Abstract
Statins are widely used to prevent cardiovascular disease. In addition to their inhibitory effects on cholesterol synthesis, statins have beneficial effects in patients with sepsis and pneumonia, although molecular mechanisms have mostly remained unclear. Using human airway epithelial cells as a proper in vitro model, we show that prior exposure to physiological nanomolar serum concentrations of simvastatin (ranging from 10–1,000 nM) confers significant cellular resistance to the cytotoxicity of pneumolysin, a pore-forming toxin and the main virulence factor of Streptococcus pneumoniae. This protection could be demonstrated with a different statin, pravastatin, or on a different toxin, α-hemolysin. Furthermore, through the use of gene silencing, pharmacological inhibitors, immunofluorescence microscopy, and biochemical and metabolic rescue approaches, we demonstrate that the mechanism of protection conferred by simvastatin at physiological nanomolar concentrations could be different from the canonical mevalonate pathways seen in most other mechanistic studies conducted with statins at micromolar levels. All of these data are integrated into a protein synthesis–dependent, calcium-dependent model showing the interconnected pathways used by statins in airway epithelial cells to elicit an increased resistance to pore-forming toxins. This research fills large gaps in our understanding of how statins may confer host cellular protection against bacterial infections in the context of airway epithelial cells without the confounding effect from the presence of immune cells. In addition, our discovery could be potentially developed into a host-centric strategy for the adjuvant treatment of pore-forming toxin associated bacterial infections.
Keywords: airway epithelium, pneumolysin, simvastatin, cholesterol, pneumonia
Clinical Relevance
Our study dissects the cellular and molecular protection mechanisms of statins in an isolated airway epithelial cell–bacterial pore-forming toxin context. This research can fill large gaps in our understanding of how statins contribute to the reduced pathology observed during pneumonia and other bacterial infections. The better understanding of the molecular mechanisms in the statin-mediated protection will further affect the therapeutic development of host-centric approach therapies in combating microbial infections.
Pneumonia is a major cause of infant mortality in developing countries, causing more than 1.2 million infant deaths per year and approximately 25% of all preventable deaths in young children (1, 2). Streptococcus pneumoniae is the most common human bacterial respiratory infectious agent and often results in community-acquired pneumonia (3). Currently, antibiotic administration is the most common treatment for pneumonia, but some unintended consequences of antibiotic use, including perturbations on host microbiota and the emergence of multi–drug-resistant bacterial strains, are growing problems (4). To prevent pneumonia, yearly vaccinations against S. pneumoniae were introduced in the past decade. However, these do not cover all 90 serotypes of pneumococcal strains or other pathogens such as Staphylococcus aureus (5). Today there is a desperate need for novel strategies to prevent or treat these infections.
Statins are competitive inhibitors of 3-hydroxy 3-methylglutaryl coenzyme A (HMG-CoA) reductase, a key enzyme regulating cholesterol biosynthesis (6). Due to their ability to inhibit cholesterol production and increase LDL uptake, these compounds are predominantly used for hyperlipidemia treatment, with an estimated 32 million Americans taking statins (7). Clinical and experimental evidence shows that statins have significant pleiotropic effects beyond the lowering of lipid levels. These include antiinflammatory and immune-modulatory effects, such as decreased leukocyte recruitment and edema during acute infection in animal models, reduced graft rejection in patients taking statins after heart transplantation, and reduced inflammation in several autoimmune diseases (8–11). In clinical epidemiological studies, statins have been suggested to have a strong beneficial effect against pneumonia- and sepsis-related mortality (12).
Animal studies of S. aureus infection, a major cause of pneumonia and sepsis, have shown protection against bacterial infections under statin administration. In rats, simvastatin was able to alleviate inflammation from staphylococcal α-hemolysin (Hla) injection (13). In C57BL/6 mice, simvastatin pretreatment in conjunction with antibiotic treatment increased survival rates from S. aureus infections (14). Furthermore, in another mouse study of S. aureus infection, statins were shown to increase the production of antibacterial DNA-based extracellular traps in neutrophils and macrophages, and this was dependent upon sterol pathway inhibition (15). It has also recently been reported that simvastatin at a range of high doses (50–100 μM) has protective effects against listeriolysin O–mediated Listeria invasion in macrophages (16). Therefore, it is evident that statins trigger immune responses in animals and work directly on immune cells to confer some beneficial effects against bacteria infection and pore-forming toxin (PFT) intoxication. However, it is unclear whether these protective effects can occur in the airway epithelium, the main physiological target of S. pneumoniae and S. aureus infections.
Airway epithelial cells play a critical role in host defenses by providing a physical barrier to microbial invasion and by acting as sentinels via signaling to immune cells, ultimately resulting in the killing of pathogens (17, 18). When these defense mechanisms fail, the consequence is pneumonia: lung colonization, pathogen-induced injury to the epithelium, and continuous inflammation. S. pneumoniae and S. aureus can secrete pore-forming toxins during infection that aid in bacterial invasion. PFTs are the largest single class of proteinaceous bacterial toxins (19, 20), and many PFTs gain access to the host cell through binding to cholesterol or lipid derivatives in lipid rafts on the cell surface, resulting in subsequent oligomerization and pore formation (21, 22). Pneumolysin (PLY), a member of the cholesterol-dependent cytolysin family, is a major virulence factor that is expressed by virtually all clinical isolates of S. pneumoniae. Genetic deletion of PLY from S. pneumoniae results in reduction of virulence by several orders of magnitude (23–25). In addition, PLY has been reported to be a critical virulence factor involved in pneumonia, acute lung injury, and pulmonary permeability edema (26–29). Hla is another PFT expressed in many strains of S. aureus. Similarly, deletion of Hla from S. aureus also results in a significant reduction in virulence (30, 31). The prevalence of PFT production by many bacterial strains, as well as a demonstrated role in bacterial infection, clearly delineate PFTs as an important target for antibacterial agents. There have been increasing efforts to target PFTs in the treatment or prevention of bacterial infections, such as vaccinations directed to target the α-hemolysin and nanoparticle-detained α-hemolysin strategies (32, 33).
In this study, we investigated whether statins at doses in physiological serum concentration ranges could protect human airway epithelial cells against PFTs from bacteria that commonly cause pneumonia. Because we intended to segregate out the immune response triggered by statin from the respiratory epithelial cellular defense against PFTs, which is not feasible in the whole animal setting, we examined the protection mechanism in an isolated airway epithelial cell–bacterial pore-forming toxin context. We found that simvastatin could protect human airway epithelial cells from PLY and Hla cytotoxicity. Because of the various known pleiotropic effects of statin use, we further applied biochemical and pharmacological approaches to understand the mechanisms behind this protective role in vitro.
Materials and Methods
Additional information about the reagents and protocols is included in the online supplement.
Cell Culture
The Human Bronchial Epithelial (HBE1) cell is a human papillomavirus immortalized bronchial epithelial cell line and was a gift from J.R. Yankaskas (University of North Carolina). Normal Human Bronchial Epithelial (NHBE) cells were isolated from human tracheal and primary bronchial tissues that were obtained from the National Institutes of Health–sponsored National Disease Research Interchange (Philadelphia, PA) procurement through consent. HBE1, NHBE, and A549 cells were maintained at 37°C in a humidified incubator with 5% CO2 as described previously (34).
Cytotoxicity Assay
In general, cells were seeded at 10,000 (HBE1) or 20,000 cells (NHBE) per well in 96-well plates and grown overnight. NHBE cells were maintained in bronchial epithelial growth medium with the addition of 5 μM ROCK inhibitor Y-27632 (35) and then seeded for all described assays without the ROCK inhibitor. Cells were exposed to simvastatin, pharmacological inhibitors, and/or metabolic supplementation at the indicated concentrations in growth medium for 24 hours. The exposed cells were then treated with toxins at the indicated concentrations for 4 hours, and cell viability was analyzed with CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI) following the recommended protocol. Simvastatin LC50 values in HBE1 cells were determined using the publicly available ED50Plus v1.0 software program.
Real-Time RT-PCR
The procedures of RNA extraction and cDNA generation were described in our previous study (36). A more detailed protocol and a list of the primers can be found in the online supplement.
