Abstract
Intracellular Ca2+ dynamics of airway smooth muscle cells (ASMCs) are believed to play a major role in airway hyperresponsiveness and remodeling in asthma. Prior studies have underscored a prominent role for inositol 1,4,5-triphosphate (IP3) receptors in normal agonist-induced Ca2+ oscillations, whereas ryanodine receptors (RyRs) appear to remain closed during such Ca2+ oscillations, which mediate ASMC contraction. Nevertheless, RyRs have been hypothesized to play a role in hyperresponsive Ca2+ signaling. This could be explained by RyRs being “sensitized” to open more frequently by certain compounds. We investigate the implications of RyR sensitization on Ca2+ dynamics in ASMC using a combination of mathematical modeling and experiments with mouse precision-cut lung slices. Caffeine is used to increase the sensitivity of RyRs to cytosolic Ca2+ concentration ([Ca2+]i) and sarcoplasmic reticulum Ca2+ ([Ca2+]SR). In ASMCs, high caffeine concentrations (>10 mM) induce a sustained elevation of [Ca2+]i. Our mathematical model accounts for this by the activation of store-operated Ca2+ entry that results from a large increase in the RyR sensitivity to [Ca2+]SR and the associated Ca2+ release, which leads to a reduction of [Ca2+]SR. Importantly, our model also predicts that: (1) moderate RyR sensitization induces slow Ca2+ oscillations, a result experimentally confirmed with low concentrations of caffeine; and (2) high RyR sensitization suppresses fast, agonist-induced Ca2+ oscillations by inducing substantial store-operated Ca2+ entry and elevated [Ca2+]i. These results suggest that RyR sensitization could play a role in ASMC proliferation (by inducing slow Ca2+ oscillations) and in airway hyperresponsiveness (by inducing greater mean [Ca2+]i for similar levels of contractile agonist).
Keywords: precision-cut lung slice, mathematical modeling, asthma, hypersensitivity, Ca2+ oscillations
Clinical Relevance
Our work shows that increased ryanodine receptor (RyR) sensitivity can underlie unexpected complications in airway smooth muscle cell Ca2+ signaling and therefore in the downstream physiology. This suggests RyR as a target for therapeutic drugs.
Intracellular Ca2+ dynamics control a wide variety of cellular processes. In particular, airway smooth muscle cell (ASMC) contraction is mediated by oscillations in cytosolic Ca2+ concentration ([Ca2+]i), which correspond to the cyclic release and reuptake of Ca2+ from the sarcoplasmic reticulum (SR). The release of Ca2+ from the SR occurs via two types of channels, the inositol 1,4,5-triphosphate (IP3) receptor (IP3R) and the ryanodine receptor (RyR), whereas Ca2+ reuptake into the SR is performed by sarcoplasmic/endoplasmic reticulum Ca2+ ATPases (SERCAs). Ca2+ may also enter the cell via receptor-operated, store-operated, and voltage-operated Ca2+ channels, and be extruded from the cell across the plasma membrane by Ca2+ ATPases (PMCAs). In ASMCs, G protein–coupled receptor stimulation (by agonists) and membrane depolarization (by KCl) induce two types of Ca2+ oscillations (1, 2). KCl-induced Ca2+ oscillations have a low frequency (∼3/min) as compared with faster agonist-induced Ca2+ oscillations (∼10/min in human and ∼30/min in mouse at room temperature; these are even faster at 37°C). In mouse ASMCs, KCl-induced Ca2+ oscillations induce twitching of the ASMCs and substantially less airway contraction as compared with agonist-induced contraction, but the relative magnitudes of KCl versus agonist-induced contraction is species dependent (2, 3).
We have previously (4) proposed a mechanism for how IP3R and RyR interact to give these two oscillatory behaviors. The pathways are summarized in Figure 1. In brief:
-
•
Agonist stimulation, and subsequent production of IP3, leads to release of Ca2+ through the IP3R, and a cycle of Ca2+ release and reuptake to and from the SR, via the mechanism of Ca2+-induced Ca2+ release. This causes sufficient depletion of the SR to inactivate the RyR, which thus play no significant role in on-going agonist-induced Ca2+ oscillations (see Figure E1A in the online supplement).
-
•
KCl stimulation causes overfilling of the SR, which activates the RyR and leads to Ca2+ release from the SR, again in a process of Ca2+-induced Ca2+ release (Figure E1B).
-
•
The IP3R and RyR interact via their joint effects on [Ca2+]i and sarcoplasmic reticulum Ca2+ ([Ca2+]SR). The fact that RyRs become desensitized when the SR is depleted plays a crucial role in determining the relative contributions of IP3R and RyR to the control of Ca2+ oscillations.
Figure 1.
Schematic diagram of the major pathways involved in the model of Ca2+ dynamics in airway smooth muscle cells (ASMCs). Solid black lines denote Ca2+ fluxes. Stimulation of cell surface G protein–coupled receptors (GPCR) leads to production of inositol 1,4,5-triphosphate (IP3) and activation of the IP3 receptor (IP3R). Subsequent release of Ca2+ from the sarcoplasmic reticulum (SR) leads to Ca2+-induced Ca2+ release (CICR) through either IP3R or ryanodine receptors (RyRs). Depletion of the SR induces the opening of store-operated Ca2+ channels (SOCCs) in the plasma membrane but also inactivates RyR. Ca2+ is removed from the cytoplasm by plasma membrane ATPase (PMCA) or by sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA). Addition of extracellular KCl leads to membrane depolarization and opening of voltage-operated Ca2+ channels (VOCCs). The consequent increase in Ca2+ influx causes (in the absence of agonist) overfilling of the SR and activation of RyR. IP3R and RyR interact via their effects on the concentration of Ca2+ ([Ca2+]) in the cytosol and the SR. Here, we focus on the predicted effects of caffeine (and other compounds), which sensitizes RyR to cytosolic and luminal Ca2+.
