll T, Levi M, Schultz MJ. Nebulized anticoagulants limit pulmonary coagulopathy, but not inflammation, in a model of experimental lung injury. J Aerosol Med Pulm Drug Deliv. 2010;23:105–111. doi: 10.1089/jamp.2009.0779. [DOI] [PubMed] [Google Scholar]
- 43.Aberg M, Siegbahn A. Tissue factor non-coagulant signaling—molecular mechanisms and biological consequences with a focus on cell migration and apoptosis. J Thromb Haemost. 2013;11:817–825. doi: 10.1111/jth.12156. [DOI] [PubMed] [Google Scholar]
- 44.Wiener-Kronish JP, Albertine KH, Matthay MA. Differential responses of the endothelial and epithelial barriers of the lung in sheep to Escherichia coli endotoxin. J Clin Invest. 1991;88:864–875. doi: 10.1172/JCI115388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaver CM, Bastarache JA. Clinical and biological heterogeneity in acute respiratory distress syndrome: direct versus indirect lung injury. Clin Chest Med. 2014;35:639–653. doi: 10.1016/j.ccm.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collier ME, Li C, Ettelaie C. Influence of exogenous tissue factor on estrogen receptor alpha expression in breast cancer cells: involvement of beta1-integrin, PAR2, and mitogen-activated protein kinase activation. Mol Cancer Res. 2008;6:1807–1818. doi: 10.1158/1541-7786.MCR-08-0109. [DOI] [PubMed] [Google Scholar]
- 48.Kocaturk B, Versteeg HH. Tissue factor-integrin interactions in cancer and thrombosis: every Jack has his Jill. J Thromb Haemost. 2013;11:285–293. doi: 10.1111/jth.12222. [DOI] [PubMed] [Google Scholar]