Immunoblot Analysis
The immunoblot analysis was performed as previously described (37) with antibodies against HMG-CoA reductase (HMGCR) (sc-271595; Santa Cruz Biotechnology, Santa Cruz, CA), pneumolysin (ab71810; Abcam, Cambridge, UK), and β-actin (A5441; Sigma-Aldrich, St. Louis, MO). A more detailed protocol can be found in the online supplement.
Animal Study and Histochemistry
C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained in a specific pathogen–free facility according to institutional guidelines. Experiments involving mice were performed with approval of the Institutional Animal Care and Usage Committee at University of California Davis and the National Health Research Institutes. Briefly, 20 mg/kg simvastatin was injected intraperitoneally into 10- to 12-week-old C57BL/6 mice for 6 days, and then 200 ng pneumolysin or PBS control was injected intratracheally. Mice were killed 16 to 18 hours later, and the lungs were paraffin embedded, sectioned, and stained with hematoxylin and eosin for histopathology.
Statistical Analyses
Results are expressed as mean ± SEM. All data were analyzed with the t test, one-way ANOVA, or two-way ANOVA followed by the appropriate post hoc test for multiple comparisons as described in the figure legends. Significance was defined as P < 0.05.
Results
Statins Confer Cellular Protection to Pneumolysin in Airway Epithelium
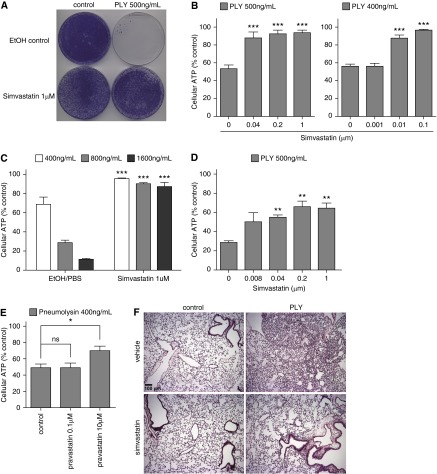
To look for pretreatment agents that protected airway epithelial cells against PFTs, we conducted a cell viability–based screen with a panel of cytokines and chemicals. One of the leading hits was simvastatin. Because statins are widely used clinically to reduce plasma cholesterol and have been shown epidemiologically to provide a better outcome for patients with pneumonia, we focused on the cellular protection of simvastatin to PFTs. We conducted CFU assays and found that the pretreatment of 1 μM simvastatin conferred remarkable resistance of HBE1 cells against a 4-hour exposure of 500 ng/ml PLY (Figure 1A). We further investigated this phenomenon with a cell viability assay by measuring cellular ATP release at a variety of simvastatin doses. Simvastatin pretreatment resulted in a significant increase in cell viability against PLY in HBE1 cells at all doses used except 1 nM, the lowest dose tested (Figure 1B). In addition, 1 μM simvastatin provided significant protection against higher doses of PLY, ranging from 0.4 to 1.6 μg/ml, with no simvastatin controls exhibiting approximately 90% loss of cell viability (Figure 1C). To examine whether this was a phenomenon specific to HBE1, we studied simvastatin protection against PLY in human primary tracheobronchial epithelial (i.e., NHBE) cells. We found that simvastatin pretreatment also resulted in a significant increase in cell viability in NHBE cells (Figure 1D). Besides lipophilic simvastatin, we found that 10 μM hydrophilic pravastatin also conferred significant protection against PLY, although a dose of 0.1 μM did not (Figure 1E). We further examined protection by statins against PFTs in vivo. Mice were injected intraperitoneally with simvastatin for 6 days and then injected intratracheally with 200 ng purified PLY or PBS control. Mice were killed 16 to 18 hours later, and the lungs were resected for histology. PLY alone caused significant immune cell accumulations in lung, whereas simvastatin pretreatment caused no obvious lung inflammation (Figure 1F). Our results support the notion that statin pretreatment can reduce host susceptibility to PLY in vivo.
Figure 1.
Statins conferred cellular protection on airway epithelial cells against pneumolysin. (A) Human Bronchial Epithelial 1 (HBE1) cells were treated with 1 μM simvastatin for 24 hours. Simvastatin was then removed from the culture, and cells were further challenged with 500 ng/ml pneumolysin (PLY) for 4 hours. Cells were cultured for 10 days to determine the CFUs. (B–E) Cellular ATP release was quantified to determine cell viability. Asterisks indicate significant statistical difference versus matching control with one-way ANOVA and Dunnet’s post test. Error bars represent SEM of three to five experiments. (B) HBE1 cells were pretreated with indicated doses of simvastatin for 24 hours and then challenged with PLY for 4 hours. (C) HBE1 cells were pretreated with 1 μM simvastatin for 24 hours and then challenged with the indicated doses of PLY for 4 hours. (D) Normal HBE (NHBE) cells were pretreated with the indicated doses of simvastatin for 24 hours and then challenged with 500 ng/ml of PLY for 4 hours. (E) HBE1 cells were pretreated with 0.1 or 10 μM pravastatin for 24 hours and then challenged with 400 ng/ml PLY for 4 hours. (F) C57BL/6 mice, 10 to 12 weeks old, were injected intraperitoneally with 20 mg/kg simvastatin for 6 days and then injected intratracheally by 200 ng/ml PLY or PBS control for overnight. Lungs were fixed and sectioned for hematoxylin and eosin staining for histopathology. Data are representative images from four mice per group. Scale bar: 100 μm. EtOH, ethanol; ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Simvastatin Does Not Reduce the Binding of Pneumolysin to HBE1 Cells
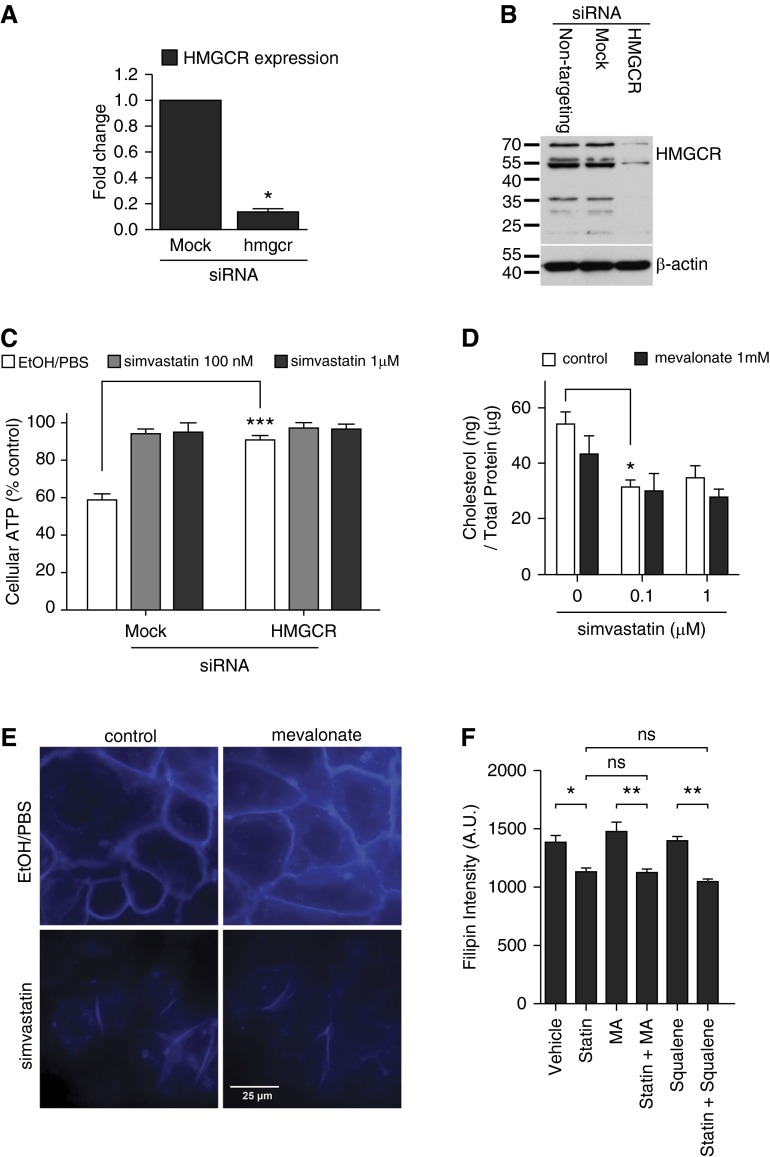
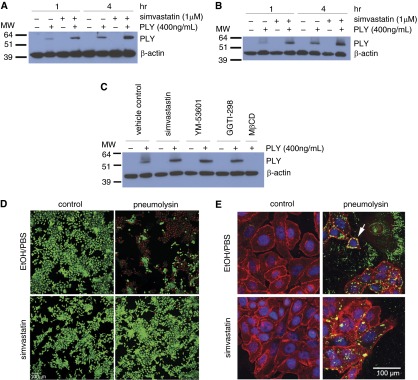
In addition to pleiotropic effects, simvastatin is known to target HMG-CoA reductase (HMGCR), affecting three canonical downstream pathways after mevalonate biosynthesis. We first examined whether HMGCR suppression in HBE1 could confer cellular resistance to PLY. The gene silencing efficacy was confirmed by quantitative RT-PCR (qRT-PCR) and immunoblot of HMGCR (Figures 2A and 2B). We found that cells with HMGCR knockdown were significantly more resistant to PLY toxicity than no-knockdown controls (Figure 2C). Because simvastatin is known to reduce cholesterol levels in the liver and blood (38), a possible cellular mechanism for its protective effect is that plasma membrane–associated cholesterol, which is essential for PLY binding, is reduced by simvastatin treatment. To test this hypothesis, we collected HBE1 cells treated with various concentrations of simvastatin for 24 hours and quantitated total cholesterol. Our results indicated that 100 nM simvastatin significantly reduced the total cholesterol content of HBE1 