As such, increasing the Ca2+ sensitivity of RyR (which we refer to in this work as RyR “sensitization”) could have important implications for the participation of RyRs in Ca2+ dynamics in hyperresponsive conditions. In addition to agonist-induced calcium signaling being intrinsically altered in asthmatic airway (e.g., Ref. 5), proinflammatory factors present in asthma enhance [Ca2+]i responses of (nonasthmatic) ASMCs to agonist stimulation and/or to store depletion (6–12). There is evidence that the RyR could play a role in these augmented Ca2+ responses (13, 14). This role for RyR sensitivity can be explored experimentally with compounds, such as caffeine, that increase the sensitivity of RyR to [Ca2+]SR and [Ca2+]i (15, 16). Therefore, in this work, we integrate both IP3R and RyR dynamics into an extension of our previous mathematical model of Ca2+ dynamics in ASMC (17), and experimentally test the predictions of this model. The consequences of RyR sensitization on ASMC Ca2+ dynamics is evaluated in the absence and presence of agonist stimulation.
Materials and Methods
Experimental Methods
Precision-cut lung slices (PCLSs) were prepared from female BALB/c mice (7–12 wk old) as described in Section E1.1 of the online supplement. All experiments were conducted at body temperature (37°C) in a custom-made, temperature-controlled microscope chamber as described in (3).
Measurement of Ca2+ oscillations: PCLSs were incubated in Hanks' buffered salt solution supplemented with 20 mM HEPES (sHBSS) containing 20 μM Oregon Green 488 BAPTA-1-AM (Invitrogen, Carlsbad, CA), a Ca2+-sensitive dye, 0.1% Pluronic F-127 (Invitrogen), and 200 μM sulfobromophthalein in the dark at 30°C for 1 hour. Subsequently, the PCLSs were incubated in 200 μM sulfobromophthalein for 30 minutes. Slices were mounted on a cover glass and held down with 200-μm nylon mesh. A smaller cover glass was placed on top of the mesh and sealed at the sides with silicone grease to facilitate solution exchange. Mounted lung slices were continuously perfused with sHBSS or sHBSS containing the required compounds. ASMC Ca2+ signals were examined with a custom-built two-photon scanning laser microscope with a 40× oil immersion objective (NA 1.35) and images recorded at 15 or 30 images s−1. Changes in fluorescence intensity (which represent changes in [Ca2+]i) were analyzed in an ASMC of interest by averaging the gray value of up to a 10 × 10-pixel region using custom written software. Relative fluorescence intensity was expressed as a ratio of the fluorescence intensity (Ft/F0; i.e., fluorescence intensity at a particular time [Ft] normalized with respect to the initial fluorescence intensity [F0]).
Statistical Analysis
Results on Ca2+ oscillation frequency are shown as mean (±SEM). Student’s t test was used to compute the 95% confidence interval of the mean (mean ±t0.975n − 1 SEM, where denotes the quantile at 97.5% of the student distribution with k degrees of freedom), and to evaluate the significance of the difference between means at different caffeine concentrations.
Mathematical Model
The model is an extension of previous work (17). In brief, the dynamics of c = [Ca2+]i and cs = [Ca2+]SR are governed by:
| (1) |
where Jin models Ca2+ influx (principally through store-operated Ca2+ channels), JPMCA and JSERCA are the Ca2+ fluxes through the PMCA and SERCA pumps, respectively, and Jrel models the flux out of the SR through IP3R and RyR. Full details of each of these fluxes are given in the online supplement (Section E1.2).
For our purposes, the most important term is Jrel, which contains a model of the RyR flux. In this flux, the open probability of the RyR is assumed to be given by:
| (2) |
Thus, the RyR sensitivity to cytosolic and luminal Ca2+ is governed by the two parameters, KcR and KsR, respectively.
Results
Effect of RyR Sensitization on Resting Ca2+
Model predictions.
Sensitization of the RyR is obtained in the model by decreasing the cytosolic Ca2+ activation threshold (KcR) or the luminal Ca2+ activation threshold (KsR) from their resting values (Equation 2 and Table E1). We define the indices, cytosolic sensitivity (rc) and luminal sensitivity (rs), to reflect the degree of cytosolic and luminal sensitization, respectively, as follows: rc = (KcR0 − KcR)/Ke and rs = (KsR0 − KsR)/Ks, where KcR0 and KsR0 are the resting values of KcR and KsR in Table E1. Thus, increasing values of rc and rs represent increasing cytosolic and luminal sensitization, respectively, of RyR to Ca2+.
