Abstract
Asthma is characterized by a T helper type 2 phenotype and by chronic allergen-induced airway inflammation (AAI). Environmental exposure to air pollution ultrafine particles (i.e., nanoparticles) exacerbates AAI, and a concern is possible exacerbation posed by engineered nanoparticles generated by emerging nanotechnologies. Signal transducer and activator of transcription (STAT) 1 is a transcription factor that maintains T helper type 1 cell development. However, the role of STAT1 in regulating AAI or exacerbation by nanoparticles has not been explored. In this study, mice with whole-body knockout of the Stat1 gene (Stat1−/−) or wild-type (WT) mice were sensitized to ovalbumin (OVA) allergen and then exposed to multiwalled carbon nanotubes (MWCNTs) by oropharygneal aspiration. In Stat1−/− and WT mice, OVA increased eosinophils in bronchoalveolar lavage fluid, whereas MWCNTs increased neutrophils. Interestingly, OVA sensitization prevented MWCNT-induced neutrophilia and caused only eosinophilic inflammation. Stat1−/− mice displayed increased IL-13 in bronchoalveolar lavage fluid at 1 day compared with WT mice after treatment with OVA or OVA and MWCNTs. At 21 days, the lungs of OVA-sensitized Stat1−/− mice displayed increased eosinophilia, goblet cell hyperplasia, airway fibrosis, and subepithelial apoptosis. MWCNTs further increased OVA-induced goblet cell hyperplasia, airway fibrosis, and apoptosis in Stat1−/− mice at 21 days. These changes corresponded to increased levels of profibrogenic mediators (transforming growth factor-β1, TNF-α, osteopontin) but decreased IL-10 in Stat1−/− mice. Finally, fibroblasts isolated from the lungs of Stat1−/− mice produced significantly more collagen mRNA and protein in response to transforming growth factor-β1 compared with WT lung fibroblasts. Our results support a protective role for STAT1 in chronic AAI and exacerbation of remodeling caused by MWCNTs.
Keywords: asthma, allergen, nanomaterials, carbon nanotubes, airway remodeling
Clinical Relevance
Our results demonstrate that deficiency in the signal transducer and activator of transcription (STAT) 1 transcription factor constitutes a susceptibility factor to occupational, consumer, or environmental exposure to carbon nanotubes in allergic airway disease. Our findings suggest that individuals with asthma who manifest reduced expression of STAT1 are at increased risk for exposure to certain engineered nanomaterials.
Asthma is a major health problem, particularly in the United States and developed countries. For example, in 2010, it was estimated that more than 25 million American adults and 7 million children suffer from asthma, and these numbers continue to grow (1). Asthma involves chronic tissue remodeling of the airways of the lung characterized by reversible and varying degrees of bronchoconstriction, hyperresponsiveness, inflammation, and remodeling of the airways, often resulting in fibrosis (2, 3). These multiple phenotypes that characterize the pathology of chronic asthma are driven partly by an imbalance of T helper (Th) type 1 and Th2 cells (4–7). Th2 cells are the primary cellular source of IL-13, which mediates the phenotypic changes that comprise airway remodeling seen in patients with asthma, including eosinophilic lung inflammation, mucous cell metaplasia (MCM) and mucus hypersecretion, airway smooth muscle cell thickening, and airway fibrosis (8).
In addition to the underlying development of allergic asthma, a variety of environmental agents exacerbate pre-existing disease. For example, ultrafine diesel exhaust particles in the nanometer range are an important factor in the exacerbation of asthma in individuals living near major roadways in metropolitan areas (9–14). A more recent concern is that engineered nanoparticles generated from emerging nanotechnologies could pose a hazard for exacerbation of asthma (15). Carbon nanotubes (CNTs) are a prototypical engineered nanoparticle composed of carbon atoms arranged in a hexagonal lattice and rolled into a tube-like structure (16). These nanomaterials can be categorized as single-walled CNTs, which consist of one tubular structure, or multiwalled CNTs (MWCNTs), which are composed of multiple layers of concentric cylinders. CNTs have several unique physical properties, including a high aspect ratio, surface area, electrical conductivity, and thermal conductivity, as well as an incredible strength-to-weight ratio, making them ideal candidates for use in electronics, engineering, and medicine (17). As they are used in increasing quantities, the potential for occupational and/or environmental exposures is inevitable. Although little is known about their ability to cause toxicity in humans, there is increasing evidence that CNTs may produce toxic effects in the lungs of rodents (17, 18). Likewise, inhalation studies in mice show that MWCNTs cause neutrophilic lung inflammation, interstitial pulmonary fibrosis, pleural immune responses, and exacerbate ovalbumin (OVA)-induced airway fibrosis (15, 19).
The signal transducer and activator of transcription (STAT) family of transcription factors includes seven family members. These transcription factors remain latent in the cytoplasm until activated by extracellular signaling proteins, typically cytokines or growth factors. STAT1 is a well-known regulator of growth arrest and apoptosis (20, 21), and functions in Th1 development and maintenance (22). Moreover, mice lacking the Stat1 gene (Stat1−/− mice) are highly susceptible to mortality caused by viral and bacterial infection due to lack of IFN signaling (23). We previously reported that Stat1−/− mice are susceptible to bleomycin-induced lung fibrosis, and this is due in part to increased sensitivity of lung fibroblasts to growth factors, such as platelet-derived growth factor (PDGF) and epidermal growth factor (24). We also demonstrated that STAT1 opposes IL-13–induced STAT6 activation to reduce PDGF production by mouse lung fibroblasts (MLFs) in vitro (25).
Because IL-13 is a key Th2 cytokine in allergic asthma, we hypothesized that STAT1 plays a central role in allergen-induced asthma and exacerbation of airway remodeling by MWCNTs. To address this issue, we investigated airway remodeling in Stat1−/− mice sensitized to OVA allergen and exposed to a single dose of MWCNTs administered by oropharyngeal aspiration. We found that OVA-sensitized Stat1−/− mice displayed increased eosinophilia, goblet cell hyperplasia, airway fibrosis, and apoptosis. Moreover, MWCNTs specifically exaggerated OVA-induced goblet cell hyperplasia and airway fibrosis. We also related these pathologic endpoints to cytokines and growth factors known to play important roles in allergic airway remodeling and found that Stat1−/− mice displayed increased IL-13 along with elevated levels of IL-1β, transforming growth factor (TGF)-β1, TNF-α, and osteopontin (OPN). Moreover, sensitization to OVA followed by MWCNT exposure increased lung mRNA levels of the antifibrogenic cytokine IL-10 in wild-type (WT) mice, but not in Stat1−/− mice. Finally, fibroblasts isolated from the lungs of Stat1−/− mice produced significantly more collagen mRNAs and soluble collagen protein in response to TGF-β1 compared with WT lung fibroblasts. These results suggest that STAT1 plays a protective role in allergen-induced airway remodeling and exacerbation by CNTs.
Materials and Methods
Animals
Male WT and Stat1−/− mice bred on a 129SV background were purchased from Taconic Laboratories (Germantown, NY) (see the online supplement).