cells (Figure 2D). We further examined the membrane-associated cholesterol using filipin III staining (39). Compared with vehicle-treated HBE1 cells, simvastatin-treated cells showed weaker staining on the plasma membrane (Figure 2E). A microplate quantification assay also demonstrated similar results, showing that simvastatin significantly reduced the level of membrane-associated cholesterol (Figure 2F) and that this inhibition was not abrogated by mevalonate or squalene. Because plasma membrane cholesterol is essential for PLY binding, oligomerization, and subsequent pore formation, we next asked whether PLY binding and pore formation were affected by simvastatin treatment. HBE1 (Figure 3A) and NHBE (Figure 3B) cells were exposed to simvastatin for 24 hours. Simvastatin was then removed, and cells were challenged with 400 ng/ml of PLY for 1 or 4 hours. After immunoblotting with an anti-PLY antibody, we found that cell lysates collected after 4 hours of toxin exposure contained more PLY than lysates collected after 1 hour (Figures 3A and 3B). Interestingly, the amount of PLY in cell lysates was not reduced by simvastatin pretreatment, suggesting that the binding of PLY is not attenuated. In contrast, 30-minute pretreatment with 2 mM methyl-β-cyclodextrin, which depletes membrane-associated cholesterol, did block binding of PLY to airway epithelial cells (Figure 3C). Because PLY binding is not attenuated by simvastatin, we next tried to examine the pore-forming ability of PLY on the simvastatin-treated HBE1 cells. Because the passage of a certain size of fluorescence dye is a well-known practical indication of pores generated by PFTs, we applied a Live/Dead assay (Life Technologies, Carlsbad, CA) that uses an ethidium homodimer to stain membrane-compromised cells (emitting red fluorescence). We found that simvastatin-pretreated cells show a drastic decrease in membrane perforation, indicated by low amounts of red fluorescence (Figure 3D). Because this evidence is an indirect demonstration of the pore formation by PLY, we further verified whether the cytotoxicity caused by PLY in HBE1 cells is mediated by pore formation. We generated a known pore-defective PLY (W433F point mutation) (40) and found that PLYW433F did not cause cytotoxicity in HBE1 cells (see Figure E1 in the online supplement). By immunofluorescence microscopy, we further observed that simvastatin does not reduce the amount of PLY puncta in HBE1 cells (47.3 ± 7.1 PLY/control cell versus 47.4 ± 10.2 PLY/statin-treated cell) (Figure 3E). These results suggest that simvastatin significantly inhibited potential PLY-mediated pore formation and membrane damage through reducing the membrane-associated cholesterol level, although the membrane-binding capacity of PLY was not alleviated.
Figure 2.
The plasma membrane cholesterol is reduced by simvastatin. (A) The efficacy of 3-hydroxy 3-methylglutaryl coenzyme A reductase (HMGCR) gene silencing in HBE1 cells was examined by quantitative qRT-PCR. Asterisks indicate significant statistical difference versus matching control with t test. Error bars represent SEM of two experiments. (B) The efficacy of HMGCR gene silencing in HBE1 cells was examined by immunoblotting. The image shown is a representative of three experiments. (C) The gene knockdown of HMGCR in HBE1 cells mimicked the simvastatin-mediated protection against PLY. Asterisks indicate significant statistical difference versus matching control with two-way ANOVA and Bonferroni’s post test. Error bars represent SEM of four to six experiments. (D) The total cholesterol content was analyzed on HBE1 cells upon simvastatin and mevalonate treatment at the indicated concentrations for 24 hours. Asterisks indicate significant statistical difference versus matching control based on one-way ANOVA with Tukey’s post test. Error bars represent SEM of four experiments. (E and F) HBE1 cells were pretreated with 1 μM simvastatin or vehicle control in the presence or absence of 1 mM mevalonate (E and F) or 1 mM squalene (F) for 24 hours. After the medium change, cells were stained with filipin to assess the cholesterol distribution of the membranes. (E) The blue fluorescence staining indicates the filipin probe binding. (F) Quantification data were obtained by fluorimetry using filipin-stained HBE1 cells grown in 96-well microplates. Asterisks indicate significant statistical difference versus matching groups with one-way ANOVA and Tukey’s post test. Error bars represent SEM of three experiments. A.U. arbitrary units; MA, mevalonate; siRNA, small interfering RNA. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 3.
The presence of pneumolysin in the cell lysate is not reduced by simvastatin. (A and B) HBE1 cells (A) and NHBE cells (B) were treated with 400 ng/ml PLY or mock control with or without 24-hour simvastatin pretreatment. Whole cell lysates were collected 1 or 4 hours after toxin treatment, and immunoblotting was conducted with antibodies against PLY or β-actin. The molecular weight markers are as indicated. Images shown are representatives of three different experiments. (C) NHBE cells were treated with 400 ng/ml pneumolysin or mock control with or without 24-hour simvastatin, YM-53601, or GGTI-298 pretreatment. The 2-mM MβCD pretreatment was done for 30 minutes before the PLY exposure. Whole cell lysates were collected 4 hours after toxin treatment, and immunoblotting was conducted with antibodies against pneumolysin or β-actin. The molecular weight markers are as indicated. The image shown is representative of three different experiments. (D) HBE1 cells were pretreated with 1 μM simvastatin for 24 hours. After the medium change, cells were incubated with 400 ng/ml of PLY or mock control for 4 hours. Cells were stained with Live/Dead assay to assess the membrane damage. Scale bar = 100 μm. (E) The cellular localization of PLY. HBE1 cells were pretreated with 1 μM simvastatin for 24 hours. After the medium change, cells were incubated with 400 ng/ml of PLY or mock control for 4 hours. Cells were then fixed and stained with PLY antibody (green), plasma membrane (red), and 4′,6-diamidino-2-phenylindole (DAPI) for nucleus (blue). The white arrow points to the representative PLY binding on the cell membrane. MβCD, methyl-β-cyclodextrin; MW, molecular weight.