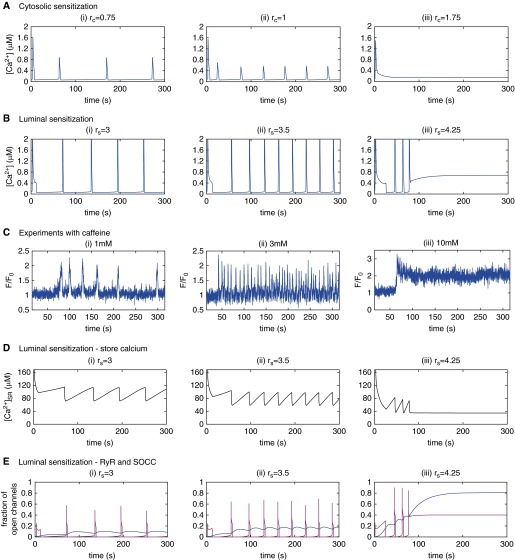
Figures 2A and 2B show the effect of increasing rc and rs, respectively, on [Ca2+]i dynamics. Both moderate cytosolic and luminal sensitization of RyR induce low-frequency RyR-mediated Ca2+ oscillations (panels i and ii), with a frequency that increases with increased sensitization. We note that the induction of Ca2+ oscillations by an increase in rs can be understood as the mirror mechanism by which KCl induces Ca2+ oscillations: the increase in [Ca2+]SR induced by membrane depolarization, which activates RyR (Figure E1B), is effectively replaced by a decrease in the [Ca2+]SR half-activation threshold, KsR (i.e., overcoming the threshold is equivalent to lowering it sufficiently).
Figure 2.
(A) Simulations of the effect of increasing levels of cytosolic RyR sensitization (rc) on cytosolic Ca2+ concentration ([Ca2+]i) dynamics, starting from (rc, rs) = (0, 1) (red dot in Figure E3A). (B) Simulations of the effect of increasing levels of luminal RyR sensitization (rs) on [Ca2+]i dynamics, starting from (rc, rs) = (0, 0) (no sensitization; black dot in Figure E3A). (C) Relative fluorescence signals indicating the [Ca2+]i responses induced by caffeine in ASMCs of mouse lung slices (see Materials and Methods) for (i) 1 mM caffeine, (ii) 3 mM caffeine, and (iii) 10 mM caffeine. (D) The predicted sarcoplasmic reticulum Ca2+ ([Ca2+]SR) associated with the [Ca2+]i dynamics modeled in B. (E) The predicted fraction of open RyRs (magenta) and open SOCCs (blue) associated with the [Ca2+]i dynamics in B.
At higher RyR sensitization (Figures 2A and 2B [iii]), the Ca2+ oscillations disappear and are replaced by a steady [Ca2+]i, with one important difference: the steady [Ca2+]i induced by high cytosolic RyR sensitization remains close to the resting [Ca2+]i, whereas the steady [Ca2+]i induced by high luminal sensitization is substantially elevated. This is worth noting for comparison with experimental observation (Ref. 1 and Figure 2C [iii]; see subsequent text). The model indicates that this difference in steady state is due to the different levels of store-operated Ca2+ entry (SOCE) activation. Indeed, as we have previously shown (17), a sustained increase in Ca2+ influx (i.e., a Ca2+ flux from the extracellular medium into the cytosol) is required for a sustained increase in [Ca2+]i. An increase in RyR rs, but not rc, induces a sustained Ca2+ influx, because it allows RyR to remain open at lower [Ca2+]SR, and thus SOCE is substantially activated. Figure 2E illustrates this mechanism: with increased RyR luminal sensitivity, the RyRs open more frequently (magenta curve), because the [Ca2+]SR (Figure 2D) reaches the lower threshold for RyR activation sooner. Simultaneously, because the [Ca2+]SR remains lower, a larger fraction of the store-operated Ca2+ channel remains open (Figure 2E, blue curve). The results of Figures 2A and 2B are summarized and extended in the online supplement (Figure E3).
Experimental validation.
Caffeine has been shown by other groups to increase RyR sensitivity to both cytosolic and luminal Ca2+ in artificial lipid bilayers (15, 16). In addition, in ASMCs, high caffeine concentrations (20 mM) induce a sustained, elevated [Ca2+]i (1), which is a signature for luminal RyR sensitization according to our model (Figure 2B [iii]). Consequently, to validate the predictions of our model that RyR sensitization influences the Ca2+ dynamics of ASMCs, we used varying concentrations of caffeine to sensitize the RyR of ASMCs in mouse lung slices (Figure 2C).
Stimulation of ASMCs with 1–4 mM caffeine (at 1-mM intervals) induced low-frequency [Ca2+]i oscillations (Figures 2C [i and ii]). The experimental dose–response curve for caffeine (for a total of four different caffeine concentrations) is given in Figure E4. It can be seen that the oscillation frequency increases as a function of caffeine concentration. Specifically, the mean oscillation frequency increased from 1.4 (±0.49)/min for 1 mM caffeine, to 3.5 (±1.1)/min for 3 mM caffeine, the increase between the two means being statistically significant at the 1% level. This is consistent with an increase in RyR sensitization, as shown by Figures 2A and 2B. We note, however, substantial variation in the Ca2+ response, shape, and frequency between cells (Figure E5). A similarly large cell variability is observed for KCl-induced Ca2+ oscillations (Figure E6).
By contrast, stimulation of ASMCs with higher concentrations of caffeine (>6 mM caffeine; 10 mM shown in Figure 2C [iii]) induced a steady, elevated [Ca2+]i. These results are explained by the model predictions for luminal sensitization of RyR (Figure 2B [iii]), and are similar to prior results obtained with 20 mM caffeine (1). In summary, these experimental results and model predictions indicate that RyR sensitization can lead to significant changes in the Ca2+ dynamics of ASMCs in resting conditions.