Experimental Design for OVA Sensitization and MWCNT Exposure
The experimental design is illustrated in Figure 1A. Mice were divided into four groups for 1- and 21-day time points (control, OVA, MWCNT, and OVA/MWCNT). Control or OVA groups contained a sample size of four and MWCNT or OVA/MWCNT groups contained a sample size of six for each genotype at each time point. Mice were sensitized to OVA on Study Days 0–32 (26) (see the online supplement for details). MWCNTs (Helix Material Solutions Inc., Richardson, TX) were characterized previously (21, 25, 27). Mice were exposed on Study Day 34 to MWCNTs (4 mg/kg) in 0.1% pluronic surfactant solution (Sigma-Aldrich, St. Louis, MO) by oropharyngeal aspiration while under isoflurane anesthesia (28–30). Control mice received 0.1% pluronic surfactant solution (see the online supplement).
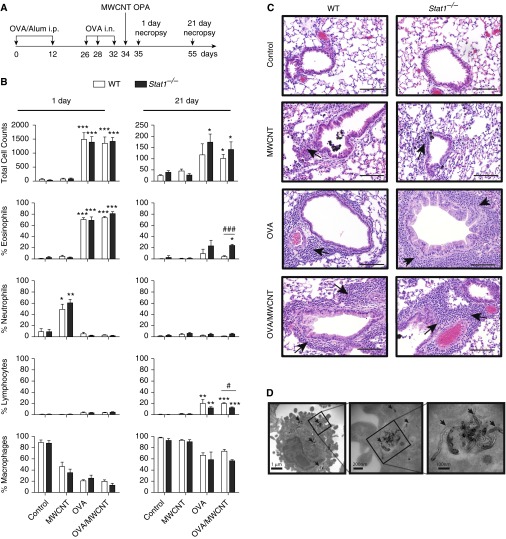
Figure 1.
Signal transducer and activator of transcription (STAT) 1 deficiency does not increase acute lung inflammation at 1 day after exposure to allergen and multiwalled carbon nanotubes (MWCNTs) but increases chronic eosinophilia at 21 days. (A) Schematic illustration of mouse exposure protocol for ovalbumin (OVA) sensitization by intraperitoneal injection (i.p.) and intranasal aspiration (i.n.) followed by oropharyngeal aspiration (OPA) of MWCNTs. (B) Total cell counts and relative percentages of inflammatory cell types in bronchoalveolar lavage fluid (BALF) at 1 and 21 days after exposure of mice to MWCNTs with or without OVA allergen sensitization. Open bars represent wild-type (WT) mice and solid bars represent Stat1−/− mice. Data are the mean ± SEM (n = 4 animals control and OVA groups, n = 6 MWCNT and OVA/MWCNT groups). *P < 0.0.05, **P < 0.01, ***P < 0.001 compared with control, as determined by one-way ANOVA; #P < 0.05 and ###P < 0.001 between genotypes within a treatment group as determined by two-way ANOVA. (C) Representative photomicrographs of hematoxylin and eosin–stained lung sections from each genotype and treatment group 1 and 21 days after MWCNT treatment. Scale bar, 200 μm. Arrows indicate inflammatory cell infiltration. (D) Transmission electron micrographs showing MWCNTs in alveolar macrophages adjacent to airways (arrows indicate MWCNT).
Necropsy and Sample Collection
Mice were killed 1 or 21 days after exposure to MWCNTs or vehicle control via intraperitoneal pentobarbital overdose. Lungs were lavaged with two aliquots of 0.5 ml PBS and the bronchoalveolar lavage fluid (BALF) saved for cell counts and ELISA. The left lung was inflated and fixed with neutral buffered formalin. The right lung lobes were used for RNA analysis.
Cell Counts
Cells from BALF were fixed and stained with the Diff-Quik Stain Set (Dade Behring Inc., Newark, DE), and differential cell counts as well as total cell counts were performed for each animal (see the online supplement).
Morphometric Analysis of Airway Remodeling
Airway fibrosis was quantified on Masson’s trichrome–stained lung sections using the area:perimeter ratio method (30, 31) or the Ashcroft scoring method (32). MCM was quantified on Alcian blue–periodic acid Schiff (AB-PAS)–stained lung sections using NIH ImageJ as previously described (30) (see the online supplement).
Transmission Electron Microscopy
Transmission electron microscopy was performed as described previously (25).
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling Staining
Apoptotic cells in lung sections were quantified using the DeadEnd Fluorometric Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) System (Promega, Madison, WI). Numbers of apoptotic cells were normalized for total 4′,6-diamidino-2-phenylindole (DAPI)–stained cells using Image J (National Institutes of Health, Bethesda, MD) (see the online supplement).
ELISA
Cytokines (IL-13, IL-5, IL-1β, TGF-β1, OPN, PDGF-AA) in BALF were measured by commercially available ELISA kits (see the online supplement).
RNA Extraction and Taqman Real-Time PCR
RNA was collected from mouse lung tissue or primary fibroblasts using the RNeasy Mini Kit (Qiagen, Valencia, CA). Taqman Gene Expression assays were performed on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) (see the online supplement).
Primary MLFs
Primary MLFs were isolated from adult male WT and Stat1−/− mice as previously published (33) (see the online supplement).
Sircol Collagen Assay
Soluble collagen secreted by MLFs in culture was measured by Sircol assay (Biocolor, Carrickfergus, UK) according to the manufacturer’s instructions (see the online supplement).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software v. 5.0 (GraphPad Software, Inc., San Diego, CA). Both a one-way ANOVA with a Tukey post hoc test and a Student’s t test were performed to identify significant differences among treatment groups. In addition, a two-way ANOVA with Bonferroni post test was used to identify significant differences between genotypes.
Results
STAT1 Promotes the Resolution of Eosinophilic Inflammation in OVA-Sensitized Mice Treated with MWCNTs
The experimental design for OVA sensitization followed by MWCNT exposure is shown in Figure 1A. To characterize the acute inflammatory response in the lungs of Stat1−/− or WT mice, we analyzed hematoxylin and eosin–stained tissue sections and performed differential cell counts on BALF. Total cell numbers in BALF were significantly increased in OVA or OVA/MWCNT groups, but were not different between genotypes at 1 day (Figure 1B). However, Stat1−/− mice had higher total cell counts after OVA or OVA/MWCNT at 21 days compared with WT mice. Exposure to MWCNTs without OVA presensitization caused an increase in neutrophils in the BALF of both WT and Stat1−/− mice at 1 day, which resolved by 21 days (Figures 1B and 1C). In contrast, OVA sensitization or OVA sensitization followed by MWCNT exposure caused a robust increase in eosinophils in the BALF of both WT and Stat1−/− mice at 1 day (Figures 1B and 1C). However, numbers of eosinophils in BALF resolved in WT mice by 21 days, but eosinophils remained significantly elevated in Stat1−/− mice in these treatment groups at 21 days (Figure 1B). Interestingly, OVA sensitization prevented MWCNT-induced neutrophilic inflammation (Figure 1B). Lymphocytes were also significantly elevated at 21 days after OVA sensitization with or without MWCNT exposure, but this chronic inflammation was seen in both WT and Stat1−/− mice (Figure 1B). MWCNTs were observed within alveolar macrophages within the bronchoalveolar region of the lung by light microscopy (Figure 1C), and MWCNT structure in macrophages verified by transmission electron microscopy (Figure 1D).