Mevalonate Supplement Does Not Abrogate Simvastatin-Conferred Resistance to Pneumolysin in HBE1 Cells
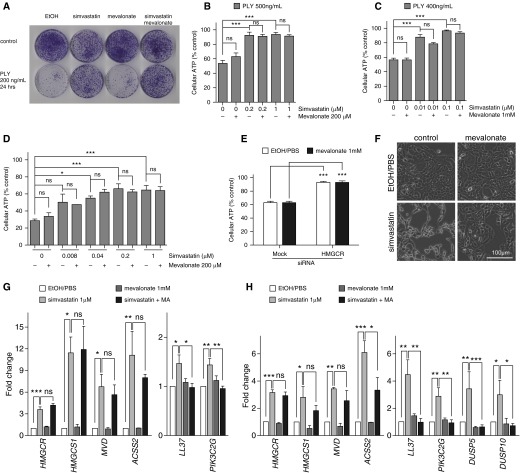
Because the knockdown of HMGCR increased cellular resistance against PLY and HMGCR is the rate-limiting enzyme in the mevalonate pathway, we next sought to examine whether mevalonate biosynthesis was involved in mediating statin-induced PFT resistance. We conducted colony forming assays with HBE1 cells exposed to simvastatin with or without mevalonate supplementation and found that the mevalonate did not restore cellular sensitivity to PLY (Figure 4A). Similarly, mevalonate did not rescue the protection conferred by simvastatin in the ATP release assay in HBE1 and NHBE cells (Figures 4B–4D). Even supplemented with a high excess (1 mM) of mevalonate, the simvastatin-mediated protection remained (Figure 4C). Mevalonate supplementation also did not reduce HMGCR-knockdown–mediated protection to PLY in HBE1 cells (Figure 4E). In addition, our results showed that mevalonate supplementation did not restore simvastatin-mediated cellular cholesterol reduction in HBE1 cells (Figure 2D). Taken together, these results implicated that mevalonate does not play a major role in simvastatin-mediated protection.
Figure 4.
Simvastatin-mediated protection against pneumolysin is mevalonate independent. (A) HBE1 cells were treated with 1 μM simvastatin with or without 200 μM mevalonate for 24 hours and then removed following 200 ng/ml PLY for 24 hours. Cells were cultured for 10 days to determine the CFUs. (B and C) HBE1 cells were pretreated with the indicted concentrations of simvastatin with or without 200 μM or 1 mM mevalonate for 24 hours and then challenged with PLY for 4 hours. Cell viability was determined by ATP release assay. (D) NHBE cells were pretreated with 1 μM simvastatin with or without 200 μM mevalonate for 24 hours and then challenged with 500 ng/ml of PLY for 4 hours. Cell viability was determined by ATP release assay. (E) The gene knockdown of HMGCR in HBE1 cells mimicked the simvastatin-mediated protection against PLY. The supplement of 1 mM mevalonate did not restore the sensitivity of HMGCR–knocked down HBE1 cells to PLY cytotoxicity. (F) HBE1 cells were treated with 20 μM simvastatin with or without 1 mM mevalonate for 48 hours. (G and H) HBE1 cells (G) and NHBE cells (H) were treated with 1 μM simvastatin with or without 1 mM mevalonate for 24 hours. Total RNA was extracted, and quantitative RT-PCR was conducted to quantify the indicated gene expressions. Asterisks indicate significant statistical difference versus matching control or groups based on one-way ANOVA with Tukey’s (B–D, G and H) or Dunnet’s (E) post test. Error bars represent SEM of at least three experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Simvastatin at higher doses has been shown to alter cellular morphology (41, 42). Although the biological significance of simvastatin-induced cellular morphological changes varies depending on cell type, we took this as one measure to evaluate the cellular uptake of mevalonate in airway epithelial cells. Indeed, we observed a significant morphology change in HBE1 cells after 48 hours of exposure to 20 μM simvastatin, and this morphology change was remarkably restored to normal conditions by the 1-mM mevalonate supplement (Figure 4F). In addition, we performed qRT-PCR on a panel of genes induced by 1 μM of simvastatin in mevalonate and/or simvastatin-treated HBE1 and NHBE cells. Furthermore, we used a panel of simvastatin-induced genes as a benchmark to examine whether the mevalonate supplementation rescues some of the effects of 1 μM simvastatin in terms of the gene regulation changes seen in our cell culture model. We found that several genes involved in lipid biosynthesis, such as HMGCR, HMG-CoA synthase (HMGCS1), acyl-CoA synthetase (ACSS2), and mevalonate decarboxylase (MVD), were up-regulated by 1 μM of simvastatin in HBE1 and NHBE cells (Figures 4G and 4H), but these inductions were not reversed by 1 mM mevalonate supplementation (Figures 4G and 4H). However, we also found that some genes, such as LL-37, PIK3C2G, DUSP5, and DUSP10, induced by 1 μM of simvastatin were significantly attenuated with mevalonate supplementation in both cell types (Figures 4G and 4H). This evidence strongly suggests that our mevalonate metabolic rescue worked on reversing certain simvastatin-induced gene expression and alteration of cell morphology but not the simvastatin-conferred protection against PLY.
Simvastatin-Mediated Protection against Pneumolysin Is Protein Synthesis and Calcium Dependent in HBE1 Cells
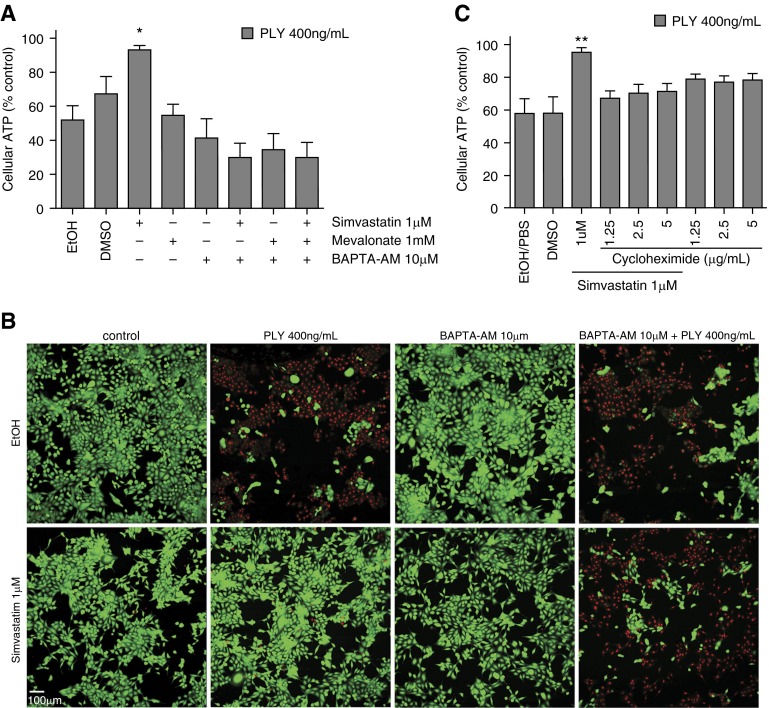
Calcium storage and influx fluctuations have been shown to be involved in statin-induced cyto-protection (43). Calcium has also been shown to play a role in repairing membrane damage caused by the cholesterol-dependent cytolysin streptolysin O (44). Here we applied [glycine, N,N′-1,2-ethanediylbis(oxy-2,1-phenylene)-bis-N-2-(acetyloxy)methoxy-2-oxoethyl]-[bis(acetyloxy)methyl ester] (BAPTA-AM), an intracellular calcium chelator, in the HBE1-PLY assays and found that 10 μM of BAPTA-AM restored cellular sensitivity to PLY in the presence of simvastatin (Figure 5A). We further found that BAPTA-AM cotreatment abrogated the simvastatin-induced reduction of potential pore formation (Figure 5B). In addition to testing the role of calcium, we cotreated HBE1 cells with simvastatin and cycloheximide (1.25–5 μg/ml) to block protein translation, followed by PLY challenge. Blocking protein translation reduced the protective effect of statins to PLY (Figure 5C). The above finding indicates that simvastatin-induced cellular protection against PLY in HBE1 cells requires calcium as well as de novo protein synthesis.
Figure 5.
Calcium and protein synthesis is required in simvastatin-conferred cellular resistance against pneumolysin. (A) HBE1 cells were treated with 1 μM simvastatin with or without various combinations of [glycine, N,N'-1,2-ethanediylbis(oxy-2,1-phenylene)-bis-N-2-(acetyloxy)methoxy-2-oxoethyl]-[bis(acetyloxy)methyl ester] (BAPTA-AM) and mevalonate for 24 hours and then challenged with 400 ng/ml PLY for 4 hours. Cellular ATP release assays were used to assess cell viability. Error bars represent SEM of three experiments. (B) HBE1 cells were pretreated with 1 μM simvastatin with or without 10 μM BAPTA-AM for 24 hours. After the medium was changed, cells were incubated with 400 ng/ml of PLY or mock control for 4 hours. Cells were stained with Live/Dead assay. Scale bar = 100 μm. (C) Protein translation is required for simvastatin-mediated protection. HBE1 cells were treated with 1 μM simvastatin with or without various doses of cycloheximide for 24 hours and then challenged with 400 ng/ml PLY for 4 hours. Cellular ATP release assays were used to assess cell viability. Error bars represent SEM of two experiments. *P < 0.05; **P < 0.01.