Effect of RyR Sensitization on Agonist-Induced Ca2+ Oscillations
Because ASMCs use Ca2+ oscillations to induce contraction, and this contraction may be related to airway hypersensitivity, we used our model to explore how RyR sensitivity may alter agonist-induced Ca2+ oscillations. There is evidence that [Ca2+]SR is reduced during agonist-induced Ca2+ oscillations (Ref. 4 and Figure E1A). We therefore expect that cytosolic sensitization of RyR, if not accompanied by substantial luminal sensitization, will have negligible effect on agonist-induced Ca2+ oscillations. This is because, in the absence of luminal sensitization, the opening probability of RyR decreases quickly with decreasing [Ca2+]SR. As a consequence, we begin the discussion of our study of the effect of RyR sensitization on agonist-induced Ca2+ oscillations by assuming luminal RyR sensitization only, and then discuss how these results are modified if there is concomitant cytosolic RyR sensitization.
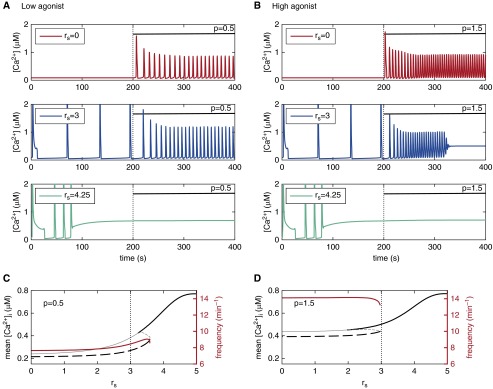
We simulate pre-existing luminal RyR sensitization by setting rs to specific values (Figures 3A and 3B) and mimic agonist application by increasing the IP3 concentration (p) from zero to either 0.5 (Figure 3A) or 1.5 (Figure 3B) at a t of 200 seconds. These simulations show that low or moderate luminal RyR sensitization has little effect on Ca2+ oscillations induced by low IP3 concentrations (p = 0.5); a small effect on Ca2+ oscillation frequency is noticeable with an rs of 3 (compare middle and bottom panels in Figure 3A; see also red curve in Figure 3C).
Figure 3.
(A and B) Effect of agonist application on ASMC Ca2+ dynamics with different levels of pre-existing luminal RyR sensitization (rs; increasing from top to bottom—red to blue to green), in the absence of cytosolic RyR sensitization (rc = 0). (A) Low [IP3] (p = 0.5), and (B) high [IP3] (p = 1.5), applied at t of 200 seconds (p = IP3 concentration). (C and D) Long-term mean [Ca2+]i (black) and Ca2+ oscillation frequency (red) as a function of luminal RyR sensitization for the two agonist levels in A and B.
By contrast, moderate luminal RyR sensitization (rs = 3) has a greater effect on Ca2+ oscillations induced by higher IP3 concentration (p = 1.5, Figure 3B). The Ca2+ oscillations are abolished and replaced with sustained elevated [Ca2+]i (blue trace). With higher levels of luminal RyR sensitization (rs = 4.25), the substantially elevated [Ca2+]i that follows the few initial slow Ca2+ oscillations is not significantly affected by an increase in [IP3] (green trace). Unfortunately, our experimental approach to test these predictions of RyR sensitization effect on agonist-induced Ca2+ oscillations with caffeine were confounded by the inhibitory action of caffeine on the IP3R (18, 19) (see also Discussion). We found the frequency of agonist-induced Ca2+ oscillations to be smaller (instead of larger) in the presence of caffeine than in its absence (Figures E7–E9), even at high agonist concentrations (800 nM methacholine [MCh]). This outcome can be reproduced with the model by assuming that caffeine inhibits the IP3R (e.g., decreases IP3R conductance) in addition to increasing luminal RyR sensitization (simulations not shown).
A summary of the interaction between luminal RyR sensitization and agonist stimulation is shown in Figures 3C and 3D. The frequency of Ca2+ oscillations (red curve) at low or high IP3 concentrations is relatively constant for increasing RyR sensitivity up to an rs of 2.5. The same applies for mean [Ca2+]i (dashed black curve). However, at higher RyR sensitivities (rs > 3), the mean [Ca2+]i increases gradually with increasing rs, and Ca2+ oscillations are suppressed.
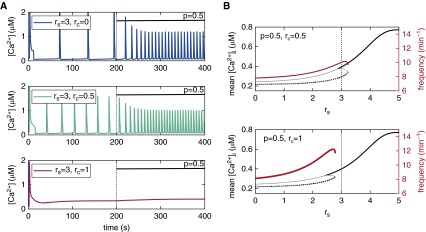
Figure 4 shows how Figure 3A (middle panel) and Figure 3C are modified if we assume cytosolic RyR sensitization (rc = 0.5 or rc = 1) in addition to luminal RyR sensitization (rs = 3 in Figure 4A). Moderate cytosolic sensitization (rc = 0.5) increases the frequency of agonist-induced Ca2+ oscillations (Figure 4A, cyan trace), whereas large cytosolic sensitization (rc = 1) suppresses the Ca2+ oscillations (Figure 4A, magenta trace). Figure 4B shows that cytosolic sensitization reduces the range of luminal sensitization (rs) in which agonist-induced Ca2+ oscillations persist, and renders the Ca2+ oscillation frequency sensitive to luminal sensitization (compare with Figure 3C).