STAT1 Suppresses Acute IL-13 Expression and Chronic IL-1β Levels in Mice Sensitized with OVA and Exposed to MWCNTs
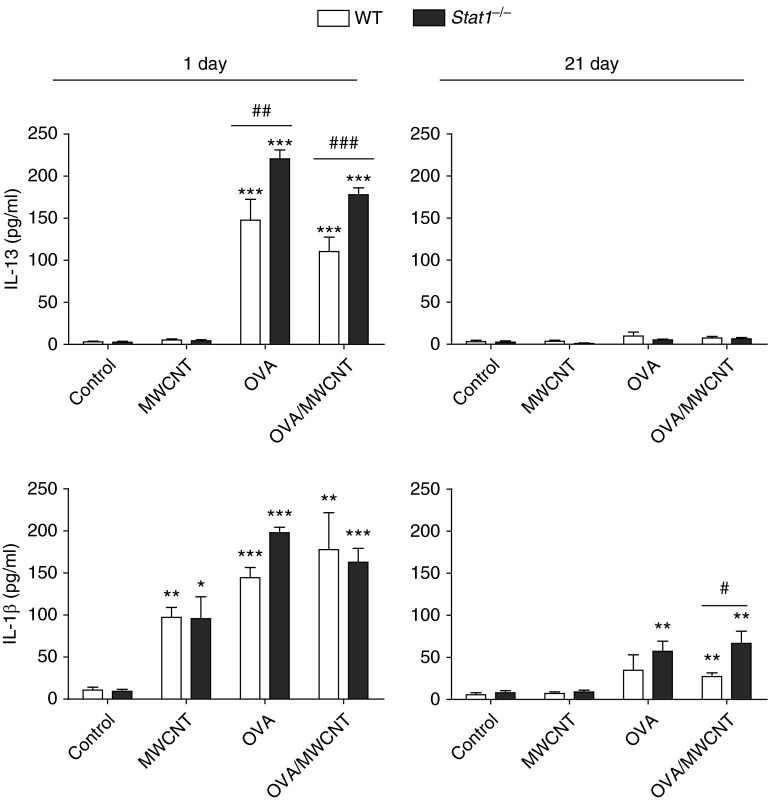
To better understand the role of STAT1 in suppressing chronic airway inflammation, we measured protein levels of proinflammatory cytokines, IL-13 and IL-1β. Levels of IL-13 in the BALF of mice were increased by OVA at 1 day after exposure, and significantly higher levels were observed in Stat1−/− mice compared with WT mice (Figure 2). MWCNT exposure alone did not increase IL-13, and treatment with MWCNTs after OVA slightly reduced OVA-induced IL-13 levels (Figure 2). IL-13 levels returned to control levels in both genotypes by 21 days. IL-5 levels were induced similarly at 1 day in Stat1−/− and WT mice after OVA sensitization with or without MWCNTs and returned to control levels by 21 day after exposure (data not shown). IL-β levels were induced by OVA, MWCNTs or the combination of OVA and MWCNTs at 1 day after exposure, and OVA-sensitized Stat1−/− mice exhibited significantly higher levels of IL-1β at 1 day compared with WT mice (Figure 2). Notably, IL-1β levels in Stat1−/− mice treated with OVA or OVA followed by MWCNT exposure were significantly higher at 21 days compared with WT mice (Figure 2). Collectively, these data revealed that STAT1 functions to suppress acute allergen–induced expression of IL-13 and chronic expression of IL-1β after allergen or MWCNT exposure.
Figure 2.
STAT1 deficiency increases levels of IL-13 at 1 day and IL-1β in the BALF at 21 days after exposure to allergen and MWCNTs. IL-13 (upper panels) and IL-1β (lower panels) in BALF were measured by ELISA. Open bars represent WT mice and solid bars represent Stat1−/− mice. Data are the mean ± SEM (n = 4 animals control and OVA groups, n = 6 MWCNT and OVA/MWCNT groups). *P < 0.05, **P < 0.01, ***P < 0.001 compared with control as determined by one-way ANOVA; #P < 0.05, ##P < 0.01, ###P < 0.001 between genotypes within the same treatment group as determined by two-way ANOVA.
STAT1 Reduces MCM and Airway Fibrosis in OVA-Sensitized Mice and Suppresses Exacerbation of MCM by MWCNTs
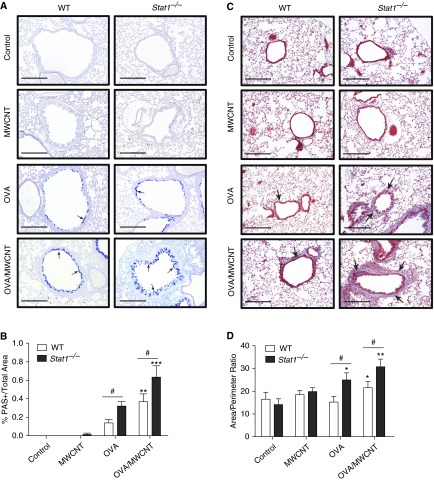
MCM is a prominent characteristic of allergic airway remodeling that can result in airway obstruction. AB-PAS staining of lung sections from WT and Stat1−/− mice showed that OVA sensitization or OVA followed by MWCNT exposure resulted in MCM in small airways at 21 days after exposure (Figure 3A). Semiquantitative morphometric analysis revealed significantly greater MCM in the lungs of Stat1−/− mice exposed to OVA at 21 days after exposure (Figure 3B). MWCNTs significantly exacerbated OVA-induced MCM in WT and Stat1−/− mice at 21 days (Figure 3B), yet MWCNTs did not acutely exacerbate OVA-induced MCM at 1 day after exposure (data not shown). MCM in OVA or OVA/MWCNT groups was significantly greater in Stat1−/− mice compared with WT mice that received the same treatments (Figure 3B). Airway fibrosis is an important chronic feature in the pathogenesis of asthma that contributes to airway resistance and impaired function. To determine the role of STAT1 in chronic remodeling and fibrosis, we assessed airway fibrosis in Masson’s trichrome–stained lung sections at 21 days after MWCNT exposure using an area:perimeter ratio method (30). Photomicrographs of trichrome-stained lung sections from WT or Stat1−/− mice showed that MWCNTs alone did not cause airway fibrosis, yet MWCNT after OVA increased airway fibrosis (Figure 3C). Area:perimeter scoring performed in a blinded manner showed that OVA or MWCNT treatments caused no significant fibrosis in WT mice, yet the combination of OVA and MWCNTs caused a significant increase in airway fibrosis in WT mice (Figure 3D). Airway fibrosis was significantly increased in Stat1−/− mice sensitized with OVA or sensitized with OVA then treated with MWCNTs (Figure 3D). Moreover, airway fibrosis in OVA or OVA/MWCNT groups was significantly greater in Stat1−/− mice compared with WT mice that received the same treatments (Figure 3D). The Ashcroft scoring method gave results similar to those of the area:perimeter scoring method (data not shown).
Figure 3.
STAT1 deficiency increases mucous cell metaplasia (MCM) and airway fibrosis after allergen sensitization and exposure to MWCNTs. (A) Representative photomicrographs of Alcian blue–periodic acid Schiff (AB-PAS) staining mucus (arrows) in lung sections from WT and Stat1−/− mice 21 days after MWCNT exposure and presensitization to OVA allergen. Scale bars, 500 μm. (B) Quantification of MCM showing the percentage of epithelial area positive for AB-PAS divided by total airway epithelial area at 21 days measured by the Image J protocol described in Materials and Methods. Open bars represent WT mice and solid bars represent Stat1−/− mice. Data are the mean ± SEM (n = 4 animals control and OVA groups, n = 6 MWCNT and OVA/MWCNT groups). **P < 0.01, ***P < 0.001 compared with control for each genotype as determined by one-way ANOVA; #P < 0.05 between genotypes as determined by two-way ANOVA. (C) Representative photomicrographs of trichrome-positive airway fibrosis (arrows) in the lungs of mice from each genotype and treatment group 21 days after MWCNT treatment. Scale bars, 500 μm. (D) Quantification of airway fibrosis by area:perimeter ratio method described in Materials and Methods. Data are the mean ± SEM (n = 4 animals control and OVA groups, n = 6 MWCNT and OVA/MWCNT groups). *P < 0.05, **P < 0.01 compared with control for each genotype as determined by one-way ANOVA; #P < 0.05 between genotypes within the same treatment group as determined by two-way ANOVA.