Squalene Synthase and GGTase Inhibition Conferred Resistance to Pneumolysin in HBE1 and NHBE Cells
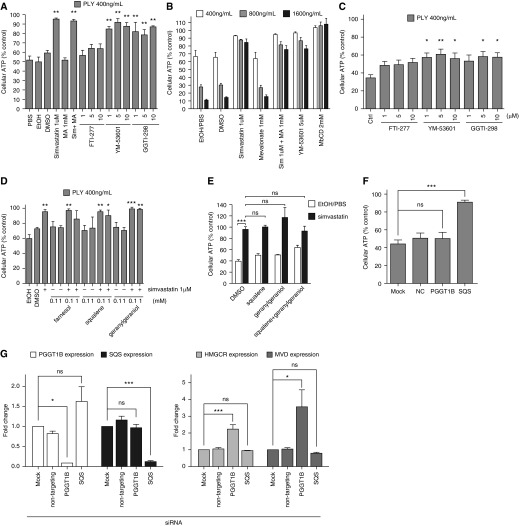
Because mevalonate supplementation does not rescue the simvastatin-mediated protection against PLY, we investigated whether other members of three canonical pathways downstream of mevalonate are involved in this protection. We found that YM-53601 (squalene synthase inhibitor) and GGTI-298 (geranylgeranyl transferase inhibitor) pretreatment mimicked the protection of HBE1 cells to a level similar to simvastatin treatment, whereas FTI-277 (farnesyl transferase inhibitor) had no significant effect (Figure 6A). We further tested YM-53601 with higher doses of PLY and found that pretreatment could still provide significant protection (Figure 6B). We similarly found that only YM-53601 and GGTI-298 could provide protection in NHBE cells (Figure 6C). We performed metabolic rescue assays with farnesol, squalene, and geranylgeraniol in HBE1 cells to examine these pathways. Surprisingly, we found that none of these supplementations could abrogate the simvastatin-mediated protection to PLY (Figure 6D). We then further conducted cosupplement experiments to examine the possibility that squalene and geranylgeraniol are unable to rescue simvastatin’s protective effect singly. The results have shown that the cotreatment of squalene and geranylgeraniol did not reverse simvastatin’s cellular protection effect to PLY in HBE1 cells (Figure 6E). To further determine whether simvastatin confers cellular protection via geranylgeranyl pyrophosphate and the squalene pathway, we knocked down two key enzymes of the HMGCR pathway, geranylgeranyl transferase (via protein geranylgeranyltransferase type I, beta subunit [PGGT1B]) and squalene synthase (SQS) via small interfering RNA (siRNA) transfection in HBE1 cells, and then challenged the cells with PLY. Our results demonstrate that the knockdown of SQS rendered the HBE1 cells more resistant to PLY cytotoxicity but that the knockdown of PGGT1B did not (Figure 6F). We confirmed siRNA knockdown efficiency of PGGT1B and SQS by qRT-PCR. However, knockdown of PGGT1B caused significant induction of HMGCR and MVD (Figure 6G), which suggests that selective compensatory gene induction caused by PGGT1B knockdown neutralizes the protection.
Figure 6.
YM-53601 and GGTI-298 treatment mimicked simvastatin-mediated protection against pneumolysin. (A and C) HBE1 cells (A) and NHBE cells (C) were treated with 1 μM simvastatin with or without 1 mM mevalonate or various doses of mevalonate downstream pharmacological inhibitors for 24 hours and then challenged with 400 ng/ml PLY for 4 hours. Asterisks in (A) indicate significant statistical difference versus matching control based on one-way ANOVA with Dunnet’s post test. (B) HBE1 cells were pretreated with 1 μM simvastatin with or without 1 mM mevalonate or with the indicated doses of YM-53601 or MβCD as a cholesterol-depleting positive control for 24 hours and then challenged with PLY at the indicated doses for 4 hours. Cellular ATP release assays were used to assess cell viability. All the treatments except MA alone have significant increases in cell viability compared with individual control treatment by two-way ANOVA and Bonferroni’s post test. (D) HBE1 cells were pretreated with simvastatin with or without farnesol, squalene, or geranylgeraniol for 24 hours and then challenged with PLY for 4 hours. (E) HBE1 cells were pretreated with simvastatin with or without 0.1 mM squalene and with or without 0.1 mM geranylgeraniol for 24 hours and then challenged with PLY for 4 hours. Cellular ATP release assays were used to assess cell viability. Asterisks indicate significant statistical difference versus matching groups based on one-way ANOVA with Tukey’s post test from four experiments. (F) The gene knockdown of squalene synthase in HBE1 cells mimicked the simvastatin-mediated protection against PLY. For D and F, asterisks indicate significant statistical difference versus matching control based on one-way ANOVA with Dunnet’s post test. (G) The efficacy and effect on HMGCR and MVD gene expression of PGGT1B and squalene synthase gene silencing in HBE1 cells were examined by quantitative RT-PCR. Asterisks indicate significant statistical difference versus matching groups based on one-way ANOVA with Tukey’s post test. Error bars represent SEM of three experiments in A, B, D, F, and G and two experiments in C. MVD, mevalonate decarboxylase; PGGT1B, protein geranylgeranyltransferase type I, beta subunit; SQS, squalene synthase. *P < 0.05; **P < 0.01; ***P < 0.001. NC, negative control; Sim, simvastatin.
Cholesterol Partially Rescued Simvastatin-Mediated Cellular Protection against PFT
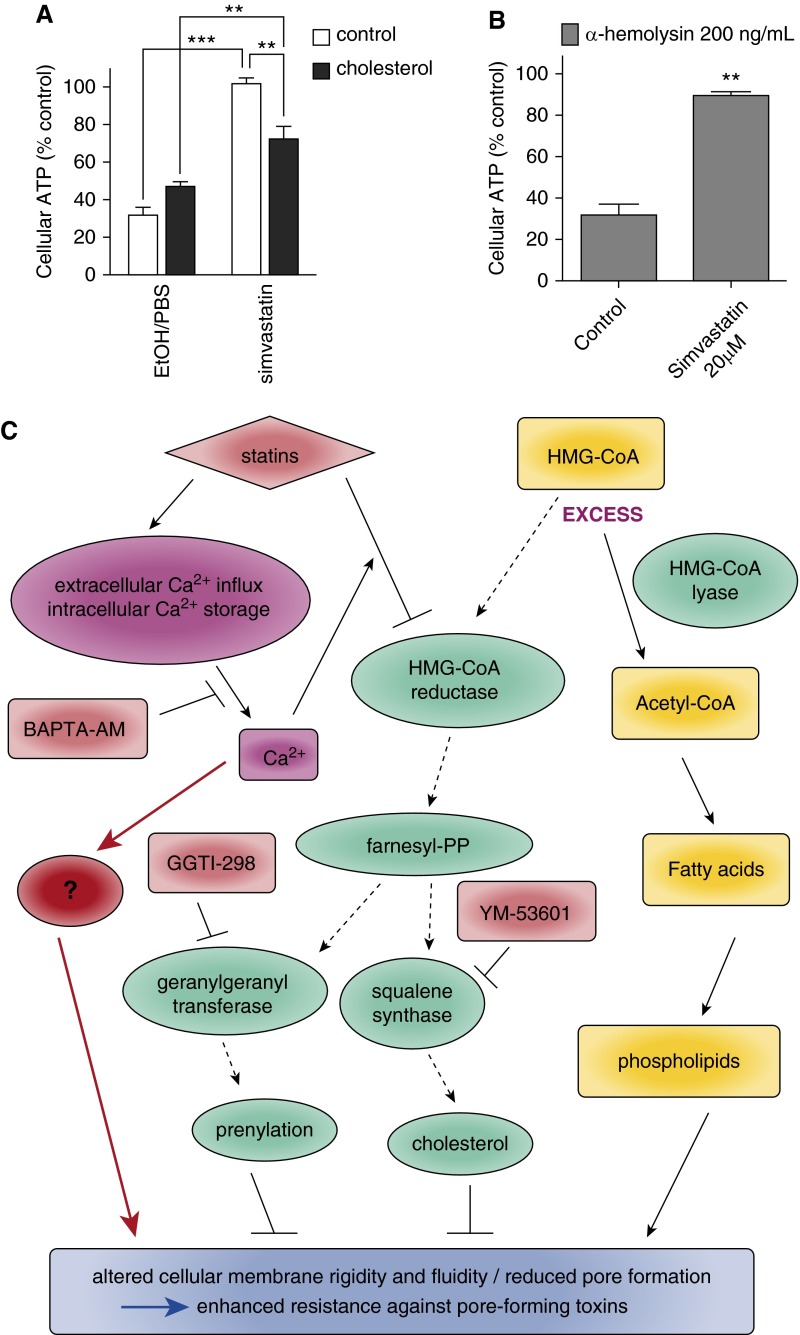
Because both pharmacological inhibition and siRNA knockdown of squalene synthase, an enzyme that controls the critical step in sterol synthesis, mimicked simvastatin protection, we investigated the role of cholesterol in this system. Cholesterol is usually present in supplemented serum of culture media but both our media used for HBE1 and NHBE cells are chemically defined and serum-free. Furthermore, we do not add cholesterol as a routine supplementation to these cultures and also confirmed that the basal cholesterol level in our medium is undetectable. We have shown above that pretreatment of methyl-β-cyclodextrin, a plasma membrane cholesterol-depleting drug, led to cellular protection (Figure 6B) due to a lack of PLY binding (Figure 3C). This led us to hypothesize that supplementing cholesterol may reduce simvastatin protection against PFT. To test this, HBE1 cells were treated with 100 nM simvastatin with or without 30 μg/ml cholesterol for 24 hours and then challenged with PLY. Indeed, supplemented cholesterol significantly reduced simvastatin protection (Figure 7A), although not to the level of the untreated controls. We then analyzed the cellular cholesterol level in HBE1 cells treated with simvastatin and cholesterol supplementation and found that the cellular cholesterol content in HBE1 cells supplemented with 30 μg/ml cholesterol for 24 hours did not increase beyond baseline (Figure E2). In addition, we found that the cholesterol supplementation normalized cellular cholesterol and significantly rescued simvastatin-mediated cellular cholesterol reduction (Figure E2). These results suggest that cholesterol might play certain roles in simvastatin-mediated protection to pneumolysin cytotoxicity. To further investigate the potential role of cellular cholesterol in the simvastatin-mediated protection in airway epithelial cells, we performed similar assays with human alveolar basal epithelial cells A549 and S. aureus α-hemolysin, which is typically not classified as a cholesterol-dependent cytolysin. Our results indicated that 20 μM simvastatin pretreatment significantly enhanced A549 cell viability, specifically against α-hemolysin (Figure 7B).
Figure 7.