Figure 4.
Effect of simultaneous cytosolic and luminal RyR sensitization on agonist-induced Ca2+ oscillations for low [IP3] (p = 0.5). (A) Increasing levels of cytosolic sensitization (from top to bottom) with RyR luminal sensitization (rs = 3; top panel is identical to middle panel in Figure 3A). (B) Long-term mean [Ca2+]i (black curves) and oscillation frequency (red curve) as a function of luminal RyR sensitization for the two non-zero sensitization levels used in A (compare with Figure 3C).
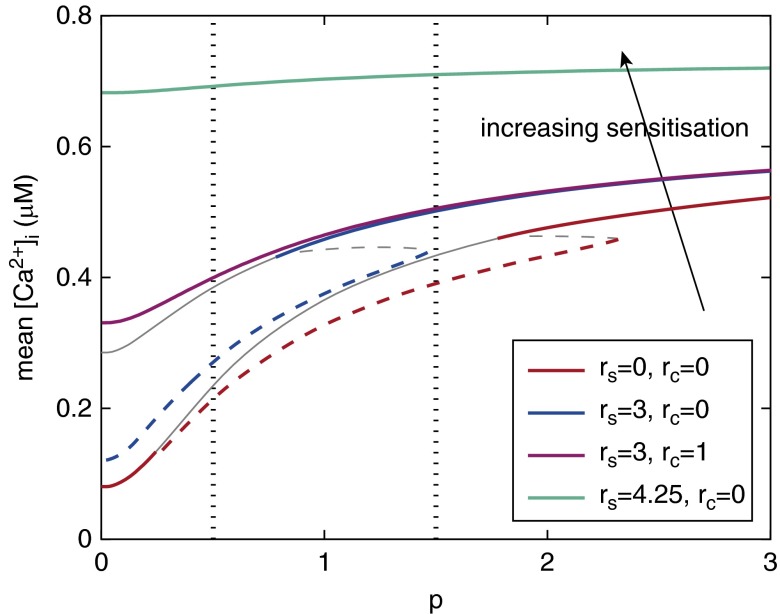
Figure 5 underscores the important implications of our model findings by showing how the mean [Ca2+]i as a function of [IP3] is modified by increasing RyR sensitization. For any given agonist concentration (dotted vertical lines represent p = 0.5 and 1.5, used in Figure 3), the mean [Ca2+]i is always higher in the presence of RyR sensitization; that is, the greater the RyR sensitization, the greater the mean [Ca2+]i. These response curves also show that the range of IP3 concentrations that can induce Ca2+ oscillations (dashed lines) shrinks and disappears as luminal RyR sensitization is increased. In particular, the overlap between Ca2+ oscillations for an rs of 0 (dashed blue curve) and steady Ca2+ for an rs of 3 (solid red curve) over a large range of [IP3] (p = 0.8–2.3 for rc = 0) implies that moderate changes in RyR sensitivity can have a large impact on the dynamics of ASMC Ca2+ signaling. In summary, increasing luminal RyR sensitization progressively impairs agonist-induced Ca2+ oscillations and increases mean [Ca2+]i. A simultaneous increase in cytosolic RyR sensitization accelerates the suppression of the Ca2+ oscillations, but does not induce an additional increase in mean [Ca2+]i at high IP3 concentrations, where [Ca2+]i is already steady (compare blue and magenta curves).
Figure 5.
Effect of different levels of RyR sensitization (increasing as indicated by the arrow) on the entire dose–response curve relating mean [Ca2+]i to [IP3] during agonist stimulation. Dashed curves correspond to Ca2+ oscillations and solid curves to steady Ca2+. Dotted vertical lines indicate the [IP3] values used in Figures 3A and 3B. The bottom (red) curve is the dose–response curve in the absence of sensitization; hence, it corresponds to the response curve in Figure E2A. Gray curves represent unstable solutions.
Discussion
In this work, we have investigated the effect of RyR sensitization on Ca2+ dynamics in ASMCs using a dual approach of mathematical modeling and experimental observation of ASMCs in mouse lung slices. Our objective was to use our model to explore the hypothesis that an increase in RyR sensitivity, which may result from on-going airway inflammation, will alter the basic Ca2+ dynamics of ASMCs and their responses to contractile agonists. Any changes in the Ca2+ dynamics of ASMCs would be expected to have consequences for ASMC proliferation and airway hyperresponsiveness that are typical of asthma.
A key advance in our model of the Ca2+ dynamics of ASMCs that allows us to explore the effect of RyR sensitization is the incorporation of SOCE and the interaction of the luminal Ca2+ concentration of the SR with RyR. The regulation of SOCE is determined by [Ca2+]SR, which, in turn, is highly dependent on the open probability of the RyR, which is itself dependent on [Ca2+]SR. Agonists that produce IP3 cause Ca2+ release from the SR, a gradual decrease of [Ca2+]SR and gradual buildup of SOCE. KCl, on the other hand, causes increased Ca2+ influx, overloading of the SR, and release of Ca2+ through activated RyR. In this latter case, the SR is overloaded, and so SOCE does not occur; even the Ca2+ spikes do not deplete the SR enough to cause significant SOCE. Caffeine, however, enables RyR activation in the absence of SR overloading. Hence, the Ca2+ spikes induced by caffeine may deplete the SR sufficiently to result in increased SOCE. Thus, although caffeine and KCl both induce RyR opening, they have quite different effects on SOCE.