Stat1−/− Mice Display Increased Cytokines That Mediate Fibrosis after OVA Sensitization and Treatment with MWCNTs
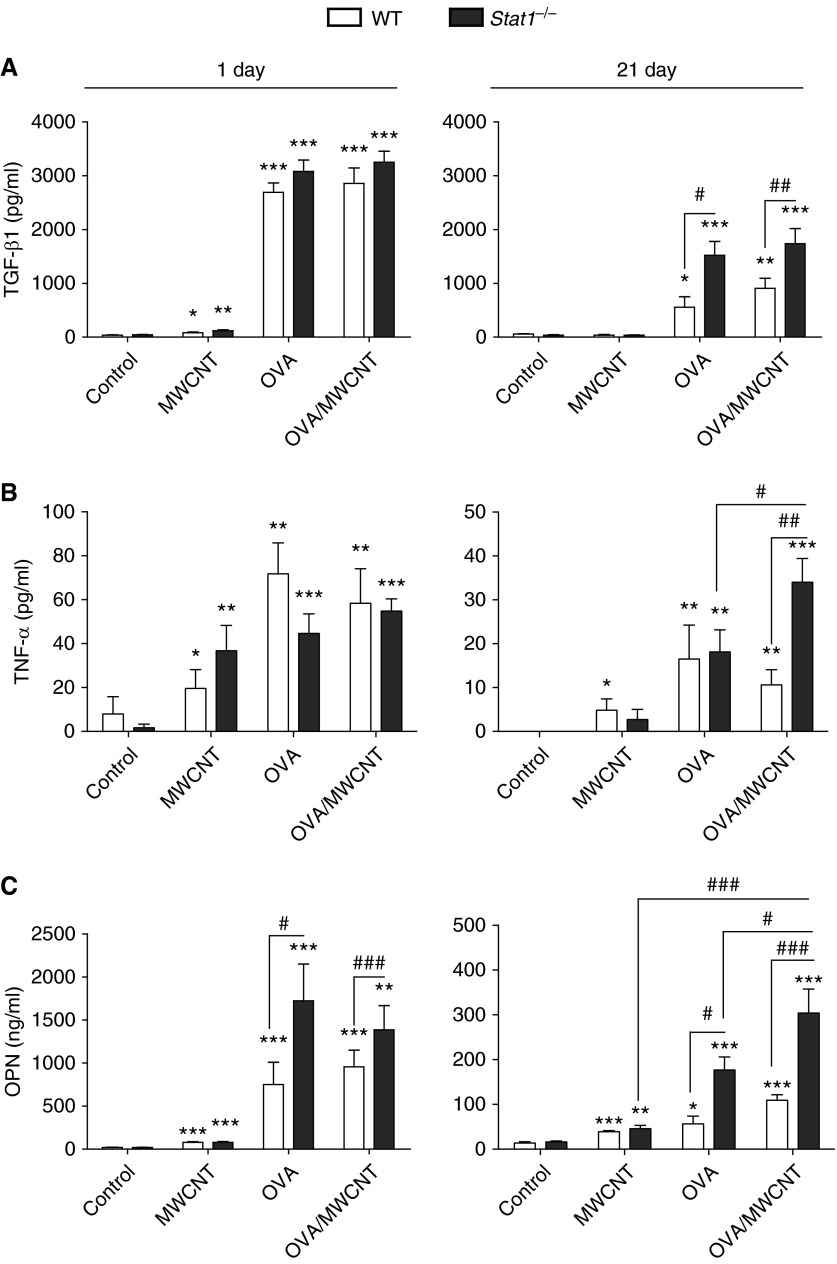
TGF-β1, TNF-α, PDGF-AA, and OPN are all important mediators of fibrotic lung disease that mediate myofibroblast collagen production, growth, and migration. TGF-β1 protein levels in BALF were strongly induced by OVA or OVA and MWCNTs in either WT or Stat1−/− mice at 1 day after exposure, yet TGF-β1 was significantly higher in OVA- or OVA/MWCNT–exposed Stat1−/− mice at 21 days after exposure compared with WT (Figure 4A). Stat1−/− mice treated with OVA or OVA/MWCNTs also exhibited higher levels of TNF-α at 21 days compared with WT mice, and levels of TNF-α were significantly higher in the OVA/MWCNT group compared with OVA group (Figure 4B). OPN protein levels in BALF were significantly higher at both 1 and 21 days in WT and Stat1−/− mice treated with OVA or OVA and MWCNTs, and levels of OPN in the OVA and OVA/MWCNT groups were significantly higher in Stat1−/− mice compared with WT mice at both 1 and 21 days (Figure 4C). PDGF-AA protein levels in BALF were also increased significantly at 21 days in Stat1−/− mice compared with WT mice, although not different between OVA and OVA/MWCNT groups (see Figure E1 in the online supplement). Collectively, these data indicate that STAT1 plays an important role in suppressing the chronic expression of profibrogenic cytokines in the lung after allergen and MWCNT exposure.
Figure 4.
STAT1 suppresses mediators of fibrosis in BALF after OVA sensitization and exposure to MWCNTs. Protein levels of (A) transforming growth factor (TGF)-β1, (B) TNF-α, and (C) osteopontin (OPN) were assessed in BALF using ELISA. Open bars represent WT mice and solid bars represent Stat1−/− mice. Data are the mean ± SEM (n = 4 animals, control and OVA groups; n = 6 animals, MWCNT and OVA/MWCNT groups). *P < 0.0.05, **P < 0.01, ***P < 0.001 compared with control as determined by one-way ANOVA; #P < 0.05, ##P < 0.01, ###P < 0.001 between genotypes within the same treatment group as determined by two-way ANOVA.
STAT1 Suppresses Collagen mRNAs and Soluble Collagen Protein in Response to TGF-β1 in Primary MLFs
To establish whether there is a relationship between increased TGF-β1 protein (Figure 4A) and increased collagen deposition and fibrosis observed in the Stat1−/− mice (Figures 3C and 3D), we isolated and cultured primary fibroblasts from the lungs of WT and Stat1−/− mice, then treated confluent cultures of the MLFs with 10 ng/ml recombinant TGF-β1 protein for 48 hours. We then evaluated collagen (Col) 1A1 and Col1A2 mRNA levels by Taqman quantitative real-time PCR and soluble collagen protein at 72 hours by Sircol assay. TGF-β1 treatment increased Col1A1 and Col1A2 mRNAs in WT MLFs (Figures 5A and 5B) and levels of soluble collagen protein in supernatants from WT MLFs (Figure 5C). However, Stat1−/− MLFs exhibited increased basal expression of Col1A1 and Col1A2 mRNAs, and, importantly, the induction of these mRNAs at 48 hours, as well as consequent secretion of soluble collagen into cell culture supernatants at 72 hours, was significantly increased by TGF-β1 in Stat1−/− MLFs compared with WT MLFs (Figure 5). Together, these data support our hypothesis that STAT1 suppresses airway fibrosis after exposure to allergen and nanoparticles by inhibiting TGF-β1–induced collagen mRNA and protein expression in lung fibroblasts.