Cholesterol is partially involved in simvastatin-mediated protection against pore-forming toxins (PFTs). (A) HBE1 cells were treated with 100 nM simvastatin with or without 30 μg/ml cholesterol for 24 hours and then washed. After 1 hour, cells were challenged with 400 ng/ml PLY for 3 to 4 hours. Asterisks indicate significant statistical difference between groups based on one-way ANOVA with Tukey’s post test. Error bars represent SEM of four experiments. (B) A549 cells were pretreated with 20 μM simvastatin for 24 hours and then challenged with 200 ng/ml α-hemolysin for 4 hours. Error bars represent SEM of three experiments. (C) The overview of our proposed pathway of how simvastatin acts via an alternative pathway (red) that bypasses the HMGCR–mevalonate pathway in addition to the HMGCR downstream pathway to confer PFT protection in airway epithelial cells. **P < 0.01; ***P < 0.001.
Discussion
Statins’ effects are multifaceted and include substantial pleiotropic effects. However, it was not clear if the known effects of reduced bacterial infection conferred by statins are also mediated via cellular protection against bacterial virulence factors in the context of epithelium. Our findings demonstrate that simvastatin can solely protect human airway epithelial cells against pneumolysin and α-hemolysin. Our results have shown it is plausible that the protection mechanism of simvastatin is not simply mediated by cholesterol reduction. Using PLY as an example, our data clearly suggest that the protective effects are not likely due to the blocking of PLY binding to the airway epithelial cells at the molecular level. Possibilities include, but are not limited to, reduced pore formation after toxin binding to membrane-associated cholesterol and enhanced cellular membrane repair or the intracellular sequestration of toxins. Interestingly, we observed a slight increase of PLY in the lysates from simvastatin-pretreated cells compared with control on immunoblots (Figures 3A and 3B). Our results suggest that PLY accumulates in a quarantined compartment inside the cells or might still bind to simvastatin-pretreated cells without forming proper pores. Our studies with immunofluorescence staining of PLY (Figure 3E) also suggest that the binding of PLY to the HBE1 plasma membranes is not blocked by simvastatin, again suggesting a potential pore-formation blockade mechanism by simvastatin.
Although simvastatin has pleiotropic effects independent of HMGCR suppression, many studies have shown enormous variety of in vitro effects of statins in a mevalonate-dependent manner. For example, it has been demonstrated that 10 to 40 μM of simvastatin exhibited a dose-dependent growth inhibition in A549 cells and that 500 μM mevalonate partially rescued this growth inhibitory effect (45). In another study, several doses of simvastatin (5, 10, 20, 50, and 100 μM) were used to examine the proliferative capacity of A549 cells after hypoxia-reoxygenation injury (46). The authors showed that the treatment with 20 μM simvastatin has the best cell proliferative effect and the best induction of surfactant protein-C–positive cells and surfactant protein-C levels in A549 cells. They further showed that these effects are mevalonate dependent by the cotreatment of 20 μM of mevalonate and 20 μM of simvastatin in A549 cells. We have also performed simvastatin treatment to examine its effect against PLY in A549. However, simvastatin did not protect A549 cells from PLY intoxication (Figure E3). This result suggests that simvastatin may affect different molecular mechanisms in A549 and HBE1 cells.
Recently, another study found that PLY-mediated microlesion formation within the myocardium during S. pneumoniae infection might contribute to adverse cardiac events seen in humans with invasive pneumococcal disease (47). They have previously reported that simvastatin protected against pneumococcal infections in a mouse sickle cell disease model and that this protection was mediated by reduced PLY-dependent cytotoxicity to endothelial cells (48). However, their minimum simvastatin protective concentration reported is 1 μM, and the protection is mevalonate dependent. This discrepancy may have arisen from the different mechanisms involved in different cell types.
Although the concentrations of simvastatin used in most of these in vitro mechanistic studies are in 10 to 100 micromolar levels, the physiological serum concentrations of simvastatin are usually detected in the nanomolar range (49–51). This argument makes it debatable whether the effects of simvastatin at real physiological serum nanomolar concentrations are mevalonate dependent. Although we have shown that the gene suppression of HMG-CoA reductase via siRNA in HBE1 cells mimics simvastatin-mediated protection against PLY, surprisingly we found that the addition of high excess doses of mevalonate did not reverse the enhanced PLY resistance. In addition, although YM-53601 and GGTI-298 mimic simvastatin-mediated cellular protection, the supplementation of geranylgeraniol or squalene could not reverse the protection. This is of great interest because it has never been reported. The simplest explanation is that airway epithelial cells do not uptake these supplementations effectively. This explanation seems less likely because mevalonate did completely reverse some simvastatin-induced genes in these airway epithelial cells (Figures 4G and 4H). Another possibility is that supplementation with mevalonate, geranylgeraniol, or squalene did not get into the precise airway epithelial cell compartment with the optimal kinetics or concentrations to reverse the effect of simvastatin on HMG-CoA reductase. All these possibilities of cellular uptake compartmentation or kinetics of mevalonate, geranylgeraniol, or squalene will take a much more extensive investigation that is outside of the scope of this study. One more intriguing interpretation is that there is a potential alternative pathway that bypasses the mevalonate pathway and that this mevalonate-independent mechanism confers PLY protection in addition to the HMGCR-mevalonate–dependent pathway. The release of inhibition of the latter pathway, through the supplementation of exogenous mevalonate or related sterols, does not restore PFT sensitivity because the mevalonate-independent pathway is still intact. To date, our work is one of the few studies using simvastatin concentrations at 0.01 to 1 μM; at these concentrations, we could still observe the beneficial effect in airway epithelial cells. It is plausible that simvastatin at 0.01 to 1 μM levels works through different mechanisms compared with simvastatin at higher concentrations (5–40 μM) in a cell type–dependent manner. The finding from Medina and colleagues showed strong support of this possibility (52). They observed a biphasic dose-related response in retinal microvascular endothelial cells treated with 0.01 to 10 μM simvastatin. Simvastatin at 0.1 μM could enhance microvascular repair, and 0.01 μM simvastatin could promote migration, sprouting, and tubulogenesis in a mevalonate-independent manner. However, they also found that 10 μM simvastatin had the opposite effect, yet in a mevalonate-dependent fashion. We propose a model of how simvastatin might protect airway epithelial cells against PFTs, as illustrated in Figure 7C.