It is also important to note that, in our model, SOCE acts like a low-pass filter, responding not to each individual Ca2+ spike, but to mean levels of SR depletion or overfilling. Thus, individual Ca2+ spikes do not activate spikes of Ca2+ entry through store-operated Ca2+.
The idea that the RyR may be involved in on-going inflammatory processes and may influence airway responsiveness has been previously suggested. For example, the cytokines, TNF-α and IL-13, were reported to increase the [Ca2+]i responses of ASMC to contractile agonists, a mechanism that correlates with activation of CD38, the production of cyclic ADP-ribose, and the modification of the RyR (8, 12). Experimental results obtained with β-escin–permeabilized ASMCs suggest that cyclic ADP-ribose has a direct effect on RyR (13).
RyR Sensitization Stimulates Slow Ca2+ Oscillations
The first prediction of the model that is experimentally confirmed is that small increases in cytosolic and/or luminal RyR sensitivity result in low-frequency Ca2+ oscillations. Although the consequences of these Ca2+ oscillations for ASMC physiology are not currently known, we propose two possibilities. The first and most obvious would be an increase in ASMC tone. However, in mouse airways, low-frequency Ca2+ oscillations do not induce substantial contraction; each ASMC is observed to twitch asynchronously. This is a result of the combination of a low sensitivity of mouse ASMCs to Ca2+ and the short duration of myosin light-chain kinase activation. By contrast, other species, including human, show greater contraction to low-frequency Ca2+ oscillations. The second, but less obvious, consequence of low-frequency oscillations could be a change in gene expression. In previous studies, Ca2+ oscillations were generated with the repetitive photolytic release of IP3 (20) or the alternate application of calcium and calcium-free solutions (21) to demonstrate that gene expression was initiated by low-frequency Ca2+ oscillations. Ca2+ oscillations generated by IP3 at 1/min stimulated more gene expression than at 0.5/min or 2/min or by a sustained plateau (20). The effectiveness of Ca2+ oscillations with a period of over 100 seconds declined when using alternating Ca2+ solutions (21). In a similar manner, Ca2+ oscillations may also affect enzyme activity as part of a frequency-modulated control system (22). Thus, the stimulation of Ca2+ oscillations may have significant effects on the basic phenotype of the ASMCs. The idea that slow Ca2+ oscillations are associated with asthma is supported by the finding of spontaneous Ca2+ oscillations in human ASMCs from biopsies (23).
RyR Sensitization Increases Mean [Ca2+]i
A second major prediction of the model is that moderate or high luminal RyR sensitization results in higher mean (or elevated) [Ca2+]i when the ASMCs are exposed to any concentration of contractile agonist. This prediction is consistent, irrespective of whether luminal RyR sensitization is moderate, and does not abolish the agonist-induced Ca2+ oscillations, or whether the RyR sensitization is high and converts the Ca2+ oscillations into a sustained [Ca2+]i elevation. Cytosolic RyR sensitization can enhance the effect of luminal RyR sensitization on mean [Ca2+]i when luminal sensitization is moderate and not capable by itself of abolishing agonist-induced Ca2+ oscillations. The immediate implication of a higher mean [Ca2+]i is that, in the presence of agonist, RyR sensitization could generate greater ASMC contraction. This, in turn, suggests a possible mechanism for airway hyperresponsiveness.
The concept that increased (sustained) mean [Ca2+]i results in increased contraction is demonstrated in Ca2+-permeabilized human lung slices (2), where, for a given agonist concentration, airway contraction increases with constant [Ca2+]i imposed. Thus, with RyR sensitization, the mean [Ca2+]i would be higher than normal, resulting in additional contraction.
Another potential consequence of the replacement of agonist-induced Ca2+ oscillations with a sustained elevation in [Ca2+]i is stimulation of Ca2+-dependent mechanisms that have slow response kinetics. It is already well known that the frequency and shape of Ca2+ oscillations are important controllers of contraction (24). However, there are potentially many other ways in which the frequency of the Ca2+ oscillations can control cellular processes; in the presence of RyR sensitization, recurrent exposure to agonist is likely to stimulate the ASMCs in ways other than simple contraction.
Unfortunately, however, we were unable to test this second prediction. Although caffeine is known to increase the sensitivity of RyR to luminal Ca2+, it also inhibits the IP3R. It is, thus, not possible to test the effects of RyR sensitization in the absence of other confounding effects. Experimental results are shown in Figures E7–E9. Addition of MCh and caffeine results in a plateau of increased Ca2+, but this plateau has superimposed slow oscillations, a result that does not agree with our model predictions. It is possible to modify our model to include the potential effects of caffeine inhibition of the IP3R, and simulations with such a modified model display much better agreement with the experimental results shown in Figures E7–E9. However, such modifications are speculative and outside the scope of the present study. It is left to future work to develop, more rigorously, a model of caffeine inhibition of IP3R.
Given the impossibility of testing the effect of RyR sensitization on agonist-induced Ca2+ oscillations with caffeine, due to its inhibitory effect on the IP3R, we have looked for other drugs that could possibly sensitize RyR without inhibiting IP3R. We found that pentifylline induces slow Ca2+ oscillations similar to those induced by caffeine (Figures E10 and E11), but it is not clear whether pentifylline does so via luminal sensitization of RyR (which is necessary to prevent RyR inhibition during agonist-induced Ca2+ oscillations) or via another mechanism.