Figure 5.
Primary lung fibroblasts from Stat1−/− mice display increased collagen mRNAs and soluble collagen protein after treatment with recombinant TGF-β1. Confluent, quiescent primary WT or Stat1−/− mouse lung fibroblasts (MLFs) were treated with recombinant TGF-β1 for 48 or 72 hours before collecting RNA from cells and harvesting cell supernatants, respectively. Collagen mRNAs (collagen [Col] 1A1 and Col1A2) were measured by Taqman real-time RT-PCR at 48 hours, and collagen protein levels were measured by Sircol assay at 72 hours. Open bars represent WT MLF and solid bars represent Stat1−/− MLF. (A) Col1A1 mRNA levels at 48 hours in WT and Stat1−/− MLF. (B) Col1A2 mRNA levels at 48 hours in WT and Stat1−/− MLF. (C) Soluble collagen levels in supernatants from WT and Stat1−/− MLF 72 hours after treatment with 10 ng/ml TGF-β1 or medium alone (control). Collagen mRNA data are the mean ± SEM of four separate dishes of cells from two experiments, and collagen protein data are the mean ± SEM from three separate dishes from a single experiment. *P < 0.0.05, **P < 0.01, ***P < 0.001 compared with control as determined by one-way ANOVA; #P < 0.05 and ###P < 0.001 between genotypes as determined by two-way ANOVA.
Stat1−/− Mice Display Reduced Lung IL-10 mRNA, but Increased Foxp3 mRNA after OVA Sensitization and MWCNT Exposure
STAT1 could also regulate T-regulatory cells (Tregs) through altered production of soluble mediators (e.g., IL-10) and transcription factors (e.g., Foxp3). Therefore, we measured mRNA levels of IL-10 and Foxp3 in lung tissue. IL-10 mRNA levels were increased in OVA and OVA/MWCNT groups in Stat1−/− mice compared WT mice, yet this effect was not significantly different between genotypes at 1 day. However, exposure of mice to OVA and MWCNT significantly increased IL-10 mRNA at 21 days in WT mice, but not in Stat1−/− mice (Figure 6A). In contrast to IL-10, Foxp3 mRNA levels were significantly induced by OVA or OVA/MWCNT treatments at 21 days in Stat1−/− mice, but not in WT mice (Figure 6B). We also measured lung mRNA levels of T-bet, a transcription factor that is required for Th1 development. Although there was a trend for T-bet mRNA levels to increase with OVA or OVA/MWCNT treatment at 1 day, this effect was not significant, nor was there a significant difference between genotypes. T-bet mRNA levels in lung tissue returned to control levels by 21 days after exposure to MWCNT (Figure E2). Finally, we measured levels of T-bet and Foxp3 mRNAs in WT and Stat1−/− MLF treated with recombinant IL-13 (10 ng/ml), MWCNTs (10 μg/cm2), or both. Foxp3 mRNA levels in WT MLF were approximately 50% higher than in Stat1−/− MLF with or without IL-13 or MWCNTs, albeit not to a significant extent (data not shown). In contrast, T-bet mRNA levels were detectable in WT MLF, but not in Stat1−/− MLF, and were induced by IL-13, but not MWCNT or the combination of IL-13 and MWCNT (data not shown).
Figure 6.
STAT1 mediates IL-10 mRNA expression but suppresses Foxp3 mRNA levels in lung tissue after OVA sensitization and exposure to MWCNTs. Levels of IL-10 and Foxp3 mRNA in lung tissue from WT mice (open bars) or Stat1−/− mice (solid bars) were measure by Taqman real-time RT-PCR. (A) IL-10 mRNA levels at 1 and 21 days after exposure to MWCNT after OVA sensitization. (B) Foxp3 mRNA levels at 1 and 21 days after exposure to MWCNT after OVA sensitization. Data are the mean ± SEM (n = 3 animals for each group). *P < 0.0.05, **P < 0.01 compared with control as determined by one-way ANOVA; ##P < 0.01, ###P < 0.001 between genotypes within the same treatment group as determined by two-way ANOVA.
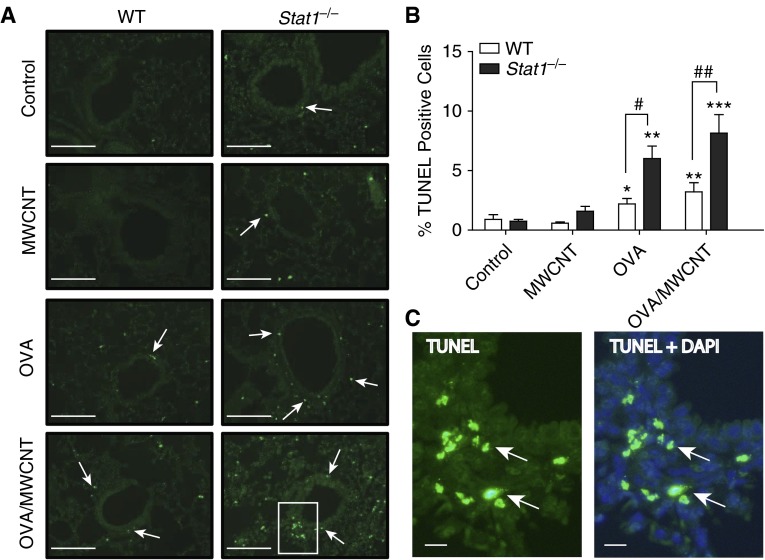
Stat1−/− Mice Display Increased Apoptosis after OVA Sensitization and Treatment with MWCNTs
Due to the well-established relationship between apoptosis and lung fibrosis, we quantitated apoptosis in paraffin-embedded lung tissue from control and treated animals using TUNEL staining. Representative images are shown in Figure 7A, and Figure 7C shows green fluorescent TUNEL cells around the airways that are predominantly interstitial cells. Although the identity of these cells has not been clarified, the location of these cells beneath the airway epithelium and their irregular nuclear profiles suggest that they are infiltrating inflammatory cells. Quantitation of apoptosis was accomplished by the Image J program described in Materials and Methods, wherein TUNEL-positive fluorescent cells were counted relative to the total number of DAPI-stained cells within the same microscopic field. Very few TUNEL-positive cells were observed in control and MWCNT-treated mice (Figure 7B). However, statistically significant increases were found after OVA sensitization and OVA sensitization with MWCNT treatment (Figure 7B). Interestingly, among these treatment groups, the Stat1−/− mice displayed significantly more apoptotic cells than similarly treated WT mice (Figure 7B).
Figure 7.
Stat1−/− mice display significant increases in apoptosis compared with WT mice after OVA sensitization and treatment with MWCNTs. (A) Representative photomicrographs of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining in lung tissue of mice from each genotype and treatment group at 21 days after MWCNT exposure (20× magnification). Arrows indicate TUNEL-positive cells. Scale bars, 500 μm. (B) Quantification of the percentages of TUNEL-positive cells relative to total number of 4′,6-diamidino-2-phenylindole (DAPI)–positive cells 21 days after MWCNT exposure using the Image J protocol described in Materials and Methods. Open bars represent WT mice and solid bars represent Stat1−/− mice. Data are the mean ± SEM (n = 4 animals, control and OVA groups; n = 6 animals, MWCNT and OVA/MWCNT groups). Significant differences compared with controls were determined by one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001). Significant differences between genotypes were determined by two-way ANOVA (#P < 0.05, ##P < 0.01). (C) Higher magnification of inset image in A for Stat1−/− group after OVA/MWCNT treatment showing TUNEL image and merged TUNEL + DAPI. Arrows indicate TUNEL-positive cells. Scale bars, 50 μm.