The gene induction of HMGCR and HMGCS1 by simvastatin is potentially due to a feedback regulation, but the biological significance of other gene induction has merited further investigation. We found that 1 μM simvastatin induced LL-37 expression in a mevalonate-dependent manner in NHBE and HBE1 cells. It has been shown (53) that a range of antimicrobial peptides, including LL-37, could kill various S. pneumoniae isolates. Because the simvastatin-induced LL-37 (and maybe other antimicrobial peptides) is not likely associated with the simvastatin-conferred pneumolysin resistance, it is possible that simvastatin’s confounding effects on pneumolysin resistance and antimicrobial peptide induction could both contribute to the overall respiratory epithelial cell susceptibility to S. pneumoniae infection. The concentrations of pneumolysin used here could be roughly estimated to 2 to 5 × 107 CFU/ml of S. pneumoniae, based on a relationship between the amount of pneumolysin protein and the bacterial number (54). We think it will be valuable to know if statin administration can protect the host from respiratory S. pneumoniae infection, but it would be more informative to investigate simvastatin’s effects on pneumolysin intoxication and S. pneumoniae infection individually.
There are a handful of intrinsic cellular pathways, including the mitogen-activated protein kinase (MAPK), unfolded protein response (UPR), autophagy, and calcium-dependent vesicle trafficking pathways, that have been shown to protect cells from PFTs in various organisms or cell types (55–61), although the molecular mechanisms of how these cellular pathways confer cellular protection remain largely unclear. It has been recently shown that statins may trigger bifurcated mevalonate–independent pathways partially via a Ca2+ increase and p38 MAPK activation, which induces UPR and cytoprotection in RAW264.7 cells (43). It is plausible that calcium-mediated MAPK/UPR activation is enhanced by statins as a mevalonate-independent upstream mechanism for PFT protection because BAPTA-AM, an intracellular calcium chelator, blocked statin-mediated protection. These potential molecular mechanisms could be further elucidated in the future.
Although our NHBE experiments were performed in submerged conditions, it would be valuable to investigate statin protection in air–liquid interfaced culture of differentiated airway epithelial cells. S. pneumoniae commonly resides asymptomatically in the respiratory tract of healthy carriers but only becomes pathogenic in susceptible individuals with a loss in barrier integrity. We suspect that, at this high cell density and tight, polarized, three-dimensional culture, it would take a large amount of pneumolysin to penetrate this barrier and attack the cells. Because the main purpose of this study was to show a proof of concept via one mechanism that illustrated the known statin-mediated benefits regarding pneumonia, we thought it was not technically practical to examine the protection effect in air–liquid interfaced culture of differentiated airway epithelial cells.
In short, we used a top-down approach, starting with the known metabolic pathways targeted by simvastatin, the signaling transduction pathways involved, and the final events that render the cells resistant to specify the mechanism(s) involved in statin-induced protection of airway epithelial cells against PFT cytotoxicity. Our molecular findings on this protective effect against PFTs may function synergistically with enhanced formation of extracellular traps, induced by statins in phagocytic cells, to serve as one basis for explaining the clinical data that point to a lower risk of severe bacterial infection and sepsis in patients receiving statin therapy and the beneficial effect of statin against pneumonia-related mortality. Besides providing mechanistic insight, the future in vivo and translational studies in the context of our novel molecular findings could be potentially developed into a host-centric strategy for the adjuvant treatment of PFT-associated bacterial infections, including pneumonia.
Acknowledgments
Acknowledgments
The authors thank Ferdinand C. O. Los in Columbia University for critically editing the manuscript and providing comments.
Footnotes
This work was supported by National Institutes of Health grants R01-HL-077902 (S.S., L.-Y.H., C.-Y.C., R.W., C.-Y.K.), T32-HL-007013 (S.S., R.W., C.-Y.K.), R01-DC-005843 (J.-D.L.), and R01-DC-004562 (J.-D.L.); by NRF in Korea grant NRF-2012R1A1A104154 (J.H.L.); by NHRI grant IM-103-PP-05 (J.-W.R., C.-T.H., C.-Y.K.); and by NSC grant 102–2320-B-400–008- (J.-W.R., C.-T.H., C.-Y.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions: S.S., J.-W.R., R.W., and C.-Y.K. designed research and wrote the paper. S.S., J.-W.R., L.-Y.H., C.-Y.C., C.-T.H., and C.-Y.K. performed experiments and analyzed data. J.H.L. and J.-D.L. provided pneumolysin construct and suggestions for experiments.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0391OC on April 15, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Denny FW, Loda FA. Acute respiratory infections are the leading cause of death in children in developing countries. Am J Trop Med Hyg. 1986;35:1–2. doi: 10.4269/ajtmh.1986.35.1. [DOI] [PubMed] [Google Scholar]
- 2.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 3.Irfan M, Farooqi J, Hasan R. Community-acquired pneumonia. Curr Opin Pulm Med. 2013;19:198–208. doi: 10.1097/MCP.0b013e32835f1d12. [DOI] [PubMed] [Google Scholar]
- 4.Low DE, Pichichero ME, Schaad UB. Optimizing antibacterial therapy for community-acquired respiratory tract infections in children in an era of bacterial resistance. Clin Pediatr (Phila) 2004;43:135–151. doi: 10.1177/000992280404300203. [DOI] [PubMed] [Google Scholar]
- 5.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (usage): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6:208–215. doi: 10.1016/j.jacl.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 9.Sparrow CP, Burton CA, Hernandez M, Mundt S, Hassing H, Patel S, Rosa R, Hermanowski-Vosatka A, Wang PR, Zhang D, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21:115–121. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 10.Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 11.McCarey DW, McInnes IB, Madhok R, Hampson R, Scherbakov O, Ford I, Capell HA, Sattar N. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–2021. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruefer D, Makowski J, Schnell M, Buerke U, Dahm M, Oelert H, Sibelius U, Grandel U, Grimminger F, Seeger W, et al. Simvastatin inhibits inflammatory properties of Staphylococcus aureus alpha-toxin. Circulation. 2002;106:2104–2110. doi: 10.1161/01.cir.0000034048.38910.91. [DOI] [PubMed] [Google Scholar]
- 14.Burns EM, Smelser LK, Then JE, Stankiewicz TE, Kushdilian M, McDowell SA, Bruns HA. Short term statin treatment improves survival and differentially regulates macrophage-mediated responses to Staphylococcus aureus. Curr Pharm Biotechnol. 2013;14:233–241. doi: 10.2174/138920113805219395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8:445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parihar SP, Guler R, Lang DM, Suzuki H, Marais AD, Brombacher F. Simvastatin enhances protection against Listeria monocytogenes infection in mice by counteracting Listeria-induced phagosomal escape. PLoS One. 2013;8:e75490. doi: 10.1371/journal.pone.0075490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu JH, Kim CH, Yoon JH. Innate immune responses of the airway epithelium. Mol Cells. 2010;30:173–183. doi: 10.1007/s10059-010-0146-4. [DOI] [PubMed] [Google Scholar]
- 18.Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alouf JE. Pore-forming bacterial protein toxins: an overview. Curr Top Microbiol Immunol. 2001;257:1–14. [PubMed] [Google Scholar]
- 20.Los FC, Randis TM, Aroian RV, Ratner AJ. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev. 2013;77:173–207. doi: 10.1128/MMBR.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benazet JD, Bischofberger M, Tiecke E, Goncalves A, Martin JF, Zuniga A, Naef F, Zeller R. A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science. 2009;323:1050–1053. doi: 10.1126/science.1168755. [DOI] [PubMed] [Google Scholar]