However, like caffeine, pentifylline impairs agonist-induced Ca2+ oscillations (Figures E12 and E13); this could be due to an inhibition of IP3R, similar to that induced by caffeine, or possibly to the absence of luminal RyR sensitization. We have also tried the drug, chlorocresol (25), but it did not induce Ca2+ oscillations.
Limitations of the Model
In this work, we have modeled ASMC Ca2+ dynamics in a deterministic and homogenous manner, although Ca2+ channels operate stochastically, and are not distributed homogeneously on the SR membrane. The extent to which this stochasticity translates into “macroscopic” (cell-level) Ca2+ dynamics depends on the cell type, the Ca2+ channels involved, and the level of stimulation (e.g., agonist concentration). The spatial distribution of Ca2+ channels on the SR membrane could also be modified in disease. In ASMCs, as well as in other cell types, the distribution of the occurrence of Ca2+ spikes during agonist-induced Ca2+ oscillations is well described by a Poisson process, characterized by a linear dependence between the SD and the mean of the interspike interval (26). However, the main properties of these stochastic Ca2+ oscillations can be predicted by a deterministic mathematical model (27). Hence, the stochastic nature of the process does not preclude the value of a deterministic description of Ca2+ dynamics as used in this study.
The mathematical model used here was originally designed to account for agonist-induced Ca2+oscillations and SOCE in ASMCs of human lung slices at room temperature. These Ca2+ oscillations are much slower than Ca2+ oscillations in mice (10/min versus 30/min). The model is therefore qualitative in this respect. Similarly, the RyR-mediated Ca2+ oscillations have a profile that is not fully reproduced by the model. Some differences in the Ca2+ profile of the model and the data are likely the result of the affnity of Oregon Green BAPTA-1-AM (OGB) that was used to report changes in Ca2+ (Kd ∼ 0.2 μM). This reporter is saturated at [Ca2+] above 1 μM. Hence, peak amplitudes of Ca2+ oscillations are unlikely to be reproduced in the fluorescent signal. Similarly, the kinetics of OGB may not be fast enough to track the rapid changes in [Ca2+]i. This could explain the elevated fluorescent baseline observed during agonist-induced Ca2+ oscillations, which is not apparent during RyR-mediated Ca2+ oscillations.
However, our model accounts for a key feature of Ca2+ oscillation frequencies in ASMCs; that is, IP3R-mediated Ca2+ oscillations are faster than RyR-mediated Ca2+ oscillations. This is because IP3R gating is mainly governed by inhibition of the receptor by [Ca2+]i, whereas RyR gating is essentially governed by the level of Ca2+ store depletion. Because the refilling of the SR with Ca2+ occurs on a longer timescale than recovery of IP3R from inhibition, RyR-mediated oscillations have a lower frequency than IP3R-mediated oscillations. The frequencies of these two types of oscillations are closer in human ASMCs (10/min [IP3R] versus 3/min [RyR]) than in mouse ASMC (30/min [IP3R] versus 3/min [RyR]), which suggests that the effect of RyR sensitization on agonist-induced Ca2+ oscillations could be larger in human ASMCs (because oscillations of closer frequency are more likely to interact).
Scope of the Experimental Results
As mentioned previously, we have used mouse ASMCs in this study. These exhibit faster IP3R-mediated Ca2+ oscillations than human ASMCs upon agonist stimulation, but both species exhibit maximum contraction (∼55% in both cases) at the highest end of the frequency spectrum (e.g., figure 6 in Ref. 28). Hence, the two species respond differently to a given Ca2+ oscillation frequency. Our predictions are, however, independent of this quantitative difference, because, in both cases, we expect RyR sensitization–induced Ca2+ oscillations to enhance agonist-induced Ca2+ oscillations; the size of the effect will, of course, depend on the species, but the prediction is qualitatively the same.
The key observation, that increased RyR sensitivity can underlie unexpected complications in ASMC Ca2+ signaling and downstream physiology, suggests RyR as a target for therapeutic drugs. However, the exact nature of such a drug is diffcult to predict, as the mechanism of luminal Ca2+ sensitivity is not well understood. Compounds that could prevent Ca2+ binding to RyR would serve to reduce its Ca2+ sensitivity. This might take the form of a Ca2+ buffer within the SR; the protein, calsequestrin, serves such a process in the SR. Because the SR Ca2+ content is a key parameter, drugs reducing SERCA pump activity or SOCE may also counteract increased RyR sensitivity by helping to maintain reduced SR Ca2+ content.
Supplementary Material
Footnotes
This work was supported by Medical Research Council New Investigator Grant G0901174 (B.S.B. and H.C.) and National Institutes of Health grant HL103405 (J.S. and M.J.S.).