Discussion
In this study we showed that STAT1 is a key regulator of allergen-induced airway inflammation and exacerbation by MWCNTs in mice. We previously reported that Stat1−/− mice are susceptible to bleomycin-induced pulmonary fibrosis (24). However, this is the first study, to our knowledge, that addresses mechanisms of susceptibility to allergen-induced airway inflammation or nanoparticle exposure in Stat1−/− mice. We observed that Stat1−/− mice developed more severe airway chronic remodeling (MCM, airway fibrosis, apoptosis) compared with WT mice after OVA sensitization, and all of these components of OVA-induced airway remodeling were further exacerbated by exposure to MWCNTs in Stat1−/− mice compared with WT mice. Profibrogenic cytokines (TGF-β1, TNF-α, OPN) were exaggerated in the lungs of Stat1−/− mice by OVA and further increased 21 days after MWCNT exposure. The mechanism of susceptibility to OVA and MWCNTs in Stat1−/− mice not only involved increased production of profibrogenic cytokines, but also increased responsiveness to cytokines, because we demonstrated that MLFs isolated from Stat1−/− mice exhibited elevated collagen mRNA and protein after stimulation with TGF-β1 in vitro. Collectively, these novel findings support a protective role for STAT1 in chronic allergen–induced airway remodeling and exacerbation of airway remodeling by nanoparticles.
Allergen-induced lung inflammation is characterized by eosinophilic lung inflammation. We observed that the relative percentages of eosinophils were similar in Stat1−/− and WT mice at 1 day after OVA or OVA/MWCNT exposure, indicating that acute eosinophilia was STAT1 independent. However, a significantly greater percentage of eosinophils were observed in Stat1−/− mice compared with WT at 21 days after OVA sensitization with or without MWCNT exposure. These data indicate that chronic eosinophilia induced by allergen challenge is STAT1 dependent, but that MWCNTs do not exacerbate allergen-induced eosinophilia. Alternatively, there might be a delay in clearance or resolution of eosinophils in Stat1−/− mice, rather than a chronic sustained increase in eosinophils. MWCNTs caused neutrophilic lung inflammation as we have reported previously (15, 30). Interestingly, OVA sensitization completely prevented MWCNT-induced neutrophilia in either WT or Stat1−/− mice, while not affecting OVA-induced eosinophilia. The STAT1-independent inhibition of MWCNT-induced neutrophilia by OVA sensitization suggests that individuals with allergic asthma exposed to MWCNTs would not mount a neutrophilic inflammatory response in the lung. This could be significant for understanding the exacerbation of allergen-induced airway remodeling by MWCNTs, as neutrophils play an important role in host defense and resolution of inflammation (34).
Many of the chronic phenotypic changes that define airway remodeling in asthma, particularly MCM and airway fibrosis, are driven by IL-13 (35). Semiquantitative morphometry of AB-PAS–stained lung sections demonstrated that MCM was significantly increased in the airways of Stat1−/− mice compared with WT mice after OVA sensitization. Moreover, MCM was further enhanced by MWCNT exposure after OVA sensitization in WT mice, and even more so in Stat1−/− mice. Therefore, our data show that STAT1 suppresses both MCM and eosinophilia, yet MWCNTs only exacerbated MCM. Eosinophilia has been reported as a marker of airway epithelial cell damage, but is unrelated to MCM (36). Our data support this concept, because MWCNTs exacerbated OVA-induced MCM, but not OVA-induced eosinophilia. Most importantly, our findings show that STAT1 plays a key role in suppressing allergen-induced MCM as well as in the exacerbation of allergen-induced MCM by MWCNTs. IL-13 has also been shown to play a prominent role in the development of airway fibrosis. Transgenic mice that overexpress IL-13 develop nearly all of the chronic remodeling phenotypes present in patients with severe asthma, including MCM and airway fibrosis (37). IL-13 likely mediates its profibrogenic effects by serving as an upstream regulator of TGF-β1 (38) and PDGF-AA (39) to promote collagen deposition and fibroblast proliferation, respectively.
Studies with mice have shown that STAT1 is protective in suppressing lung fibrogenesis. For example, we previously reported that Stat1−/− mice are susceptible to bleomycin-induced lung fibrosis (24). Enhanced susceptibility to bleomycin-induced lung fibrosis was due in part to increased epidermal growth factor– or PDGF-induced proliferation of fibroblasts isolated from the lungs of Stat1−/− mice. In the present study, we discovered that Stat1−/− lung fibroblasts also have increased responsiveness to recombinant TGF-β1, and these cells produced significantly higher levels of collagen mRNAs as well as more soluble collagen protein than WT MLFs treated with TGF-β1. Therefore, STAT1 suppresses lung fibrosis at least in part by reducing the activity of several growth factors that mediate fibroblast proliferation and collagen production. It has also been reported that Stat1−/− mice are highly susceptible to severe acute respiratory syndrome coronavirus infection, which results in end-stage lung disease that features pulmonary fibrosis (40). In that study, the absence of STAT1 impaired viral clearance, but also stimulated the development of alternatively activated macrophages that mediated a profibrogenic microenvironment within the lung. Although Stat1−/− mice sensitized with OVA and exposed to MWCNTs had increased profibrogenic cytokines (e.g., OPN, TGF-β1), we also showed that mRNA encoding IL-10, an antifibrogenic cytokine, was completely suppressed in Stat1−/− mice after OVA sensitization and MWCNT exposure. These data are in agreement with previous work showing that STAT1 promotes IL-10 production in CD4+ T cells from naive mice (41). In contrast to our findings of suppressed IL-10 in lung tissue from Stat1−/− mice, mRNA levels of the transcription factor Foxp3 were significantly enhanced in lung tissue from Stat1−/− mice compared with WT mice. Others have shown that STAT1 deficiency in donor splenocytes from mice correlated with expansion of CD4+/Foxp3+ Tregs (42). Two major subsets of Tregs are Foxp3+ Tregs and IL-10–producing Tregs (43). Although our study only evaluated mRNA levels of IL-10 and Foxp3 in total lung tissue and not Treg populations, our data suggest that STAT1 serves to suppress Foxp3+ Tregs induced by allergen and MWCNTs while enhancing IL-10+ Tregs.
A significant body of work has explored the relationship between apoptosis and fibrotic lung disease. Not only is it well documented that increased apoptosis of airway epithelial cells can contribute to fibrosis, but analysis of human lung biopsies and small animal models of lung fibrogenesis also reveal apoptotic cells associated with fibrotic foci (44, 45). It is notable, then, that we observed significant increases in apoptosis in Stat1−/− animals sensitized to OVA and/or sensitized to OVA and treated with MWCNTs (Figure 7). However, very few airway or alveolar epithelial cells were TUNEL positive, suggesting that another cell type was undergoing cell death in the lungs of Stat1−/− mice. Although the identity of the TUNEL-positive cells has not been clarified, the location of these cells beneath the airway epithelium and nuclear profiles suggest that they are most likely infiltrating inflammatory cells, such as neutrophils or eosinophils. This is also consistent with observations that some TUNEL-positive cells were observed within and surrounding the pulmonary vasculature (data not shown). It remains unclear whether it is the absence of STAT1 that directly results in this increase in apoptosis, or whether increased apoptosis in Stat1−/− mice is due to an increase in secondary factors, such as increased levels of cytokines, growth factors, or oxidants, which then serve to increase the fibrotic response.