- 22.Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Berry AM, Yother J, Briles DE, Hansman D, Paton JC. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubins JB, Charboneau D, Paton JC, Mitchell TJ, Andrew PW, Janoff EN. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J Clin Invest. 1995;95:142–150. doi: 10.1172/JCI117631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med. 2008;8:497–509. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 26.Cockeran R, Anderson R, Feldman C. Pneumolysin as a vaccine and drug target in the prevention and treatment of invasive pneumococcal disease. Arch Immunol Ther Exp (Warsz) 2005;53:189–198. [PubMed] [Google Scholar]
- 27.Witzenrath M, Gutbier B, Hocke AC, Schmeck B, Hippenstiel S, Berger K, Mitchell TJ, de los Toyos JR, Rosseau S, Suttorp N, et al. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit Care Med. 2006;34:1947–1954. doi: 10.1097/01.CCM.0000220496.48295.A9. [DOI] [PubMed] [Google Scholar]
- 28.Lim JH, Stirling B, Derry J, Koga T, Jono H, Woo C-H, Xu H, Bourne P, Ha U-H, Ishinaga H, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007;27:349–360. doi: 10.1016/j.immuni.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Lucas R, Yang G, Gorshkov BA, Zemskov EA, Sridhar S, Umapathy NS, Jezierska-Drutel A, Alieva IB, Leustik M, Hossain H, et al. Protein kinase C-α and arginase I mediate pneumolysin-induced pulmonary endothelial hyperpermeability. Am J Respir Cell Mol Biol. 2012;47:445–453. doi: 10.1165/rcmb.2011-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bubeck Wardenburg J, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: α-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 31.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thai P, Statt S, Chen CH, Liang E, Campbell C, Wu R. Characterization of a novel long noncoding RNA, SCAL1, induced by cigarette smoke and elevated in lung cancer cell lines. Am J Respir Cell Mol Biol. 2013;49:204–211. doi: 10.1165/rcmb.2013-0159RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol. 2013;49:341–347. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kao C-Y, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κB signaling pathways. J Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 37.Kao C-Y, Kim C, Huang F, Wu R. Requirements for two proximal NF-κB binding sites and IκB-ζ in IL-17A-induced human β-defensin 2 expression by conducting airway epithelium. J Biol Chem. 2008;283:15309–15318. doi: 10.1074/jbc.M708289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering: are they clinically relevant? Eur Heart J. 2003;24:225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 39.Castanho MA, Coutinho A, Prieto MJ. Absorption and fluorescence spectra of polyene antibiotics in the presence of cholesterol. J Biol Chem. 1992;267:204–209. [PubMed] [Google Scholar]
- 40.Korchev YE, Bashford CL, Pederzolli C, Pasternak CA, Morgan PJ, Andrew PW, Mitchell TJ. A conserved tryptophan in pneumolysin is a determinant of the characteristics of channels formed by pneumolysin in cells and planar lipid bilayers. Biochem J. 1998;329:571–577. doi: 10.1042/bj3290571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park DS, So HS, Lee JH, Park HY, Lee YJ, Cho JH, Yoon KH, Park C, Yun K, Park R. Simvastatin treatment induces morphology alterations and apoptosis in murine cochlear neuronal cells. Acta Otolaryngol. 2009;129:166–174. doi: 10.1080/00016480802163358. [DOI] [PubMed] [Google Scholar]
- 42.Menter DG, Ramsauer VP, Harirforoosh S, Chakraborty K, Yang P, Hsi L, Newman RA, Krishnan K. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PLoS One. 2011;6:e28813. doi: 10.1371/journal.pone.0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J-C, Wu M-L, Huang K-C, Lin W-W. HMG-CoA reductase inhibitors activate the unfolded protein response and induce cytoprotective GRP78 expression. Cardiovasc Res. 2008;80:138–150. doi: 10.1093/cvr/cvn160. [DOI] [PubMed] [Google Scholar]
- 44.Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol. 2008;180:905–914. doi: 10.1083/jcb.200708010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodenak Kladniew B, Polo M, Montero Villegas S, Galle M, Crespo R, Garcia de Bravo M. Synergistic antiproliferative and anticholesterogenic effects of linalool, 1,8-cineole, and simvastatin on human cell lines. Chem Biol Interact. 2014;214:57–68. doi: 10.1016/j.cbi.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Lv J, Feng D, Jiang F, Fan X, Zhang Z, Yin R, Xu L. Restoration of alveolar type II cell function contributes to simvastatin-induced attenuation of lung ischemia-reperfusion injury. Int J Mol Med. 2012;30:1294–1306. doi: 10.3892/ijmm.2012.1161. [DOI] [PubMed] [Google Scholar]
- 47.Brown AO, Mann B, Gao G, Hankins JS, Humann J, Giardina J, Faverio P, Restrepo MI, Halade GV, Mortensen EM, et al. Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog. 2014;10:e1004383. doi: 10.1371/journal.ppat.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosch JW, Boyd AR, Hinojosa E, Pestina T, Hu Y, Persons DA, Orihuela CJ, Tuomanen EI. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest. 2010;120:627–635. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Backman JT, Kyrklund C, Kivisto KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68:122–129. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- 50.Ucar M, Neuvonen M, Luurila H, Dahlqvist R, Neuvonen PJ, Mjorndal T. Carbamazepine markedly reduces serum concentrations of simvastatin and simvastatin acid. Eur J Clin Pharmacol. 2004;59:879–882. doi: 10.1007/s00228-003-0700-5. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Ahn BJ, Chae HS, Han S, Doh K, Choi J, Jun YK, Lee YW, Yim DS. A population pharmacokinetic-pharmacodynamic model for simvastatin that predicts low-density lipoprotein-cholesterol reduction in patients with primary hyperlipidaemia. Basic Clin Pharmacol Toxicol. 2011;109:156–163. doi: 10.1111/j.1742-7843.2011.00700.x. [DOI] [PubMed] [Google Scholar]
- 52.Medina RJ, O'Neill CL, Devine AB, Gardiner TA, Stitt AW. The pleiotropic effects of simvastatin on retinal microvascular endothelium has important implications for ischaemic retinopathies. PLoS One. 2008;3:e2584. doi: 10.1371/journal.pone.0002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Habets MG, Rozen DE, Brockhurst MA. Variation in Streptococcus pneumoniae susceptibility to human antimicrobial peptides may mediate intraspecific competition. Proc Biol Sci. 1743;2012:3803–3811. doi: 10.1098/rspb.2012.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houldsworth S, Andrew PW, Mitchell TJ. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, Aroian RV. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc Natl Acad Sci USA. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bischof LJ, Kao C-Y, Los FCO, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, Aroian RV. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 2008;4:e1000176. doi: 10.1371/journal.ppat.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellier A, Chen C-S, Kao C-Y, Cinar HN, Aroian RV. Hypoxia and the hypoxic response pathway protect against pore-forming toxins in C. elegans. PLoS Pathog. 2009;5:e1000689. doi: 10.1371/journal.ppat.1000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kao C-Y, Los FCO, Huffman DL, Wachi S, Kloft N, Husmann M, Karabrahimi V, Schwartz J-L, Bellier A, Ha C, et al. Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 2011;7:e1001314. doi: 10.1371/journal.ppat.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Los FCO, Kao C-Y, Smitham J, Mcdonald KL, Ha C, Peixoto CA, Aroian RV. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe. 2011;9:147–157. doi: 10.1016/j.chom.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kloft N, Neukirch C, Bobkiewicz W, Veerachato G, Busch T, von Hoven G, Boller K, Husmann M. Pro-autophagic signal induction by bacterial pore-forming toxins. Med Microbiol Immunol (Berl) 2010;199:299–309. doi: 10.1007/s00430-010-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Husmann M, Dersch K, Bobkiewicz W, Beckmann E, Veerachato G, Bhakdi S. Differential role of p38 mitogen activated protein kinase for cellular recovery from attack by pore-forming S. aureus alpha-toxin or streptolysin O. Biochem Biophys Res Commun. 2006;344:1128–1134. doi: 10.1016/j.bbrc.2006.03.241. [DOI] [PubMed] [Google Scholar]