Author Contributions: Conception and design—H.C., J.S., M.J.S., and B.S.B.; analysis and interpretation—H.C., X.T., J.S., and B.S.B.; drafting the manuscript for important intellectual content—H.C., J.S., M.J.S., and B.S.B.; performed the experiments—X.T., J.C., and M.J.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0386OC on April 15, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol. 2005;125:535–553. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ressmeyer AR, Bai Y, Delmotte P, Uy KF, Thistlethwaite P, Fraire A, Sato O, Ikebe M, Sanderson MJ. Human airway contraction and formoterol-induced relaxation is determined by Ca2+ oscillations and Ca2+ sensitivity. Am J Respir Cell Mol Biol. 2010;43:179–191. doi: 10.1165/rcmb.2009-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Y, Sanderson MJ. The contribution of Ca2+ signaling and Ca2+ sensitivity to the regulation of airway smooth muscle contraction is different in rats and mice. Am J Physiol Lung Cell Mol Physiol. 2009;296:L947–L958. doi: 10.1152/ajplung.90288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang IY, Bai Y, Sanderson MJ, Sneyd J. A mathematical analysis of agonist- and KCl-induced Ca2+ oscillations in mouse airway smooth muscle cells. Biophys J. 2010;98:1170–1181. doi: 10.1016/j.bpj.2009.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204:3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White TA, Xue A, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-α–enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol. 2006;35:243–251. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moynihan B, Tolloczko B, Michoud MC, Tamaoka M, Ferraro P, Martin JG. MAP kinases mediate interleukin-13 effects on calcium signaling in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L171–L177. doi: 10.1152/ajplung.00457.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieck GC, White TA, Thompson MA, Pabelick CM, Wylam ME, Prakash YS. Regulation of store-operated Ca2+ entry by CD38 in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294:L378–L385. doi: 10.1152/ajplung.00394.2007. [DOI] [PubMed] [Google Scholar]
- 9.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci USA. 2009;106:10775–10780. doi: 10.1073/pnas.0902295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sathish V, Thompson MA, Bailey JP, Pabelick CM, Prakash YS, Sieck GC. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297:L26–L34. doi: 10.1152/ajplung.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sathish V, Delmotte PF, Thompson MA, Pabelick CM, Sieck GC, Prakash YS. Sodium–calcium exchange in intracellular calcium handling of human airway smooth muscle. PLoS One. 2011;6:e23662. doi: 10.1371/journal.pone.0023662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshpande DA, Dogan S, Walseth TF, Miller SM, Amrani Y, Panettieri RA, Kannan MS. Modulation of calcium signaling by interleukin-13 in human airway smooth muscle: role of CD38/cyclic adenosine diphosphate ribose pathway. Am J Respir Cell Mol Biol. 2004;31:36–42. doi: 10.1165/rcmb.2003-0313OC. [DOI] [PubMed] [Google Scholar]
- 13.Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am J Physiol. 1998;274:C1653–C1660. doi: 10.1152/ajpcell.1998.274.6.C1653. [DOI] [PubMed] [Google Scholar]
- 14.Jude J, Dileepan M, Panettieri R, Walseth TF, Kannan MS. Altered CD38/cyclic ADP-ribose signaling contributes to the asthmatic phenotype. J Allergy (Cairo) 2012;2012:289468. doi: 10.1155/2012/289468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SRW. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J. 2008;414:441–452. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porta M, Zima AV, Nani A, Diaz-Sylvester PL, Copello JA, Ramos-Franco J, Blatter LA, Fill M. Single ryanodine receptor channel basis of caffeine’s action on Ca2+ sparks. Biophys J. 2011;100:931–938. doi: 10.1016/j.bpj.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croisier H, Tan X, Perez-Zoghbi JF, Sanderson MJ, Sneyd J, Brook BS. Activation of store-operated calcium entry in airway smooth muscle cells: insight from a mathematical model. PLoS One. 2013;8:e69598. doi: 10.1371/journal.pone.0069598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker I, Ivorra I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol. 1991;433:229–240. doi: 10.1113/jphysiol.1991.sp018423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezprozvanny I, Bezprozvannaya S, Ehrlich BE. Caffeine-induced inhibition of inositol(1,4,5)-trisphosphate-gated calcium channels from cerebellum. Mol Biol Cell. 1994;5:97–103. doi: 10.1091/mbc.5.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 21.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 22.Berridge MJ. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney D, Hollins F, Gomez E, Saunders R, Challiss RAJ, Brightling CE. [Ca2+]i oscillations in ASM: relationship with persistent airflow obstruction in asthma. Respirology. 2014;19:763–766. doi: 10.1111/resp.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roux E, Guibert C, Savineau JP, Marthan R. [Ca2+]i oscillations induced by muscarinic stimulation in airway smooth muscle cells: receptor subtypes and correlation with the mechanical activity. Br J Pharmacol. 1997;120:1294–1301. doi: 10.1038/sj.bjp.0701061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Mousa F, Michelangeli F. Commonly used ryanodine receptor activator, 4-chloro-m-cresol (4CmC), is also an inhibitor of SERCA Ca2+ pumps. Pharmacol Rep. 2009;61:838–842. doi: 10.1016/s1734-1140(09)70139-2. [DOI] [PubMed] [Google Scholar]
- 26.Skupin A, Kettenmann H, Winkler U, Wartenberg M, Sauer H, Tovey SC, Taylor CW, Falcke M. How does intracellular Ca2+ oscillate: by chance or by the clock? Biophys J. 2008;94:2404–2411. doi: 10.1529/biophysj.107.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao P, Tan X, Donovan G, Sanderson MJ, Sneyd J. A deterministic model predicts the properties of stochastic calcium oscillations in airway smooth muscle cells. PLoS Comput Biol. 2014;10:e1003783. doi: 10.1371/journal.pcbi.1003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauzon AM, Bates JHT, Donovan G, Tawhai M, Sneyd J, Sanderson MJ. A multi-scale approach to airway hyperresponsiveness: from molecule to organ. Front Physiol. 2012;3:191. doi: 10.3389/fphys.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.