Together, our results suggest that the presence of STAT1 is critical in tempering the immediate and long-term severity of allergic airway remodeling. There are likely multiple explanations for these findings. One explanation involves the lack of STAT-1 resulting in a Th1/Th2 imbalance, toward a Th2 phenotype. This explanation is consistent with our data that show prolonged presence of eosinophils and increased levels of IL-13, a key cytokine responsible for many of the pathologic abnormalities that are characteristic of asthma. An additional explanation and/or contributor to the increased sensitivity of Stat1−/− mice to airway remodeling after allergen sensitization may be that the lack of STAT1 results in increased activity of STAT6 (46). STAT6 is often seen increased in the lung epithelium of patients with severe asthma (47), and is known to be downstream of several Th2 cytokines, including IL-13. IL-13 signals to STAT6 in a pathway leading to increased TGF-β1 by epithelial cells and macrophages, which stimulates fibroblast collagen production in a paracrine manner (37, 38). Past studies have revealed a function for STAT1 in the negative regulation of STAT6 through its ability to up-regulate the suppressor of cytokine signaling protein 1 (46).
MWCNTs were found to exacerbate only some allergen-induced phenotypic endpoints of chronic airway remodeling; namely, MCM and airway fibrosis. Moreover, exacerbation of OVA-induced MCM and airway fibrosis by MWCNTs was enhanced in Stat1−/− mice. Although several mediators in the BALF of Stat1−/− mice were increased after allergen challenge (IL-13, IL-1β, TGF-β1), we found that OPN and TNF-α were significantly induced by MWCNTs in the OVA-sensitized animals at 21 days. OPN is a matricellular protein that has numerous associations with various pathologic processes, such as inflammation, tissue repair/fibrosis, and angiogenesis (48). OPN contributes to the pathogenesis of airway remodeling, as well as lung fibroblast activation and differentiation in allergen-induced asthma. When OPN-deficient mice were sensitized to OVA, they were found to have a reduction in eosinophils, a known cellular source of TGF-β1 as well as Th2 cytokines (49). In addition, these mice were found to produce less TGF-β1 and have less subepithelial fibrosis. TNF-α also promotes fibroblast activation for collagen production via TGF-β1 production (50). Collectively, our data show that STAT1 suppresses the production and activity of a network of profibrogenic cytokines. Deficiency in STAT1 results in susceptibility to chronic airway remodeling by increased production of profibrogenic cytokines and enhanced collagen production by fibroblasts.
In the present study, we used a relatively high dose of MWCNTs (4 mg/kg). However, we previously reported that this dose of MWCNT did not cause significant fibrosis in mice in the absence of allergen challenge (30). Porter and colleagues (28) used an MWCNT dose range of 10–80 μg/mouse. Our dose approximates the high end of this dose range (i.e., 80 μg delivered to a 20 g mouse = 4 mg/kg). Furthermore, it has been estimated that a dose of 10 μg in mice approximates human deposition for a person performing light work for between 9 months and 7.5 years, based on average daily MWCNT workplace exposure data (28). Therefore, the dose used in our study would likely represent a relatively high occupational exposure. It is important to note that even the relatively high dose of MWCNTs used in our study did not cause pathology in the lungs of WT or Stat1−/− mice in the absence of allergen. Nevertheless, further investigation should focus on lower dose, repeated exposures to MWCNTs.
In summary, we report that OVA-sensitized Stat1−/− mice have increased chronic eosinophilia, MCM, airway fibrosis, and apoptosis, and also produce higher levels of cytokines and growth factors that are known to mediate asthma and fibrosis. The enhancement of chronic eosinophilia in OVA-sensitized Stat1−/− mice was not further increased by MWCNT exposure. However, MWCNTs significantly enhanced OVA-induced MCM and airway fibrosis, suggesting that STAT1 is also important in the exacerbation of allergic airway inflammation by inhaled nanoparticles. The mechanism of susceptibility of Stat1−/− mice to exacerbation of allergic airway remodeling by MWCNTs appears to be due to both increased production of profibrogenic cytokines in the lung and increased collagen production by lung fibroblasts. Our findings identify STAT1 as an important protective factor in allergic airway remodeling, and suggest that deficiency of STAT1 is a susceptibility factor to allergic airway disease and exposure to MWCNT.
Footnotes
This work was supported by U.S. Public Health Service grants R01-ES020897 and RC2-ES018772.
Author Contributions: Conception and design: E.A.T. and J.C.B.; experimentation, analysis, and interpretation: E.A.T., B.C.S., E.E.G.-B., K.A.S., M.D.I., K.S.D., A.J.T., and J.C.B.; drafting the manuscript for important intellectual content: E.A.T. and J.C.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0221OC on March 25, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akinbami OJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS. 2012;94:1–8. [PubMed] [Google Scholar]
- 2.Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402:B2–B4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 3.Drazen JM. Asthma and the human genome project: summary of the 45th Annual Thomas L. Petty Aspen Lung Conference. Chest. 2003;123:447S–449S. doi: 10.1378/chest.123.3_suppl.447s. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 5.Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991;12:256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- 6.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 7.Finotto S, Glimcher L. T cell directives for transcriptional regulation in asthma. Springer Semin Immunopathol. 2004;25:281–294. doi: 10.1007/s00281-003-0143-1. [DOI] [PubMed] [Google Scholar]
- 8.Wills-Karp M. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 9.Stenfors N, Nordenhäll C, Salvi SS, Mudway I, Söderberg M, Blomberg A, Helleday R, Levin JO, Holgate ST, Kelly FJ, et al. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur Respir J. 2004;23:82–86. doi: 10.1183/09031936.03.00004603. [DOI] [PubMed] [Google Scholar]
- 10.Nordenhäll C, Pourazar J, Blomberg A, Levin JO, Sandström T, Adelroth E. Airway inflammation following exposure to diesel exhaust: a study of time kinetics using induced sputum. Eur Respir J. 2000;15:1046–1051. doi: 10.1034/j.1399-3003.2000.01512.x. [DOI] [PubMed] [Google Scholar]
- 11.Nordenhäll C, Pourazar J, Ledin MC, Levin JO, Sandström T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J. 2001;17:909–915. doi: 10.1183/09031936.01.17509090. [DOI] [PubMed] [Google Scholar]
- 12.Pereira P, Saldiva PH, Sakae RS, Bohm GM, Martins MA. Urban levels of air pollution increase lung responsiveness in rats. Environ Res. 1995;69:96–101. doi: 10.1006/enrs.1995.1030. [DOI] [PubMed] [Google Scholar]
- 13.Ohta K, Yamashita N, Tajima M, Miyasaka T, Nakano J, Nakajima M, Ishii A, Horiuchi T, Mano K, Miyamoto T. Diesel exhaust particulate induces airway hyperresponsiveness in a murine model: essential role of GM-CSF. J Allergy Clin Immunol. 1999;104:1024–1030. doi: 10.1016/s0091-6749(99)70084-9. [DOI] [PubMed] [Google Scholar]
- 14.Hao M, Comier S, Wang M, Lee JJ, Nel A. Diesel exhaust particles exert acute effects on airway inflammation and function in murine allergen provocation models. J Allergy Clin Immunol. 2003;112:905–914. doi: 10.1016/j.jaci.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009;40:349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shvedova AA, Kisin ER, Porter D, Schulte P, Kagan VE, Fadeel B, Castranova V. Mechanisms of pulmonary toxicity and medical applications of carbon nanotubes: two faces of Janus? Pharmacol Ther. 2009;121:192–204. doi: 10.1016/j.pharmthera.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson K. Carbon Nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci. 2006;92:5–22. doi: 10.1093/toxsci/kfj130. [DOI] [PubMed] [Google Scholar]
- 18.Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc. 2010;7:138–141. doi: 10.1513/pats.200907-061RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephanou A, Latchman DS. STAT-1: a novel regulator of apoptosis. Int J Exp Pathol. 2004;84:239–244. doi: 10.1111/j.0959-9673.2003.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439–454. doi: 10.1089/1079990041689665. [DOI] [PubMed] [Google Scholar]
- 22.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 23.Durbin JEJ, Hackenmiller RR, Simon MCM, Levy DED. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 24.Walters DM, Antao-Menezes A, Ingram JL, Rice AB, Nyska A, Tani Y, Kleeberger SR, Bonner JC. Susceptibility of signal transducer and activator of transcription-1–deficient mice to pulmonary fibrogenesis. Am J Pathol. 2005;167:1221–1229. doi: 10.1016/S0002-9440(10)61210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingram JL, Antao-Menezes A, Mangum JB, Lyght O, Lee PJ, Elias JA, Bonner JC. Opposing actions of Stat1 and Stat6 on IL-13–induced up-regulation of early growth response-1 and platelet-derived growth factor ligands in pulmonary fibroblasts. J Immunol. 2006;177:4141–4148. doi: 10.4049/jimmunol.177.6.4141. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto A, Hiramatsu K, Li Y, Azuma A, Kudoh S, Takizawa H, Sugawara I. Repeated exposure to low-dose diesel exhaust after allergen challenge exaggerates asthmatic responses in mice. Clin Immunol. 2006;121:227–235. doi: 10.1016/j.clim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Lee JK, Sayers BC, Chun KS, Lao HC, Shipley-Phillips JK, Bonner JC, Langenbach R. Multi-walled carbon nanotubes induce COX-2 and iNOS expression via MAP kinase-dependent and -independent mechanisms in mouse RAW264.7 macrophages. Part Fibre Toxicol. 2012;9:14. doi: 10.1186/1743-8977-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S. Mouse pulmonary dose- and time course–responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res. 2006;32:181–199. doi: 10.1080/01902140600817465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayers BC, Taylor AJ, Glista-Baker EE, Shipley-Phillips JK, Dackor RT, Edin ML, Lih FB, Tomer KB, Zeldin DC, Langenbach R, et al. Role of COX-2 in exacerbation of allergen-induced airway remodeling by multi-walled carbon nanotubes. Am J Respir Cell Mol Biol. 2013;49:525–535. doi: 10.1165/rcmb.2013-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brass DM, Savov JD, Gavett SH, Haykal-Coates N, Schwartz DA. Subchronic endotoxin inhalation causes persistent airway disease. Am J Physiol Lung Cell Mol Physiol. 2003;285:L755–L761. doi: 10.1152/ajplung.00001.2003. [DOI] [PubMed] [Google Scholar]
- 32.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice AB, Ingram JL, Bonner JC. p38 mitogen-activated protein kinase regulates growth factor–induced mitogenesis of rat pulmonary myofibroblasts. Am J Respir Cell Mol Biol. 2002;27:759–765. doi: 10.1165/rcmb.2002-0070OC. [DOI] [PubMed] [Google Scholar]
- 34.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 35.Firszt R, Francisco D, Church TD, Thomas JM, Ingram JL, Kraft M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-β1 in airway fibroblasts in asthma. Eur Respir J. 2013;43:464–473. doi: 10.1183/09031936.00068712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson SJ, Rigden HM, Ward JA, Laviolette M, Jarjour NN, Djukanović R. The relationship between eosinophilia and airway remodelling in mild asthma. Clin Exp Allergy. 2013;43:1342–1350. doi: 10.1111/cea.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingram JL, Rice AB, Geisenhoffer K, Madtes DK, Bonner JC. IL-13 and IL-1beta promote lung fibroblast growth through coordinated up-regulation of PDGF-AA and PDGF-Ralpha. FASEB J. 2004;18:1132–1134. doi: 10.1096/fj.03-1492fje. [DOI] [PubMed] [Google Scholar]
- 40.Zornetzer GA, Frieman MB, Rosenzweig E, Korth MJ, Page C, Baric RS, Katze MG. Transcriptomic analysis reveals a mechanism for a prefibrotic phenotype in STAT1 knockout mice during severe acute respiratory syndrome coronavirus infection. J Virol. 2010;84:11297–11309. doi: 10.1128/JVI.01130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma H, Lu C, Ziegler J, Liu A, Sepulveda A, Okada H, Lentzsch S, Mapara MY. Absence of Stat1 in donor CD4+ T cells promotes the expansion of Tregs and reduces graft-versus-host disease in mice. J Clin Invest. 2011;121:2554–2569. doi: 10.1172/JCI43706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C, Pot C, Apetoh L, Thalhamer T, Zhu B, Murugaiyan G, Xiao S, Lee Y, Rangachari M, Yosef N, et al. Metallothioneins negatively regulate IL-27–induced type 1 regulatory T-cell differentiation. Proc Natl Acad Sci USA. 2013;110:7802–7807. doi: 10.1073/pnas.1211776110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson DS. Regulatory T cells and asthma. Clin Exp Allergy. 2009;39:1314–1323. doi: 10.1111/j.1365-2222.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 44.Uhal BD, Joshi I, Hughes WF, Ramos C, Pardo A, Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275:L1192–L1199. doi: 10.1152/ajplung.1998.275.6.L1192. [DOI] [PubMed] [Google Scholar]
- 45.Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127:266–274. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- 46.Yu C-R, Mahdi RM, Ebong S, Vistica BP, Chen J, Guo Y, Gery I, Egwuagu CE. Cell proliferation and STAT6 pathways are negatively regulated in T cells by STAT1 and suppressors of cytokine signaling. J Immunol. 2004;173:737–746. doi: 10.4049/jimmunol.173.2.737. [DOI] [PubMed] [Google Scholar]
- 47.Mullings RE, Wilson SJ, Puddicombe SM, Lordan JL, Bucchieri F, Djukanović R, Howarth PH, Harper S, Holgate ST, Davies DE. Signal transducer and activator of transcription 6 (STAT-6) expression and function in asthmatic bronchial epithelium. J Allergy Clin Immunol. 2001;108:832–838. doi: 10.1067/mai.2001.119554. [DOI] [PubMed] [Google Scholar]
- 48.O’Regan A. The role of osteopontin in lung disease. Cytokine Growth Factor Rev. 2003;14:479–488. doi: 10.1016/s1359-6101(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 49.Kohan M, Breuer R, Berkman N. Osteopontin induces airway remodeling and lung fibroblast activation in a murine model of asthma. Am J Respir Cell Mol Biol. 2009;41:290–296. doi: 10.1165/rcmb.2008-0307OC. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan DE, Ferris M, Pociask D, Brody AR. Tumor necrosis factor-α induces transforming growth factor-β1 expression in lung fibroblasts through the extracellular signal–regulated kinase pathway. Am J Respir Cell Mol Biol. 2005;32:342–349. doi: 10.1165/rcmb.2004-0288OC. [DOI] [PubMed] [Google Scholar]